Abstract
Senior-Løken syndrome (SLS) is an autosomal recessive disease characterized by development of a retinitis pigmentosa (RP)- or Leber congenital amaurosis (LCA)-like retinal dystrophy and a medullary cystic kidney disease, nephronophthisis. Mutations in several genes (called nephrocystins) have been shown to cause SLS. The proteins encoded by these genes are localized in the connecting cilium of photoreceptor cells and in the primary cilium of kidney cells. Nephrocystins are thought to have a role in regulating transport of proteins bound to the outer segment/primary cilium; however, the precise molecular mechanisms are largely undetermined. This review will survey the biochemistry, cell biology and existing animal models for each of the nephrocystins as it relates to photoreceptor biology and pathogenesis of retinal degeneration.
Keywords: Senior-Løken syndrome, nephronophthisis, nephrocystins, retinal degeneration, ciliopathy, primary cilium
1. Introduction
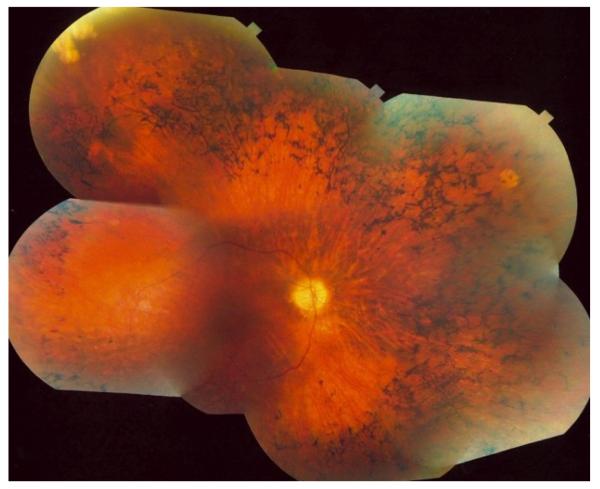
Inherited retinal diseases are estimated to affect up to 200,000 people in the United States (http://www.mdsupport.org/library/numbers.html). A vast majority of these cases are retinitis pigmentosa (RP), which accounts for 40% or 80,000 people. RP is a disease where the photoreceptors, the light-sensing cells of the eye, die (Hartong et al., 2006). RP clinically presents with nyctalopia (night blindness) and progresses to significant peripheral visual field reduction (tunnel vision). Central visual acuity and cone function are often preserved until late in the course of the disease. Patients with end stage disease will have no light perception at all. Other clinical findings on a retinal exam include bone spicule formation (retinal pigmentation), peripheral retinal atrophy, waxy pallor of the optic disk and optic nerve head drusen and vascular attenuation (Fig. 1). Diagnosis is done using a detailed clinical and family history, a dilated retinal examination and photography, visual field testing, electroretinography, optical coherence tomography and genetic testing. Currently, there is no therapy that stops progression or restores vision in patients with RP, and therefore, prognosis is poor.
Fig. 1.
Fundus photograph of a patient diagnosed with Senior-Løken syndrome. Shown are classic findings in RP: bone-spicule shaped deposits, attenuation of blood vessels with macular sparing.
RP may associate with development of pathology in other organs (Sattar and Gleeson, 2011). Examples of syndromic RP include Bardet-Biedl syndrome (BBS), Joubert syndrome JBTS), Meckel syndrome (MKS), Usher syndrome (USH) and Senior-Løken syndrome (SLS) (Table 1). Bardet-Biedl syndrome is characterized by RP, obesity, polydactyly, cognitive impairment, male infertility, and renal abnormalities, among other pathologies (Zaghloul and Katsanis, 2009). Joubert syndrome presents with hypotonia and ataxia early in life. Other characteristic features are breathing problems, abnormal eye movements, and mental retardation. Joubert syndrome is sometimes associated with RP, kidney, and liver disease, and polydactyly (Juric-Sekhar et al., 2012; Doherty, 2009) (Table 1). Usher syndrome affects mostly hair cells in the ear and photoreceptors in the retina causing hearing loss and RP (Kremer et al., 2006).
Table 1.
Clinical syndromes associated with development of retinitis pigmentosa. Column 1 shows various clinical syndromes. Column 2 shows mutations in different NPHP genes (with the exception in Usher syndrome - not associated with development of nephronophthisis). Column 3 shows other pathologies in different organs. Links to the sources are shown in blue in column 3.
| Syndrome | Gene Mutations | Other pathologies besides RP |
|---|---|---|
| Bardet Biedl | CEP290 (NPHP6), SDCAAG8 (NPHP10) |
Truncal obesity, postaxial polydactyly, cognitive impairment, male hypogonadotropic hypogonadism, complex female genitourinary malformations, and renal abnormalities http://omim.org/entry/209900 |
| COACH | NEK8 (NPHP9), TMEM67 (NPHP11) |
Cerebellar vermis hypoplasia/aplasia, Oligophrenia, Ataxia, Coloboma, and Hepatic fibrosis http://omim.org/entry/216360 |
| Jeune | TTC21B (NPHP12), WDR19 (NPHP13) |
Severely constricted thoracic cage (thoracic hypoplasia), short- limbed (brachydactyly), short stature, and polydactyly. It often leads to death in infancy due to respiratory insufficiency (chronic nephritis) http://omim.org/entry/612014 http://omim.org/entry/608151 |
| Joubert | NPHP1, CEP290 (NPHP6), RPGRIP1L (NPHP8), NEK8 (NPHP9), TMEM67 (NPHP11), TTC21B (NPHP12) |
Abnormally rapid breathing (hyperpnea), decreased muscle tone (hypotonia), jerky eye movements (oculomotor apraxia), mental retardation, inability to coordinate voluntary muscle movements (ataxia). http://omim.org/entry/213300 |
| Meckel | NPHP3, CEP290 (NPHP6), RPGRIP1L (NPHP8), NEK8 (NPHP9), TMEM67 (NPHP11) |
Encephalocele, hepatic ductal dysplasia and cysts, and polydactyly http://omim.org/entry/249000 |
| Renal-Hepatic- Pancreatic dysplasia (RHPD) |
NPHP3 | Pancreatic fibrosis, renal dysplasia, hepatic dysgenesis. http://omim.org/entry/208540 |
| Senior-L0ken | NPHP1, INVS (NPHP2), NPHP3, NPHP4, IQCB1 (NPHP5), CEP290 (NPHP6), SDCAAG8 (NPHP10) |
Nephronophthisis and Leber congenital amaurosis (LCA) http://www.omim.org/entry/266900 |
| Sensenbrenner | WDR19 (NPHP13) | Microcephaly, narrow thorax, heart defects, liver fibrosis, hypoplasia of the corpus callosum, skeletal abnormalities, including craniosynostosis, narrow rib cage, short limbs, and brachydactyly, and ectodermal defects. http://omim.org/entry/614378 |
| Usher | MYO7A, USH1C, CDH23, PCDH15, SANS, USH2A, VLGR1, WHRN, USH3A |
Hearing loss and severe balance problems http://omim.org/entry/276900 |
Patients with Senior-Løken syndrome develop RP and a kidney pathology called nephronophthisis. Nephronophthisis (NPHP) is an autosomal recessive cystic kidney disease and is the most frequent genetic cause of end-stage renal disease (ESRD) in children and adolescents. A recent review on nephronophthisis can be found in Wolf and Hildebrandt, 2011. The median age for ESRD is around 13 years old. Children clinically present with polyuria, nocturia or secondary enuresis. Renal ultrasound scans may initially show normally sized kidneys with increased echogenicity, poor corticomedullary differentiation, and corticomedullary cysts and can progress to atrophic kidneys with more prominent cysts. A kidney biopsy may show the triad of corticomedullary cysts, tubular basement membrane disruption and interstitial fibrosis. NPHP is diagnosed using renal biopsy or by mutational analysis. So far, mutations in 13 genes (NPHP1-13) are known to cause nephronophthisis, and nine mutant genes are associated with development of retinal degeneration (Table 2). However, most of the gene mutations are also associated with development of pathology in other organs besides the kidneys and the retina (Table 1 and Table 2). A majority of the nephrocystins associated with development of retinal degeneration or Senior-Løken syndrome are now known to be expressed in the photoreceptor connecting cilium, a structural counterpart of the primary cilium found in most cell types.
Table 2. Human and mouse NPHP genes associated with various syndromes or retinal degeneration (RD).
