Abstract
Objective
The objective of this study was to define the musculoskeletal syndrome associated with use of Aromatase Inhibitors (AIs). The specific aims are to describe its incidence, time to onset, risk factors and clinical presentation.
Methods
Post-menopausal women with hormone-sensitive, non-metastatic breast cancer starting AI therapy were enrolled in this prospective cohort study. They underwent complete rheumatologic evaluation and contrast-enhanced MRIs of the hands and wrists prior to starting AI, at 3 and 6 months. The primary outcome was change in grip strength.
Results
Twenty-eight of 52 women (54%) reported new or worsening musculoskeletal symptoms. Two discontinued AIs due to pain. Mean time to symptom onset was 6 weeks (range 2 to 18) and 75% of symptomatic patients developed symptoms by 8 weeks. Later stage cancer and worse quality of life (QOL) pre-treatment were significantly associated with symptom development. Sixty-eight percent of symptomatic subjects had involvement of the hands however, there was no difference in the mean change in grip strength (−2.9 kg vs −1.3 kg, p-value=0.6). Among symptomatic subjects, 46% had evidence of focal tenosynovitis of the hands and feet on examination. Although some symptomatic subjects had new MRI abnormalities, RAMRIS scores did not significantly change.
Conclusion
The incidence of AI associated musculoskeletal syndrome is over 50% with most women developing symptoms by 8 weeks. The key finding in symptomatic women was focal tenosynovitis of the hands and feet, without evidence of autoimmune disease or systemic inflammation. Later stage cancer and poorer QOL were predictive of symptom development.
Third generation aromatase inhibitors (AIs) are quickly replacing tamoxifen as the standard adjuvant endocrine therapy for hormone receptor positive, post-menopausal breast cancer. Adherence to this crucial therapy has been limited by debilitating musculoskeletal side effects. Adverse event reporting from large randomized controlled trials (RCTs) has consistently shown higher rates of arthralgia in patients taking AIs.[1-7] Of RCT participants, 5-10% discontinued therapy due to musculoskeletal symptoms.[8] In several practice based studies, the incidence rates of AI-associated arthralgia were higher than those reported in RCTs ranging from 32-47% with a concerning rate of discontinuation of 13-50%.[9-11]
Despite its clinical significance, the AI-associated musculoskeletal syndrome remains poorly defined. Reports on time to onset of symptoms have a broad range from 6 weeks to 6 months.[7,9] Case series have described arthralgia, myalgia and stiffness in women on AIs, predominantly involving the hands and wrists.[12-14] In 2 small studies, Morales et. al describe tenosynovitis presenting as trigger finger, carpal tunnel syndrome (CTS) and deQuervain’s tendonitis. [15-16] Other studies however, describe a more widespread anatomic involvement presenting as osteoarthritis and tendonitis [9]. Risk factors for symptom development have also been discordant in the literature. While some studies have suggested an increased risk with lower BMI, chemotherapy and previous hormone replacement therapy [7,10] others have not demonstrated this relationship. [9]
We conducted a 6-month, prospective, pilot study of breast cancer patients starting adjuvant AI therapy with the goal of describing the associated musculoskeletal syndrome. In this study we aim specifically to define the incidence, time to onset and risk factors for symptoms development as well as to describe the clinical presentation.
To achieve our objective, and provide a comprehensive description of the syndrome, we performed and extensive rheumatologic work-up including QOL questionnaires, physical examination, laboratory studies and MRI. Grip strength, a well-validated, objective measure of hand impairment was chosen as the primary outcome measure based on the existing AI literature which suggests that the musculoskeletal effects of AIs are seen predominantly in the hands. [15,16] Choosing the same outcome measure allowed calculation of a rational sample size.
We anticipate that description of this syndrome will guide physicians in counseling and treating patients with the hope of improving compliance with this important therapy.
PATIENTS AND METHODS
Patients
Post-menopausal women with stage I - III hormone receptor positive breast cancer, starting adjuvant AI therapy were enrolled. Exclusion criteria included previous use of AIs, corticosteroid use in the 4 weeks prior to enrollment, other active cancer not in remission (excluding non-melanomatous skin cancer) or previous diagnosis of any systemic rheumatic diseases or crystal-induced arthritides.
Study Design
In this prospective, single center, observational, cohort study patients were evaluated by a rheumatologist before initiating AI therapy, at 3 months and at 6 months. Symptomatic patients were defined as those who either: (1) responded in the affirmative at their 3 or 6 months scheduled study visit to the question “Have you developed new or worsening muscle and/or joint symptoms since starting AIs?” (2) contacted an investigator to report new or worsening symptoms at any time. If symptoms were reported between scheduled study visits, the patient was brought in for an additional interim visit within 7 days of symptom report.
