Abstract
The midbrain periaqueductal gray (PAG) plays a central role in the descending control of vocalization across vertebrates. The PAG has also been implicated in auditory-vocal integration, though its precise role in such integration remains largely unexplored. Courtship and territorial interactions in plainfin midshipman fish depend on vocal communication, and the PAG is a central component of the midshipman vocal-motor system. We made focal neurobiotin injections into the midshipman PAG to both map its auditory-vocal circuitry and enable evolutionary comparisons with tetrapod vertebrates. These injections revealed an extensive bidirectional pattern of connectivity between the PAG and known sites in both the descending vocal-motor and ascending auditory systems, including portions of the telencephalon, dorsal thalamus, hypothalamus, posterior tuberculum, midbrain and hindbrain. Injections in the medial PAG produced dense label within hindbrain auditory nuclei, while those confined to the lateral PAG preferentially labeled hypothalamic and midbrain auditory areas. Thus, the teleost PAG may have functional subdivisions playing different roles in vocal-auditory integration. Together, the results confirm several pathways previously identified by injections into known auditory or vocal areas and provide strong support for the hypothesis that the teleost PAG is centrally involved in auditory-vocal integration.
Keywords: hindbrain, audio-vocal, midshipman fish, vocal pattern generator, torus semicircularis
INTRODUCTION
The role of the midbrain periaqueductal gray (PAG) in vocal production across vertebrate species has been well-established, by stimulation and lesion studies, by electrophysiological studies examining PAG activity during vocal production, and by anatomical studies revealing descending connections from the PAG to phonatory motoneurons (as reviewed by Jurgens, 1994, 2002, 2009). Together, these studies demonstrate that PAG function is essential for the production, particularly, of innate unlearned vocalizations such as calls involved in courtship, territorial defense, and aggression, in a diverse array of species. More generally, the PAG is thought to integrate motivational / limbic inputs and sensory inputs to select and initiate specific “emotional” motor behaviors (e.g., courtship, mating, parental care, defense, hunting), many of which have a social dimension, relevant for an individual's motivational state and current environment (Holstege, 1998; Sewards and Sewards, 2003; Sukikara et al., 2006; O'Connell and Hofmann, 2011). This general model implies that the PAG's role in vocal production includes the integration of auditory information about the vocal behavior of conspecifics, to initiate appropriate vocal-motor responses. However, comparatively little is known about the specific role of the PAG in auditory-vocal integration. Anatomical data reveals relatively dense inputs from known auditory areas, including auditory cortex and the midbrain inferior colliculus, to the PAG in multiple species, including squirrel monkeys (Dujardin and Jurgens, 2005), horseshoe bats (Metzner, 1996), ferrets (Bajo et al., 2007), gerbils (Bajo and Moore, 2005) and pigeons (Wild et al., 1997). Limited neurophysiological data reveal a small number of neurons in the PAG of squirrel monkeys that may act as an audio-vocal interface, showing greater activity when an animal both hears a conspecific call and responds vocally than when either just hearing calls or just vocalizing (Dusterhoft et al., 2004).
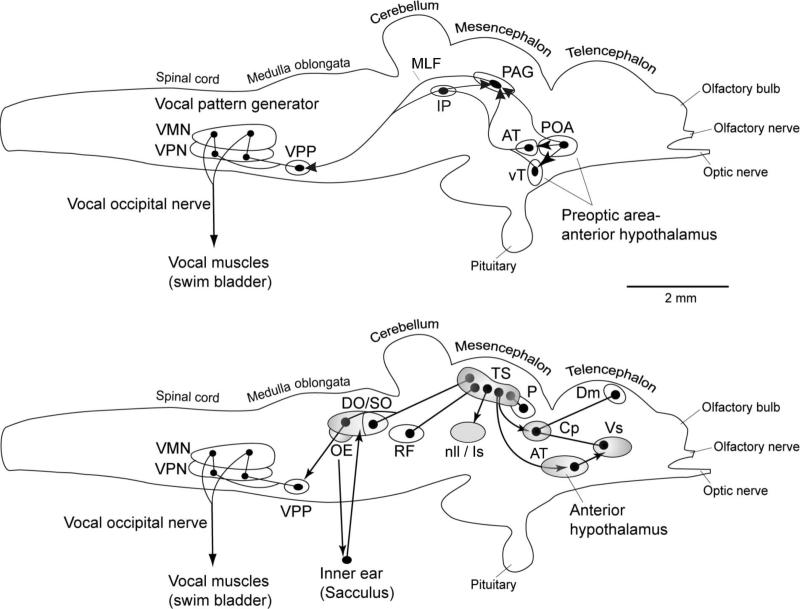
Here, we examine the connectivity of the presumptive homolog of the periaqueductal gray in a vocal teleost fish, the plainfin midshipman (Porichthys notatus), with a particular focus on auditory and vocal-motor connectivity. These fish represent a well-studied model system for examining questions related to the evolution and function of vocal-motor systems, auditory systems, and auditory-vocal integration. Midshipman communicate extensively by vocalization in nest sites in the intertidal zone of the Pacific coast of the United States (Bass, 1996). Sonic muscles surrounding the swim bladder produce sound, and are innervated by a hindbrain pattern-generating circuit (Bass and Baker, 1990; Bass et al., 1994; Chagnaud et al., 2011). Males establish and defend nests, from which they hum to attract females, and grunt and growl in various defensive contexts (i.e., when startled, and to defend their nests from encroaching males) (Brantley and Bass, 1994; Bass et al., 1999). The vocal-motor pathways in the midshipman brain have been well-mapped (Fig. 1, top) (Bass and Baker, 1990; Bass et al., 1994; Goodson and Bass, 2002; Kittelberger et al., 2006; Chagnaud et al., 2011), and strong evidence exists for homologies between the hindbrain vocal pattern-generating circuitry and hindbrain vocal circuits in other vertebrates, including birds and mammals (Bass and Baker, 1997; Bass et al., 2008). The midshipman vocal circuit also includes a presumptive PAG homolog (Goodson and Bass, 2002), identified as well in closely related toadfish species Opsanus tau and O. beta that are members of the same family (Batrachoididae) as midshipman (e.g., Demski and Gerald, 1972, 1974; Fine, 1979). As in terrestrial vertebrates, stimulation of this site in batrachoidid fish elicits vocalization (Demski and Gerald, 1972, 1974; Fine, 1979; Goodson and Bass, 2002), inactivation blocks vocal production (Kittelberger et al., 2006), and the activity of PAG neurons predicts vocal output (Kittelberger et al., 2006). Furthermore, the structure and function of the midshipman auditory system has been well characterized (Fig. 1, bottom) (Bass et al., 1994, 2000; Bass and Lu, 2007). Connections between the midshipman PAG and other components of the vocal-motor circuit have previously been described (Goodson and Bass, 2002; Kittelberger et al., 2006), but the auditory connectivity of the PAG has been only partially assessed (Goodson and Bass, 2002).
Figure 1.
Schematic sagittal view of the midshipman brain showing the vocal motor (top) and central auditory (bottom) systems (modified from Bass and McKibben, 2003; Forlano et al., 2010; Kittelberger et al., 2006). Solid dots represent neuronal cell bodies. Lines represent axonal connections: lines connecting two dots indicate reciprocal connections while lines with arrowheads indicate uni-directional connections. Our results indicate that most of the major structures in the central auditory pathway are also interconnected with the PAG (shaded areas, bottom, and see also Fig. 7). For clarity, some minor nodes and pathways in these two systems have been omitted. Descending vocal motor pathways shown here are based on Bass et al., 1994; Goodson and Bass, 2002; and Kittelberger, et al., 2006. Central auditory pathways are based on Bass et al., 2000.
To more completely map the anatomical basis for auditory-vocal integration occurring in the midshipman PAG, as well as to better understand evolutionary relationships between the PAG in midshipman and in terrestrial vertebrates, we characterized the complete connectivity of the midshipman PAG, by making focal injections of the neuronal tracer neurobiotin. As it is well established that the mammalian PAG contains functionally and anatomically distinct subdivisions, with different patterns of afferent and efferent connectivity (Bandler and Shipley, 1994; Cameron et al., 1995a, 1995b), we also sought to determine whether different portions of the midshipman PAG have different connectivity patterns.
MATERIALS AND METHODS
Subjects
Plainfin midshipman fish (Porichthys notatus) have two male reproductive morphs that differ in vocal and spawning behaviors (Brantley and Bass, 1994). All males used in the experiments described here were adult type I (territorial) males, which have the most dynamic vocal repertoire (Bass et al., 1999). A total of 12 type I males and 5 females were used. Fish were collected from tidal pool nesting sites or by offshore trawls in northern California and Washington, shipped to Cornell University or Gettysburg College, and maintained in artificial seawater tanks at ~15° C. Experimental procedures were approved by the Cornell University and Gettysburg College Institutional Animal Care and Use Committees. Note that some data from five of these fish have previously been published (Kittelberger et al., 2006), describing a subset of the vocal-motor connections of the PAG.
Surgeries
Surgical procedures were similar to those described previously (Bass and Baker 1990; Goodson and Bass 2000c). Fish were anesthetized by immersion in 0.025% benzocaine (ethyl p-amino benzoate, Sigma, St. Louis, MO). Local anesthetic (0.2 ml of 0.25% bupivacaine (Abbott Labs, N. Chicago, IL) with 0.01mg/ml epinephrine (International Medication Systems, South El Monte, CA)) was then injected subdermally to the top of the head. The midbrain was exposed by dorsal craniotomy. Before transfer to the experimental apparatus, fish were immobilized with an intramuscular injection of pancuronium bromide (~5 mg/kg, Baxter Healthcare, Deerfield, IL). Fish were then suspended in a parafilm sling in a plexiglass tank with their head stabilized, and artificial seawater at ~15° C was perfused continuously across their gills. Exposed portions of the brain were kept covered with an inert, electrically conductive fluorocarbon (Fluorinert, 3M, St. Paul, MN).