Column 1, human gene symbols and most common aliases. Column 2, mouse genes. Column 3, human and mouse gene loci. Column 4, approximate frequency of patients affected by RD. Column 4, naturally occurring or laboratory generated mouse models. Column 5, expression of mouse genes in photoreceptors and phenotype of mutants. CC, connecting cilium; BB, basal bodies. Column 6, associated human diseases. Abbreviations of human pathologies also serve as aliases.
| Human Gene (aliases) | Mouse genes (aliases) |
Locus Human/mouse | RD | Mouse model |
Photoreceptor expression/phenotype |
Associated Human Diseases |
|---|---|---|---|---|---|---|
|
NPHP1 (Nephrocystin 1) |
Nphp1 (Nph1) |
2q13 / 2 F3 | <10% | Nphpf −/− | CC and BB / RD | Joubert syndrome (JBTS4) Nephronophthisis, juvenile Senior-Løken syndrome (SLSN1) |
|
INVS (Inversion of embryo turning protein) (NPHP2) |
invs (Nphp2) |
9q22-q31 / 4 B | 10% | inv/inv | CC / unknown | Nephronophthisis, infantile Senior-Løken syndrome (SLSN2) |
| NPHP3 |
Nphp3 (Pcy) |
3q22 / 9 | <20% | pcy | CC / unknown | Nephronophthisis, adolescent Senior-Løken syndrome (SLSN3)Meckel Syndrome (MKS7) Renal-hepatic-pancreatic dysplasia |
| Nphp3 −/− | (embryonic lethal) | |||||
|
NPHP4 (POC10) |
Nphp4 | 1p36/4 | <40% | Nphp4−/− | BB / RD | Nephronophthisis Senior-Løken syndrome (SLSN4) Cogan Syndrome |
|
IQCB1 (IQ motif containing B1) (NPHP5) |
Iqcb1 | 3q21.1 / 16 B3 | 100% | None | CC and OS / RD | Senior-Løken syndrome (SLSN5)Leber congenital amaurosis (LCA10) |
|
CEP290 (centrosomal protein 290) (NPHP6) |
Cep290 Nphp6, rd16 |
12q21.32 / 10 D1 | 100% | rd16 | CC and BB / RD | Nephronophthisis Meckel Syndrome (MKS4) Senior-Løken syndrome (SLSN6)Leber congenital Amaurosis (LCA 10) Bardet Biedl Syndrome (BBS14) Joubert syndrome (JBTS5) |
|
GLIS2 ( GLIS family zinc finger 2) (NPHP7) |
Glis2 (Nphp7) |
16p13.3 / 16 | 0% | Nphp7 −/− | unknown | Nephronophthisis |
|
RPGRIP1L (RP GTPase regulator- interacting 1 like protein) (NPHP8) |
Rpgrip1l (Nphp8, ftm) |
16q12.2 / 8 | <10% | ftm | CC / none | Nephronophthisis Meckel syndrome (MKS5) Joubert syndrome (JBTS7) |
| Rpgrip1l −/− | (embryonic lethal) | |||||
|
NEK8 (NIMA (never in mitosis gene a)- related kinase 8)(NPHP9) |
Nek8 (Nphp9; jck) |
17q11.1 / 11B5 | 0 | jck | CC / none | Nephronophthisis COACH syndrome Meckel Syndrome Joubert Syndrome |
|
SDCAAG8 (serologically defined colon cancer antigen 8) (NPHP10) |
Sdcaag8 Nphp10 |
1q43/1H3 | 100% | No | BB / unknown | Nephronophthisis Senior-Løken syndrome (SLSN7) Bardet Biedl Syndrome (BBS16) |
|
TMEM67 (NPHP11) (Meckelin) |
Tmem67 (Bpck) |
8q21.1 / 4 | 0 | Bpck | unknown / RD | Meckel syndrome (MKS3) Joubert syndrome (JBTS6) Nephronophthisis COACH Syndrome |
| wpk (rat) | unknown / RD | |||||
|
TTC21B (Tetratricopeptide repeat domain 21B) (NPHP12) |
Ttc21b (aln) |
2q24.3 / 2 | 0 | aln | (embryonic lethal)* | Nephronophthisis Jeune Syndrome (ATD4) Joubert Syndrome (JBTS11) |
|
WDR19 ( WD repeat domain 19) (NPHP13) (IFT144) |
Wdr19 | 4p14-p11 / 5 | <50% | No | unknown | Jeune syndrome (ATD5) Sensenbrenner syndrome Nephronophthisis |
The aln mouse is lethal at E18.5 so retinal and renal phenotype cannot be determined; there is a knockdown model in the rat that has retinal degeneration
2. The Photoreceptor Connecting Cilium
Cilia are composed of nine microtubule doublets (called the axoneme) in a cylindrical arrangement that emanate from basal bodies, the microtubule organizing centers of the cell. There are two types of cilia: motile cilia that are necessary for locomotion and fluid movement, and non-motile (primary) cilia that serve mainly sensory functions. Motile cilia have an additional microtubule doublet found in the center of the axonemal cylinder giving it a characteristic 9+2 pattern unlike the 9+0 pattern of doublets found in primary cilia. Nodal cilia (a type of motile cilia) that are found on cells of the embryonic node were initially thought to have 9+0 arrangement but some have recently been found to be 9+2 (Caspary et al., 2007; Novarino et al., 2011; Okada and Hirokawa, 2009). In addition to the central pair of doublets, motile cilia also have inner and outer dynein arms on the outer doublets that are required for generation of force necessary for motility. The axonemal structure is anchored at the base by the basal body or mother centriole.
The connecting cilium (CC) of vertebrate photoreceptors is a structural homolog of the primary cilium containing the 9+0 pattern of microtubule doublets. The CC is 0.5-1.2μm in length and approximately 0.2μm in diameter, connects the biosynthetic inner segment (IS) to the sensory outer segment (OS). The entire structure consisting of CC and OS is also called the “photosensitive cilium”. It is now recognized that mutations in numerous genes, which are expressed in the photoreceptor connecting cilium cause a group of heterogeneous diseases called ciliopathies. Because of the ubiquitous expression of these genes in primary cilia, ciliopathies often affect multiple organs including kidney, retina, brain, or spermatozoa. The most prominent syndromic diseases that affect the retina are Joubert Syndrome, Bardet-Biedl syndrome, and Senior Løken syndrome (Table 1).
Senior-Løken syndrome and the mutations in the genes that are associated to the development of this disease will be important to study to understand the basic mechanisms of protein transport through the connecting cilium. Therefore, it is important to understand the normal function of each of the nephrocystins to understand pathogenesis of retinal degeneration in these patients.
3. Genes Associated with Development of Senior-Løken Syndrome
By positional cloning, several mutations in NPHP genes have been found to cause nephronophthisis (Hildebrandt et al., 2009). The mutations which cause disease are inherited in an autosomal recessive pattern. Transmission can be monogenic (i.e. homozygous and compound heterozygous mutations) or digenic (i.e., heterozygous mutations in two different genes). These genes include NPHP1, Inversin/NPHP2, NPHP3, NPHP4, IQCB1/NPHP5, CEP290/NPHP6, GLIS2/NPHP7, RPGRIP1L/NPHP8, NEK8/NPHP9, SDCAAG8/NPHP10, TMEM67/NPHP11, TTC21B/NPHP12, and WDR19/NPHP13. The frequency of RP with nephronophthisis (Senior-Løken syndrome) depends on which nephrocystin gene is mutated. The frequency of retinal dystrophy in NPHP1-4 is less than 40% and reaches 100% in NPHP5 and NPHP6 mutations (Table 2). It should be noted that mutations in each of the nephrocystin genes may give rise to heterogeneous phenotypes and even different syndromes. Abnormalities in multiple organs may develop including the retina, brain, liver and heart (Table 2). For example, NPHP6/CEP290 mutations may be associated with isolated Leber congenital amaurosis (LCA), Senior-Løken syndrome (SLS) or the lethal multi-systemic Meckel Gruber syndrome (MKS) (Table 1). The presence of modifier alleles (quantitative trait loci, QTL) may further contribute to the complexity of the ciliopathies. In contrast, mutations in NPHP5 are only associated with development of the retinal-renal disease phenotype making this gene the classic Senior-Løken gene (Wolf and Hildebrandt, 2011).
3.1. NPHP1
Nephronophthisis, first described by Fanconi (FANCONI et al., 1951) in1951, is caused by mutations in 13 distinct and unrelated genes (Table 2) (Wolf and Hildebrandt, 2011). Juvenile nephronophthisis is caused by mutations in nephrocystin-1 (NPHP1), the first of the nephronophthisis genes discovered (Hildebrandt et al., 1997). End stage renal disease (ESRD) develops at an age of approximately 13 years. NPHP1 is located on chromosome 2q13, contains 20 exons, and produces a 4.5kb-transcript encoding an 83kDa protein. NPHP1 contains an SH3 domain (Hildebrandt et al., 1997), a coiled-coil domain in the N-terminus and a highly conserved C-terminal domain called a nephrocystin homology domain (NHD) (Donaldson et al., 2002). The major gene defect observed in juvenile nephronophthisis is a large homozygous deletion in the NHD region of NPHP1 (Otto et al., 2000a). Deletion of this region is also associated with development of Cogan syndrome with nephronophthisis (Betz et al., 2000).
In the mouse embryo, NPHP1 is widely and uniformly expressed; in the adult mouse, there is strong expression in testes (Otto et al., 2000). In retina, NPHP1 is expressed in photoreceptors and localizes to the connecting cilium in close proximity to the basal body (Fliegauf et al., 2006). This may explain why a subset of patients with NPHP1 mutations develops retinal degeneration. Targeted disruption of NPHP1 in the mouse (deletion of the last C-terminal exon 20) did not produce nephronophthisis, but exhibited rapid retinal degeneration starting at P14-P21(Jiang et al., 2008) and caused male infertility (Jiang et al., 2009). The Nphp1−/− mice failed to develop normal outer segment (OS) but the axoneme of the connecting cilium was still present in the mutant photoreceptors. At 8 months of age, there is an almost complete absence of the OS and inner segment (IS) (Jiang et al., 2008). Rhodopsin, transducin and other phototransduction proteins destined for the OS were predominantly located in the IS at P14, before significant degeneration of photoreceptor cells began (Jiang et al., 2008). However, myosin VIIA, RPGR, KIF3A, centrin-1, and WDR19 localized normally to the connecting cilium. At P14, there was significant increase of apoptosis in photoreceptor cells which correlates to the increased amount of rhodopsin mislocalized in the IS (Jiang et al., 2008).