All patients without contraindication had contrast-enhanced magnetic resonance imaging (MRI) of bilateral hands and wrists at baseline and 6 months. Symptomatic participants had an additional interim MRI of the hands and wrists only if they also had an abnormal hand or wrist rheumatologic exam. The interim MRI was performed within 7 days of symptom report.
Time to symptom onset was calculated from the start date of AIs to the first day the patient noticed symptoms, regardless of when they reported the symptoms. Patients were censored once they became symptomatic.
Clinical Outcome Measures
The primary outcome measure was grip strength measured in kilograms using a JAMAR hydraulic hand dynamometer® (Sammons Preston Rolyan, Bolingbrook, IL). Three readings were taken for each hand and were added together for a total score. This outcome measure was chosen based on findings that patients started on AIs were more than 2 times more likely to have a decrease in grip strength than those started on tamoxifen. Grip strength was a clinically and statistically significant correlate of tenosynovial changes on MRI.[16] Furthermore, this well-validated tool is an objective marker of change in function in the hands, the site most commonly involved according to the existing data.
Secondary outcome measures included a standardized 66/68 swollen and tender joint count and a non-standardized symptom-directed rheumatologic examination (in symptomatic patients) performed by board certified rheumatologists, a visual analogue scale (VAS) for global pain, Health Assessment Questionnaire-Disease Index (HAQ-DI), Short Form-36 (SF 36), Australian-Canadian Hand Index (AUSCAN), Functional Assessment of Cancer Quality of Life Instrument Breast subset with Endocrine Subscale (FACTB-ES), serum markers for creatine phosphokinase (CPK), estimated sedimentation rate (ESR), C-reactive protein (CRP), anti-nuclear antibodies (ANA), anti-SSA antibodies, anti –SSB antibodies, rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA).
MRI
MRI of both hands and wrists was performed at 1.5 Tesla (GE, Excite HDx) simultaneously using a body phase-array coil. Images were acquired using axial and coronal fast spin echo T1, coronal and sagittal fast spin echo inversion recovery, and axial and coronal LAVA before and after contrast administration with fat saturation. Magnevist (gadopentetate dimeglumine) contrast was used at 0.5-mol/L weight based at 0.2 mL/kg.
MRIs were interpreted using a modified OMERACT Rheumatoid Arthritis MRI Scoring system with Tenosynovitis Subscale (RAMRIS-TS). The RAMRIS-TS has 4 scores; erosion, edema, synovitis and tenosynovitis. At each joint in the hand and wrist, erosion is graded from 0-10; and edema, synovitis and tenosynovitis 0-3. Combined (right and left) scores range from 0-460, 0-130, 0-42, and 0-60, respectively. Based on the distribution of scores in our population we used a modified RAMRIS scoring system; combined erosion, edema and synovitis scores >6 and combined tenosynovitis score >1 were considered positive.
Statistical analysis
Descriptive baseline analyses included all enrolled patients. Prospective analyses were performed on all patients who were started on an AI and were seen at least once in follow up. Baseline MRI analyses were performed on all patients with a baseline MRI. Prospective imaging analyses were performed on all patients with either a paired interim or a 6-month MRI. For prospective analyses, last value carried forward was used to impute missing data.
Descriptive statistics were calculated for variables of interest. Univariate analyses included the Wilcoxon rank-sum test for continuous and ordinal variables and Fisher’s exact test for categorical variables. All analyses were performed using SAS Software version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient disposition
Patients were enrolled between January 2009 and March 2010. Ninety-eight patients were referred to the study; 55 were enrolled. Three patients were not included in the prospective analysis – 2 did not start AIs and 1 was lost to follow-up before the 3-month visit. Of 43 referred but not enrolled, 26 patients declined enrollment and 17 were ineligible (12 previous AI use, 2 Lyme disease, 1 psoriatic arthritis, 1 polymyalgia rheumatica, and 1 steroid use). Fifty-two patients were analyzed; 51 completed 6 months of follow up and 1 patient was lost to follow up after the 3-month visit.
Baseline Demographics
The mean age of enrollees was 60.3 years, and 87% of participants were Caucasian (Table 1). Fifty-four percent had switched from tamoxifen to AIs. Ninety-two percent were prescribed anastrozole and 8% letrozole. Sixty-one percent had Stage I disease. Patient demographics were similar between groups.
Table 1.