Tracer Injections
To characterize the connectivity, both antero- and retrograde, of neurons in the PAG, we made focal iontophoretic injections of the neuronal tracer Neurobiotin (Vector Labs, Burlingame, CA). Surface landmarks and micromanipulator coordinates were used to guide glass micropipettes (A-M Systems), pulled to a tip resistance of 10-15 MΩ on a Flaming/Brown micropipette puller (Model P-97, Sutter Instruments, Novato, CA), to sites defined previously by electrophysiological recordings and anatomical labeling to lie in the PAG (Kittelberger, et al., 2006). Neurobiotin (5%, in 2M KCl) was iontophoresed for 5-10 min using +3 μA pulsed current (15 s on / 15 s off). Only a single injection was made in each fish. After a 10 h survival time to allow transport of the tracer, fish were perfused and brains processed as described below.
Histology and Image Processing
Fish were deeply anesthetized (0.025% benzocaine) and perfused with ice-cold, teleost Ringer's solution with 10 units/ml of heparin (Elkins-Sinn, Cherry Hill, NJ), followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed, post-fixed overnight, and transferred to 0.1 M PB (pH 7.2) for storage. Brains were equilibrated in 30% sucrose for 24 h and then sectioned transversely at 50 μm on a freezing microtome. Neurobiotin injections were visualized using a standard avidin-biotin-peroxidase protocol. Briefly, sections were collected in PBS, permeabilized for 30 min in PBS with 0.04% Triton-X100 (Sigma), incubated for 3h at room temperature in avidin-biotin-peroxidase in PBS (Vectastain ABC Elite, Vector Labs), rinsed twice in PB, reacted with 0.05% diaminobenzidine (Sigma) and 0.0072% H2O2 in PB, rinsed twice more in PB, and mounted on gelatin subbed slides. Alternate sections were mounted on separate slides, dried overnight, cleared in xylene, and coverslipped. Later, one set of sections was stained with 0.5% cresyl violet (Sigma) to delineate the borders of the different cell clusters. One type I male was perfused as described above, without neurobiotin injection, and the tissue processed for cresyl violet staining to delineate the cytoarchitecture of the PAG.
Sections were examined and photomicrographs taken on a Nikon Eclipse E-800 microscope with a Nikon FDX 35mm camera or a Nikon Eclipse 90i microscope with a Nikon DS-Fi1 digital camera and NIS-Elements (v 3.10) imaging software. Camera lucida drawings were made using a Leitz Dialux 22 microscope. Photomicrographs and line drawings were digitally scanned, and images were processed in Adobe Photoshop (v. 5.5 and 10.0.1) and Corel Draw Graphics Suite 12. Photomicrograph images presented in the figures were cropped, scaled and labeled, and the brightness, contrast, and hue/saturation were adjusted. Otherwise, we did not further manipulate or retouch any of the photomicrographs. Cell bodies and axon terminals in the line drawings were enlarged in Photoshop to make them more visible.
RESULTS
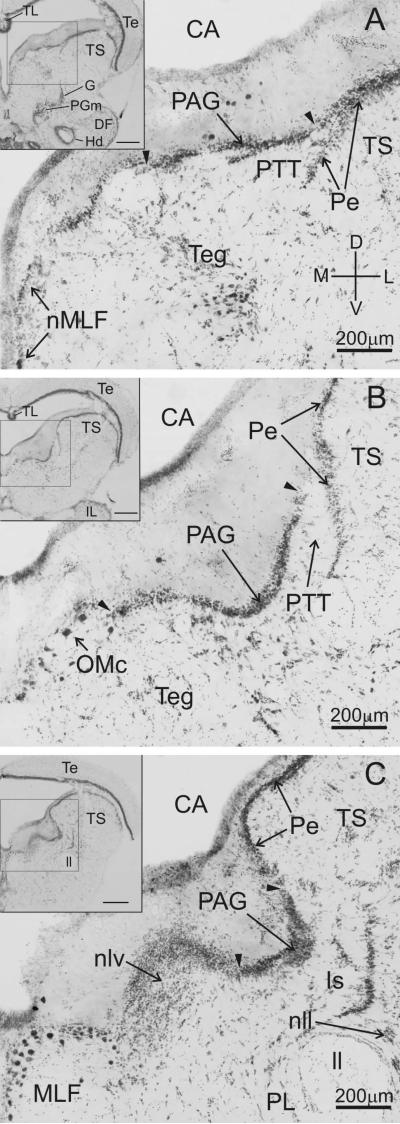
The midbrain periaqueductal gray (PAG) in plainfin midshipman fish forms a discrete cell layer just ventral to the cerebral aqueduct, and just medial of the torus semicircularis (TS) (Fig. 2; see Bass et al., 1994 and 2000 and Goodson and Bass, 2002, for detailed descriptions of the cytoarchitecture of vocal and auditory nuclei in midshipman hindbrain, midbrain including the PAG, and forebrain). The PAG cell layer appears through essentially the entire rostro-caudal extent of the midbrain (~1.0 to 1.5mm for animals ranging in length from 13.1 to 26.7 cm). The lateral border of the PAG is defined by its relationship to the periventricular cell layer (Pe) of the TS (Fig. 2A-C). Rostrally, the PAG merges with the Pe (lateral arrowhead, Fig. 2A), but more caudally is separated by a clear break between the two (lateral arrowhead, Fig. 2B, C). The medial border of the PAG is defined rostrally by a distinct interruption of the cell layer (medial arrowhead, Fig. 2A, B), with the nucleus of the medial longitudinal fasciculus (nMLF) lying on the midline well medial of the PAG at this level (Fig. 2A). Further caudal, the PAG is bordered medially by the oculo-motor complex (OMc, Fig. 2B), and at the caudal end by the adjacent nucleus lateralis valvulae (nlv, Fig. 2C). Neurobiotin injections were targeted to the PAG in a total of 16 fish (11 type I males and 5 females). Of these, 7 (6 males and 1 female) were found to have focal injections confined entirely to the cytoarchitectonically-defined boundaries of the PAG cell body layer with strong labeling of both fibers and cell bodies throughout the brain. Within the PAG, three of these injections were confined to the medial and rostral portion of the PAG, two to the lateral PAG, and the remaining two were centrally located. Injection sites were scattered across the rostro-caudal extent of the PAG, except that no injections were made in the caudal-most tail (~200-300 μm; ~ 20% of the length of the PAG, though the cell body layer is especially narrow medio-laterally at this level, see Fig. 2C).
Figure 2.
Cytoarchitecture of the periaqueductal gray (PAG). Cresyl-violet stained coronal sections (60 μm thick) of the PAG from a type I male midshipman at rostral (A), central (B), and caudal (C) levels. The PAG appears as a dense layer of cell bodies ventral to the cerebral aqueduct (CA) and medial to the periventricular cell layer (Pe) of the torus semicircularis (TS). Arrowheads denote the medial and lateral borders of the PAG at each level. The rostral PAG section shown in A is comparable to the level of the rostral-medial PAG injection depicted in Figs. 5 and 6. The central PAG section shown in B is comparable, or just caudal, to the level of the lateral PAG injection depicted in Figs. 3 and 4. Insets depict low-power images of each section. Inset scale bars = 500 μm. Sections A and B are separated by 540 μm; sections B and C are separated by 480 μm. Dorsal (D), ventral (V), medial (M), and lateral (L) directions as indicated in A.
Focal neurobiotin injections confined to the PAG resulted in both anterograde labeling of axon fibers and terminals and retrograde labeling of cell bodies. Generally, this pattern of label defined a network of brain structures, some of which are involved in the motor production of vocalization and others of which are important nodes in the ascending auditory pathway.
Connectivity to Vocal and Auditory Areas
Labeled axons and terminals had a rostral-caudal extent from telencephalic to hindbrain levels following neurobiotin injections into either the lateral (Figs. 3A-G; 4A-I) or medial (Figs. 5A-J; 6A-K) PAG. Injections into the lateral PAG were centered between the auditory-recipient TS and the remaining midbrain tegmentum (Teg, Fig. 4A), while those in the medial PAG were closer to the midline distant from the TS (Fig. 6A). All PAG injections resulted in retrogradely labeled cell bodies in the ventral tuberal nucleus of the anterior hypothalamus (vT; Figs. 3A, 4B, 5B) and the posterior parvocellular preoptic nucleus (PPp; Figs. 3A, 5B), both of which are structures where focal electrical stimulation reliably elicits output from the hindbrain vocal pattern generator (Goodson and Bass, 2000a; Kittelberger et al., 2006). Labeled terminals and a scarcity of cell bodies relative to other labeled regions were observed in the paralemniscal midbrain tegmentum (PL) (Figs. 3C; 5D, E), another known component of the midshipman vocal-motor circuit (Goodson and Bass, 2000b, c, 2002). Presumptive labeled PAG dendrites were present in and around the PL following lateral PAG injections (e.g., Fig. 3C), often making it difficult to unambiguously discriminate labeled terminals in this area. Clear terminals were also identified, however, in the PL after injections to the medial-rostral PAG (e.g., Fig. 5D, E), where PAG dendrites were not observed in the vicinity of the PL. Labeled cell bodies, but few terminals, were found in the isthmal paraventricular group of the caudal midbrain (IP, Figs. 3D, 4G, I; 5F; 6C) that is also part of the brainstem vocal system (Bass et al., 1994). The final structure in the descending neural circuit driving vocal-motor production is the vocal motor nucleus (VMN) that innervates the paired sonic muscles on the wall of the swim bladder (Bass and Baker, 1990; Bass et al., 2008). Presynaptic to the VMN lie, in turn, the vocal pacemaker nucleus (VPN), and the vocal prepacemaker nucleus (VPP; formerly ventral medullary nucleus). These three hindbrain structures comprise the vocal pattern generator (Fig. 1) (Bass and Baker, 1990; Bass et al., 1994; Chagnaud et al., 2011). All injections into the PAG resulted in labeled axon terminals throughout VPP (Figs. 3G, 5J, 6K), but no label in either the VPN or the VMN.