The Nphp1−/− retinal phenotype was rescued after crossing the NPHP1 mutant mice with a transgenic mouse expressing GFP-tagged NPHP1 (Jiang et al., 2008) showing specificity of the effect of a targeted disruption in NPHP1. Another important observation in these mice was the mislocalization of specific IFT (intraflagellar transport) particles along the connecting cilium. These include IFT88 but not IFT122 which suggests that NPHP1 may regulate a specific subset of IFT particles and their associated cargos destined to the OS (Jiang et al., 2008; Donaldson et al., 2002). Importantly, nephrocystin-1 depletion does not completely abolish photoreceptor ciliogenesis and IFT, it only affects the transportation efficiency and sorting mechanism of IFT (Jiang et al., 2008).
3.2. INVS/NPHP2
Genetic linkage to infantile nephronophthisis type 2, which is a form of nephronophthisis that clinically presents before the age of 5 years, has been mapped to 9q31 (Haider et al., 1998). The gene associated with this disease was identified as INVS and shown to produce a 5.5kb transcript encoding a 1065 amino acid protein, inversin/NPHP2 (Otto et al., 2003). In human, two transcript variants encoding distinct isoforms (long form, 1065 amino acids, and short form, 895 amino acids) have been identified for this gene. The shorter form lacks an in-frame segment in the coding region. The mouse genome contains only the long form transcript (1062 amino acids) (Morgan et al., 1998). The protein contains multiple domains and protein-binding motifs including 16 tandem ankyrin repeats in the N-terminus, 2 putative nuclear localization signals (Morgan et al., 1998), 2 IQ-type calmodulin-binding domains (Morgan et al., 2002b), and 2 D-box domains (one is important for binding Apc2, a subunit of the anaphase promoting complex, APC) (Morgan et al., 2002a). The NPHP2 gene is conserved in chimpanzee, dog, cow, mouse, rat, chicken, zebrafish, and C. elegans.
The inv/inv mouse, which has a deletion of exons 4-12 (of 17 total) in the Invs gene (Morgan et al., 1998), resembles the human disease with renal cysts, hepatobiliary duct malformations and other renal histopathological features (Phillips et al., 2004). It also has been shown that recessive mutations in INVS cause infantile nephronophthisis with or without presence of situs inversus in patients, a classic finding in ciliopathies (Otto et al., 2003). Moreover, disruption of invs in zebrafish also causes renal cyst formation (Otto et al., 2003). NPHP2/Inversin is expressed in the retina as shown by fluorescence microscopy using an inversin:GFP transgenic mouse line (Watanabe et al., 2003). However, there is no report of a retinal phenotype in the loss-of-function inv/inv mouse and zebrafish models. There has only been one known case of a mutation in NPHP2 (in exon 13, R907X) causing retinal degeneration (SLS) suggesting that there may be genetic modifiers in the background of NPHP2 mutations which lead to development of retinal pathology (O’Toole et al., 2006).
Inversin/Nphp2 accumulates at the base of the primary cilium (termed the Inv compartment) and acts as an anchor for NPHP3 and NPHP9/NEK8 in this compartment (Sang et al., 2011; Shiba et al., 2010). Inversin/Nphp2 has not been found to affect formation or maintenance of the renal primary cilia in the inv/inv mouse. This suggests that Inversin/Nphp2 is not necessary for regulating structure of the primary cilia (Morgan et al., 2002b). Inversin also colocalizes with NPHP1 and β-tubulin in the primary cilium of MDCK cells (Otto et al., 2003), and with IQCB1/NPHP5 and B9d2, a MKS1-related protein, in zebrafish photoreceptors (Zhao and Malicki, 2011).
The ERK pathway, known to regulate cell proliferation, has been thought to play a role in cyst formation. In the inv/inv mouse expressing a C-terminal truncated form of INVS, the ERK pathway is activated (Watanabe et al., 2003). After administration of a MAP kinase inhibitor there was decreased ERK phosphorylation and inhibition of renal cyst progression (Watanabe et al., 2003). Finally, the canonical Wnt/β-catenin signaling pathway is also activated in renal cells of a patient with NPHP2 suggesting a role of this pathway in cyst formation (Simons et al., 2005; Bellavia et al., 2010). However, there is very little data on the role of the ERK and Wnt/β-catenin signaling pathways in photoreceptor cells, cells that are no longer proliferating.
3.3. NPHP3
NPHP3 is associated with adolescent nephronophthisis that causes ESRD at a median age of 19 years old (Morgan et al., 1998). The NPHP3 gene was mapped on chromosome 3q22 (Omran et al., 2000). The NPHP3 gene (28 exons) generates a 6.5kb transcript encoding a 1,330-amino acid protein (Olbrich et al., 2003). It is expressed in the retina suggesting that it contributes to development of Senior-Løken syndrome (Olbrich et al., 2003). Linkage to the NPHP3 locus has been shown in families with Senior-Løken syndrome (Omran et al., 2002).
NPHP3 contains several protein-protein interaction domains including an N-terminal coiled-coil domain, a C-terminal tetratrico peptide repeat domain and a tubulin-tyrosine kinase domain (Olbrich et al., 2003). Furthermore, it is N-terminally myristoylated and interacts with the acyl-binding proteins UNC119A and UNC199B (Wright et al., 2011). Both UNC119A and UNC119B contain immunoglobulin-like hydrophobic binding pocket to accommodate acyl side chains (Wright et al., 2011). Targeting to primary cilia is controlled by N-terminal myristoylation as a NPHP3 (G2A) mutant did not target to cilia (Nakata et al., 2012). Knockdown of UNC119B also prevented cilia targeting, suggesting that both myristoylation of NPHP3 and interaction with the myristoyl-binding protein UNC119B is important for targeting (Wright et al., 2011; Nakata et al., 2012). Furthermore, Wright et al. showed that the GTP-binding protein ARL3 and its GTPase activating protein (GAP), Retinitis Pigmentosa 2 protein (RP2), are involved in release of cargo from UND119B (Wright et al., 2011). Nephrocystin-3 also interacts with NPHP2/inversin, and like inversin, can inhibit canonical Wnt signaling (Bergmann et al., 2008).
The pcy mouse, a spontaneous mouse cystic kidney disease model, produces a phenotype that resembles the disease progression of NPHP3 in humans(Omran et al., 2001). Synteny has been demonstrated in the human NPHP3 and mouse pcy loci (Omran et al., 2001). However, there is no report of retinal pathology in the pcy mouse. The pcy mutation is a hypomorphic most likely caused by a missense mutation (Olbrich et al., 2003). Generation of an NPHP3 loss-of-function mouse is embryonic lethal with additional phenotypes of situs inversus and congenital heart defects (Bergmann et al., 2008). In contrast to NPHP1 and NPHP2, loss of function of NPHP3 shows a defect in the primary cilium length suggesting a role in regulating the structure of the primary cilium (Bergmann et al., 2008). Knockdown of nephrocystin-3 in Xenopus laevis or zebrafish showed defective convergent extension, which is regulated by non-canonical Wnt pathway activation (planar cell polarity defects) (Bergmann et al., 2008; Zhou et al., 2010).
3.4. NPHP4
NPHP4 is located in chromosome 1p36 and encodes a 1,250-amino acid protein called nephrocystin-4 (Mollet et al., 2002; Schuermann et al., 2002). Development of Senior-Løken syndrome has been shown to associate to this region (Mollet et al., 2002; Otto et al., 2002). NPHP4 has a proline-rich region known to bind SH3 domains and is thought to mediate its binding with NPHP1 (Mollet et al., 2002).
Nephrocystin-4 interacts with p130Cas and Pyk2, proteins involved in cell-cell adhesion (Mollet et al., 2005). It is also known that NPHP4 negatively regulates Pyk2-dependent tyrosine phosphorylation of NPHP1, which is important for localization of NPHP1 to a trans-Golgi interacting protein (Liebau et al., 2011). NPHP4 localizes to the renal epithelial cell’s periphery, primary cilium (where it colocalizes with α-tubulin) and the basal body (Keller et al., 2005; Mollet et al., 2005). NPHP1 and NPHP4 also associate with other tight junction proteins such as PALS1/PATJ and Par6 (Delous et al., 2009). Similar to NPHP2/inversin, NPHP4 is thought to regulate the canonical and noncanonical Wnt-signaling pathways (Burckle et al., 2011).
NPHP4 interacts with the Retinitis GTPase Regulator (RPGR) and RPGR-Interacting Protein 1 (RPGRIP1) and may provide the link to development of retinal degeneration in patients with a mutation in NPHP4 (Murga-Zamalloa et al., 2009; Roepman et al., 2005). RPGR mutations cause over 70% of X-linked RP cases (Murga-Zamalloa et al., 2009). Mutations in RPGRIP1 are linked to development of Leber congenital amaurosis (LCA) where both rod and cone photoreceptors degenerate early in life (Roepman et al., 2005). RPGRIP1 is localized to the photoreceptor connecting cilia associated with the ciliary axoneme (Hong et al., 2001). Mutations in either NPHP4 or RPGRIP1 abolish this interaction (Roepman et al., 2005).