Demographic and baseline cancer characteristics
| Characteristic | Total (n=55)* |
Symptomatic (n=28) |
Asymptomatic (n=24) |
PŦ |
|---|---|---|---|---|
| Age, years | 60.3 (44-76) | 59.8 (44-76) | 61.5 (45-76) | 0.52 |
|
| ||||
| Race | 0.06 | |||
| White | 48 (87.3) | 22 (78.6) | 23 (95.8) | |
| Asian | 4 (7.3) | 4 (14.3) | 0 | |
| African American | 1 (1.8) | 0 | 1 (4.2) | |
| Other | 2 (3.6) | 2 (7.1) | 0 | |
|
| ||||
| Hispanic | 4 (7.3) | 3 (10.7) | 1 (4.2) | 0.61 |
|
| ||||
| BMI | 0.40 | |||
| ≤25 | 28 (50.9) | 12 (42.9) | 14 (58.3) | |
| ≥26 | 27 (49.1) | 16 (57.1) | 10 (41.7) | |
|
| ||||
| Education | 0.72 | |||
| < HS Graduate | 2 (3.6) | 1 (3.4) | 1 (4.2) | |
| HS Graduate | 12 (21.8) | 5 (17.8) | 7 (29.2) | |
| College graduate | 20 (36.6) | 11 (39.4) | 8 (33.3) | |
| Graduate degree | 21 (38.2) | 11 (39.4) | 8 (33.3) | |
|
| ||||
| Drug | 1.00 | |||
| Anastrazole | 49 (89.1) | 26 (92.9) | 22 (91.7) | |
| Letrozole | 4 (7.3) | 2 (7.1) | 2 (8.3) | |
| Exemestane | 0 | 0 | 0 | |
|
| ||||
| Previous Tamoxifen | 20 (36.4) | 10 (35.7) | 9 (37.5) | 1.00 |
|
| ||||
| Cancer Stage | 0.04 | |||
| 1 | 34 (61.8) | 13 (46.3) | 19 (79.1) | |
| 2 | 17 (30.9) | 12 (42.9) | 4 (16.7) | |
| 3 | 4 (7.3) | 3 (10.7) | 1 (4.2) | |
|
| ||||
| Her2/Neu Positive | 7 (12.7) | 4 (14.3) | 3 (12.5) | 1.00 |
|
| ||||
| Surgery | 0.39 | |||
| Lumpectomy | 35 (63.5) | 16 (57.1) | 17 (70.9) | |
| Mastectomy | 20 (36.4) | 12 (42.9) | 7 (29.1) | |
|
| ||||
| Lymph Node DissectionΨ – no. (%) | 20 (36.6) | 13 (46.4) | 6 (25) | 0.15 |
|
| ||||
| Chemotherapy | 0.17 | |||
| Any | 24 (43.6) | 15 (53.6) | 8 (33.3) | |
| Taxol based | 15 (27.3) | 10 (35.7) | 4 (16.7) | |
|
| ||||
| Radiation | 39 (70.9) | 19 (67.9) | 18 (75) | 0.76 |
55 patients enrolled; 2 did not start AI and 1 lost to follow up. 52 patients were analyzed at 6 months (28 symptomatic and 24 asymptomatic).
p-value is comparing the symptomatic to asymptomatic
Lymph node dissection was defined as ≥ 5 lymph nodes removed
Twenty-eight patients (54%) developed new or worsening musculoskeletal symptoms and 2 (3.8%) stopped AIs due to musculoskeletal ailments. Five participants discontinued the AI for non-musculoskeletal complaints: 2 for uterine bleeding, 3 for hot flashes. Sixty-eight percent of symptomatic participants had involvement of their hands and/or wrists and 32% of their feet and/or ankles. Relatively few subjects had hip, knee or back pain. Mean time to symptom onset was 6 weeks, (median 4 weeks, range 2 to 18 weeks). Seventy-five percent of symptomatic patients presented with their complaints by 8 weeks.
In univariate analysis, having Stage 2 or 3 cancer was significantly associated with developing musculoskeletal symptoms (p-value=0.04, Table 1). There was no association between development of AI-associated pain and age, education level, body mass index (BMI), previous tamoxifen use, Her2/Neu positivity, or exposure to any chemotherapy, including Taxol.
All subscales of the FACT and the SF 36, were lower at baseline in the symptomatic group (lower scores indicate poorer QOL, Table 2). The Physical Component Score (PCS) of the SF36 and both the general FACT G and the breast cancer-specific FACT B were statistically significantly associated with developing musculoskeletal symptoms (P-value=0.01 and 0.03 respectively).
Table 2.
