Abstract
Aims
Previous data suggest heterogeneity in laminar distribution of the pathology in the molecular disorder frontotemporal lobar degeneration (FTLD) with transactive response (TAR) DNA-binding protein of 43kDa (TDP-43) proteinopathy (FTLD-TDP). To study this heterogeneity, we quantified the changes in density across the cortical laminae of neuronal cytoplasmic inclusions (NCI), glial inclusions (GI), neuronal intranuclear inclusions (NII), dystrophic neurites (DN), surviving neurons, abnormally enlarged neurons (EN), and vacuoles in regions of the frontal and temporal lobe.
Methods
Changes in density of histological features across cortical gyri were studied in ten sporadic cases of FTLD-TDP using quantitative methods and polynomial curve-fitting.
Results
Our data suggest that laminar neuropathology in sporadic FTLD-TDP is highly variable. Most commonly, NCI, DN, and vacuolation were abundant in the upper laminae and GI, NII, EN, and glial cell nuclei in the lower laminae. TDP-43-immunoreactive inclusions affected more of the cortical profile in longer duration cases, their distribution varied with disease subtype, but was unrelated to Braak tangle score. Different TDP-43-immunoreactive inclusions were not spatially correlated.
Conclusions
Laminar distribution of pathological features in ten sporadic cases of FTLD-TDL is heterogeneous and may be accounted for, in part, by disease subtype and disease duration. In addition, the feed-forward and feed-back cortico-cortical connections may be compromised in FTLD-TDP.
Keywords: Frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP), FTLD with ubiquitin-positive inclusions (FTLD-U), Transactive response TAR DNA-binding protein of 43 kDa (TDP-43), Neuronal cytoplasmic inclusions (NCI), Laminar distribution
Introduction
Frontotemporal lobar degeneration (FTLD) is the second most common form of cortical dementia of early-onset after Alzheimer’s disease (AD) [1]. The disorder is associated with a heterogeneous group of clinical syndromes including behavioural variant frontotemporal dementia (bvFTD), FTD with motor neuron disease (FTD-MND), progressive non-fluent aphasia (PNFA), semantic dementia (SD), and progressive apraxia (PAX) [2].
FTLD with transactive response (TAR) DNA-binding protein of 43kDa (TDP-43) proteinopathy (FTLD-TDP), previously called FTLD with ubiquitin-immunoreactive inclusions (FTLD-U) [3,4], is characterized by a variable neocortical and allocortical atrophy principally affecting the frontal and temporal lobes. In addition, there is neuronal loss, microvacuolation largely affecting the superficial cortical laminae, and a reactive astrocytosis [5,6]. A variety of TDP-43-immunoreactive inclusions are present including neuronal cytoplasmic inclusions (NCI), neuronal intranuclear inclusions (NII), dystrophic neurites (DN), and glial inclusions (GI) [6]. Based on the distribution and density of inclusions, there have been attempts to classify FTLD-TDP into subtypes [7–10]. Most schemes define four pathological subtypes, based originally on ubiquitin immunohistochemistry (IHC) but extended to cases of FTLD-TDP, and which utilize the distribution and density of the pathological changes in neocortical regions. The same descriptors have been used to define subtypes but the numbering of each subtype varies between different schemes. Using a consensus system proposed by Cairns et al. [10] and based on both genetics and previous schemes: type 1 cases (Mackenzie-type 2) are characterized by long DN in superficial cortical laminae with few or no NCI or NII, type 2 (Mackenzie-type 3) by numerous NCI in superficial and deep cortical laminae with infrequent DN and sparse or no NII, type 3 (Mackenzie-type 1) by pathology predominantly affecting the superficial cortical laminae with numerous NCI, DN and varying numbers of NII, and type 4 by numerous NII, and infrequent NCI and DN especially in neocortical areas. Although there may be no clear distinction between the various subtypes [6], it is still possible that there are different distribution patterns of pathology that characterize FTLD.
In many neurodegenerative disorders, the density of pathological inclusions varies significantly across the cortical laminae from pia mater to white matter [11–15]. The laminar distribution of an inclusion may reflect degeneration of neural pathways that have their cells of origin or axon terminals located within one or more laminae and can therefore indicate the pattern of cortical degeneration in a disorder [16,17]. Previous studies based on subjective and semi-quantitative estimates of inclusion density, suggest heterogeneity in laminar distribution of the pathology in FTLD-TDP [7–10] but these differences have not been systematically investigated using quantitative methods. To study this heterogeneity, we quantified the changes in density across the cortical laminae of the TDP-43-immunoreactive inclusions, abnormally enlarged neurons (EN), surviving neurons, vacuoles, and glial cell nuclei in gyri of frontal and temporal cortex in ten cases of the disease. Quantitative methods and a polynomial curve-fitting procedure [18] were used to study changes in density of each histological feature across the gyri. The specific objectives were: (1) to describe the changes in density of histological features across the laminae, (2) to determine whether the distribution of the TDP-43-immunoreactive inclusions was correlated with disease duration, stage of disease, and consistent with previously assigned disease subtypes, and (3) to determine the spatial correlations of the pathological features.
Materials and methods
Cases
Ten cases of sporadic FTLD-TDP (see Table 1) were obtained from dementia centers in the USA and Canada: Vancouver General Hospital, Vancouver, Canada (5 cases), University of Pittsburgh, Pittsburgh, PA (3 cases), and Harvard Brain Tissue Resource Center, Belmont, MA (2 cases). All cases were without a family history of neurodegenerative disease and none had mutations in genes known to cause TDP-43 proteinopathy, viz., progranulin (GRN) [19–24], valosin-containing protein (VCP) [25], TAR DNA-binding protein (TARDBP) [26–29], or C90RF72 [30, 31]. All cases exhibited FTLD with neuronal loss, microvacuolation of the superficial cortical laminae, and a reactive astrocytosis consistent with proposed diagnostic criteria for FTLD-TDP [10]. A variety of TDP-43-immunoreactive inclusions was present including NCI, NII, DN, and GI consistent with a diagnosis of TDP-43 proteinopathy [10]. One case had coexisting motor neuron disease (FTLD-MND) [32,33] but none met 'Consortium to Establish a Registry of Alzheimer's Disease' (CERAD) [34], ‘National Institute on Aging (NIA)-Reagan Institute’ criteria [35,36], or NIA-Alzheimer’s Association Guidelines [37] for a neuropathological diagnosis of Alzheimer’s disease (AD) and none had associated hippocampal sclerosis (HS). Staging of cases was based on the Braak tangle score [38]. Cases had been assigned previously to the four proposed subtypes of FTLD-TDP by an experienced neuropathologist using the composite (‘harmonized’) scheme of Cairns et al. [10]. Only subtypes 1, 2 and 3 were represented in the present sample as subtype 4 may be more frequently associated with familial FTLD-TDP with VCP gene mutation [25].
Table 1.
Demographic features, gross brain weight (BW), Braak tangle stage, and disease subtype of the ten cases of frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP).
| Case | Centre | Sex | Onset | Death | Duration | BW | Braak | Assigned |
|---|---|---|---|---|---|---|---|---|
| stage | ST | |||||||
| A | H | F | 80 | 88 | 8 | 1035 | III | 2 |
| B | H | M | 73 | 79 | 6 | 1180 | II | 3 |
| C | V | F | - | 82 | - | 940 | 0 | 1 |
| D | V | M | 69 | 72 | 3 | - | 0 | 2 |
| E | V | M | 66 | 71 | 5 | 1230 | II | 3 |
| F | V | F | 55 | 70 | 15 | - | III | 1 |
| G* | V | M | 70 | 71 | 1 | 1140 | IV | 2 |
| H | P | M | 61 | 65 | 4 | 1100 | II | 2 |
| I | P | M | - | 76 | - | 1440 | III | 1 |
| J | P | M | 61 | 64 | 3 | 1330 | I | 2 |
Abbreviations: H = Harvard Brain Tissue Resource Center, V = Vancouver General Hospital, P = Department of Pathology, University of Pittsburgh, M = Male, F = Female, Disease onset, age at death, and disease duration are given in years, BW = Brain weight (gm), Braak stage represents staging of Alzheimer disease-associated neurofibrillary pathology, ST = previously assigned disease subtype according to Cairns et al (2007).