A loss-of-function mouse model of NPHP4 (Nphpnmfl92) shows severe retinal degeneration reminiscent of LCA, with mislocalization of rhodopsin and ROM1 to the IS (Won et al., 2011). Connecting cilia and ribbon synapses developed normally, but OS failed to develop and ribbon structures eventually degenerated. However, as in NPHP1 and NPHP6 mouse mutants, no renal pathology was observed in the Nphpnmfl92 mouse (Chang et al., 2006; Jiang et al., 2008; Won et al., 2011).
3.5. IQCB1/NPHP5
Located on human chromosome 3, the NPHP5 gene encodes a 69kD protein called nephrocystin-5 or NPHP5 (also referred to as IQCB1) (Otto et al., 2005). By sequence analysis, it was found that NPHP5 contains two IQ-calmodulin binding regions (similar to inversin/NPHP2) and several coiled-coiled domains thought to be important for protein-protein interactions (Otto et al., 2005). In contrast to NPHP1-4 where approximately 10-33% of patients develop retinal degeneration, Senior-Løken syndrome is thought to develop in 100% of patients with NPHP5 mutations (Hildebrandt et al., 2009). Moreover, unlike the other nephrocystin genes, which may involve multiple organs, NPHP5 mutations only associate with the retinal-renal phenotype, making this the classic Senior-Løken gene (Hildebrandt et al., 2009). NPHP5 mutations in isolated cases of LCA (without nephronophthisis) have also been found (Estrada-Cuzcano et al., 2011; Stone et al., 2011).
NPHP5 is expressed in the photoreceptor connecting cilia (Otto et al., 2005). It is known to interact with calmodulin, NPHP6 and RPGR, other proteins involved in development of retinal degeneration (Schafer et al., 2008; Murga-Zamalloa et al., 2010). Knockdown of NPHP5 in zebrafish causes mislocalization of opsin suggesting its role in specific transport of proteins to the OS (Zhao and Malicki, 2011).
3.6. CEP290/NPHP6
The NPHP6/Cep290 gene spans nearly 100 kb and consists of 54 exons, and is located in chromosome 12q21.32 (Sayer et al., 2006). Null mutations in NPHP6 have been linked in a cohort of patients with nephronophthisis, Joubert syndrome, Meckel syndrome or Senior-Løken syndrome (Baala et al., 2007a; Helou et al., 2007; Sayer et al., 2006) (Table 1). Hypomorphic NPHP6 mutations have been found in isolated LCA patients (den Hollander et al., 2006). The NPHP6/Cep290 gene encodes a 2,472-amino acid protein, which contains several putative coiled-coil domains, an ATP/GTP binding loop (P-loop) and a C-terminal myosin-tail homology domain among others (Chang et al., 2006; Sayer et al., 2006). NPHP6 is localized in the photoreceptor connecting cilium and associates with dynein-dynastic and kinesin-II molecular motor subunits, RPGR, RPGRIP, and NPHP5 (Chang et al., 2006; Moradi et al., 2011; Murga-Zamalloa et al., 2010; Schafer et al., 2008). NPHP6 also directly interacts with the transcription factor ATF4 and mediates activation of ATF4 targets (Sayer et al., 2006).
Loss-of-function cep290 morpholino knockdowns in zebrafish exhibited renal, retinal and cerebellar anomalies reminiscent of the clinical findings in Joubert syndrome (Sayer et al., 2006), such as reduced Kupffer’s vesicle size, delays in melanosome transport and visual impairments (Baye et al., 2011). Interestingly, the N-terminal region of human CEP290 was able to restore vision (Baye et al., 2011). In a C. reinhardtii null mutant, flagella were shown to have abnormal levels of IFT and ciliopathy-associated proteins (Craige et al., 2010). The rd16 mouse, which has an in-frame deletion of exons 35-39 also develops an early-onset retinal degeneration phenotype (CEP290-LCA) associated with kidney and cerebellar abnormalities (Chang et al., 2006). This deletion removes part of the C-terminal myosin-tail homology domain of NPHP6 which interacts with MKKS, a protein associated with BBS6 (Rachel et al., 2012). Another naturally occurring Cep290 mutant is the Abyssinian retinal degeneration cat model (rdAc) (Narfstrom, 1983). The retina phenotype of the mutant cat resembles a slowly developing recessive RP, with reduced ERG a-wave amplitudes at 7 months, and complete photoreceptor degeneration at 3-5 years of age. The CEP290 gene defect was recently identified as a missense mutation in intron 50, generating a new splice site, a 4 bp insertion and a CEP protein that is shortened by 159 amino acids (Menotti-Raymond et al., 2007).
3.7. GLIS2/NPHP7
NPHP7/GLIS2 encodes a 3.8kb transcript containing 6 exons. The protein, a 55kDa Krupel-like transcription factor is important for kidney development (Zhang et al., 2002). Mutations in GLIS2/NPHP7 cause nephronophthisis in humans (Attanasio et al., 2007) without reports of association of retinal degeneration. The GLIS2/NPHP7 loss-of-function mouse recapitulates the human disease with atrophic kidneys and presence of fibrosis (Attanasio et al., 2007). It is not known whether GLIS2/NPHP7 is expressed in the mammalian retina and is currently not known whether it has an important role in photoreceptor development and maintenance.
3.8. RPGRIP1L/NPHP8
The NPHP8/RPGRIP1L (retinitis pigmentosa GTPase regulator-interacting protein-1 like) is located on chromosome 16q12.2 with 35 exons. It encodes a protein with 1,315 amino acids. RPGRIP1L/NPHP8 shares two protein kinase C conserved regions (C2 domains) with RPGRIP, but overall sequence similarity is relatively low (31%) (Arts et al., 2007). The protein further contains five coiled-coil domains in the N-terminus and an RPGR-interacting domain (RID) in the C-terminus (Delous et al., 2007). By RT-PCR, RPGRIP1L is expressed in retina, kidney and brain (Arts et al., 2007).
RPGRIP1L/NPHP8 is known to interact with NPHP4 and NPHP6/CEP290. When expressed individually in COS cells, NPHP8/RPGRIP1L is soluble, but when co-expressed with NPHP4, it localizes to basal bodies and cilia suggesting that NPHP4 recruits RPGRIP1L/NPHP8 to these structures. In photoreceptors, RPGRIP1L/NPHP8 localizes to the photoreceptor connecting cilium, basal body, in the ONL and the synaptic region, areas where other NPHP gene products, particularly NPHP4 are located (Arts et al., 2007; Delous et al., 2007).
Loss-of-function mutations in RPGRIP1L/NPHP8 cause Joubert syndrome (JBTS) or Meckel syndrome (MKS), ataxia and abnormal eye movements (Arts et al., 2007; Delous et al., 2007; Wolf et al., 2007) (Table 1) consistent with the notion that it is required for normal brain and kidney development. In Rpgrip1l knockout mice (ftm, fantom or fused tow mouse), fetuses had head abnormalities and very small or non-existing eyes (Delous et al., 2007). Patients with NPHP8/RPGRIP1L null alleles do not develop RP; however, an A229T variant is associated with photoreceptor cell loss (Khanna et al., 2009). This mutation compromises interaction with RPGR and causes degeneration. Absence of the mutation is thought to have a protective effect from RP (Khanna et al., 2009).
3.9. NEK8/NPHP9
Mutations in human NEK8/NPHP9 are associated with development of nephronophthisis (Otto et al., 2008). It is not clear whether mutations in NEK8/NPHP9 cause Senior-Løken syndrome. Of the 3 patients reported with mutations in this gene, one patient with a heterozygote mutation in NEK8/NPHP9 developed RP and was blind at 24 years of age; however, this patient also contained an additional homozygous mutation in NPHP5 (Otto et al., 2008).
NEK8/NPHP9 is a serine/threonine protein kinase and a member of the NIMA (never in mitosis-A)-related kinase (Nek) family. NEK8/NPHP9 encodes a 692 amino acid protein, which contains an N-terminal catalytic (kinase) domain, several RCC1-like domains, (RCC1 is a guanine nucleotide exchange factor for Ran GTPase) and a coiled-coil domain in the C-terminus (Holland et al., 2002). Members of this family are thought to regulate coordination of cilium formation and the cell cycle. Like other nephrocystin proteins, NEK8/NPHP9 are localized to the primary cilium. It is not known whether it is expressed in photoreceptor cells of the mammalian retina.
Mutations in NPHP9/NEK8 occur in the juvenile cystic kidney (jck) mouse, a model of autosomal recessive cystic kidney disease (Atala et al., 1993). Knockdown studies in zebrafish produce development of pronephric cysts (Liu et al., 2002); however, in both jck and zebrafish models, retinal pathology has not been reported. It is more likely that mutations in NPHP9/NEK8 are not associated with development of retinal degeneration in humans, which correlates well with the vertebrate models.
3.10. SDCCAG8/NPHP10
Truncating mutations in SDCCAG8/NPHP10 have been found in several families with nephronophthisis and associated pathologies including retinal degeneration (Otto et al., 2010). Mutations in SDCCAG8/NPHP10 have also been found in Bardet-Biedl syndrome (BBS) (Schaefer et al., 2011). The SDCCAG8/NPHP10 gene produces a 3.3kb transcript encoding an 88kDa protein containing an N-terminal globular domain, a nuclear localization signal and eight putative coiled-coil domains (Otto et al., 2010).