Baseline Quality of Life Questionnaire Scores, joint counts and serologic evaluation
| Total (n=55) |
Symptomatic (n=28) |
Asymptomatic (n=24) |
P | |
|---|---|---|---|---|
| VAS Pain (0-100) | 9.67 | 10.33 | 9 | 0.45 |
|
| ||||
| Grip Strength, Kg | 44.8 | 44.6 | 44.9 | 0.96 |
|
| ||||
| HAQ-DI (0-3) | 0.11 | 0.17 | 0.05 | 0.09 |
|
| ||||
| SF-36 | ||||
| PCS (0-100) | 56.8 | 55.9 | 61.9 | a0.01 |
| MCS (0-100) | 53.8 | 54.1 | 54.4 | 0.69 |
|
| ||||
| FACT | ||||
| FACT G Total (0-108) | 92.6 | 91.4 | 95.5 | 0.03 |
| FACT B TOI (0-92) | 75.6 | 75.78 | 77.9 | 0.12 |
| FACT B Total (0-144) | 119.6 | 117.8 | 123.9 | 0.03 |
| FACT ES Total (0-176) | 152.6 | 152.4 | 154.8 | 0.56 |
|
| ||||
| AUSCAN | ||||
| Pain (0-20) | 1.25 | 1.25 | 0.96 | a0.78 |
| Stiffness (0-4) | 0.35 | 0.29 | 0.33 | 0.75 |
| Physical Function (0-36) | 2.4 | 2.57 | 1.83 | 0.84 |
| Total (0-60) | 4 | 4.11 | 3.13 | 0.75 |
|
| ||||
| 66/68 Joint Count * | ||||
| Swollen | 8 (14.6) | 3 (10.7) | 5 (20.8) | 0.45 |
| Tender | 12 (21.8) | 6 (21.4) | 5 (20.8) | 1.00 |
|
| ||||
| Serologic Analysis, mean, n, (%)Ŧ | ||||
| ESR, mm/hr | 15.87, 4 (7.3) | 18.25, 3 (10.1) | 13.7, 1 (4.2) | 0.61 |
| CRP, mg/dl | 0.38, 0 (0) | 0.5, 0 (0) | 0.29, 0, (0) | |
| CPK, U/L | 129.6, 3 (5.5) | 114, 2 (7.1) | 151, 1 (4.2) | a1.00 |
| ANAΨ | 5 (9.1) | 2 (7.1) | 3 (12.5) | 0.65 |
| RF | 4 (7.3) | 2 (7.1) | 2 (8.3) | 1.00 |
| ACPA | 0 (0) | 0 (0) | 0 (0) | |
| SSA | 0 (0) | 0 (0) | 0 (0) | |
| SSB | 0 (0) | 0 (0) | 0 (0) | |
VAS=Visual Analogue Scale, HAQ-DI=Health Assessment Questionnaire-Disability Index, PCS=Physical component Score, MCS=Mental Component Score, FACT-Functional Assessment Cancer G=General, B=Breast cancer, TOI=Trial Outcome Index
Number of patients with abnormal joint count (>1 swollen and/or tender joint)
Number and percentage of participants with lab value outside the limits of normal
Abnormal ANA titer defined as ≥ 1:80
Clinical outcomes
Grip Strength
There was a trend toward a greater decrease in combined grip strength in the symptomatic group, but these results did not meet statistical significance (−2.89 kg versus −1.39 kg, P-value=0.6, Table 3).
Table 3.
Change in outcome variables at 6-months or time of symptom onset
| Characteristic | Symptomatic (n=28) |
Asymptomatic (n=24) |
P |
|---|---|---|---|
| VAS Pain (0-100) | 14.6 | −2.6 | 0.002 |
|
| |||
| Grip Strength, Kg | −2.89 | −1.39 | 0.6025 |
|
| |||
| HAQ-DI | 0.17 | 0.03 | 0.1126 |
|
| |||
| SF-36 | |||
| PCS (0-100) | −2.09 | −1.8 | 0.87 |
| MCS (0-100) | −0.42 | 0.64 | 0.45 |
|
| |||
| FACT | |||
| FACT G Total (0-108) | −0.9 | 0.39 | 0.63 |
| FACT B TOI (0-92) | −1.48 | 1.56 | 0.37 |
| aFACT B Total (0-144) | −0.4 | 0.8 | 0.56 |
| FACT ES Total (0-176) | −1.76 | −0.58 | 0.78 |
|
| |||
| AUSCAN | |||
| Pain (0-20) | 2.25 | −0.17 | <0.001 |
| Stiffness (0-4) | 0.68 | −0.308 | <0.001 |
| Physical Function (0-36) | 1.86 | 0 | 0.03 |
| Total (0-60) | 4.79 | −0.25 | <0.001 |
|
| |||
| 66/68 Joint Count – N abnormal, (%)* | |||
| Swollen | 4 (14.3) | 2 (8.3) | 0.67 |
| Tender | 9 (32.1) | 2 (8.3) | 0.04 |
|
| |||
| Serologic AnalysisŦ – Mean, N abnormal, (%) | |||
| ESR, mm/hr | 4 (14.3) | 1 (4.2) | 0.36 |
| CRP, mg/dl | 0 | 0 | |
| CPK, U/L | 5 (17.9) | 3 (12.5) | 0.71 |