(-)= data not available,
Case with associated motor neuron disease (MND)
Histological methods
After death, the consent of the next of kin was obtained for brain removal following local Ethical Committee procedures and the 1995 Declaration of Helsinki (as modified Edinburgh, 2000). Tissue blocks were taken from the frontal lobe at the level of the genu of the corpus callosum to study the middle frontal gyrus (MFG) and the temporal lobe at the level of the lateral geniculate body to study the inferior temporal gyrus (ITG), and parahippocampal gyrus (PHG). Tissue was fixed in 10% phosphate buffered formal-saline and embedded in paraffin wax. Following formic acid (95%) pretreatment for 5 minutes, IHC was performed on 4 – 10µm sections with a rabbit polyclonal antibody that recognizes physiologic TDP-43 epitopes (dilution 1:1000; ProteinTech Inc., Chicago, IL). Sections were also stained with haematoxylin.
Morphometric methods
In gyri with sufficient densities of inclusions, the distribution of the NCI, GI, NII, and DN together with the surviving neurons, EN, vacuoles, and glial cell nuclei was studied from pia mater to white matter using methods described previously [39]. TDP-immunoreactive pathology is not distributed evenly along the gyri. Hence, five traverses from pia mater to the edge of the white matter were located randomly along each gyrus. All histological features were then counted in 50 × 250µm sample fields arranged contiguously, the larger dimension of the field being located parallel with the surface of the pia mater. An eye-piece micrometer comprised the sample field and was moved down each traverse one step at a time from pia mater to the edge of the white matter. Histological features of the section were used to correctly position the field.
The NCI were rounded, spicular, or skein-like [6,40,41], while the GI morphologically resembled the ‘coiled bodies’ reported in tauopathies such as corticobasal degeneration (CBD) [42,43], progressive supranuclear palsy (PSP) [44–46], and argyrophilic grain disease (AGD) [47]. The NII were lenticular, spindle-shaped, or circular in shape [6,48] and the DN were long and contorted [6,49]. It can be difficult to identify surviving neurons without special stains. Hence, surviving neurons were identified in the TDP-43 sections as cells containing at least some stained cytoplasm in combination with larger shape and non-spherical outline [50]. All perikarya meeting these criteria were counted not just the pyramidal cells. Small spherical or asymmetrical nuclei without cytoplasm, but with the presence of a thicker nuclear membrane and more heterogeneous chromatin, were identified as glial cells. EN had enlarged perikarya, lacked NCI, had a shrunken nucleus displaced to the periphery of the cell, and a maximum cell diameter at least three times that of the nucleus [50]. The number of discrete vacuoles that were greater than 5µm in diameter was also counted in each sample field [51,52]. It can be difficult to differentiate microvacuolation of the neuropil from vacuolation around neurons and blood vessels attributable to artifacts of processing. Hence, vacuoles clearly associated with such structures were not counted. The mean of the counts from the five traverses was calculated to study variations in density of each histological feature across the gyrus.
Data analysis
No attempt was made to locate precisely the boundaries between individual cortical laminae. First, the degree of cortical degeneration present in many gyri made laminar identification difficult. Second, identification was especially difficult in the frontal cortex because it exhibits a heterotypical structure, i.e., six laminae cannot always be clearly identified and vary in prominence from case to case. Third, inclusions appeared to exhibit complex patterns of distribution across the cortex rather than being confined to specific laminae. Hence, variations in lesion density with distance across the cortex were analyzed using a polynomial curve-fitting procedure (STATISTICA software, Statsoft Inc., 2300 East 14th St, Tulsa, OK, 74104, USA) [11,18,53]. For each gyrus, polynomials were fitted successively to the data. Hence, quadratic curves are parabolic, cubic curves are ‘S’ shaped and quartic curves often appear as ‘double-peaked’ or ‘bimodal’. With each fitted polynomial, the correlation coefficients (Pearson’s ‘r’), regression coefficients, standard errors (SE), values of t, and the residual mean square were obtained [18]. At each stage, the reduction in the sums of squares (SS) was tested for significance. The analysis was continued until either a non-significant value of F was obtained or there was little gain in the explained variance [18]. Polynomials greater than the fourth order did not fit any of the distributions. The shape of the fitted polynomial curve was used to classify the distribution of each histological feature across the cortex. Hence, histological features exhibiting a single peak of density were described as ‘unimodal’, peak density being located either in the upper (approximating to laminae I,II,III) or lower (approximating to laminae V,VI) cortex while those with two peaks as ‘bimodal’, peaks of density occurring in the upper and lower cortex. These histological features were then classified further according to whether the density peaks were of similar or different magnitude in upper and lower cortex. To examine the relationship between laminar distribution, disease duration, Braak tangle stage [38], and disease subtype [7–10], the frequencies of the different types of distribution exhibited by the TDP-43-immunoreactive inclusions were compared using chi-square (χ2) contingency table tests.
To determine the degree to which different histological features occurred within the same or different regions of the cortex or were distributed independently of each other, correlations between the densities of all histological features were tested using Pearson's correlation coefficient (‘r’).
Results
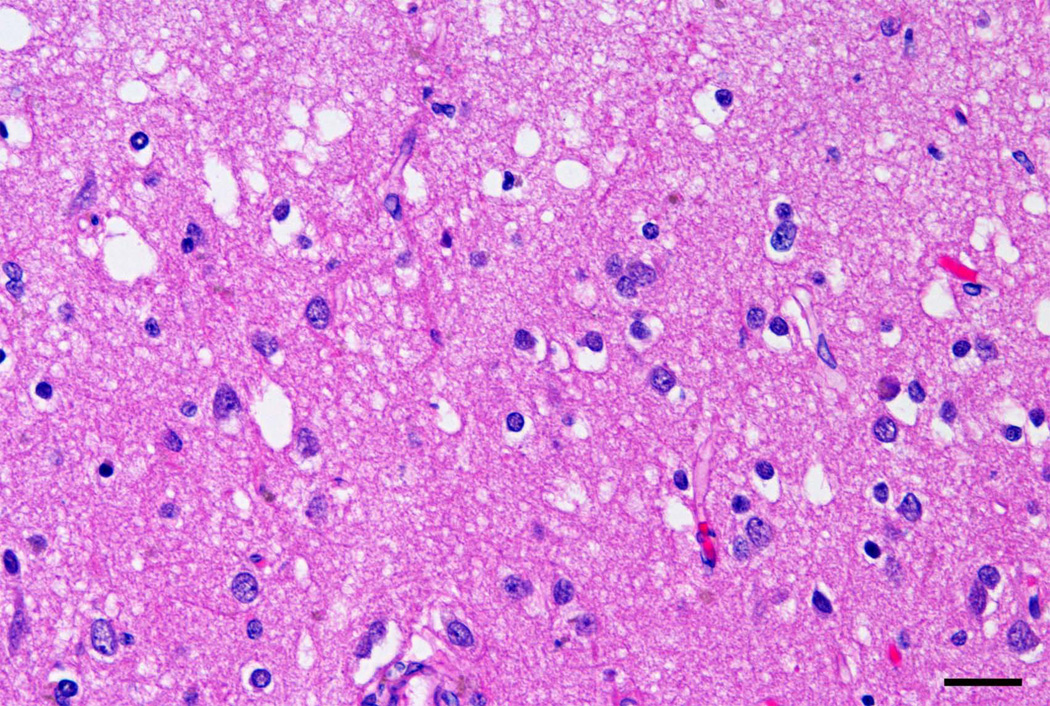
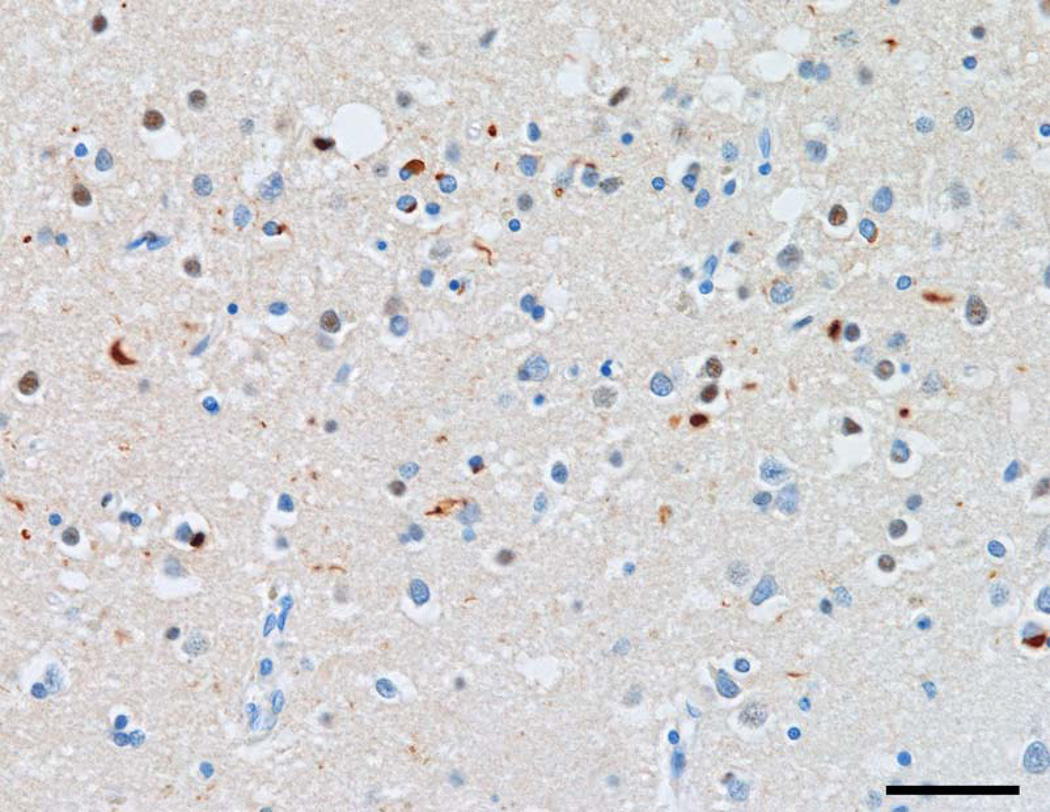
Examples of the distribution of the pathology in sporadic FTLD-TDP cases are shown in Figs 1 and 2. Fig 1 shows extensive vacuolation in the ITG, typically present in the superficial laminae of many gyri, while Fig 2 shows TDP-43-immunoreactive inclusions in the upper laminae of the ITG.