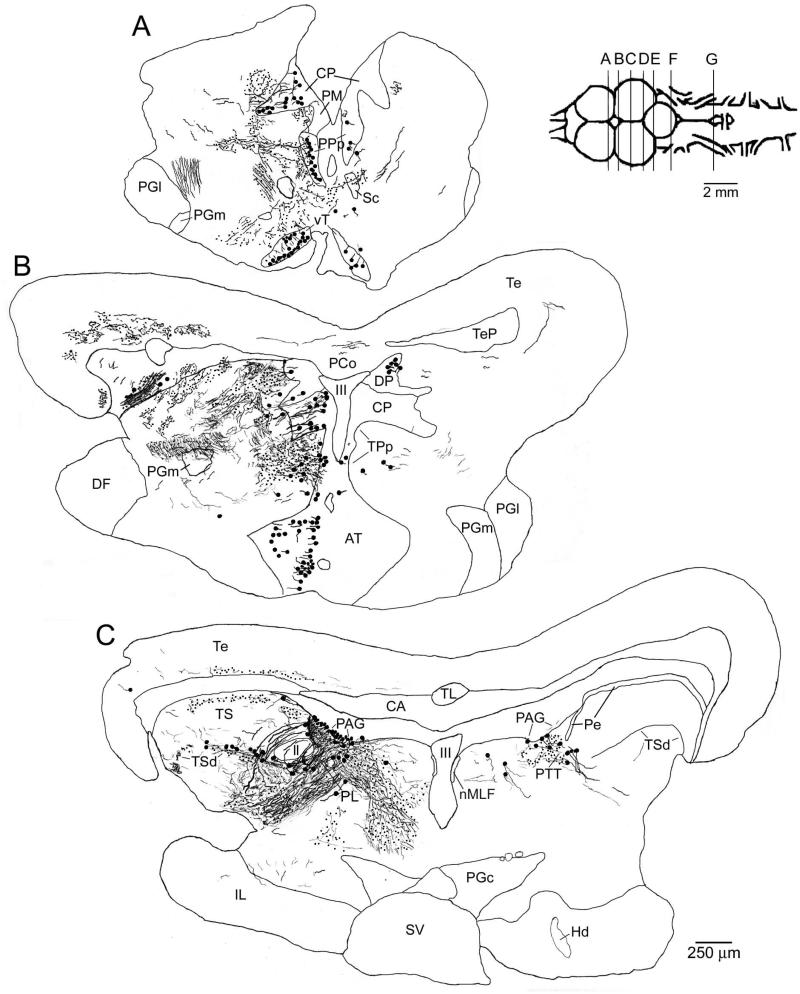
Figure 3.
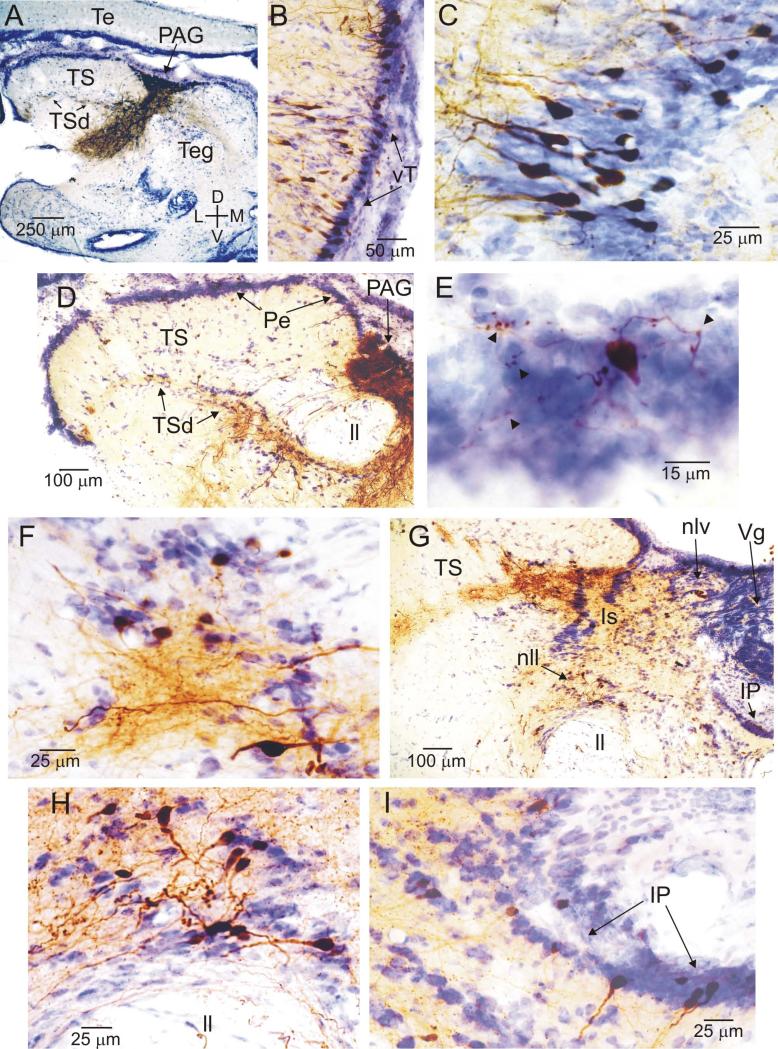
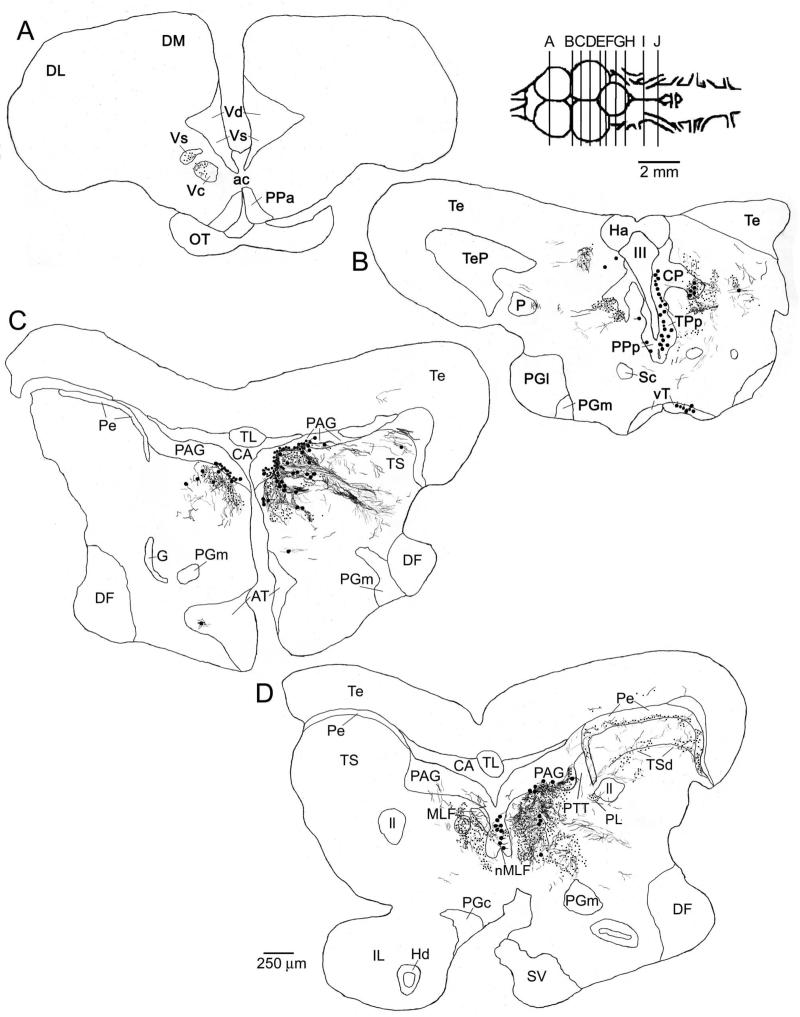
Charting of label resulting from a neurobiotin injection to the lateral PAG. Labeled cells are indicated as large dots, fibers as thin lines, and putative terminals as small dots. All sections are coronal, with planes of section as indicated in the inset to right of A. The injection site in the PAG is shown in C. Some of the labeled fibers running ventral and lateral to the injection site (e.g., in and around the paralemniscal tegmentum (PL) in C) are likely to reflect filled PAG neuron dendrites. Labeling was observed in known vocal-motor structures including the ventral tuberal hypothalamus (vT, cell bodies, A), the posterior parvocellular preoptic nucleus (PPp, cell bodies, A), the isthmal paraventricular nucleus (IP, cell bodies, D), and the vocal prepacemaker nucleus (VPP, terminals, G). Labeling was also found in a number of known auditory structures including the periventricular (Pe, terminals and a few cell bodies) and deep (TSd, terminals and cell bodies) layers of the torus semicircularis (TS, C and D), the central posterior nucleus of the thalamus (CP, cell bodies, A and B), the anterior tuberal hypothalamus (AT, cell bodies and terminals, B), and the nucleus of the lateral lemniscus (nll, cell bodies and terminals, D).
Figure 4.
Photomicrographs of label resulting from a neurobiotin injection to the lateral PAG. All sections are coronal, oriented as indicated in A, and have been counterstained with cresyl violet. A, low power image showing the injection site in the lateral PAG. B, dense cluster of labeled cell bodies in the ventral tuberal hypothalamus (vT). C, cluster of labeled cell bodies in the central posterior nucleus of the thalamus (CP), with fibers and terminals lateral (left) of the cell body layer. D, low power overview of label in the torus semicircularis (TS), slightly caudal of the section shown in A. E, a higher power image of a portion of the medial periventricular cell layer (Pe) of the TS, showing a labeled cell body and putative terminal boutons (arrowheads). F, dense fibers, terminals, and cell bodies in the medial half of the deep layer of the TS (TSd). G, overview of labeling in the caudal midbrain, including the nucleus of the lateral lemniscus (nll), the isthmal nucleus of the midbrain (Is), and the isthmal paraventricular nucleus (IP). H, higher power image of labeled cells and surrounding fibers and terminals in the nll. I, cell bodies labeled in IP. A full charting of label resulting from this injection is shown in Figure 3.