| ANAΨ | 2 (7.10 | 3 (12.5) | 0.65 |
| RF | 2 (7.1) | 2 (8.3) | 1.00 |
| ACPA | 0 (0) | 0 (0) | |
| SSA | 0 (0) | 0 (0) | |
| SSB | 0 (0) | 0 (0) | |
VAS=Visual Analogue Scale, HAQ-DI=Health Assessment Questionnaire-Disability Index, PCS=Physical component Score, MCS=Mental Component Score, FACT-Functional Assessment Cancer G=General, B=Breast cancer, TOI=Trial Outcome Index
Number of patients with abnormal joint count (>1 swollen and/or tender joint)
Number and percentage of participants with lab value outside the limits of normal
Abnormal ANA titer defined as ≥a 1:80
Quality of Life
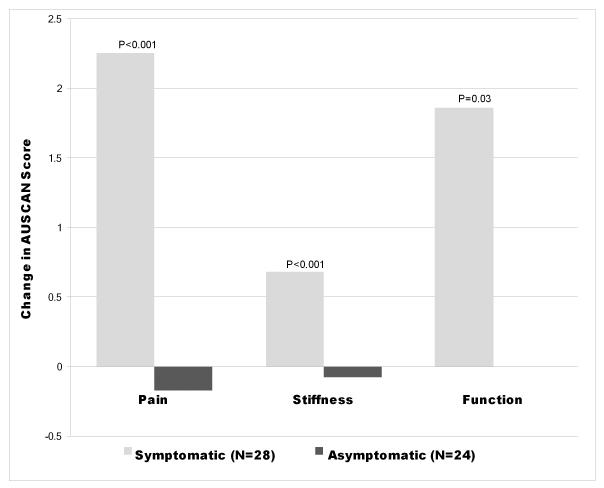
Symptomatic patients had a 14.6-point worsening from baseline on the VAS global pain scale (0-100), compared to a 2.73-point improvement in the asymptomatic group (p-value=0.002, Table 3). The symptomatic group had a greater worsening in all components of the AUSCAN Hand Index (Figure 1). There was no significant difference in the changes in HAQ-DI, SF 36 and FACT scores between symptomatic and asymptomatic groups (Table 3). There was no statistically significant association between musculoskeletal symptoms and endocrine symptoms as measured by the FACT Endocrine Subscale (FACT-ES). Furthermore, of the 5 participants who stopped AIs due to endocrine symptoms, none had musculoskeletal complaints.
Figure 1.
| Pain | Stiffness | Function | |
|---|---|---|---|
| Symptomatic (N=28) | 2.25 | 0.68 | 1.86 |
| Asymptomatic (N=24) | −0.17 | −0.08 | 0 |
Change in AUSCAN Hand Index scores at 6 months
Physical Examination and Laboratory Findings
A greater proportion of symptomatic patients had an increase in their tender joint count (32.1% vs. 8.3%, p-value 0.04). There was no difference in swollen and total joint counts. Symptom-directed physical examination revealed tenosynovitis in 13 patients defined as signs of tendon sheath inflammation and/or enthesitis. A positive Finkelstein test for deQuervain’s tendinitis in 3 patients, tenderness over the digital flexor tendons in 4 patients with locking of the digit in 1, tenderness over the plantar fascia in 4 patients and over the Achilles tendon in 1. There was no association between clinical symptoms and any change in serologic markers (Table 3).
MRI Findings
Forty-three of the 52 enrolled patients had baseline MRIs performed (9 patients had a contraindication to MRI). Five patients with baseline MRIs refused the follow-up MRI, leaving 38-paired MRIs available for analysis. Twenty-one MRIs were performed on symptomatic patients. Of those, 15 were performed at 6 months and 6 were performed as an interim MRI. Mean time from symptom onset to MRI for all patients was 13.5 weeks (range 1-21), and 12.5 weeks (range 1-21) for patients with hand symptoms.
There was no difference in either the mean or the proportion of abnormal baseline MRI RAMRIS-TS scores between symptomatic and asymptomatic groups (data not shown). Furthermore, there was no difference in the change in modified RAMRIS-TS scores between groups (Table 4). In a sub-analysis comparing patients with symptoms localizing to the hands to all other patients (symptomatic without hand involvement and asymptomatic), there was likewise no difference in MRI scores.