Fig 1.
Vacuolation in lamina II of the inferior temporal gyrus (ITG) in a case of frontotemporal lobar degeneration with TDP proteinopathy (FTLD-TDP) (H/E, bar = 20µm).
Fig 2.
TDP-43-immunoreactive inclusions in laminae II/III of the inferior temporal gyrus (ITG) in a case of frontotemporal lobar degeneration with TDP proteinopathy (FTLD-TDP) (TDP-43 immunohistochemistry, bar = 20µm).
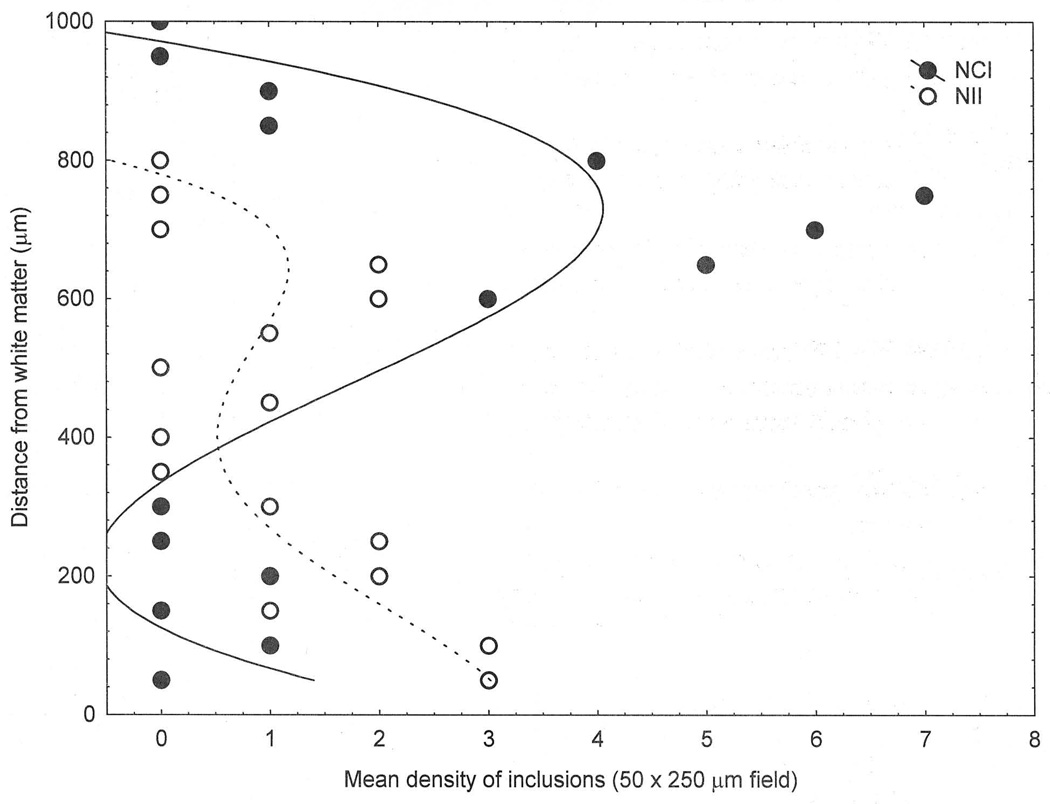
Examples of the changes in density of NCI and NII across the cortex and the curve-fitting procedure are shown in Fig 3. The distribution of NCI in the MFG of case D was fitted by a third-order polynomial (r = 0.70, P < 0.001), a large density peak being present in the upper cortex, and significantly lower densities of NCI in the lower cortex. The distribution of NII in the ITG of case F was also fitted by a third-order polynomial (r = 0.65, P < 0.01). The distribution of the NII overlapped with the NCI, but the greatest densities of NII occurred in the lower cortex.
Fig 3.
Examples of the distribution of the neuronal cytoplasmic inclusions (NCI) and neuronal intranuclear inclusions (NII) in the middle frontal gyrus (MFG) of a case of frontotemporal lobar dementia with TDP-43 proteinopathy (FTLD-TDP). Curve of best fit: NCI, third order polynomial (r = 0.70, P < 0.001); NII, third-order polynomial (r = 0.65, P < 0.01).
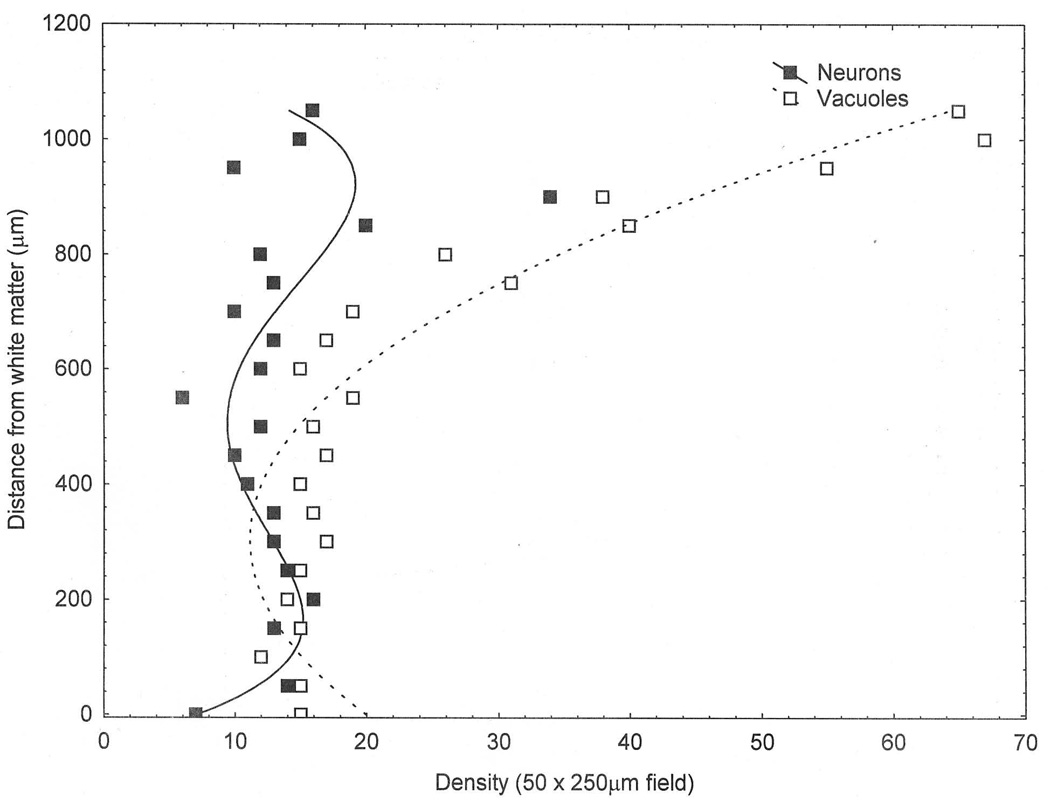
Examples of the distribution of surviving neurons and vacuoles are shown in Fig 4. The distribution of surviving neurons in the MFG of case B was fitted by a fourth-order polynomial (r = 0.61, P < 0.01) indicating a bimodal distribution, i.e., the distribution was double-peaked, densities in the upper laminae being slightly greater, with the exception of a single datum point, than those in the lower laminae. The distribution of the vacuoles in the PHG of case A was fitted by a second-order polynomial (r = 0.96, P < 0.001). Hence, although some vacuolation is evident throughout the cortical profile, the density of the vacuoles was greatest in the superficial laminae declining markedly with distance across the cortex.