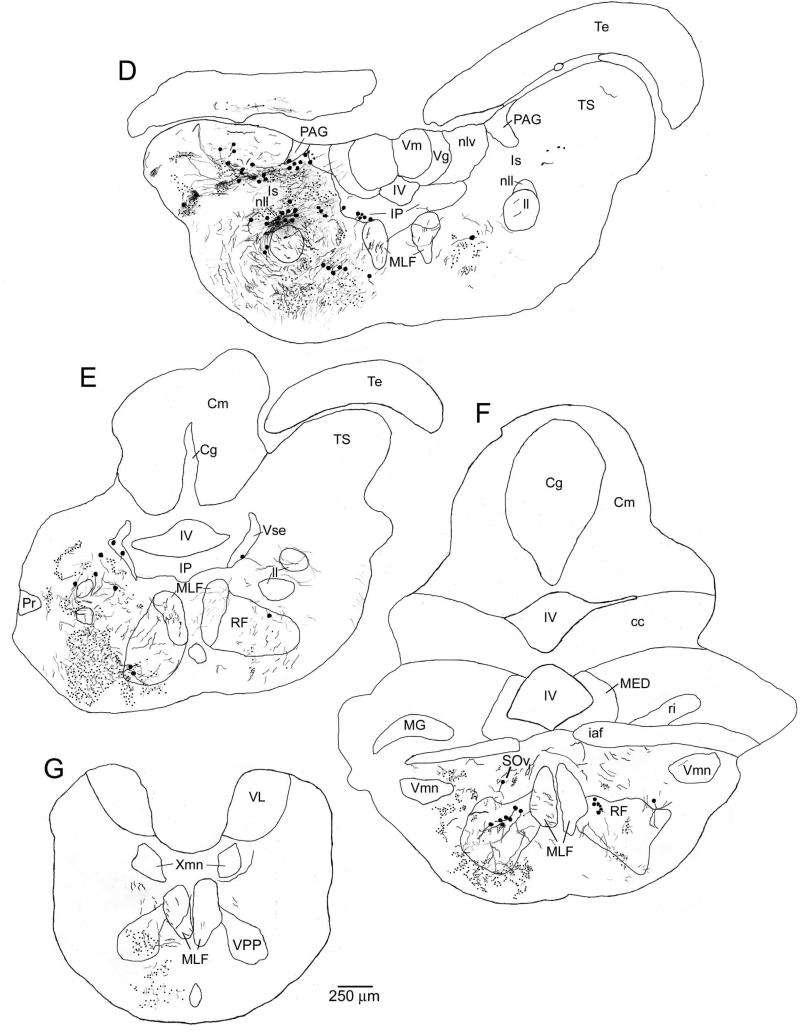
Figure 5.
Charting of label resulting from a neurobiotin injection to the medial PAG. Labeled cells are indicated as large dots, fibers as thin lines, and putative terminals as small dots. All sections are coronal, with planes of section as indicated in the inset to right of A. The injection site in the medial / rostral PAG is shown in C and D. As with injections to the lateral PAG (as shown in Fig. 3), labeling was observed in known vocal-motor structures including the ventral tuberal hypothalamus (vT, cell bodies, B), the posterior parvocellular preoptic nucleus (PPp, cell bodies, B), the paralemniscal midbrain tegmentum (PL, terminals, D and E), the isthmal paraventricular nucleus (IP, cell bodies, F), and the vocal prepacemaker nucleus (VPP, terminals, J). Again, similar to more lateral injections, labeling was found in known auditory structures including the periventricular (Pe) and deep (TSd) layers of the torus semicircularis (TS, terminals and a few cell bodies, D and E), the central posterior nucleus of the thalamus (CP, cell bodies, B), the anterior tuberal hypothalamus (AT, terminals and a few cell bodies, C), and the nucleus of the lateral lemniscus (nll, cell bodies and terminals, F). In addition, unlike injections to the more lateral PAG, label was found here in medullary auditory structures including terminals in the rostral intermediate division of the descending octaval nucleus (ri, H and I), the ventral tegmental nucleus (VT, H), and the rostral division of the octavolateralis efferent nucleus (OEr, I), and cell bodies in the anterior octaval nucleus (AO, H). Discrete terminal fields were also observed here, but not after more lateral PAG injections, in the supracommisural (Vs) and central (Vc) nuclei of area ventralis of the telencephalon (A).
Figure 6.
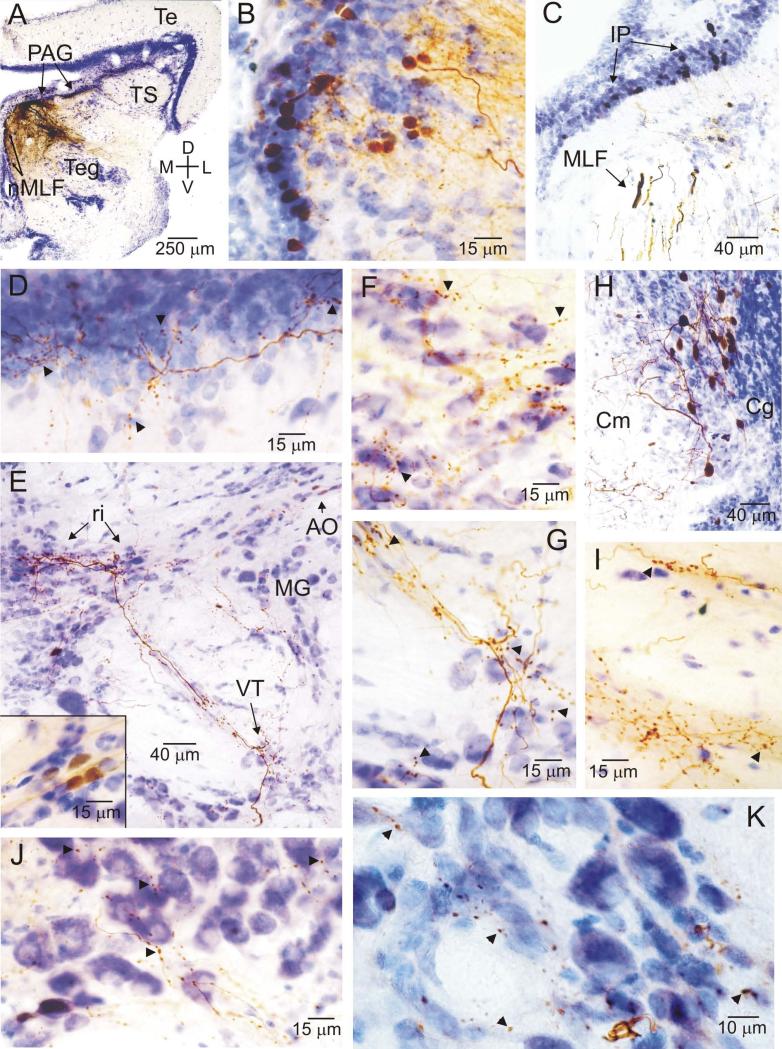
Photomicrographs of label resulting from a neurobiotin injection to the medial PAG. All sections are coronal, oriented as indicated in A, and have been counterstained with cresyl violet. Examples of putative terminal boutons are indicated with arrowheads. Note, in particular, the staining in various medullary auditory structures (E-G, I, J). A, low power image showing the injection site in the medial / rostral PAG, and labeled neurons in the nucleus of the medial longitudinal fasciculus (nMLF) on the midline medial and ventral of the injection site. B, dense cluster of labeled cell bodies in the periventricular nucleus of the posterior tuberculum (TPp), with fibers and terminals lateral (right) of the cell body layer. C, cell bodies labeled in isthmal paraventricular nucleus in the caudal midbrain (IP), and fibers in the medial longitudinal fasciculus (MLF). D, labeled fibers and terminals in the medial portion of the periventricular cell layer (Pe) of the torus semicircularis (TS). E, overview of label in several medullary auditory structures, including fibers and terminals in the rostral intermediate division of the descending octaval nucleus (ri) and in the ventral tegmental nucleus (VT) and cell bodies in the anterior octaval nucleus (AO, inset lower left). F, G, higher power views showing labeled fibers and terminals in ri (F) and VT (G). H, a discrete cluster of labeled cell bodies found caudally and laterally within the granule cell layer of the cerebellum (Cg), contralateral to the injection site. I, J, high power views of sparse terminals around cell bodies in the rostral division of the octavolateralis efferent nucleus (OEr, J) and much denser terminal labeling ventral / medial of the OEr proper, in the region where OEr dendrites arborize (I). K, labeled terminals around cell bodies in the vocal prepacemaker nucleus (VPP), a component of the hindbrain vocal pacemaker circuit. A full charting of label resulting from this injection is shown in Figure 5.
In addition to these presumptive vocal-motor areas, label was found following PAG injections in a variety of different brain regions involved in auditory processing. Dense terminal label and a small number of labeled cell bodies were found throughout the periventricular layer (Pe) of the TS and adjacent neuropil of nucleus centralis, which is the primary auditory relay in the midbrain (Figs. 3C, D; 4D, E; 5D, E; 6D) (Bodnar and Bass, 1997, 1999; Bass et al., 2000). A dense band of terminal label and a small number of labeled cell bodies were also found in a region that has been referred to as the deep cell layer of the TS in midshipman (TSd, Fig. 3C, D; 4D, F; 5D, E; see Fig. 3 in Bass et al., 2000). Portions of the torus between the Pe and TSd were relatively free of label; this area contains the ventrolateral nucleus, which receives ascending inputs from the lateral line recipient nuclei of the hindbrain (Weeg and Bass, 2000). Labeled fibers and terminals, varying in density depending on the exact location of the injection site, were consistently found in two targets of nucleus centralis (Bass et al., 2000), the paratoral tegmentum (PTT; Figs. 3C, 5D) and, more caudally, the isthmal nucleus of the midbrain (Is, not to be confused with the visual nucleus isthmi in many vertebrates; Figs. 3D, 4G, 5E).
Dense label of both terminals and cell bodies was observed in the central posterior nucleus of the thalamus (CP), the primary dorsal thalamic recipient of ascending auditory input from the torus (Figs. 3B, 4C, 5B) (Bass et al., 2000). The anterior tuberal nucleus (AT) contained labeled cell bodies (Figs. 3B, 5C), and the nucleus of the lateral lemniscus (nll) contained both cell bodies and terminals (Figs. 3D; 4G, H; 5E), though in the case of both nuclei the density of the label varied depending on the exact location of the injection within the PAG (see below). Both AT and nll receive auditory input from the nucleus centralis of the torus (Bass et al., 2000); the lateral-most portions of AT, which receive the densest toral input, were the most consistently labeled following PAG injections. Thus, in addition to the known vocal-motor structures outlined above, a variety of midbrain and thalamic auditory areas were also consistently labeled following injections to the PAG.