SDCCAG8/NPHP10 is localized to the connecting cilium area in photoreceptor cells particularly in the basal body and transition zone (Otto et al., 2010). It also has been shown that it co-localizes with other proteins associated with development of retinal degeneration including NPHP5, RPGRIP and RP1 (Otto et al., 2010). Knockdown of SDCCAG8/NPHP10 in zebrafish resulted in kidney cysts, hydrocephalus as well as other developmental problems but no retinal phenotype has been reported (Otto et al., 2010).
3.11. TMEM67/NPHP11
Hypomorphic mutations in TMEM67/NPHP11 cause nephronophthisis with associated liver fibrosis (Otto et al., 2009) as well as other diseases in the ciliopathy spectrum including Meckel-Gruber syndrome (Smith et al., 2006) and Joubert syndrome (Baala et al., 2007b). Out of the 12 individuals found with a mutation in TMEM67/NPHP11, 3 patients developed retinal degeneration/blindness (Otto et al., 2009).
TMEM67/NPHP11 is located in chromosome 8q22 and transcribed into 2 isoforms. The longer isoform 1 (28 exons) contains 995 amino acids and the shorter isoform 2 (29 exons) contains 914 amino acids. In the shorter isoform, the three N-terminal exons of isoform 1 are replaced by two alternate exons. The protein encoded by the isoforms, called Meckelin, is a transmembrane protein predicted to have an extracellular region with four N-liked glycosylation sites, seven transmembrane regions and a short cytoplasmic tail (Smith et al., 2006).
The TMEM67/NPHP11 locus is syntenic to the Wpk (Wistar polycystic kidney) locus in rat, which carries a hypomorphic mutation (P394L) that produces polycystic kidney disease, agenesis of the corpus callosum and hydrocephalus (Gattone et al., 2004; Nauta et al., 2000). Further, the Wpk photoreceptors showed advanced retinal degeneration at 3 weeks of age (Tammachote et al., 2009).
A spontaneous deletion of TMEM67/NPHP11 in mouse (bpck mouse - bilateral polycystic kidneys) has also been reported with phenotypes that resemble the human disease (polycystic kidney and hydrocephalus) (Cook et al., 2009). The photoreceptors in bpck mice degenerate very early with complete absence of the OS at P24 and reduction of the axonemal tip in the connecting cilia at P14 (Collin et al., 2012). However, at P14, the structure of the CC and the basal body was normal. Rhodopsin and ROM1 were mislocalized at P12, but RPGR, RPGRIP1L and ALMS1 located normally to the CC. At this age, mutant OS were disorganized and arrestin and transducin failed to translocate in the photoreceptors when exposed to light (Collin et al., 2012). Cone photoreceptors were also affected in the bpck retina. Both ML-opsin and S-opsin were mislocalized and cone outer segments appeared shortened (Collin et al., 2012). These results suggest that NPHP11, like other of the nephrocystin proteins in photoreceptors are important in regulation of protein trafficking.
3.12. TTC21B/NPHP12
TTC21B/NPHP12 (tetratricopeptide repeat domain 21B) located on chromosome 2q24.3 contains 29 coding exons and encodes IFT139, a ~150kDa retrograde intraflagellar transport (IFT) protein (Davis et al., 2011). TTC21B/NPHP12 contains eleven tetratricopeptide (TPR) domains and is important for mediating protein-protein interactions and assembly of multimeric complexes. Mutations in NPHP12/TTC21B/THM1 cause disease phenotypes in the ciliopathy spectrum including isolated nephronophthisis and Jeune syndrome (asphyxiating thoracic dystrophy) (Davis et al., 2011) (Table 1). Mutations in this gene also contribute as a modifier allele with other disease-causing genes (Davis et al., 2011).
In IMCD3 cells, NPHP12/TTCB21B colocalizes with Acetyl-tubulin in cilia, and was shown to be present throughout the axoneme, reminiscent of IFT88 localization (Tran et al., 2008). Live cell imaging in NPHP12/TTCB21B-deficient IMCD3 cells revealed IFT88-EYFP at the distal ends of cilia, consistent with impairment of retrograde IFT (Tran et al., 2008).
The alien mouse (aln), an NPHP12/TTC21B null mutant generated by ENU mutagenesis, is embryonic lethal at E18.5. Aln shows skeletal abnormalities, neural tube defects as well as delayed development of the eye and brain (Tran et al., 2008), similar to the hallmark features in individuals with Jeune syndrome. In rodents, specific knockdown of NPHP12/TTC21B show abnormal photoreceptor structure (Davis et al., 2011).
3.13. WDR19/NPHP13
WDR19/NPHP13 belongs to the family of WD-repeat proteins. The WDR domain consists of a conserved 40 amino acids domain flanked by glycine-histidine and tryptophan-aspartic acid dipeptides (WD) (Lin et al., 2003). WD-repeat containing proteins are thought to be important in vesicle formation and vesicular trafficking (Lin et al., 2003). The WDR19/NPHP13 gene contains 36 exons located on chromosome 4p15-4p11. It encodes IFT144, a protein containing six WD repeats, a clathrin heavy-chain repeat and three transmembrane domains (Lin et al., 2003). IFT144 is a member of the intraflagellar transport (IFT) complex A (Bredrup et al., 2011). Proteins in the IFT-A complex regulate retrograde ciliary transport in contrast to the IFT-B complex, which regulate anterograde transport.
Compound heterozygous mutations in WDR19/NPHP13 have been shown to associate with development of Sensenbrenner syndrome characterized by renal insufficiency leading to chronic renal failure (nephronophthisis-like), RP and various skeletal anomalies including polydactyly, short fingers, and other bone abnormalities (Bredrup et al., 2011). Compound heterozygous and homozygous missense mutations in WDR19/NPHP13 have also been identified in isolated nephronophthisis and in Jeune syndrome, a syndrome with similar clinical findings as that of Sensenbrenner syndrome (Bredrup et al., 2011).
WDR19/NPHP13 localizes to the primary cilium of fibroblast cells and when absent, negatively affects cilia formation (Bredrup et al., 2011). In disease-causing mutations, which still express the protein, IFT-B particles accumulate in the tip of cilia highlighting the importance of WDR19/NPHP13 in retrograde trafficking.
Of the six patients with WDR19/NPHP13 mutations associated with disease development (Sensenbrenner, isolated nephronophthisis and Jeune syndrome), three develop retinal abnormalities (50%) with two being diagnosed with RP (Bredrup et al., 2011). It is not known whether NPHP13/WDR19 localizes to the photoreceptor connecting cilium; however, presumably, it may have an important role in retrograde protein trafficking in photoreceptor cells, although retrograde IFT in photoreceptors may be less significant as outer segments are constantly phagocytosed (Kevany and Palczewski, 2010). It will be important to elucidate proteins which NPHP13/WDR19 bind and transport from the photoreceptor OS to IS. Also, animal models will be important in elucidating the molecular mechanism of NPHP13/WDR19.
4. Conclusions and Perspectives
Thirteen nephrocystin (NPHP) genes have been identified to date and shown to be associated with nephronophthisis, Senior-Løken, Bardet-Biedl, Joubert and Meckel syndromes, among others (Table 1). These NPHP genes express a variety of proteins which are unrelated by sequence, domain structure, or protein interaction motifs. The proteins have been exhaustively analyzed for motifs to gather clues about their function, and their genes have been deleted or knocked down in mouse, zebrafish, worm and algae to study the effect of loss of function. Despite all these efforts, the functions of most NPHP genes are still largely unknown.
What do NPHP gene products have in common? First, nephrocystins localize to primary cilia and centrosomes in renal epithelial cells. This observation strengthens the ‘unifying theory’ of ciliopathies (Hildebrandt and Zhou, 2007), which suggests that mutant gene products associated with cystic kidney diseases in humans, mice, or zebrafish are expressed in primary cilia or centrosomes of renal epithelial cells. As the eight nephrocystins that are associated with retinitis pigmentosa are also found in the photoreceptor connecting cilium and basal body, the unifying theory may be expanded to include photosensitive cilia in the retina.
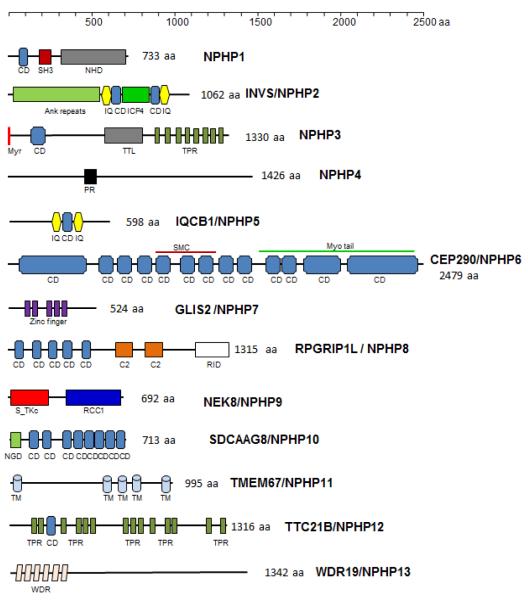
Second, nine of thirteen nephrocystins carry one or more protein interacting domains. The most frequent protein-protein interaction motifs identified in NPHP protein are the coiled coil structural motif, carried by 7 of 13 nephrocystins, followed by a IQ calmodulin interacting motif present in NPHP2 and NPHP5, a tetratricopeptide motif in NPHP3 and NPHP12, a large ankyrin repeat in NPHP2 and a SH3 interacting motif in NPHP1 (Figure 2). A key example of a protein with large numbers coiled-coils is CEP290/NPHP6, known to interact with multiple partners including kinesin motors and transcription factors.