Fig 4.
Examples of the distribution of the surviving neurons and vacuolation in the middle frontal gyrus (MFG) in a case of frontotemporal lobar dementia with TDP-43 proteinopathy (FTLD-TDP). Curve of best fit: Surviving neurons, fourth-order polynomial (r = 0.0.61, P < 0.01); Vacuolation, second order polynomial (r = 0.96, P < 0.001).
The results of the curve fitting procedure for each histological feature in all gyri studied are shown in Table 2 and a summary of these distributions in Table 3. The NCI were distributed largely in the upper laminae in 7/23 (30%) of gyri studied and in further 8 gyri, a bimodal distribution was present, the density peak in the upper cortex being larger or of similar magnitude to that in the lower cortex. In 6/23 (26%) gyri, there was no significant change in the density of NCI across the cortex. In 4/6 (67%) gyri, the density of GI was greatest in the lower cortex. Similarly, the NII frequently exhibited greater densities in the lower cortex, 11/21 (52%) gyri showing this pattern. In addition, NII exhibited a smaller density peak in the upper cortex and in 8 gyri, there were no differences in NII density across the cortex. In 6/13 (46%) gyri, the density of DN was greatest in the upper cortex. The density of the EN was greatest in the lower cortex in 6/20 (30%) gyri but in the remaining gyri, EN were more uniformly distributed across the cortex. The distribution of the surviving neurons was highly variable. In 17/30 (57%) gyri, the distribution of the surviving neurons was bimodal, the density in the upper cortex being either greater than or similar to that in the lower cortex. In 8 gyri, there were no significant differences in the density of surviving neurons across the cortex. The greatest density of the vacuolation was present in the upper cortex, 25/30 (83%) gyri showing this pattern. In 17/30 (57%) of these gyri, the vacuoles also exhibited a smaller density peak in the lower cortex. Glial cell nuclei were more abundant in the lower cortex and in many gyri, glial cell density increased linearly across the cortex from pia mater to white matter. There were no consistent differences in the distribution of histological features between gyri of the frontal and temporal cortex, or in the case with associated MND compared with the cases without MND.
Table 2.
Results of the polynomial curve-fitting procedure for each histological feature features
| Case | Feature | MFG | ITG | PHG |
|---|---|---|---|---|
| A | NCI | 4, 0.45* (U) | 1, 0.45*(U) | 3, 0.61*(U) |
| GI | 4, 0.80**(L) | NS | 3, 0.67*(L) | |
| NII | - | 2, 0.60*(L) | 3, 0.66*(L) | |
| EN | 1,0.61*(U) | 4,0.42*(U = L) | - | |
| SN | 1,0.72***(U) | NS | NS | |
| V | 4,0.77(U) | 1,0.59*(U) | 2,0.96*(U) | |
| DN | - | - | - | |
| GL | 1,0.88**(L) | 1,0.80***(L) | 1,0.82***(L) | |
| B | NCI | NS | - | 3, 0.48*(U) |
| GI | - | NS | 3, 0.67*(L) | |
| NII | - | 2, 0.60*(L) | 3, 0.66*(L) | |
| EN | 1,0.61*(U) | 4,0.42*(U = L) | - | |
| SN | 1,0.72***(U) | NS | NS | |
| V | 4,0.77(U) | 1,0.59*(U) | 2,0.96***(U) | |
| DN | - | - | - | |
| GL | 1,0.88**(L) | 1,0.80***(L) | 1,0.82***(L) | |
| C | NCI | - | NS | - |
| GI | - | - | 4, 0.61*(L) | |
| NII | - | - | - | |
| EN | NS | NS | - | |
| SN | 1,0.52**(U) | 3,0.64** (U = L) | 1,0.70***(U) | |
| V | 1,0.79***(U) | 3,0.70**(U) | 4,0.72**(U = L) | |
| DN | 4,0.76***(U) | 2.0.49*(L) | 2,0.73**(U = L) | |
| GL | 1,0.86**(L) | 1,0.73***(L) | 4,0.63*(L) | |
| D | NCI | 3,0.70***(U) | 4,0.52*(U) | 4, 0.59**(U) |
| GI | NS | - | - | |
| NII | 3,0.48*(L) | - | 1,0.49*(L) | |
| EN | 4,0.47*(U) | NS | - | |
| SN | 4,0.62**(U) | 3,0.59**(U) | 4,0.56**(U = L) | |
| V | 4,0.91***(U) | 3,0.88***(U) | 3,0.92***(U) | |
| DN | - | - | - | |
| GL | 1,0.82**(L) | 1,0.67***(L) | 2,0.69***(L) | |
| E | NCI | NS | 4,0.90***(U) | 4, 0.49*(U = L) |
| GI | 1, 0.42*(L) | - | - | |
| NII | NS | NS | - | |
| EN | NS | 2,0.42*(L) | NS | |
| SN | NS | NS | 2,0.49*(U) | |
| V | 3,0.90***(U) | 2,0.57** | 4,0.64**(U) | |
| DN | - | 3,o.38*(U) | - | |
| GL | 1,0.59***(L) | 1,0.69***(L) | 3,0.78***(L) | |
| F | NCI | - | - | - |
| GI | - | 4,0.57* (L) | - | |
| NII | 3,0.52*(L) | 3.0.65*(L) | 3,0.54*(L) | |
| EN | - | - | - | |
| SN | 4,0.53**(U) | 3,0.83***(U) | 4,0.44*(U = L) | |
| V | 2,0.97***(U) | 3,0.90***(U) | 2,0.91***(U) | |
| DN | 3,0.59*(U) | 2,0.78***(U) | 3,0.57** (U = L) | |
| GL | 1,0.85**(L) | 3,0.88***(L) | 1,0.86***(L) | |
| G | NCI | - | 4,0.56**(U = L) | - |
| GI | - | - | - | |
| NII | NS | 4,0.54**(L) | NS | |
| EN | 2,0.46**(L) | 4,0.39*(U) | 3,0.41*(U = L) | |
| SN | 4,0.39*(U) | 3,0.41*(U = L) | 3,0.87***(U) | |
| V | 2,0.92**(U) | 4,0.88**(U) | 3,0.90**(U) | |
| DN | - | NS | 3,0.80** (L) | |
| GL | 1,0.78***(L) | 3,0.88***(L) | 2,0.83***(L) | |
| H | NCI | 3,0.69***(U) | 3,0.73***(U) | 3,0.85***(U) |
| GI | - | - | - | |
| NII | - | - | - | |
| EN | NS | 2,0.40*(L) | - | |
| SN | NS | 4,0.80***(U) | NS | |
| V | 4,0.60**(U) | 2,0.75**(U) | 2,0.79***(U) | |
| DN | - | - | - | |
| GL | 1,0.88***(L) | 1,0.84***(L) | 1,0.95***(L) | |
| I | NCI | 1,0.43*(L) | NS | 4,0.71***(U) |
| GI | - | - | - | |
| NII | 4,0.82***(L) | NS | NS | |
| EN | 4,0.59**(L) | NS | 1,0.50**(L) | |
| SN | 3,0.70***(U = L) | 4,0.63**(U = L) | 4,0.57***(U) | |
| V | 2,0.76***(U) | 2,0.42*(U = L) | 4,0.67***(U = L) | |
| DN | 3,0.72***(L) | - | - | |
| GL | 1,0.91***(L) | 1,0.78***(L) | 1,0.83***(L) | |
| J | NCI | 3,0.61**(U) | NS | NS |
| GI | - | - | - | |
| NII | NS | 4,0.54**(L) | NS | |
| EN | - | 4,0.55*(U) | NS | |
| SN | NS | NS | 2,0.70***(U = L) | |
| V | 2,0.84***(U) | 2,0.86***(U) | 4,0.95**(U) | |
| DN | NS | 3,0.78***(L) | - | |
| GL | 1,0.84***(L) | 1,0.80***(L) | 2,0.73**(L) |
(NCI = Neuronal cytoplasmic inclusions, GI = Glial inclusions, NII = Neuronal intranuclear inclusions, DN = Dystrophic neurites, EN = Abnormally enlarged neurons, V = Vacuolation, SN = Surviving neurons, GL = Glial cell nuclei) in each cortical gyrus. The first figure of each entry is the order of polynomial fitted to the data (1 = linear, 2 = quadratic, 3 = cubic, 4 = quartic), the second figure is the statistical significance of the fitted curve (*P < 0.05, **P < 0.01, ***P < 0.001, NS = no significant curve fitted the data), and the third letter in parentheses, whether a density peak was located in the upper (U) or lower (L) cortex (- indicates insufficient density of a histological feature to quantify its distribution).