Finally, a number of areas, which can neither be defined as strictly vocal nor as strictly auditory, were also consistently labeled by focal injections into the PAG. These included the periventricular nucleus of the posterior tuberculum (TPp), where we observed dense cell body and terminal labeling (Figs. 3B, 5B, 6B). Further caudal, the medial portion of the midbrain tegmentum consistently contained a diffuse spread of terminals and some labeled cells (Figs. 3C, D; 4A; 5D, E; 6A). Diffuse terminals and an occasional cell body were also observed in the ventral layers of the tectum (Figs. 3B-D; 5D, E). A dense bundle of axons was consistently observed entering the medial longitudinal fasciculus (MLF) and running caudally toward the hindbrain (Figs. 3D-G, 5D-J). In contrast, the lateral lemniscus (ll) generally contained relatively few labeled fibers. Labeled fibers were observed to exit the MLF at different levels of the brainstem, to labeled terminal fields or cell bodies scattered throughout much of the rostro-caudal extent of the reticular formation (Figs. 3E, F; 5F-J) or to VPP (see above). Few, if any, labeled axons were found within the MLF or reticular formation at levels caudal to VPP. A summary of these results, showing the major vocal, auditory, and other connections of the midshipman PAG, is displayed in Figure 7A.
Figure 7.
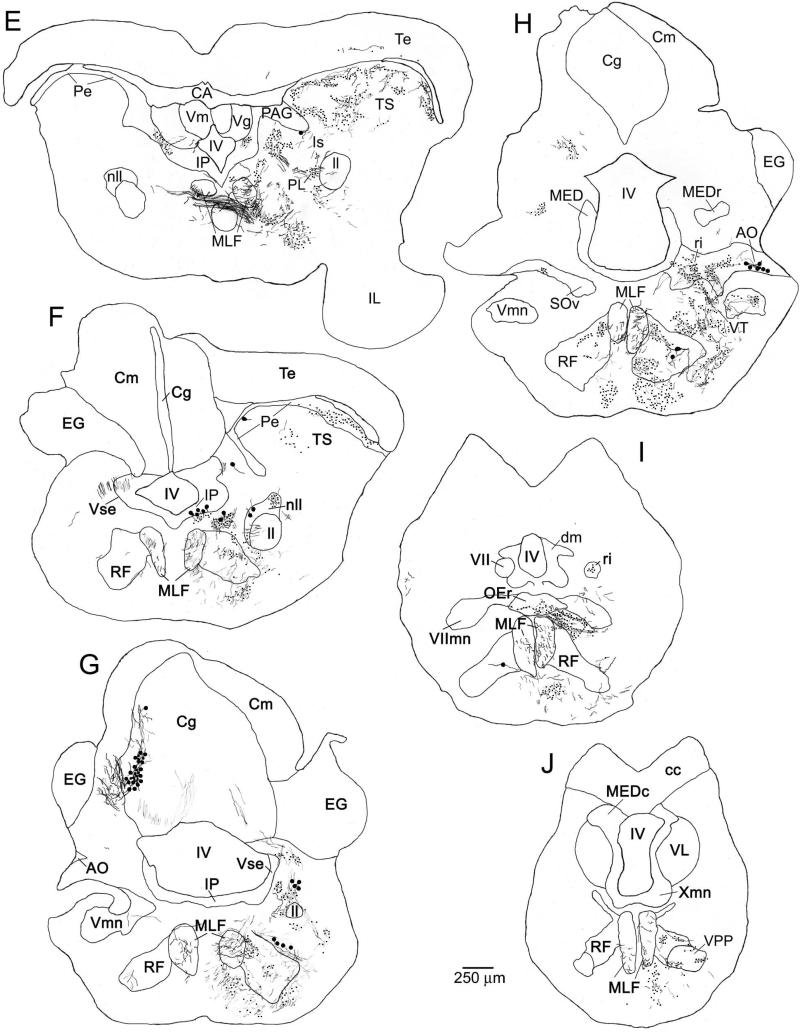
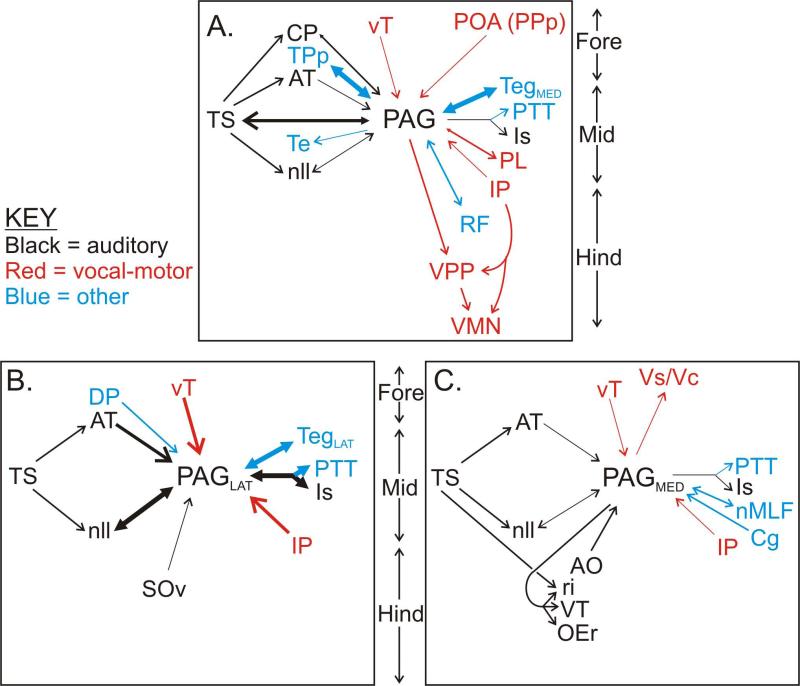
Summary of the major afferent and efferent connections of the PAG, as revealed by neurobiotin injections. Auditory, vocal-motor and other structures are shown separately, as indicated in the key. Relative density of each connection is indicated by the thickness of the lines. Forebrain structures (“Fore”) are at the top of each panel, with midbrain (“Mid”) and hindbrain (“Hind”) structures towards the bottom. A. Consensus connectivity pattern revealed by all injections to the PAG (n = 7). B and C. Connectivity patterns specific only to the lateral (B, n = 2) or medial (C, n = 3) PAG. Note several apparent differences in the relative density of connections between B and C. See Fig. 1 for a more complete outline of the midshipman vocal-motor and auditory systems. See Table for abbreviations.
Specific Connectivity of Lateral vs. Medial PAG
The preceding descriptions outline the pattern of label observed after all injections to the PAG, regardless of where within the PAG the injection site was localized. In addition, however, we observed some specific differences in the pattern of label observed after injections confined to the lateral versus the medial portions of the PAG cell layer. These included areas that were only labeled following injections to one or the other portion of the PAG, as well as areas where the density of label was consistently different depending on the site of the injection. These medio-lateral differences in PAG connectivity are outlined in Figure 7B, C.
Injections confined solely to the medial / rostral portion of the PAG (n = 3), such as that illustrated in Figures 5 and 6, revealed connections to a number of structures not observed following injections to other parts of the PAG. These included a number of known auditory structures in the hindbrain, such as the rostral intermediate division of the descending octaval nucleus (ri), which is a major target of auditory input from the VIIIth cranial nerve and also part of the hindbrain vocal system (Fig. 1, bottom) (Highstein et al., 1992; Bass et al., 1994, 2000). Labeled fine fibers, with swellings indicative of presynaptic boutons, were observed throughout ri following injections to the medial, but not lateral, PAG (compare Figs. 5H; 6E, F with 3F). Similar terminal fibers were labeled in and around the ventral tegmental cell group (VT; Figs. 5H; 6E, G), another structure that, like ri, is within the complex of auditory-vocal hindbrain nuclei (Bass et al., 1994, 2000). A discrete cluster of labeled neurons was observed in the anterior octaval nucleus (AO) (Fig. 5H, 6E and inset), another major target of VIIIth nerve afferents (Highstein et al., 1992; Bass et al., 1994; Edds-Walton et al., 1999). Finally, dense fiber and terminal labeling was observed both within the rostral division of the octaval efferent nucleus (OEr), and also ventral and medial to this nucleus, overlapping extensively with the region occupied by dendrites of OEr neurons (Figs. 5I; 6I, J) (see Bass et al., 1994 for OEr dendritic arbor). None of these hindbrain auditory structures were stained following injections to the lateral PAG (n = 2), though some sparse staining in these structures was detected after injections to the central PAG (n = 2).
In addition to these hindbrain auditory structures, injections to the medial / rostral PAG also specifically labeled several other structures not observed following injections to the lateral PAG. These include sparse, but focal, clusters of terminal fibers in the supracommisural (Vs) and central (Vc) nuclei of area ventralis of the telencephalon (Fig. 5A); a dense cluster of labeled cell bodies in the nMLF on the midline of the rostral midbrain (Fig. 5D, 6A, compare with Fig. 3C); another dense focal cluster of labeled cell bodies in the lateral, caudal portion of the contralateral cerebellum at the interface between the granule cell and molecular layers (Cg; Figs. 5G, 6H); and dense terminals surrounding the large neurons of the oculomotor complex (OMc) in the dorsal-medial mesencephalon.
By contrast, injections to the lateral PAG (n = 2) resulted in label in only three structures not seen following injections to the medial PAG. In addition to cell body labeling in the auditory thalamus (CP), which was observed after all PAG injections, injections to the lateral PAG resulted in cell body labeling in the adjacent dorsal posterior nucleus of the thalamus (DP, Fig. 3B). A few labeled cells were observed in the vicinity of either the ventral division of the secondary octaval nucleus (SOv, Fig. 3F) or in the reticular formation rostral to SOv, only following lateral PAG injections. The SOv, as well as the surrounding reticular formation, project to the auditory torus (Fig. 1, bottom) (Bass et al., 2000). In addition to these novel projections only observed following injections to the lateral PAG, such injections also yielded an apparent increase in the density of label observed in several auditory-vocal areas, relative to the density observed after injections to the central or rostral / medial PAG (highlighted in Fig. 7B, C by differential arrow thickness). Specifically, denser cell body labeling was observed following injections to the lateral PAG in vT (Figs. 3A, 4B versus 5B), AT (Figs. 3B versus 5C), and nll (Figs. 3D, 4G, H versus 5F). Lateral PAG injections resulted in a dense cluster of labeled cell bodies in Is (Figs. 3D, 4G), but injections to the medial PAG resulted in only labeled fibers in this area (Fig. 5E).