Figure 2.
Motif and domain structure of NPHP proteins. The nephrocystins contain the following predicted motifs: NPHP1, coiled-coil (CD), SRC Homology 3 (SH3) and a nephrocystin homology domain (NHD) (Otto et al., 2000b). INVS/NPHP2, ANK, N-terminal Ankyrin repeat, ICP4, transcriptional regulator domain, flanked by CD and IQ calmodulin-binding motifs (IQ) (Otto et al., 2003; Lienkamp et al., 2012). NPHP3, myr, N-terminal myristoylation signal; CD; TTL, tubulin-tyrosine ligase domain; TPR, tetratricopeptide repeat domains (Olbrich et al., 2003; Wright et al., 2011). NPHP4, PR, proline-rich region (Mollet et al., 2002). NPHP5, a CD domain flanked by 2 IQ calmodulin binding motifs (Otto et al., 2005). NPHP6, multiple CDs throughout the molecule; myo tail, myosin tail homology domain (Chang et al., 2006). NPHP7, several Zinc finger motifs (Zhang et al., 2002). NPHP8, S_TKc, N-terminal serine/threonine protein kinase catalytic domain; RCC1, C-terminal Regulator of chromosome condensation 1 domain (Zalli et al., 2012). NPHP10, NGD, N-terminal globular domain; eight CDs (Otto et al., 2010). NPHP11, TM, transmembrane domains (Otto et al., 2009). NPHP12, TPR, tetratricopeptide domains (Davis et al., 2011). NPHP13, WDR, WD repeats (Lin et al., 2003).
Third, mutations in nephrocystins affect trafficking of proteins in cilia. In photoreceptors, NPHP1 colocalizes with Ac-tubulin in the connecting cilium. In Nphp1−/− mice (del20) (Jiang et al., 2008) the structure of the cilium was present, but IFT88 and IFT144 accumulated at the cilium suggesting that NPHP1 may facilitate the movement of cargo through the cilium by molecular motors. Consistent with this, rhodopsin and transducin accumulate in the inner segments and outer segments are not formed. The accumulation of protein in the inner segment is presumed to trigger apoptotic signals, which leads to retinal cell death and ultimately to widespread retinal degeneration. An excellent example for involvement of nephrocystins in trafficking was recently described in zebrafish. It was shown that opsin transport was dependent on Inversin/NPHP2 and IQCB1/NPHP5 suggesting that a NPHP1/2/5 complex may interact with the IFT cargo to facilitate transport of opsin through the connecting cilium (Zhao and Malicki, 2011). It is conceivable that nephrocystins together with IFT particles, molecular motor subunits, and adaptor proteins like B9d2 may form large complexes as observed for components to the BBSome (Nachury et al., 2007).
Finally, it should be noted that the inheritance pattern of nephronophthisis is exclusively recessive suggesting that mutations in NPHP genes generate null alleles. In the human retina, CEP290/NPHP6 and IQCB1/NPHP5 mutations cause recessive RP or recessive LCA (Cideciyan et al., 2011), and mouse models with retina phenotype (Nphp1−/−, rd16) are available for gene therapy rescue experiments. Using AAV2/5 vectors, it was shown recently that up to 8.9 kb of DNA can be packaged into viral particles, large enough for murine Abca4 and human MYO7A proteins (Allocca et al., 2008). Therefore, even CEP290 mutants such as rd16 may serve as suitable animal models to attempt to rescue of LCA in preclinical trials.
Highlights.
Thirteen nephrocystin genes (NPHP1-13) and their gene products are reviewed
Particular emphasis is given to mutant genes associated with retina degeneration
Existing naturally occurring or laboratory generated animal models are discussed
Highlights.
Senior-Løken syndrome (SLS) is an autosomal recessive disease
SLS develops retinitis pigmentosa (RP) or Leber congenital amaurosis (LCA) and a medullary cystic kidney disease, nephronophthisis
Mutations in 13 unrelated nephrocystin genes have been shown to cause SLS
This review will survey the biochemistry, cell biology and existing animal models for each of the nephrocystins
Acknowledgements
This work was supported by National Institute of Health grants EY08123, EY019298, EY014800-039003 (NEI core grant to the University of Utah), by a grant of the Foundation Fighting Blindness, Inc., and unrestricted grants to the Departments of Ophthalmology at the University of Utah from Research to Prevent Blindness (RPB; New York). WB is a recipient of a Research to Prevent Blindness Senior Investigator Award.
Abbreviations
- NPHP
nephronophthisis
- NPHP5
nephrocystin-5
- SLS
Senior-Løken Syndrome
- RP
retinitis pigmentosa
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, Kim SR, Maguire A, Rex TS, Di VU, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin. Invest. 2008;118:1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Marker T, Voesenek K, Kartono A, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat. Genet. 2007;39:882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- Atala A, Freeman MR, Mandell J, Beier DR. Juvenile cystic kidneys (jck): a new mouse mutation which causes polycystic kidneys. Kidney Int. 1993;43:1081–1085. doi: 10.1038/ki.1993.151. [DOI] [PubMed] [Google Scholar]
- Attanasio M, Uhlenhaut NH, Sousa VH, O’Toole JF, Otto E, Anlag K, Klugmann C, Treier AC, Helou J, Sayer JA, et al. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat. Genet. 2007;39:1018–1024. doi: 10.1038/ng2072. [DOI] [PubMed] [Google Scholar]
- Baala L, Audollent S, Martinovic J, Ozilou C, Babron MC, Sivanandamoorthy S, Saunier S, Salomon R, Gonzales M, Rattenberry E, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am. J. Hum. Genet. 2007a;81:170–179. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, et al. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am. J. Hum. Genet. 2007b;80:186–194. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baye LM, Patrinostro X, Swaminathan S, Beck JS, Zhang Y, Stone EM, Sheffield VC, Slusarski DC. The N-terminal region of centrosomal protein 290 (CEP290) restores vision in a zebrafish model of human blindness. Hum. Mol. Genet. 2011;20:1467–1477. doi: 10.1093/hmg/ddr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavia S, Dahan K, Terryn S, Cosyns JP, Devuyst O, Pirson Y. A homozygous mutation in INVS causing juvenile nephronophthisis with abnormal reactivity of the Wnt/beta-catenin pathway. Nephrol. Dial. Transplant. 2010;25:4097–4102. doi: 10.1093/ndt/gfq519. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kranzlin B, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am. J. Hum. Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz R, Rensing C, Otto E, Mincheva A, Zehnder D, Lichter P, Hildebrandt F. Children with ocular motor apraxia type Cogan carry deletions in the gene (NPHP1) for juvenile nephronophthisis. J. Pediatr. 2000;136:828–831. [PubMed] [Google Scholar]
- Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D, Leh SM, Midtbo M, Filhol E, Bole-Feysot C, et al. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am. J. Hum. Genet. 2011;89:634–643. doi: 10.1016/j.ajhg.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckle C, Gaude HM, Vesque C, Silbermann F, Salomon R, Jeanpierre C, Antignac C, Saunier S, Schneider-Maunoury S. Control of the Wnt pathways by nephrocystin-4 is required for morphogenesis of the zebrafish pronephros. Hum. Mol. Genet. 2011;20:2611–2627. doi: 10.1093/hmg/ddr164. [DOI] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum. Mol. Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Rachel RA, Aleman TS, Swider M, Schwartz SB, Sumaroka A, Roman AJ, Stone EM, Jacobson SG, Swaroop A. Cone photoreceptors are the main targets for gene therapy of NPHP5 (IQCB1) or NPHP6 (CEP290) blindness: generation of an all-cone Nphp6 hypomorph mouse that mimics the human retinal ciliopathy. Hum. Mol. Genet. 2011;20:1411–1423. doi: 10.1093/hmg/ddr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin GB, Won J, Hicks WL, Cook SA, Nishina PM, Naggert JK. Meckelin is necessary for photoreceptor intraciliary transport and outer segment morphogenesis. Invest Ophthalmol. Vis. Sci. 2012;53:967–974. doi: 10.1167/iovs.11-8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SA, Collin GB, Bronson RT, Naggert JK, Liu DP, Akeson EC, Davisson MT. A mouse model for Meckel syndrome type 3. J. Am. Soc. Nephrol. 2009;20:753–764. doi: 10.1681/ASN.2008040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV, et al. TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat. Genet. 2011;43:189–196. doi: 10.1038/ng.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat. Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Delous M, Hellman NE, Gaude HM, Silbermann F, Le BA, Salomon R, Antignac C, Saunier S. Nephrocystin-1 and nephrocystin-4 are required for epithelial morphogenesis and associate with PALS1/PATJ and Par6. Hum. Mol. Genet. 2009;18:4711–4723. doi: 10.1093/hmg/ddp434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KE, Zonneveld MN, Strom TM, Meitinger T, Brunner HG, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J Hum. Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D. Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin. Pediatr. Neurol. 2009;16:143–154. doi: 10.1016/j.spen.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JC, Dise RS, Ritchie MD, Hanks SK. Nephrocystin-conserved domains involved in targeting to epithelial cell-cell junctions, interaction with filamins, and establishing cell polarity. J. Biol. Chem. 2002;277:29028–29035. doi: 10.1074/jbc.M111697200. [DOI] [PubMed] [Google Scholar]