Table 3.
Summary of the various types of distribution exhibited by the histological features
| Unimodal distribution | Bimodal distribution | ||||||
|---|---|---|---|---|---|---|---|
| Lesion | N | Upper | Lower | Upper > | Upper = | Upper < | NS |
| cortex | cortex | lower | lower | lower | |||
| NCI | 23 | 7 | 1 | 5 | 3 | 1 | 6 |
| GI | 6 | 1 | 1 | 0 | 0 | 3 | 1 |
| NII | 21 | 1 | 6 | 0 | 1 | 5 | 8 |
| DN | 13 | 2 | 2 | 4 | 2 | 2 | 1 |
| EN | 20 | 2 | 6 | 1 | 1 | 0 | 10 |
| SN | 30 | 4 | 0 | 9 | 8 | 1 | 8 |
| V | 30 | 8 | 0 | 17 | 4 | 0 | 1 |
| GL | 30 | 0 | 28 | 0 | 0 | 2 | 0 |
(NCI = Neuronal cytoplasmic inclusions, GI = Glial inclusions, NII = Neuronal intranuclear inclusions, DN = Dystrophic neurites, EN = Abnormally enlarged neurons, V = Vacuolation, SN = Surviving neurons, GL = Glial cell nuclei) across the cortex in gyri of the frontal and temporal lobe in ten cases of sporadic frontotemporal lobar dementia with TDP-43 proteinopathy (FTLD-TDP) (N = number of gyri with sufficient densities of lesions to study laminar distribution). Where a bimodal distribution is present, data indicate whether the density peak in the upper laminae was greater, equal to, or smaller than the density peak in the lower laminae, NS = no significant change in density from pia mater to white matter.
The relationship between the distribution of the TDP-43-immunoreactive inclusions, disease duration, and Braak tangle stage is shown in Table 4. Inclusions affecting either upper or lower cortex alone were more frequent in shorter duration cases while bimodal distributions affecting upper and lower cortex were more frequent in longer duration cases (χ2 = 10.63, 4DF, P < 0.05). There were no significant differences in the frequencies of laminar distribution when cases were classified according to Braak tangle stage (χ2 = 9.46, 6DF, P > 0.05).
Table 4.
Relationship between the distribution of TDP-43-immunoreactive inclusions, disease duration, and Braak stage in frontotemporal lobar dementia with TDP-43 proteinopathy (FTLD-TDP). Data show the frequency of gyri in which collectively, the TDP-43-immunoreactive inclusions exhibited a unimodal or a bimodal distribution, NS = No significant change in abundance across the cortex.
| Laminar distribution | |||
|---|---|---|---|
| Variable | Categories | Unimodal | Bimodal |
| Disease duration | 1 – 4 | 11 | 5 |
| (years) | 5 – 8 | 4 | 11 |
| 15 | 1 | 5 | |
| Braak stage | 0/1 | 10 | 4 |
| 2 | 4 | 6 | |
| 3 | 5 | 13 | |
| 4 | 0 | 3 | |
Chi-square (χ2) contingency table tests: Disease duration χ2 = 10.63 (4DF, P < 0.05), Braak score χ2 = 9.46 (6DF, P > 0.05), Disease subtype χ2 = 3.71 (4DF, P > 0.05)
The relationship between the distributions of NCI, NII, and DN and previously assigned disease subtypes is shown in Table 5. In cases assigned to subtype 1, NCI and NII were relatively infrequent while DN were more abundant and more commonly distributed in the upper cortex. In cases assigned to subtype 2, pathology affected both the upper and lower cortices and NCI and DN were abundant either in the upper cortex alone or a bimodal distribution was present, inclusions being present in both upper and lower cortices, and with NII predominant in the lower cortex. In cases assigned to subtype 3, the TDP-43-immunoreactive inclusions were predominantly located in the upper cortex.
Table 5.
Comparison of the distribution of the neuronal cytoplasmic inclusions (NCI), neuronal intranuclear inclusions (NII), and dystrophic neurites (DN) based on quantitative data with previously assigned disease subtypes of frontotemporal lobar degeneration with TDP proteinopathy (FTLD-TDP) classified according to the consensus scheme of Cairns et al. [10].
| Frequency of laminar distribution | |||||
|---|---|---|---|---|---|
| Inclusion | Subtype | Upper | Lower | Bimodal | NS |
| cortex | cortex | distribution | |||
| NCI | 1 | 1 | 1 | 0 | 1 |
| 2 | 6 | 0 | 5 | 2 | |
| 3 | 2 | 0 | 1 | 0 | |
| NII | 1 | 0 | 2 | 2 | 2 |
| 2 | 0 | 2 | 5 | 3 | |
| 3 | 2 | 0 | 0 | 3 | |
| DN | 1 | 4 | 0 | 0 | 0 |
| 2 | 1 | 0 | 2 | 2 | |
| 3 | 3 | 1 | 1 | 1 | |
NS = No significant change in abundance across the cortex.
A summary of the spatial correlations between the densities of the various histological features across the cortex is shown in Table 5. In the majority of gyri, there were no significant spatial correlations between the different TDP-43-immunoreactive inclusions. NCI density was positively correlated with surviving neuron density in 9/24 (38%) gyri and surviving neuron and vacuole densities were positively correlated in 14/31 (45%) gyri. Vacuoles and glial cell nuclei densities were negatively correlated in 12/30 (40%) gyri.
Discussion
Both phosphorylation-dependent (pTDP-43) and independent (iTDP-43) antibodies have been used to study TDP-43- immunoreactive pathological changes in FTLD-TDP [54–58]. Initially many groups used iTDP-43 which immunolabels normal physiological TDP-43 as well as pathological inclusions. This is a particularly useful attribute as this antibody shows clearly that in most neurons there is reduced staining in the nucleus and increased staining of the cytoplasm leading to the hypothesis that the protein is abnormally translocated in disease. However, it is often difficult to distinguish the presence of an abnormal inclusion, or pre-inclusion, in the nucleus, or even the cytoplasm where normal and abnormal staining may admix. The advantage of pTDP-43 antibodies is that they do not immunolabel normal physiological TDP-43 [54,56], especially in the nucleus, thus enabling the TDP-43-immunoreactive lesions to be more clearly visualized and quantified. In a previous study [58], we compared he densities of TDP-43-immunoreactive inclusions using iTDP-43 and pTDP-43 antibodies. On average, the pTDP-43 antibody revealed slightly more NCI and GI per sample field than iTDP-43 while on average pTDP-43 revealed slightly fewer NII than the iTDP-43 antibody. For the DN, very similar densities of DN were revealed by both antibodies. Hence, although there are some differences in density revealed by both types of antibody, these differences are unlikely to significantly affect the laminar distributions as revealed by the present iTDP-43 antibody.