Table 4.
Change in MRI scores at 6-months or time of symptom onset
| Hand Involvement | No Hand Involvement | |||
|---|---|---|---|---|
|
|
||||
| Score | Symptomatic (n=21) |
Asymptomatic (n=17) |
Hand Symptoms (n=16) |
No Hand Symptoms (n=22) |
| Edema | 0 | 0 | 0 | 0 |
|
| ||||
| Erosion | 0 | 2 (11.8) | 0 | 2 (9.1) |
|
| ||||
| Synovitis | 1 (4.8) | 4 (23.5) | 1 (6.25) | 4 (18.2) |
|
| ||||
| Tenosynovitis | 0 | 0 | 0 | 0 |
Number and percentage of participants with worsening in their MRI scores at 6-months or at time of symptom onset. No difference in scores met P<0.05
Clinical Course
Of the 2 patients who stopped AI due to musculoskeletal symptoms, one developed deQuervain’s tenosynovitis at week 10. Alhough the RAMRIS-TS scores were not different between groups, in this particular patient, an interim MRI showed fluid within the abductor pollicis longus and extensor pollicus brevis tendons (Figure 2). Symptoms continued for one month off AIs despite conservative therapy, and resolved after corticosteroid injection. The second patient developed generalized body aches at 8 weeks. She stopped AIs at 5 months. She remained symptomatic at her final visit 1 month after AI discontinuation.
Figure 2.
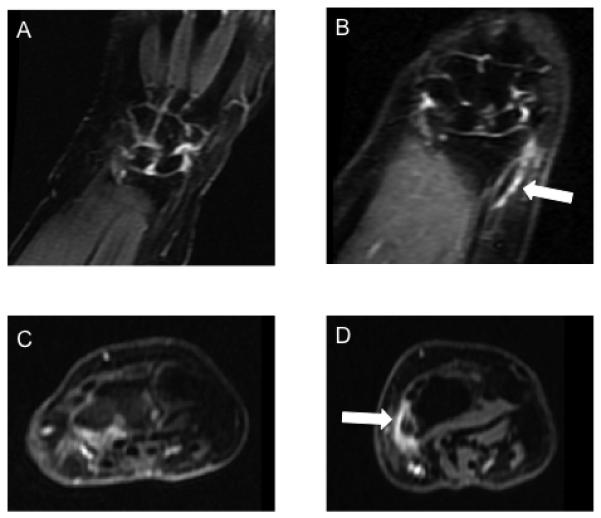
MRI of the hands at baseline (A & C) and 6 months (B & D) in a patient with new onset DeQuervain’s tenosynovitis. Demonstrates abnormal increased T2 signal on coronal stir (B) and enhancement on axial post-contrast images (D) in the first extensor compartment including the right abductor pollicis longus and extensor pollicis brevis tendons
DISCUSSION
In this prospective study we describe the AI-associated musculoskeletal syndrome in a large cohort of women. Over half of enrolled women developed new or worsening musculoskeletal symptoms. Seventy-five percent of symptomatic women presented within 8 weeks of initiation of AI. Our incident rate was consistent with other practice-based studies but higher than those from RCTs.[1-10] In a cross-sectional study, 47% of 200 women questioned in an oncology waiting room reported AI associated joint symptoms.[9] This is comparable to findings from a nested cohort study of the Consortium on Breast Cancer Pharmacogenetics RCT were 45% of participants developed musculoskeletal symptoms.[8] The increased publicity surrounding the musculoskeletal side effects of AIs and the heightened awareness of those willing to participate in a study of these symptoms may account for the higher incident rates than those seen in RCTs.
Non-adherence with AIs due to musculoskeletal symptoms was lower in our study (3.8%) than previously reported (5-13%).[8,11] This may be in part due to women’s unwillingness to stop hormonal therapy given the increasing awareness of the benefit of AIs on breast cancer outcomes.
We were able to identify several possible predictors of symptom development. Later stage cancer correlated with symptom development. However, contrary to findings from previous studies, development of musculoskeletal symptoms did not seem to be related to chemotherapy or in particular taxol exposure. [7,10]
Women with poorer baseline quality of life as measured by the SF 36 and FACT-B were also more likely to develop musculoskeletal symptoms. Interestingly, the mean difference in baseline SF-36 PCS of 6 between symptomatic and asymptomatic women is larger than the minimum clinically important difference.[17] If women who develop pain have worse physical function at baseline, this is a potentially modifiable risk factor for the AI syndrome.
In our study, greater than 50% of symptomatic patients had involvement of the hand and wrist. Despite this, there was no difference in change in grip strength, our primary outcome chosen based on existing literature.[10] However, the profound effects on the hands were evident by the impressive change in AUSCAN Hand index.