Lateralization of PAG Connectivity
Almost all of the connections to and from the midshipman PAG described above were ipsilateral to the injection site. However, a few connections were either bilateral or contralateral. Specifically, labeled cells were observed bilaterally in both PPp and vT, though contralateral labeling was clearly sparser than that found ipsilaterally (Figs. 3A, 5B). Injections to the PAG consistently resulted in labeled neurons and terminals in the contralateral PAG, and neurons and terminals in the nearby PTT and medial tegmentum were also observed bilaterally (Figs. 3C, D; 5C, D). The diffuse label of neurons, terminals, and fibers in the reticular formation (Figs. 3E, F; 5G, H) was consistently present bilaterally, though labeled fibers in the VPP (Figs. 3G, 5J) were only observed ipsilaterally. Labeled neurons in DP, only observed after more lateral PAG injections, were bilateral, and with possibly a denser distribution contralateral to the injection site (Fig. 3B). AT label followed an interesting pattern, being entirely ipsilateral to the more lateral PAG injection sites (Fig. 3B), but entirely contralateral (and much sparser) to the medial PAG injection sites (Fig. 5C). Medial PAG injections also resulted in apparently entirely contralateral terminal label in Vs / Vc (Fig. 5A) and cell body label in the cerebellum (Cg; Fig. 5G). An obvious fiber bundle was traceable from the labeled contralateral cluster of cerebellar neurons running ventral and rostral, just lateral to the trigeminal sensory nucleus (Vse, Fig. 5F), to cross the midline dorsal to the MLF at the level of IP in the caudal midbrain (Fig. 5E), where it turned and ran rostral through the medial tegmentum towards the injection site.
DISCUSSION
Our results, summarized in Figure 7, confirm and extend previous findings (Goodson and Bass, 2002; Kittelberger et al., 2006) regarding vocal-motor connectivity of the PAG; reveal a more extensive pattern of auditory connections, particularly in the auditory hindbrain, to and from the PAG than previously appreciated (Goodson and Bass, 2002); and show several differences in the connectivity of rostral / medial portions of the PAG versus more central, caudal, and lateral PAG (Fig. 7B, C), suggesting the possibility of functionally distinct subdivisions.
Methodological Considerations
A concern regarding the current results is the degree to which the observed labeling represents the labeling of fibers of passage through the injection site, or spread of tracer into adjacent structures, rather than anterograde labeling of PAG neuronal axons or retrograde label of fibers terminating in the PAG. These concerns can be discounted for several reasons. First, Neurobiotin, the tracer used here, was specifically chosen due to the very limited degree to which it labels fibers of passage (Vercelli et al., 2000). Second, close examination of the injection sites reveals no obvious labeled fibers of passage, or spread beyond the PAG, particularly laterally into the TS. Third, several of the specific connections we describe here, between the PAG and AT, CP, vT, VM and cerebellum, have been confirmed in the opposite direction (Goodson and Bass, 2002; A. Bass, unpublished observations). Fourth, the most obvious source for fibers of passage (or concern about tracer spread) is the TS, yet we observe very few retrogradely-labeled neurons in the TS. Furthermore, the main output pathways for the midshipman TS pass fairly far ventral and lateral of all the injection sites (Bass et al., 2000). Fifth, the observed pattern of label diverges substantially from the pattern of label observed after injections to the medial torus (the nucleus centralis, the auditory portion of the torus and the portion immediately adjacent to the PAG). Specifically, we observe label in a number of structures not labeled by nucleus centralis injections, including vT, VPP, and IP (Bass et al., 2000). Furthermore, we see no labeling of a number of structures which are labeled after injections to the nucleus centralis, including the medial pretoral nucleus, tectum, the dm subdivision of the descending octaval nucleus, and the contralateral TS (Bass et al., 2000). We also observe retrogradely labeled neurons in CP and nll, where nucleus centralis injections only yield labeled terminals (Bass et al., 2000). Sixth, neither the MLF nor lateral lemniscus was close to any of our injection sites. Seventh, given that we observed labeled neurons in nMLF, which is medial to the rostral PAG, after medial PAG injections, perhaps some of the terminal arbors which we claim here to be evidence of efferent connections of the medial PAG are in actuality efferents of the nMLF. We think this is not likely to be the case for several reasons. The nMLF in midshipman is well medial of medial PAG injections (Figs. 2A, 6A; see also Forlano, et al., 2005, Fig. 8), and close inspection indicates no spread of dye from the injection sites into nMLF. Therefore, nMLF neuron labeling results from either dendrites or axon collaterals extending into the medial PAG, indicating connectivity between nMLF and the medial PAG. This does not exclude the possibility that labeled nMLF axons could contribute to axon terminal labeling caudal to the injection sites, particularly since we do see thick axons in the MLF running caudally. However, there are a number of distinct differences between the pattern of axon terminal labeling we describe for the medial PAG and the pattern of connectivity of the nMLF in teleosts as described by others. For example, there is a major pathway from the nMLF to the spinal cord in several species (Oka et al., 1986; Rao et al., 1993; Gahtan and O'Malley, 2003). After medial PAG injections, however, we observed no labeled fibers running caudal to the level of VPP in the central hindbrain. Similarly, while the trochlear nucleus has been described as a major output of the nMLF (e.g., Torres et al., 1995), we observed no labeled fibers in the trochlear nucleus after medial PAG injections. These discrepancies argue against the possibility that what we describe as efferents of the medial PAG are significantly contaminated by nMLF labeling. Thus, it seems unlikely that the pattern of label we describe here, particularly the auditory connectivity of the PAG, is due to labeling of structures adjacent to the PAG (i.e., the TS, nMLF) or of fibers of passage through or near the PAG.
Vocal-Motor Connectivity of Midshipman Periaqueductal Gray
The major components of the midshipman vocal-motor circuit (Fig. 1, top) to which the PAG is connected include afferents from the hypothalamus (vT), the preoptic area (PPp) and the isthmal region (IP), and efferents to the tegmentum (PL) and the vocal prepacemaker neurons in the caudal hindbrain (VPP). The VPP is a central component of the hindbrain vocal pattern generator, projecting to vocal pacemaker neurons (VPN), which, in turn, innervate the vocal motor nucleus (VMN) (Fig. 1, top) (Bass et al., 1994; Goodson and Bass, 2002; Chagnaud et al., 2011). We observed no label in either the VPN or the VMN following injections to the PAG, implying that descending information from the PAG to the hindbrain vocal pattern generator is received solely by VPP. The vT and PPp are components of the anterior hypothalamus / preoptic vocal network identified by Goodson and Bass (2002). The vT is considered to be homologous to the anterior hypothalamus, and PPp to the paraventricular nucleus of the mammalian preoptic area, and interconnections between these areas and the PAG are widely conserved across vertebrates (Goodson, 2005; Forlano and Bass, 2011; O'Connell and Hofmann, 2011). Stimulation of these sites elicits vocal production (Fine and Perini, 1994; Goodson and Bass, 2000a; Kittelberger et al., 2006), which is blocked by inactivation of the PAG (Kittelberger et al., 2006). The pathways from vT to the PAG and from the PAG to VPP have been confirmed bi-directionally by injections to those structures (Goodson and Bass, 2002). Intracellular recordings in VPP neurons reveal short-latency, apparently mono-synaptic, EPSPs after stimulation of midbrain sites including the PAG (Chagnaud et al., 2011). All of the vocal-motor PAG connections described here were identified previously by Goodson and Bass (2002), though the PAG injections in that study were made only to the very caudal-most portion of the PAG. Our results thus confirm that these vocal-motor PAG connections are ubiquitous to all portions of the PAG, and establish vT / PPp to PAG to VPP as the major descending vocal-motor pathway.
IP, another site where electrical stimulation in toadfish can produce vocal output (Demski and Gerald, 1972), sends inputs to both the PAG, as shown here and by Goodson and Bass (2002), and to the hindbrain vocal pattern generator (Bass et al., 1994; Goodson and Bass, 2002), suggesting that IP activity may influence vocal production at several levels. PL, another midbrain site where stimulation reliably evokes vocal output (Goodson and Bass, 2000b, c), is also heavily interconnected with the PAG. We observed mostly terminal fibers and a few retrogradely labeled neurons in the PL following injections to the PAG, while Goodson and Bass (2002) observed stronger retrograde labeling of PL cell bodies following injections to the more caudal PAG and the immediately adjacent tegmentum (PTT). We observed little, if any, label in the anterior parvocellular preoptic nucleus (PPa) after injections to the PAG, representing another difference between our results and those of Goodson and Bass (2002). Further slight differences between our current results and those of Goodson and Bass (2002) include: 1) we observed only retrogradely-labeled neurons in vT, PPp, and AT, while Goodson and Bass observed both neurons and terminals in those locations; 2) conversely, in VPP we observed only labeled terminals while Goodson and Bass observed occasional labeled cell bodies as well; 3) Goodson and Bass observed labeled cell bodies and terminals in two sites (the posterior nucleus of the ventral telencephalon and the paraventricular hindbrain cell group) where we found no label; and 4) as discussed further below, we identified here a number of novel connections of the PAG (auditory nuclei, cerebellum, nMLF, OMc) not described by Goodson and Bass. We presume that these various differences are attributable to the fact that the PAG injections made by Goodson and Bass were made in the extreme caudal portion of the PAG, and also intruded upon the adjacent tegmentum (PTT). Our injections, by contrast, spanned the entire rostro-caudal and medio-lateral extent of the PAG with the exception of the caudal-most tail, were smaller, restricted to the PAG cell layer, and did not encroach into the PTT.