The data suggest significant changes in density of histological features across the cortex in sporadic FTLD-TDP. Changes in density were variable with significant differences between gyri and cases. The most consistent patterns of distribution were: (1) significant vacuolation was present in the superficial laminae, declining in importance with distance below the pia mater and (2) a greater abundance of glial cell nuclei in the lower cortex. Of the TDP-43-immunoreactive inclusions, the NCI and DN were most frequently abundant in the upper cortex and NII and GI in the lower cortex. The quantitative data support some aspects of the classification of cases into subtypes based on subjective and semi-quantitative assessment of the pathology [7–10]. Hence, DN and NCI were more frequent in the superficial cortical laminae especially in cases assigned to subtypes 1 and 3. In addition, numerous NCI were present in superficial and deep cortical laminae in cases assigned to subtype 2. Hence, previously reported differences associated with disease subtype [7–10] could account for some of the variation in laminar distribution observed between the 10 cases. However, a much larger series of cases would need to be studied to establish whether different subtypes consistently exhibit a specific laminar distribution of inclusions. If this hypothesis is shown to be correct, it raises the possibility that different anatomical pathways may be affected in the various subtypes. Hence, both the feedforward and feedback cortico-cortical pathways may be affected in subtype 2 while the feedforward pathways may be predominantly affected in subtypes 1 and 3.
Distribution across the cortex was also related to disease duration, inclusions in shorter duration cases being more likely to be unimodal and restricted either to upper or lower laminae, while a bimodal distribution was more common in longer duration cases. These data suggest that as the disease progresses, inclusions could spread vertically within modules or columns to affect more of the cortical profile.
The distribution of the surviving neurons was especially variable in our cases. In normal elderly brain, pyramidal neurons in frontal and temporal cortex often exhibit a bimodal distribution in which peak density in the upper cortex (corresponding to laminae II/III) is often larger than in the lower cortex (corresponding to laminae V/VI) [17]. A similar bimodal distribution was observed in some gyri in FTLD-TDP, but in eight gyri, peak densities were similar in the upper and lower cortex, and in eight further gyri, surviving neurons were uniformly distributed down the cortex consistent with greater neuronal loss in the upper cortex. Pathological changes in the upper cortex could be associated with degeneration of the feed-forward cortico-cortical projections, which have their cells or origin in laminae II/III as shown in other disorders [16]. By contrast, EN, which may reflect either a stress response [58] or axonal degeneration [60,61], and glial cell nuclei were more abundant in the lower cortex consistent with degeneration of the lower laminae in FTLD-TDP. Pathological changes in lower cortex could result from degeneration of either the feed-back cortico-cortical projections [16] or the afferent and efferent cortical-subcortical projections. As in other neurodegenerative disorders, it is possible that the pathology spreads between cortical regions via cell to cell contact [16,62] and observed variations in laminar distribution between cases and gyri could represent different stages of this process.
The various TDP-43-immunoreactive inclusion were not spatially correlated in the majority of gyri suggesting that NCI, NII, DN, and GI do not affect the same cortical laminae. NCI and surviving neuron densities, however, were positively correlated in over a third of gyri suggesting that a constant proportion of all surviving neurons develop NCI in some gyri. Vacuole and surviving neuron densities were also positively correlated in nearly half of the gyri studied. Vacuolation also occurs in Creutzfeldt-Jakob disease (CJD), dementia with Lewy bodies (DLB), and advanced AD [6]. In sporadic CJD (sCJD), for example, vacuoles are often clustered around neuronal perikarya [51] and in the cerebellum of variant CJD (vCJD), clusters of vacuoles in the molecular layer are negatively correlated with surviving Purkinje cells [52]. Hence, the relationship between surviving neurons and vacuolation is complex. An initial positive correlation may arise if vacuoles develop in association with the dendrites of degenerating neurons. However, if the neuronal perikarya subsequently disappear but the vacuoles remain or vice versa, a negative correlation may result. Vacuolation within the superficial cortical laminae in FTLD-TDP could be the result of neuronal degeneration within laminae II/III subsequently affecting the ascending projections. Variation in correlation in different gyri could reflect the stage of the disease, e.g., a positive correlation between surviving neurons and vacuolation might disappear as significantly more neurons are lost. Vacuole and glial cell densities were negatively correlated in approximately half the gyri studied reflecting their relative abundance in the upper and lower laminae respectively.
In conclusion, no single pattern of laminar distribution is characteristic of the pathology of the ten sporadic FTLD-TDP cases studied. Most commonly, the pathology affected all laminae with significant vacuolation of the superficial cortical laminae and a gliosis largely affecting the lower laminae. Of the TDP-43-immunoreactive inclusions, most frequently the NCI and DN were abundant in the upper cortex and NII and GI in the lower cortex. Variations in laminar distribution of the TDP-43 immunoreactive inclusions may be explained in part by disease subtype and disease duration, the latter reflecting stages in the spread of the pathology possibly via the feed-forward and feed-back cortico-cortical projections.
Table 6.
Frequency of correlations (Pearson’s ‘r’) between the densities of histological features
| Frequency of significant correlations | ||||
|---|---|---|---|---|
| Variables | N | Positive correlation | Negative correlation | NS |
| NCI/GI | 8 | 0 | 0 | 8 |
| NCI/NII | 18 | 0 | 0 | 18 |
| NCI/DN | 10 | 1 | 0 | 9 |
| NCI/EN | 19 | 0 | 0 | 19 |
| NCI/SN | 24 | 9 | 0 | 15 |
| NCI/V | 24 | 4 | 0 | 20 |
| NCI/GL | 23 | 3 | 1 | 19 |
| GI/NII | 9 | 1 | 0 | 8 |
| GI/DN | 6 | 0 | 0 | 6 |
| GI/EN | 9 | 0 | 0 | 9 |
| GI/SN | 12 | 1 | 0 | 11 |
| GI/V | 11 | 0 | 0 | 11 |
| GI/GL | 12 | 3 | 0 | 8 |
| NII/DN | 11 | 0 | 0 | 11 |
| NII/EN | 16 | 0 | 0 | 16 |
| NII/SN | 21 | 0 | 0 | 21 |
| NII/V | 21 | 0 | 0 | 21 |
| NII/GL | 22 | 5 | 0 | 17 |
| DN/EN | 10 | 0 | 0 | 10 |
| DN/SN | 15 | 0 | 0 | 15 |
| DN/V | 15 | 1 | 1 | 13 |
| DN/GL | 15 | 0 | 0 | 15 |
| EN/SN | 21 | 0 | 0 | 21 |
| EN/V | 21 | 1 | 1 | 19 |
| EN/GL | 20 | 0 | 1 | 19 |
| SN/V | 31 | 14 | 0 | 17 |
| SN/GL | 30 | 0 | 8 | 22 |
| V/GL | 30 | 0 | 12 | 18 |
(NCI = Neuronal cytoplasmic inclusions, GI = Glial inclusions, NII = Neuronal intranuclear inclusions, DN = Dystrophic neurites, EN = Abnormally enlarged neurons, V = Vacuolation, SN = Surviving neurons, GL = Glial cell nuclei) across gyri of the frontal and temporal cortex from pia mater to white matter in ten cases of frontotemporal lobar dementia with TDP-43 proteinopathy (FTLD-TDP), N = number of gyri tested, NS = no significant correlations.
Acknowledgements
We thank clinical, genetic, pathology, and technical staff of Vancouver General Hospital, Vancouver, Canada; University of Pittsburgh, Pittsburgh, PA; and Harvard Brain Tissue Resource Center, Belmont, MA for making information and tissue samples available for this study and we thank the families of patients whose generosity made this research possible. Support for this work was provided by the National Institute on Aging of the National Institutes of Health to NJC (P50-AG05681, and P01-AG03991) and to RH (P50-AG05133).
List of abbreviations
- AD
Alzheimer’s disease
- AGD
Argyrophilic grain disease
- CBD
Corticobasal degeneration
- CERAD
'Consortium to Establish a Registry of Alzheimer's Disease'
- DN
Dystrophic neurites
- EN
Abnormally enlarged neurons
- FTD
Frontotemporal dementia
- FTLD
Frontotemporal lobar degeneration
- FTLD-U
Frontotemporal lobar degeneration with ubiquitin positive inclusion
- GI
Glial inclusions
- GL
Glial cell nuclei
- GRN
Progranulin gene
- IHC
Immunohistochemistry
- ITG
Inferior temporal gyrus
- MFG
Middle frontal gyrus
- MND
Motor neuron disease
- NCI
Neuronal cytoplasmic inclusion
- NIA
National Institute on Aging
- NII
Neuronal intranuclear inclusion
- NFT
Neurofibrillary tangle
- PAX
Progressive apraxia
- PHG
Parahippocampal gyrus
- PNFA
Progressive non-fluent aphasia
- PiD
Pick’s disease
- PSP
Progressive supranucuclear palsy
- SD
Semantic dementia
- SP
Senile plaque
- STG
Superior temporal gyrus
- TARDBP
TAR DNA-binding protein
- TDP-43
Transactive response (TAR) DNA-binding protein of 43kD
- VCP
Valosin-containing protein
Footnotes
The authors report no conflicts of interest.