- Estrada-Cuzcano A, Koenekoop RK, Coppieters F, Kohl S, Lopez I, Collin RW, De Baere EB, Roeleveld D, Marek J, Bernd A, et al. IQCB1 mutations in patients with leber congenital amaurosis. Invest Ophthalmol. Vis. Sci. 2011;52:834–839. doi: 10.1167/iovs.10-5221. [DOI] [PubMed] [Google Scholar]
- FANCONI G, HANHART E, von AA, UHLINGER E, DOLIVO G, PRADER A. Familial, juvenile nephronophthisis (idiopathic parenchymal contracted kidney) Helv. Paediatr. Acta. 1951;6:1–49. [PubMed] [Google Scholar]
- Fliegauf M, Horvath J, von SC, Olbrich H, Muller D, Thumfart J, Schermer B, Pazour GJ, Neumann HP, Zentgraf H, et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J. Am. Soc. Nephrol. 2006;17:2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- Gattone VH, Tourkow BA, Trambaugh CM, Yu AC, Whelan S, Phillips CL, Harris PC, Peterson RG. Development of multiorgan pathology in the wpk rat model of polycystic kidney disease. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004;277:384–395. doi: 10.1002/ar.a.20022. [DOI] [PubMed] [Google Scholar]
- Haider NB, Carmi R, Shalev H, Sheffield VC, Landau D. A Bedouin kindred with infantile nephronophthisis demonstrates linkage to chromosome 9 by homozygosity mapping. Am. J. Hum. Genet. 1998;63:1404–1410. doi: 10.1086/302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Helou J, Otto EA, Attanasio M, Allen SJ, Parisi MA, Glass I, Utsch B, Hashmi S, Fazzi E, Omran H, et al. Mutation analysis of NPHP6/CEP290 in patients with Joubert syndrome and Senior-Loken syndrome. J. Med. Genet. 2007;44:657–663. doi: 10.1136/jmg.2007.052027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J. Am. Soc. Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, Adolphs J, Hanusch H, Brandis M. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat. Genet. 1997;17:149–153. doi: 10.1038/ng1097-149. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J. Am. Soc. Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- Holland PM, Milne A, Garka K, Johnson RS, Willis C, Sims JE, Rauch CT, Bird TA, Virca GD. Purification, cloning, and characterization of Nek8, a novel NIMA-related kinase, and its candidate substrate Bicd2. J. Biol. Chem. 2002;277:16229–16240. doi: 10.1074/jbc.M108662200. [DOI] [PubMed] [Google Scholar]
- Hong DH, Yue G, Adamian M, Li T. Retinitis pigmentosa GTPase regulator (RPGRr)-interacting protein is stably associated with the photoreceptor ciliary axoneme and anchors RPGR to the connecting cilium. J. Biol. Chem. 2001;276:12091–12099. doi: 10.1074/jbc.M009351200. [DOI] [PubMed] [Google Scholar]
- Jiang ST, Chiou YY, Wang E, Chien YL, Ho HH, Tsai FJ, Lin CY, Tsai SP, Li H. Essential role of nephrocystin in photoreceptor intraflagellar transport in mouse. Hum. Mol. Genet. 2009;18:1566–1577. doi: 10.1093/hmg/ddp068. [DOI] [PubMed] [Google Scholar]
- Jiang ST, Chiou YY, Wang E, Lin HK, Lee SP, Lu HY, Wang CK, Tang MJ, Li H. Targeted disruption of Nphp1 causes male infertility due to defects in the later steps of sperm morphogenesis in mice. Hum. Mol. Genet. 2008;17:3368–3379. doi: 10.1093/hmg/ddn231. [DOI] [PubMed] [Google Scholar]
- Juric-Sekhar G, Adkins J, Doherty D, Hevner RF. Joubert syndrome: brain and spinal cord malformations in genotyped cases and implications for neurodevelopmental functions of primary cilia. Acta Neuropathol. 2012;123:695–709. doi: 10.1007/s00401-012-0951-2. [DOI] [PubMed] [Google Scholar]
- Keller LC, Romijn EP, Zamora I, Yates JR, III, Marshall WF. Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr. Biol. 2005;15:1090–1098. doi: 10.1016/j.cub.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology. (Bethesda.) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat. Genet. 2009;41:739–745. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer H, van WE, Marker T, Wolfrum U, Roepman R. Usher syndrome: molecular links of pathogenesis, proteins and pathways. Hum. Mol. Genet. 2006;15(Spec No 2):R262–R270. doi: 10.1093/hmg/ddl205. [DOI] [PubMed] [Google Scholar]
- Liebau MC, Hopker K, Muller RU, Schmedding I, Zank S, Schairer B, Fabretti F, Hohne M, Bartram MP, Dafinger C, et al. Nephrocystin-4 regulates Pyk2-induced tyrosine phosphorylation of nephrocystin-1 to control targeting to monocilia. J. Biol. Chem. 2011;286:14237–14245. doi: 10.1074/jbc.M110.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienkamp S, Ganner A, Walz G. Inversin, Wnt signaling and primary cilia. Differentiation. 2012;83:S49–S55. doi: 10.1016/j.diff.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Lin B, White JT, Utleg AG, Wang S, Ferguson C, True LD, Vessella R, Hood L, Nelson PS. Isolation and characterization of human and mouse WDR19,a novel WD-repeat protein exhibiting androgen-regulated expression in prostate epithelium. Genomics. 2003;82:331–342. doi: 10.1016/s0888-7543(03)00151-4. [DOI] [PubMed] [Google Scholar]
- Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–5846. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- Menotti-Raymond M, David VA, Schaffer AA, Stephens R, Wells D, Kumar-Singh R, O’Brien SJ, Narfstrom K. Mutation in CEP290 discovered for cat model of human retinal degeneration. J Hered. 2007;98:211–220. doi: 10.1093/jhered/esm019. [DOI] [PubMed] [Google Scholar]
- Mollet G, Salomon R, Gribouval O, Silbermann F, Bacq D, Landthaler G, Milford D, Nayir A, Rizzoni G, Antignac C, et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat. Genet. 2002;32:300–305. doi: 10.1038/ng996. [DOI] [PubMed] [Google Scholar]
- Mollet G, Silbermann F, Delous M, Salomon R, Antignac C, Saunier S. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum. Mol. Genet. 2005;14:645–656. doi: 10.1093/hmg/ddi061. [DOI] [PubMed] [Google Scholar]
- Moradi P, Davies WL, Mackay DS, Cheetham ME, Moore AT. Focus on molecules: centrosomal protein 290 (CEP290) Exp. Eye Res. 2011;92:316–317. doi: 10.1016/j.exer.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Morgan D, Eley L, Sayer J, Strachan T, Yates LM, Craighead AS, Goodship JA. Expression analyses and interaction with the anaphase promoting complex protein Apc2 suggest a role for inversin in primary cilia and involvement in the cell cycle. Hum. Mol. Genet. 2002a;11:3345–3350. doi: 10.1093/hmg/11.26.3345. [DOI] [PubMed] [Google Scholar]
- Morgan D, Goodship J, Essner JJ, Vogan KJ, Turnpenny L, Yost HJ, Tabin CJ, Strachan T. The left-right determinant inversin has highly conserved ankyrin repeat and IQ domains and interacts with calmodulin. Hum. Genet. 2002b;110:377–384. doi: 10.1007/s00439-002-0696-4. [DOI] [PubMed] [Google Scholar]
- Morgan D, Turnpenny L, Goodship J, Dai W, Majumder K, Matthews L, Gardner A, Schuster G, Vien L, Harrison W, et al. Inversin, a novel gene in the vertebrate left-right axis pathway, is partially deleted in the inv mouse. Nat. Genet. 1998;20:149–156. doi: 10.1038/2450. [DOI] [PubMed] [Google Scholar]
- Murga-Zamalloa CA, Desai NJ, Hildebrandt F, Khanna H. Interaction of ciliary disease protein retinitis pigmentosa GTPase regulator with nephronophthisis-associated proteins in mammalian retinas. Mol. Vis. 2010;16:1373–1381. [PMC free article] [PubMed] [Google Scholar]
- Murga-Zamalloa CA, Swaroop A, Khanna H. RPGR-containing protein complexes in syndromic and non-syndromic retinal degeneration due to ciliary dysfunction. J. Genet. 2009;88:399–407. doi: 10.1007/s12041-009-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nakata K, Shiba D, Kobayashi D, Yokoyama T. Targeting of Nphp3 to the primary cilia is controlled by an N-terminal myristoylation site and coiled-coil domains. Cytoskeleton (Hoboken.) 2012 doi: 10.1002/cm.21014. in press. [DOI] [PubMed] [Google Scholar]
- Narfstrom K. Hereditary progressive retinal atrophy in the Abyssinian cat. J Hered. 1983;74:273–276. doi: 10.1093/oxfordjournals.jhered.a109782. [DOI] [PubMed] [Google Scholar]
- Nauta J, Goedbloed MA, Herck HV, Hesselink DA, Visser P, Willemsen R, Dokkum RP, Wright CJ, Guay-Woodford LM. New rat model that phenotypically resembles autosomal recessive polycystic kidney disease. J. Am. Soc. Nephrol. 2000;11:2272–2284. doi: 10.1681/ASN.V11122272. [DOI] [PubMed] [Google Scholar]