Midshipman Periaqueductal Gray and Auditory-Vocal Integration
In addition to those aspects of PAG connectivity that appear to be primarily vocal-motor, we describe here dense interconnectivity between the PAG and a number of structures in the ascending auditory pathway. The midshipman auditory pathway (Fig. 1, bottom) is similar to the auditory circuit in most teleosts (e.g., Finger and Tong, 1984; Highstein et al., 1992; Bass et al., 1994; Kozlowski and Crawford, 1998; Edds-Walton et al., 1999; Weeg and Bass, 2000; Tomchik and Lu, 2005; reviewed by McCormick, 1999; Bass and Lu, 2007; Bass and McKibben, 2003). Inputs from the primary auditory organ in the inner ear, the saccule, innervate a complex of five hindbrain auditory structures (the posterior, descending, tangential, magnocellular, and anterior octaval nuclei) as well as distinct medullary lateral line nuclei. Other medullary auditory structures include a secondary octaval nucleus (with dorsal and ventral subdivisions), a nearby ventral tegmental cell cluster (VT), and an octaval efferent nucleus, which projects back to the saccule (Bass et al., 1994, 2000). The descending and secondary octaval nuclei, in turn, innervate auditory portions of the midbrain's torus semicircularis (TS), the teleost homolog of the mammalian inferior colliculus (e.g., Echteler, 1984; Kozlowski and Crawford, 1998; Bass et al., 2000; Bass and Lu, 2007). Projections arising in the auditory torus innervate the isthmal midbrain (nll, Is), the rostral midbrain (medial pretoral nucleus, P; Fig. 1, bottom), anterior hypothalamus (AT), dorsal thalamus (CP), tectum, and the preglomerulosus complex of the diencephalon (lateral division of nucleus preglomerulosus, PGl) (e.g., Echteler, 1984; Kozlowski and Crawford, 1998; Bass et al., 2000; Bass and Lu, 2007). Telencephalic auditory areas include portions of the area ventralis just lateral of the Vs and Vc, which receive inputs from CP and AT and from the auditory TS itself (Bass et al., 2000; Goodson and Bass, 2002) and portions of the dorsal telencephalon, which receive auditory input from components of the preglomerular complex including the PGl in goldfish and carp (Yamamoto and Ito, 2005, 2008; Northcutt, 2006).
Previous work has identified several possible sites for auditory-vocal integration. The vocal prepacemaker nucleus, primarily considered as a vocal-motor structure with its descending inputs from the PAG and IP and outputs to the other components of the vocal pattern generator (VPN and VMN), also receives inputs from a number of auditory structures including the nll, ri, and the ventral portion of the secondary octaval nucleus (SOv), and has outputs to VT and to the octaval efferent nucleus (OE, mainly to the caudal division), thus potentially enabling vocal information to influence auditory tuning in the saccule (Bass et al., 1994; Goodson and Bass, 2002; Weeg et al., 2005; Chagnaud et al., 2011). Area ventralis in the telencephalon may also be an important site for auditory-vocal integration, as a distinct region just lateral of Vs / Vc receives inputs from both auditory structures (CP, AT, TS) and vocal-motor structures (vT, PAG) (Bass et al., 2000; Goodson and Bass, 2002; present study). Finally, Goodson and Bass (2002) identified a number of auditory connections of the PAG, including reciprocal connections with CP, AT, the nll, and Is, making the PAG a potential site for auditory-vocal integration. Our current data confirms these projections across the entire extent of the PAG, and not just for the caudal-most tail of the PAG, as shown by Goodson and Bass (2002). It should be noted that the connections between the PAG and both CP and AT have also been confirmed bi-directionally (Goodson and Bass, 2002). In addition, we observed connections between the PAG and portions of the auditory hindbrain not found by Goodson and Bass, including efferent projections to ri, VT, and the rostral portion of the octaval efferent nucleus (OEr), and afferent projections from AO and possibly also SOv. With the exception of the sparse connection from SOv, all of these were only observed after injections to the rostral / medial portion of the PAG, an area not targeted by Goodson and Bass (2002). We observed no label following PAG injections in other portions of the auditory hindbrain, including the dorsolateral and dorsomedial (dm) subdivisions of the descending octaval nucleus, the dorsal portion of the secondary octaval nucleus, and the magnocellular octaval nucleus (MG). Interestingly, ri, SOv, VT, and OE, shown here to be connected to the PAG, are the same structures previously identified as interconnected with the hindbrain vocal pattern generator (though VPP projects to the caudal portion of OE, while PAG projects to the rostral portion) (Bass et al., 1994, 2000; Goodson and Bass, 2002; Chagnaud et al., 2011). These structures may thus be more involved in auditory-vocal integration, while other portions of the auditory hindbrain (i.e., MG, the dorsolateral and dorsomedial divisions of the descending octaval nucleus, the dorsal portion of the secondary octaval nucleus) may be more “purely” auditory in nature. The same phenomenon is found in the midbrain, diencephalon and telencephalon, where some auditory structures (i.e., CP, AT, nll, Is, Vs/Vc) also receive vocal-motor inputs (including from the PAG), while other auditory areas (i.e., the medial pretoral nucleus, the PGl, and auditory portions of the tectum and dorsal telencephalon) do not.
We observed dense fiber labeling, and a few filled neurons, in the TS after injections to the PAG, further supporting the idea that the PAG is an important site for auditory-vocal integration. This contrasts somewhat with the results of Goodson and Bass (2002), which showed terminals only in the ventral TS. Again, this may be attributable to the fact that the previous results only targeted a small portion of the PAG. We observed labeling specifically in the portions of the TS previously identified as auditory, including nucleus centralis and the deep layer (TSd) (Bass et al., 1994, 2000). We found very little labeling in other non-auditory portions of the TS, including, for example, the ventrolateral nucleus, a region shown to be involved mainly in processing information from the lateral line system (Weeg and Bass, 2000). Similarly, no label was observed in hindbrain, isthmal, or thalamic lateral line structures (i.e., the nucleus medialis, nucleus praeeminentialis, and posterior thalamic nucleus, respectively) after injections to the PAG. Finally, no label was observed in the lateral and ventral regions of the descending octaval nuclei or in the tangential octaval nucleus, which are considered to be primarily vestibular in teleosts (e.g., Highstein et al., 1992; Tomchik and Lu, 2005; Straka et al., 2006).
Together, these data imply that the TS itself may act to integrate vocal inputs coming from the PAG with ascending auditory information. Several lines of evidence support this possibility functionally in other species. In monkeys, neurons in the inferior colliculus display electrical activity related to both auditory stimuli and vocal-motor production when recorded in awake-behaving animals (Pieper and Jurgens, 2003). In frogs, immediate early gene expression in the TS tracks sexually-dimorphic responses to different call types, and predicts stimulus- and behavior-linked immediate-early gene expression patterns in forebrain motor areas, implying that the TS is a critical node between ascending auditory information and descending motor responses (Hoke et al., 2007, 2010). Vocal-motor inputs to the TS, such as demonstrated here from the PAG, would be expected to inform auditory processing of calls to avoid responses to self-generated vocalizations.
Other Connections of Midshipman Periaqueductal Gray
Several structures were labeled by PAG injections that have not been identified as being either particularly auditory or particularly vocal. The mammalian PAG is interconnected with a wide array of structures, many of which are neither vocal-motor nor auditory (see more on this below), so it is not surprising to find such connections in midshipman. These include dense clusters of retrogradely-labeled neurons in TPp, nMLF, and in a discrete portion of the contralateral cerebellum, and terminals in the OMc. None of these projections have previously been identified in midshipman. The nMLF, OMc, and cerebellar projections were only observed with rostro-medial PAG injections, likely explaining why the caudal PAG injections made by Goodson and Bass (2002) failed to label these structures. The nMLF, along with the OMc, is part of a network involved in oculomotor control in both teleosts and mammals (Torres et al., 1992, 1995; Chen and May, 2002; Luque et al., 2011), and components of this network have been shown to project to the PAG in cats, including a sparse projection from the nMLF itself (Chen and May, 2002; Klop et al., 2006). The teleost nMLF is also involved in descending control of locomotion, and has been proposed to be the equivalent of the mesencephalic locomotor region (MLR) identified in the context of lamprey locomotion (Uematsu and Todo, 1997; Sirota et al., 2000; Dubuc et al., 2008; Sankrithi and O'Malley, 2010). While the neuroanatomical connections of the teleost nMLF are not well characterized, several of the structures thought to comprise the mammalian MLR (Sinnamon and Benaur, 1997; Jordan, 1998), including nMLF, have been shown to project to the PAG, and, in particular, to vocal portions of the PAG (Mantyh, 1982; Chen and May, 2002; Dujardin and Jurgens, 2005). The cluster of labeled cerebellar neurons we observed was found at the interface between the granule cell layer and the molecular layer (that is, in the position of the Purkinje cell layer, often referred to as the ganglionic cell layer in teleosts) in the caudal cerebellum. Injections to the cerebellum in midshipman confirm this connection, revealing terminals in the PAG (A. Bass, unpublished observations). The well-defined fiber pathway from this cluster of cells to the injection site closely followed the trajectory described for the brachium conjunctivum in other teleosts (Wullimann and Northcutt, 1988; Ikenaga et al., 2002). We thus propose that these labeled neurons are most likely eurydendroid cells, the major output neurons of the teleost cerebellum (Ikenaga et al., 2005, 2006; see also Straka et al., 2006).
We observed heavy label in the TPp after all of our injections to the PAG. Goodson and Bass (2002) also showed this connection, but did not identify it as the TPp (see Fig. 7C in Goodson and Bass, 2002). Projections to the PAG from structures considered to be homologous to the TPp have been demonstrated in a number of species (reviewed in O'Connell and Hofmann, 2011). The TPp is a major dopaminergic cell group in teleosts (Rink and Wulliman, 2001; Ma, 2003; Yamamoto and Vernier, 2011), so our findings suggest the presence of dopamine inputs to the PAG. The TPp is thought to be homologous to the mammalian midbrain dopamine structures, the substantia nigra and ventral tegmental area (VTA; Rink and Wulliman, 2001; O'Connell and Hofmann, 2011), though this hypothesis is not without controversy (Yamamoto and Vernier, 2011). Afferents from the substantia nigra and VTA to the PAG have been shown in squirrel monkeys (Dujardins and Jurgens, 2005), and from the VTA to the PAG in rats (Domesick, 1988), and efferents from PAG to substantia nigra and VTA have been shown in rats (Cameron et al., 1995a; Omelchenko and Sesack, 2010).