Contributions of listed authors
Richard Armstrong designed the project, collected the quantitative data, analysed the data, and wrote the manuscript. Nigel Cairns coordinated the project, assisted in project design, contributed cases for the study, and made comments on the manuscript. Ronald Hamilton, Ian Mackenzie, and John Hedreen contributed cases for the study and commented on the manuscript.
References
- 1.Tolnay M, Probst A. Frontotemporal lobar degeneration- tau as a pied piper. Neurogenetics. 2002;4:63–75. doi: 10.1007/s10048-002-0140-x. [DOI] [PubMed] [Google Scholar]
- 2.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- 3.Woulfe J, Kertesz A, Munoz DG. Frontotemporal dementia with ubiquinated cytoplasmic and intranuclear inclusions. Acta Neuropathol. 2001;102:94–102. doi: 10.1007/s004010000346. [DOI] [PubMed] [Google Scholar]
- 4.Kovari E, Gold G, Giannakopoulos P, Bouras C. Cortical ubiquitin positive inclusions in frontotemporal dementia without motor neuron disease: a quantitative immunocytochemical study. Acta Neuropathol. 2004;108:207–212. doi: 10.1007/s00401-004-0881-8. [DOI] [PubMed] [Google Scholar]
- 5.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, III, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Steiber A, Xu Y, Forman MS, Trojanowski JQ, Lee VMY, Mackenzie IRA. TDP-43 familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007a;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong RA, Ellis W, Hamilton RL, Mackenzie IRA, Hedreen J, Gearing M, Montine T, Vonsattel J-P, Head E, Lieberman AP, Cairns NJ. Neuropathological heterogeneity in frontotemporal lobar degeneration with TDP-43 proteinopathy: a quantitative study of 94 cases using principal components analysis. J Neural Transm. 2010;117:227–239. doi: 10.1007/s00702-009-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DMA. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006a;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann M, Igaz LM, Kwong LK, Nakashima-Yasuda H, Kolb SJ, Dreyfuss G, Kretzschmar HA, Trojanowski JQ, Lee VMY. Absence of heterogeneous nuclear riboproteins and survival neuron protein (TDP-43) positive inclusions in frontotemporal lobar degeneration. Acta Neuropathol. 2007;113:543–548. doi: 10.1007/s00401-007-0221-x. [DOI] [PubMed] [Google Scholar]
- 10.Cairns NJ, Bigio EH, Mackenzie IRA, Neumann M, Lee VMY, Hatanpaa KJ, White CL, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DMA. Neuropathologic diagnostic and nosological criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007b;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong RA. β-amyloid (Aβ) deposits and blood vessels: laminar distribution in the frontal cortex of patients with Alzheimer's disease. Neurosci Res Communs. 1996a;18:19–28. [Google Scholar]
- 12.Armstrong RA, Cairns NJ, Lantos PL. Laminar distribution of cortical Lewy bodies and neurofibrillary tangles in dementia with Lewy bodies. Neurosci Res Commun. 1997;21:145–152. [Google Scholar]
- 13.Armstrong RA, Cairns NJ, Lantos PL. Laminar distribution of Pick bodies, Pick cells, and Alzheimer’s disease pathology in the frontal and temporal cortex in Pick’s disease. Neuropathol Appl Neurobiol. 1999;25:266–271. doi: 10.1046/j.1365-2990.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong RA, Lantos PL, Cairns NJ. Laminar distribution of ballooned neurons and tau positive neurons with inclusions in patients with corticobasal degeneration. Neurosci Res Communs. 2000;27:85–93. [Google Scholar]
- 15.Armstrong RA, Lantos PL, Cairns NJ. Multiple system atrophy: laminar distribution of the pathological changes in frontal and temporal neocortex. Clin Neuropathol. 2005;24:230–235. [PubMed] [Google Scholar]
- 16.De Lacoste M, White CL. The role of cortical connectivity in Alzheimer's disease pathogenesis: a review and model system. Neurobiol Aging . 1993;14:1–16. doi: 10.1016/0197-4580(93)90015-4. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong RA, Slaven A. Does the neurodegeneration of Alzheimer’s disease spread between visual cortical regions B17 and B18 via the feedforward or feedback short cortico-cortical projections. Neurodegen. 1994;3:191–196. [Google Scholar]
- 18.Armstrong RA, Hilton A. Statistical Analysis in Microbiology: Statnotes. Hoboken, NJ, USA: Wiley-Blackwell; 2011. [Google Scholar]
- 19.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 20.Cruts M, Gijselink I, van der Zee J, Engelborgs S, Wils H, Pirici D, Radamakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De pooter T, Mattheijssens M, van den BM, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee O, Pastor P, Cairns NJ, Chakraaverty S, Kauwe JSK, Shears S, Behrens MI, Budde J, Hinrichs AL, Norton J, Levitch D, Taylor-Reinwald L, Gitcho M, Tu PH, Grinberg LT, Liscic RM, Armendariz J, Morris JC, Goate AM. HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Annals of Neurology. 2006;60:314–322. doi: 10.1002/ana.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie IRA, Baker M, Pickering-Brown S, Hsinng GYR, Lindholm C, Dwosh E, Cannon A, Rademakers R, Hutton M, Feldman HH. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain. 2006b;129:3081–3090. doi: 10.1093/brain/awl271. [DOI] [PubMed] [Google Scholar]
- 23.Rademakers R, Hutton M. The genetics of frontotemporal lobar degeneration. Curr Neurol Neurosci Rep. 2007;7:434–442. doi: 10.1007/s11910-007-0067-6. [DOI] [PubMed] [Google Scholar]
- 24.Behrens MI, Mukherjee O, Tu PH, Liscic RM, Grinberg LT, Carter D, Paulsmeyer K, Taylor-Reinwald L, Gitcho M, Norton JB, Chakraverty S, Goate AM, Morris JC, Cairns NJ. Neuropathologic heterogeneity in HDDD1: a familial frontotemporal lobar degeneration with ubiquitin-positive inclusions and progranulin mutation. Alz Dis Assoc Disord. 2007;21:1–7. doi: 10.1097/WAD.0b013e31803083f2. [DOI] [PubMed] [Google Scholar]
- 25.Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA, Jhaveri BS, Karlawish JH, Pestrvik A, Smith TN, Tu PH, Watts GDJ, Markesbery WR, Smith CD, Kimonis VE. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol. 2006;65:571–581. doi: 10.1097/00005072-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, Hatanpaa K, J, White C L, III, Bigio EH, Caselli R, Baker M, Al-Lozi MT, Morris JC, Pestronk A, Rademakers R, Goate AM, Cairns NJ. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard J.-P, Lacomblez L, Pochigaeva K, Salachas F, Pradat P-F, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 28.Benajiba L, Le Ber I, Camuzat A, Lacoste M, Thomas-Anterion C, Couratier P, Legallic S, Salachas F, Hannequin D, Decousus M, Lacomblez L, Guedj E, Golfier V, Camu W, Dubois B, Campion D, Meininger V, Brice A. French Clinical and Genetic Research Network on Frontotemporal Lobar Degeneration/Frontotemporal Lobar Degeneration with Motoneuron Disease TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol. 2009;65:470–474. doi: 10.1002/ana.21612. [DOI] [PubMed] [Google Scholar]
- 29.Gitcho MA, Bigio EH, Mishra M, Johnson N, Weintraub S, Mesulam M, Rademakers R, Chakraverty S, Cruchaga C, Morris JC, Goate AM, Cairns NJ. TARDBP 3-prime-UTR variant in autopsy-confirmed frontotemporal lobar degeneration with TDP-43 proteinopathy. Acta Neuropath. 2009;118:633–645. doi: 10.1007/s00401-009-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luty AA, Kwok JBJ, Thompson EM, Blumsbergs P, Brooks WS, Loy CT, Dobson-Stone C, Panegyres PK, Hecker J, Nicholson GA, Halliday GM, Schofield PR. Pedigree with frontotemporal lobar degeneration-motor neuron disease and Tar DNA binding protein-43 positive neuropathology: genetic linkage to chromosome 9. BMC Neurology. 2008;8:32. doi: 10.1186/1471-2377-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetou A, Abramzon Y, Remes AM, Kaganovitch A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Ricahrdson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kalvorinne AL, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, chio A, Restagno G, Borghero G, Sabatelli M, The ITALSGEN Consortium, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Josephs KA, Whitwell JL, Jack CR, Parisi JE, Dickson DW. Frontotemporal lobar degeneration without lobar atrophy. Arch Neurol. 2006;63:1632–1638. doi: 10.1001/archneur.63.11.1632. [DOI] [PubMed] [Google Scholar]