A key finding in our study is that symptoms appeared to reflect tenosynovial pathology that was localized to the hands and feet. This presentation seems to be the defining clinical feature of this syndrome. Thirteen of twenty-eight patients with symptoms had evidence of tenosynovitis. The absence of autoantibody development or correlation between pain and inflammatory markers suggests a localized enthesitis/tenosynovitis rather than a systemic inflammatory process. Furthermore, the low frequency of lower back and knee complaints, which are typical sites of post-menopausal pain, suggest pathology other than osteoarthritis of menopause.
Previous studies have also described tenosynovitis of the hands.[10,15-16] In a cross-sectional study of 12 symptomatic women on AIs, the most common symptoms were those of carpal tunnel syndrome and trigger finger.[15] MRIs showed tendon sheath enhancement. In a follow up prospective study by the same authors, 12 women on AIs were more likely to have MRI changes at 6 months consistent with tendon pathology of the hands than the 5 control patients on tamoxifen.[16] A subsequent review of the Anastrozole alone or in combination with Tamoxifen versus Tamoxifen Alone (ATAC) trial showed that clinical carpal tunnel syndrome was reported as an adverse effect more frequently in the anastrozole group compared with the tamoxifen group (2.6% v 0.7%; P =.0001).[18] In our study, tenosynovial pathology was not isolated to the hand. To our knowledge, we are the first to describe symptoms and physical findings of the foot and ankle consistent with plantar fasciitis and Achilles tendinitis in this patient population.
Despite our clinical findings, as well as MRI changes in individual symptomatic patients, tenosynovial pathology was not consistently demonstrated on MRI using the RAMRIS-TS-even among patients with tenosynovial symptoms. There are several possible explanations. There were delays between the onset of musculoskeletal symptoms and both the reporting of thise symptoms as well as obtaining MRIS. Therefore, MRIs may not have been performed at the time of peak symptoms. Additionally, our protocol was limited to bilateral hand images using a 1.5 Tessla MRI. Three Tessla MRI imaging of each hand and wrist individually may have improved capture image quality and increased the radiologist’s ability to identify subtle tenosynovial changes. Furthermore, we chose to use a validated scoring system for musculoskeletal MRI of the hands the RAMRIS however, may not have been the ideal scoring system. The RAMRIS, was designed and validated for use in rheumatoid arthritis, a systemic disease. It includes evaluation of multiple anatomic sites resulting in a wide range for total scores. Though we used our data to derive meaningful cut-offs to dichotomize positive and negative scores, inflammation in our population was focal and the RAMRIS-TS may not provide enough discrimination for such relatively localized tenosynovial process.
The etiology of AI-associated arthralgia remains elusive. Estrogen deprivation has been proposed as the major contributor to joint symptoms. Low estrogen states have been associated with arthralgias as early as 1925 when Cecil and Archer first described “arthralgia of menopause.”[19] In a retrospective analysis of the ATAC trial, participants who reported musculoskeletal and vasomotor symptoms at their 3-month study visit had lower cancer recurrence rates. The authors suggest that this indicates lower estrogen levels achieved in this population account for their musculoskeletal symptoms.[20] Contrary to this hypothesis, we found no correlation between endocrine symptoms on the FACT-B ES and musculoskeletal symptoms. In fact, none of the women who stopped AIs due to endocrine side effects developed musculoskeletal pain. Given these findings, it is important to entertain the idea that other mechanisms aside from estrogen depletion contribute to symptoms. More research needs to be done to pursue alternate etiologies.
There are several limitations to our study. Recruitment was from an oncology unit at a single institution. The relatively homogenous patient group reflects the local patient mix, which limits generalizability. Due to prescribing patterns at that center, the majority of participants were taking anastrozole and no conclusions can be made about the other third generation AIs. Another limitation is the lack of a control group. Though women being started on tamoxifen as well as those with hormone receptor negative breast cancer were entertained as possible controls, at our center, the former are pre-menopausal and younger and the latter undergo more aggressive treatment regimens. Both were not considered an appropriate control group.
In conclusion, the results presented from this large prospective study describe the incidence, time to onset, risk factors and clinical characteristics of the AI associated musculoskeletal syndrome.. Over half of women prescribed this important hormonal therapy develop musculoskeletal symptoms, most by 8 weeks after starting AIs. Our data suggest that poorer QOL may be a risk factor for symptom development and it is tempting to speculate there may be a benefit of improving physical function before starting AIs. Oncologists prescribing AIs can use this information to counsel women prior to initiation of these drugs.