- Novarino G, Akizu N, Gleeson JG. Modeling human disease in humans: the ciliopathies. Cell. 2011;147:70–79. doi: 10.1016/j.cell.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole JF, Otto EA, Frishberg Y, Hildebrandt F. Retinitis pigmentosa and renal failure in a patient with mutations in INVS. Nephrol. Dial. Transplant. 2006;21:1989–1991. doi: 10.1093/ndt/gfl088. [DOI] [PubMed] [Google Scholar]
- Okada Y, Hirokawa N. Observation of nodal cilia movement and measurement of nodal flow. Methods Cell Biol. 2009;91:265–285. doi: 10.1016/S0091-679X(08)91014-1. [DOI] [PubMed] [Google Scholar]
- Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat. Genet. 2003;34:455–459. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- Omran H, Fernandez C, Jung M, Haffner K, Fargier B, Villaquiran A, Waldherr R, Gretz N, Brandis M, Ruschendorf F, et al. Identification of a new gene locus for adolescent nephronophthisis, on chromosome 3q22 in a large Venezuelan pedigree. Am. J. Hum. Genet. 2000;66:118–127. doi: 10.1086/302705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omran H, Haffner K, Burth S, Fernandez C, Fargier B, Villaquiran A, Nothwang HG, Schnittger S, Lehrach H, Woo D, et al. Human adolescent nephronophthisis: gene locus synteny with polycystic kidney disease in pcy mice. J. Am. Soc. Nephrol. 2001;12:107–113. doi: 10.1681/ASN.V121107. [DOI] [PubMed] [Google Scholar]
- Omran H, Sasmaz G, Haffner K, Volz A, Olbrich H, Melkaoui R, Otto E, Wienker TF, Korinthenberg R, Brandis M, et al. Identification of a gene locus for Senior-Loken syndrome in the region of the nephronophthisis type 3 gene. J. Am. Soc. Nephrol. 2002;13:75–79. doi: 10.1681/ASN.V13175. [DOI] [PubMed] [Google Scholar]
- Otto E, Betz R, Rensing C, Schatzle S, Kuntzen T, Vetsi T, Imm A, Hildebrandt F. A deletion distinct from the classical homologous recombination of juvenile nephronophthisis type 1 (NPH1) allows exact molecular definition of deletion breakpoints. Hum. Mutat. 2000a;16:211–223. doi: 10.1002/1098-1004(200009)16:3<211::AID-HUMU4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Otto E, Hoefele J, Ruf R, Mueller AM, Hiller KS, Wolf MT, Schuermann MJ, Becker A, Birkenhager R, Sudbrak R, et al. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am. J. Hum. Genet. 2002;71:1161–1167. doi: 10.1086/344395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E, Kispert A, Schatzle, Lescher B, Rensing C, Hildebrandt F. Nephrocystin: gene expression and sequence conservation between human, mouse, and Caenorhabditis elegans. J. Am. Soc. Nephrol. 2000b;11:270–282. doi: 10.1681/ASN.V112270. [DOI] [PubMed] [Google Scholar]
- Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat. Genet. 2010;42:840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O’Toole JF, Helou J, Attanasio M, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat. Genet. 2005;37:282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat. Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Tory K, Attanasio M, Zhou W, Chaki M, Paruchuri Y, Wise EL, Wolf MT, Utsch B, Becker C, et al. Hypomorphic mutations in meckelin (MKS3/TMEM67) cause nephronophthisis with liver fibrosis (NPHP11) J. Med. Genet. 2009;46:663–670. doi: 10.1136/jmg.2009.066613. [DOI] [PubMed] [Google Scholar]
- Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J. Am. Soc. Nephrol. 2008;19:587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CL, Miller KJ, Filson AJ, Nurnberger J, Clendenon JL, Cook GW, Dunn KW, Overbeek PA, Gattone VH, Bacallao RL. Renal cysts of inv/inv mice resemble early infantile nephronophthisis. J. Am. Soc. Nephrol. 2004;15:1744–1755. doi: 10.1097/01.asn.0000131520.07008.b3. [DOI] [PubMed] [Google Scholar]
- Rachel RA, May-Simera HL, Veleri S, Gotoh N, Choi BY, Murga-Zamalloa C, McIntyre JC, Marek J, Lopez I, Hackett AN, et al. Combining Cep290 and Mkks ciliopathy alleles in mice rescues sensory defects and restores ciliogenesis. J. Clin. Invest. 2012;122:1233–1245. doi: 10.1172/JCI60981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepman R, Letteboer SJ, Arts HH, van Beersum SE, Lu X, Krieger E, Ferreira PA, Cremers FP. Interaction of nephrocystin-4 and RPGRIP1 is disrupted by nephronophthisis or Leber congenital amaurosis-associated mutations. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18520–18525. doi: 10.1073/pnas.0505774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S, Gleeson JG. The ciliopathies in neuronal development: a clinical approach to investigation of Joubert syndrome and Joubert syndrome-related disorders. Dev. Med. Child Neurol. 2011;53:793–798. doi: 10.1111/j.1469-8749.2011.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat. Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- Schaefer E, Zaloszyc A, Lauer J, Durand M, Stutzmann F, Perdomo-Trujillo Y, Redin C, Bennouna G,V, Toutain A, Perrin L, et al. Mutations in SDCCAG8/NPHP10 Cause Bardet-Biedl Syndrome and Are Associated with Penetrant Renal Disease and Absent Polydactyly. Mol. Syndromol. 2011;1:273–281. doi: 10.1159/000331268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer T, Putz M, Lienkamp S, Ganner A, Bergbreiter A, Ramachandran H, Gieloff V, Gerner M, Mattonet C, Czarnecki PG, et al. Genetic and physical interaction between the NPHP5 and NPHP6 gene products. Hum. Mol. Genet. 2008;17:3655–3662. doi: 10.1093/hmg/ddn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuermann MJ, Otto E, Becker A, Saar K, Ruschendorf F, Polak BC, Ala-Mello S, Hoefele J, Wiedensohler A, Haller M, et al. Mapping of gene loci for nephronophthisis type 4 and Senior-Loken syndrome, to chromosome 1p36. Am. J. Hum. Genet. 2002;70:1240–1246. doi: 10.1086/340317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba D, Manning DK, Koga H, Beier DR, Yokoyama T. Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia. Cytoskeleton (Hoboken.) 2010;67:112–119. doi: 10.1002/cm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat. Genet. 2006;38:191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- Stone EM, Cideciyan AV, Aleman TS, Scheetz TE, Sumaroka A, Ehlinger MA, Schwartz SB, Fishman GA, Traboulsi EI, Lam BL, et al. Variations in NPHP5 in patients with nonsyndromic leber congenital amaurosis and Senior-Loken syndrome. Arch. Ophthalmol. 2011;129:81–87. doi: 10.1001/archophthalmol.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammachote R, Hommerding CJ, Sinders RM, Miller CA, Czarnecki PG, Leightner AC, Salisbury JL, Ward CJ, Torres VE, Gattone VH, et al. Ciliary and centrosomal defects associated with mutation and depletion of the Meckel syndrome genes MKS1 and MKS3. Hum. Mol. Genet. 2009;18:3311–3323. doi: 10.1093/hmg/ddp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat. Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, Hamada H. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development. 2003;130:1725–1734. doi: 10.1242/dev.00407. [DOI] [PubMed] [Google Scholar]
- Wolf MT, Hildebrandt F. Nephronophthisis. Pediatr. Nephrol. 2011;26:181–194. doi: 10.1007/s00467-010-1585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MT, Saunier S, O’Toole JF, Wanner N, Groshong T, Attanasio M, Salomon R, Stallmach T, Sayer JA, Waldherr R, et al. Mutational analysis of the RPGRIP1L gene in patients with Joubert syndrome and nephronophthisis. Kidney Int. 2007;72:1520–1526. doi: 10.1038/sj.ki.5002630. [DOI] [PubMed] [Google Scholar]
- Won J, Marin de EC, Smith RS, Hicks WL, Edwards MM, Longo-Guess C, Li T, Naggert JK, Nishina PM. NPHP4 is necessary for normal photoreceptor ribbon synapse maintenance and outer segment formation, and for sperm development. Hum. Mol. Genet. 2011;20:482–496. doi: 10.1093/hmg/ddq494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, Wang W, Pretorius PR, Sheffield VC, Sengupta P, et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 2011;25:2347–2360. doi: 10.1101/gad.173443.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J. Clin. Invest. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalli D, Bayliss R, Fry AM. The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis. Hum. Mol. Genet. 2012;21:1155–1171. doi: 10.1093/hmg/ddr544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Nakanishi G, Kurebayashi S, Yoshino K, Perantoni A, Kim YS, Jetten AM. Characterization of Glis2, a novel gene encoding a Gli-related, Kruppel-like transcription factor with transactivation and repressor functions. Roles in kidney development and neurogenesis. J. Biol. Chem. 2002;277:10139–10149. doi: 10.1074/jbc.M108062200. [DOI] [PubMed] [Google Scholar]
- Zhao C, Malicki J. Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. 2011;30:2532–2544. doi: 10.1038/emboj.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Dai J, Attanasio M, Hildebrandt F. Nephrocystin-3 is required for ciliary function in zebrafish embryos. Am. J. Physiol Renal Physiol. 2010;299:F55–F62. doi: 10.1152/ajprenal.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]