- 33.Kersaitis C, Holliday GM, Xuereb JH, Pamphlett R, Bak TH, Hodges JR, Kril JJ. Ubiquitin-positive inclusions and progression of pathology in frontotemporal dementia and motor neurone disease identifies a group with mainly early pathology. Neuropathol Appl Neurobiol. 2006;32:83–91. doi: 10.1111/j.1365-2990.2005.00704.x. [DOI] [PubMed] [Google Scholar]
- 34.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G and Berg L. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 35.Jellinger KA, Bancher C. Neuropathology of Alzheimer’s disease: a critical update. J Neural Transm. 1998;54:77–95. doi: 10.1007/978-3-7091-7508-8_8. [DOI] [PubMed] [Google Scholar]
- 36.Newell KL, Hyman BT, Growden JH, Hedley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Insitute criteria for the neuropathological diagnosis of Alzheimer’s disease. J Neuropathol Exp Neurol. 1999;58:1147–1155. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Theis B, Trojanowski JQ, Vinters HV, Montine TJ. National Insitute on Aging-Alzheimer’s Association Guidelines for the neuropathological assessment of Alzheimer’s disease. Alzheimer & Dem. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duyckaerts C, Hauw JJ, Bastenaire F, Piette F, Poulain C, Rainsard V, Javoy-Agid F, Berthaux P. Laminar distribution of neocortical senile plaques in senile dementia of the Alzheimer type. Acta Neuropathol. 1986;70:249–256. doi: 10.1007/BF00686079. [DOI] [PubMed] [Google Scholar]
- 40.Yaguchi M, Fujita Y, Amari M, Takatama M, Al-Sarraj S, Leigh PN, Okamoto K. Morphological differences of intraneural ubiquitin positive inclusions in the dentate gyrus and parahippocampal gyrus of motor neuron disease with dementia. Neuropathol. 2004;24:296–301. doi: 10.1111/j.1440-1789.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 41.Davidson Y, Kelley T, Mackenzie IRA, Pickering Brown S, Du Plessis D, Neary D, Snowden JS, Mann DMA. Ubiquinated pathological lesions in frontotemporal lobar degeneration contain TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto S, Udaka F, Kameyama M, Kusaka H, Itoh H, Imai T. Subcortical neurofibrillary tangles, neuropil threads and argentophilic glial inclusions in corticobasal degeneration. Clin Neuropath. 1996;15:209–214. [PubMed] [Google Scholar]
- 43.Armstrong RA, Cairns NJ, Lantos PL. A quantitative study of the pathological lesions in neocortex and hippocampus of 12 patients with corticobasal degeneration. Exp Neurol. 2000;163:348–356. doi: 10.1006/exnr.2000.7392. [DOI] [PubMed] [Google Scholar]
- 44.Yamada T, McGeer PL, McGeer EG. Appearance of paired nucleated tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett. 1992;135:99–102. doi: 10.1016/0304-3940(92)90145-w. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda K, Akiyama H, Kondo H, Haga C, Tanno E, Tokuda T, Ikeda S. Thorn-shaped astrocytes: possibly secondarily induced tau-positive glial fibrillary tangles. Acta Neuropathol. 1995;90:620–625. doi: 10.1007/BF00318575. [DOI] [PubMed] [Google Scholar]
- 46.Komori T. Tau positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol. 1999;9:663–679. doi: 10.1111/j.1750-3639.1999.tb00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Probst A, Tolnay M. Argyrophilic grain disease, a frequent and largely underestimated cause of dementia in old patients. Rev Neurol. 2002;158:155–165. [PubMed] [Google Scholar]
- 48.Pirici D, Vandenberghe R, Rademakers R, Dermant B, Cruts M, Vennekens K, Cuijt I, Lubke U, Centerick C, Martin JJ, Van Broeckhoven C, Kumar-Singh S. Characterization of ubiquinated intraneuronal inclusions in a novel Belgian frontotemporal lobar degeneration family. J Neuropath Exp Neurol. 2006;65:289–301. doi: 10.1097/01.jnen.0000205147.39210.c7. [DOI] [PubMed] [Google Scholar]
- 49.Hatanpaa KJ, Bigio EH, Cairns NJ, Womack KB, Weintraub S, Morris JC, Foong C, Xiao GH, Hladik C, Mantanona TY, White CL. TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: A Midwest-Southwest Consortium for FTLD-U study. J Neuropathol Exp Neurol. 2008;67:271–279. doi: 10.1097/NEN.0b013e31816a12a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armstrong RA. Correlations between the morphology of diffuse and primitive β-amyloid (Aβ) deposits and the frequency of associated cells in Down’s syndrome. Neuropath Appl Neurobiol. 1996b;22:527–530. doi: 10.1111/j.1365-2990.1996.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 51.Armstrong RA, Lantos PL, Cairns NJ. Spatial correlations between the vacuolation, prion protein deposits, and surviving neurons in the cerebral cortex in sporadic Creutzfeldt-Jakob disease. Neuropathol. 2001;21:266–271. doi: 10.1046/j.1440-1789.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong RA, Ironside J, Lantos PL, Cairns NJ. A quantitative study of the pathological changes in the cerebellum of 15 cases of variant Creutzfeldt-Jakob disease. Neuropathol Appl Neurobiol. 2009;35:36–45. doi: 10.1111/j.1365-2990.2008.00979.x. [DOI] [PubMed] [Google Scholar]
- 53.Snedecor GW, Cochran WG. Statistical Methods. Ames, Iowa USA: Iowa State University Press; 1980. [Google Scholar]
- 54.Neumann M, Kwong LK, Lee EB, Kremmer E, Flately A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanoswki JQ, Lee VMY. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Annals of Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olive M, Janve A, Moreno D, Gamez J, Torrejon-Escritano B, Ferrer I. TAR DNA-binding protein 43 accumulation in protein aggregate myopathies. J Neuropathol Exp Neurol. 2009;68:262–273. doi: 10.1097/NEN.0b013e3181996d8f. [DOI] [PubMed] [Google Scholar]
- 57.Schwab C, Arai T, Hasegawa M, Akiyama H, Yu S, McGeer PL. TDP-43 pathology in familial British dementia. Acta Neuropathol. 2009;118:303–311. doi: 10.1007/s00401-009-0514-3. [DOI] [PubMed] [Google Scholar]
- 58.Armstrong RA, Carter D, Cairns NJ. A quantitative study of the neuropathology of 32 sporadic and familial cases of frontotemporal lobar degeneration (FTLD) with TDP-43 proteinopathy (FTLD-TDP) Neuropathol Appl Neurobiol. 2012;38:25–38. doi: 10.1111/j.1365-2990.2011.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minamu M, Mizutani T, Kawanishi R, Suzuki Y, Mori H. Neuronal expression of alpha B crystalline in cerebral infarction. Acta Neuropathol. 2003;105:549–554. doi: 10.1007/s00401-003-0679-0. [DOI] [PubMed] [Google Scholar]
- 60.Pierucci A, de Oliveira AL. Increased sensory neuron apoptotic death two weeks after peripheral axonotomy in C57BL/6J mice compared to A/J mice. Neurosci Lett. 2006;396:127–131. doi: 10.1016/j.neulet.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 61.Kato S, Hirano A, Umahara T, Wena JF, Herz F, Ohama E. Ultrastructural and immunohistochemical studies on ballooned cortical neurons in Creutzfeldt-Jakob disease: expression of alpha-B-crystallin, ubiquitin and stress response protein-27. Acta Neuropathol. 1992;84:443–448. doi: 10.1007/BF00227673. [DOI] [PubMed] [Google Scholar]
- 62.Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends in Neurosciences. 2010;33:317–325. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]