Most notably, the defining clinical presentation of the syndrome is that of a focal tenosynovitis, most often involving the hands and feet, not associated with evidence of systemic inflammation or autoimmune disease. Knowledge of the localized nature of this syndrome will aid treating rheumatologist streamline their evaluations and focus management of these patients.
Significance and Innovations.
Over 50% of women starting Aromatase Inhibitors develop new or worsening pain
Focal tenosynovitis of the hand and foot is the most common presentation
Later stage breast cancer and poorer QOL are predictive of symptom development
Financial Support
Funding for this study came from the Clinical Translational Science Center NIH - UL1 RR024996, NY Community Trust Grant 53273220, Anne Moore Breast Cancer Fund, NIH Mentored Patient-Oriented Research Career Development Award - K-NIAMS K23 AR050607. I, Dr. Ora Singer, received the ACR-REF Physician Scientist Development Award providing salary support in association with this work. There was no support or other benefits from commercial sources. The study sponsors had no role in the study design; in the collection, analysis and interpretation data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
None of the authors have financial interests, which could create a possible conflict of interest.
References
- 1.Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet. 2002;359:2131–9. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 2.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 3.Howell A, Cuzik J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 4.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–09. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 5.Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, et al. Survival and safety of exemestane versus tamoxifen after 2-3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–70. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 6.Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–62. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 7.Sestak I, Cuzick J, Sapunar F, Eastell R, Farbes JF, Bianco AR, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–72. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 8.Buzdar A, Howell A, Cuzick J, Wale C, Distler W, Hoctin-Boes G, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7:633–43. doi: 10.1016/S1470-2045(06)70767-7. [DOI] [PubMed] [Google Scholar]
- 9.Henry NL, Giles JT, Ang D, Mohan M, Dadabhy D, Robarge J, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111:365–72. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–83. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 11.Dizdar O, Özçakar L, Malas FU, Harputluoglu H, Bulut N, Akspy S, et al. Sonographic and electrodiagnostic evaluations in patients with aromatase inhibitor-related arthralgia. J Clin Oncol. 2009;27:1956–61. doi: 10.1200/JCO.2008.20.5435. [DOI] [PubMed] [Google Scholar]
- 12.Larche M, Borg S, Lassoued S, D LaFontain B, Roche H. Joint Pain with Aromatase Inhibitors: Abnormal Frequency of Sjögren’s Syndrome. J Rheum. 2007;34:2259–63. [PubMed] [Google Scholar]
- 13.Donnellan PP, Douglas SL, Cameron DA, Leonard RC. Aromatase Inhibitors and Arthralgia. J Clin Oncol. 2001;19:2767. Letter. [PubMed] [Google Scholar]
- 14.Ohsako T, Inoue K, Nagamoto N, Yoshida Y, Nakahara O, Sakamoto N. Joint Symptoms: A Practical Problem of Anastrozole. Breast Cancer. 2006;13:284–88. doi: 10.2325/jbcs.13.284. [DOI] [PubMed] [Google Scholar]
- 15.Morales L, Pans S, Paridaens R, Westhovens R, Timmerman D, Verhaeghe J, et al. Debilitating musculoskeletal pain and stiffness with letrozole and exemestane: associated tenosynovial changes on magnetic resonance imaging. Breast Cancer Res Treat. 2007;104:87–91. doi: 10.1007/s10549-006-9394-6. [DOI] [PubMed] [Google Scholar]
- 16.Morales L, Pans S, Verschueren K, Van Calster B, Paridaens R, Westhovens R, et al. Prospective Study to Assess Short-Term Intra-Articular and Tenosynovial Changes in the Aromatase Inhibitor-Associated Arthralgia Syndrome. J Clin Oncol. 2008;26:3147–52. doi: 10.1200/JCO.2007.15.4005. [DOI] [PubMed] [Google Scholar]
- 17.Angst F, Aeschlimann A, Stucki G. Smallest Detectable and Minimal Clinically Important Differences of Rehabilitation Intervention with their Implications for Required Sample Sizes Using WOMAC and SF-36 Quality of Life Measurement Instruments I Patients With Osteoarthritis of the Lower Extremities. Arth Care. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Sestak I, Sapunar F, Cuzak J. Aromatase inhibitor-induced carpal tunnel syndrome: results from the ATAC trial. J Clin Oncol. 2009;27:4961–5. doi: 10.1200/JCO.2009.22.0236. [DOI] [PubMed] [Google Scholar]
- 19.Cecil RL, Archer BH. Arthritis of the menopause. JAMA. 1925;84:75–79. [Google Scholar]
- 20.Cuzik J, Sestak I, Cella D. Fallowfield. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9:1143–48. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]