Differences in Connectivity between Medial and Lateral Periaqueductal Gray
As described in the Results, and summarized in Figure 7, we found several differences in the pattern of connectivity between the rostral / medial midshipman PAG and the more central, lateral, and caudal portions. These differences imply the presence of anatomical subdivisions within the PAG, reminiscent of the subdivisions found in the mammalian PAG, which has distinct columns differing in both connectivity and function (Bandler and Shipley, 1994; Cameron et al., 1995a, 1995b). In particular, we found connections between rostral / medial PAG and structures in the auditory hindbrain, the nMLF, the OMc, the cerebellum, and area ventralis in the telencephalon not labeled after injections to other portions of the PAG. One possibility is thus that the rostral / medial PAG is more involved in auditory-vocal integration than other parts of the PAG. However, it is worth noting that certain other auditory structures, particularly those above the level of the hindbrain (i.e., AT, nll, Is), provided denser inputs to central / lateral PAG than to rostral / medial PAG. It is not immediately obvious that either portion of the midshipman PAG, as described here, is directly comparable to any specific subdivision of the mammalian PAG, based at least on their patterns of connectivity (Bandler and Shipley, 1994; Cameron et al., 1995a, 1995b). Furthermore, it is worth noting that electrophysiological studies of the midshipman PAG have noted no particular differences in vocal-motor related activity between recording sites in the rostral / medial vs. the central / lateral / caudal PAG (Kittelberger et al., 2006). Recently, however, functional subdivisions of the avian PAG have been demonstrated to be homologous to specific subdivisions of the mammalian PAG, and organized into medial and lateral columns running rostro-caudally, which is highly reminiscent of the pattern we describe here for the midshipman PAG (Kingsbury et al., 2011).
Comparisons with Terrestrial Vertebrates
Notwithstanding the lack of precise congruence between the possible subdivisions of the midshipman PAG and the well-established subdivisions of the mammalian PAG, the overall pattern of connectivity of the midshipman PAG is strikingly similar to that of the PAG in both mammals and birds (Mantyh, 1982; Holstege, 1989; Cameron et al., 1995a, 1995b; Briganti et al., 1996; Wild et al., 1997; Vanderhorst et al., 2000; Dujardins and Jurgens, 2005; Kingsbury et al., 2011; O'Connell and Hofmann, 2011). As in midshipman, dense reciprocal connections are found between the PAG and hypothalamic and preoptic nuclei (Mantyh, 1982; Rizvi et al., 1992; Cameron et al., 1995a; Briganti et al., 1996; Dujardins and Jurgens, 2005; Kingsbury et al., 2011). In particular, interconnections between the anterior hypothalamus (vT in midshipman), the ventromedial hypothalamus (AT in midshipman) and the PAG are widely conserved across vertebrates (O'Connell and Hofmann, 2011). Vocal-motor connections of the mammalian PAG include inputs from anterior cingulate cortex, and outputs to vocal premotor neurons in the medullary reticular formation, the nucleus retroambiguus, and the solitary tract nucleus (Holstege, 1989; Vanderhorst et al., 2000; Jurgens, 2002). The final output structure of the vocal motor pathway in midshipman, the VMN, is homologous to mammalian and avian vocal motor nuclei (Bass et al., 2008), and in mammals, as here, the PAG does not directly innervate the vocal motor neurons but instead influences these motor neurons indirectly, via medullary premotor structures (Holstege, 1989; Vanderhorst et al., 2000; Jurgens, 2002). Birds may be an exception, as the putative avian PAG homologue, the dorsomedial nucleus of the intercollicular complex (Dubbeldam and den Boer-Visser, 2002; Kingsbury et al., 2011) projects directly to the tracheosyringeal portion of the hypoglossal nucleus (nXIIts), which contains the vocal motor neurons innervating the syrinx (Wild et al., 1997). It is worth noting, however, that the avian PAG also connects to medullary vocal-motor structures pre-synaptic to nXIIts (Wild et al., 1997), as in both mammals and midshipman. As a whole, the hypothalamic, preoptic, and hindbrain vocal-motor connectivity of the midshipman PAG is highly reminiscent of patterns described in mammals and birds.
The mammalian PAG, like the midshipman PAG, is also interconnected with a number of known auditory structures, though the details of these connections vary among species. Neurons in the auditory cortex innervate the PAG in monkeys, ferrets, and gerbils (Bajo and Moore, 2005; Dujardins and Jurgens, 2005; Bajo et al., 2007). The PAG is known to receive input from the inferior colliculus in bats (Metzner, 1996), monkeys (Dujardins and Jurgens, 2005), and also pigeons (Wild et al., 1997), while we show, predominantly, PAG output to the TS, with only sparse input (see discussion above of the implications for the TS in auditory-vocal integration). Furthermore, studies in bats and monkeys implicate a region in the paralemniscal midbrain tegmentum in auditory-vocal integration (Metzner, 1996; Schuller et al., 1997; Hannig and Jurgens, 2006) and this region is generally reciprocally connected with both the PAG and several primary auditory structures (with some variation in the reciprocity of connections with specific areas varying between species) (Metzner, 1996; Schuller et al., 1997; Hannig and Jurgens, 2006; Varga et al., 2008). Following PAG injections, we observed sparsely distributed fibers and terminals throughout the tegmentum, including the paralemniscal tegmentum regions previously identified as vocal (Goodson and Bass, 2002), but it is unclear if this region is directly comparable with the paralemniscal site described in mammals. Others have described connections between various subdivisions of the amygdala, particularly the medial amygdala, and the PAG, across a range of vertebrate species (Mantyh, 1982; Rizvi et al., 1991; O'Connell and Hofmann, 2011). Consistent with these findings, we show here labeled terminal fibers following medial / rostral PAG injections in a region of area ventralis (Vs) thought to be homologous to the medial amygdala in terrestrial vertebrates (Forlano and Bass, 2011; O'Connell and Hofmann, 2011). Finally, as discussed above, connections between the dopaminergic teleost TPp and the PAG also appear to be conserved across many vertebrates (Cameron, et al., 1995a; Dujardins and Jurgens, 2005; Omelchenko and Sesack, 2010; O'Connell and Hofmann, 2011), as are certain connections between the PAG and midbrain locomotor and oculomotor structures (Chen and May, 2002; Dujardins and Jurgens, 2005; Klop et al. 2006). Collectively, our data thus imply a similar general pattern of PAG connectivity between midshipman and other vertebrates, including both vocal and auditory interconnections. Nonetheless, it is important to note that the specific PAG inputs and outputs in midshipman by no means precisely replicate homologous structures interconnected with the mammalian, or avian, PAG.
Conclusions
In conclusion, we demonstrate here a robust pattern of connectivity between the PAG and a large number of vocal and auditory brain structures in a sound producing teleost fish. This pattern is generally, but not precisely, consistent with the known connectivity of the PAG among vertebrates, and more specifically mammals; implies the existence of functional subdivisions within the midshipman PAG; and supports the hypothesis that the midshipman PAG is involved in auditory-vocal integration. Specifically, we show vocal-motor inputs to the PAG from the anterior hypothalamus-preoptic area (vT, PPp) and the isthmal region (IP), and outputs to the ventral forebrain (Vs/Vc), paralemniscal tegmentum (PL), and hindbrain vocal pattern generator (VPP). These findings are consistent with a large body of literature supporting a central role for the PAG in descending vocal-motor pathways in a variety of species, including midshipman (Holstege, 1989; Jurgens, 1994, 2002, 2009; Goodson and Bass, 2002; Kittelberger et al., 2006). Furthermore, we show inputs to the midshipman PAG from known auditory structures in the anterior hypothalamus (AT), dorsal thalamus (CP), midbrain (nll, Is), and rostral hindbrain (AO, SOv). Outputs from the PAG to auditory areas include CP, nll, Is, auditory portions of the midbrain torus semicircularis (TS), and other hindbrain auditory nuclei (ri, VT) including the nucleus that provides direct input to the inner ear (OEr). These extensive auditory connections of the PAG are found at multiple levels, from the medulla to the midbrain, thalamus, and hypothalamus. By contrast, a number of components of the auditory system are not directly connected to the PAG, including the medial pretoral nucleus, lateral nucleus preglomerulosus and several hindbrain auditory nuclei (dorsolateral and dorsomedial divisions of the descending octaval nucleus, the magnocellular octaval nucleus, and the dorsal division of SO) – these structures may thus be more purely auditory in function. Based on this architecture, neurons in the midshipman PAG may themselves directly integrate auditory input with descending vocal-motor commands. Alternatively, or additionally, the PAG may relay efference copies of vocal-motor information to certain auditory areas (i.e., OEr, TS, VT) to shape responses to incoming auditory stimuli. Such information could be useful, for example, in decreasing auditory responsiveness to self-generated vocalizations. Finally, PAG inputs to either forebrain (Vs/Vc) or hindbrain (VPP) vocal-motor structures may influence vocal patterning, possibly enabling adjustment of vocal behavior based on perceived conspecific communication signals. Such a function would be consistent with evidence that a region of the rodent PAG plays a role in switching behavioral modes depending on both internal motivational states and external environmental stimuli (Sukikara et al., 2006). If this function of the PAG is a shared feature among vertebrates, then midshipman fish may be a useful model system for elucidating a more general role of the PAG in auditory-vocal integration during adaptive vocal communication interactions.
| Abbreviations | |||
|---|---|---|---|
| ac | anterior commissure | PGm | nucleus preglomerulosus, medial division |
| AO | anterior octaval nucleus | PL | Paralemniscal midbrain tegmentum |
| AT | anterior tuberal nucleus | PM | magnocellular preoptic nucleus |
| CA | cerebral aqueduct | POA | preoptic area |
| cc | cerebellar crest | PPa | anterior parvocellular preoptic nucleus |
| Cg | granule cell layer of the cerebellum | PPp | posterior parvocellular preoptic nucleus |
| Cm | molecular layer of the cerebellum | Pr | nucleus preeminentialis |
| CP | central posterior nucleus of the thalamus | PTT | paratoral tegmentum |
| DF | diffuse nucleus of the inferior lobe | ri | descending octaval nucleus, rostral intermediate division |
| DL | dorsolateral telencephalon | ||
| DM | dorsomedial telencephalon | RF | reticular formation |
| dm | descending octaval nucleus, dorsomedial division | Sc | suprachiasmatic nucleus |
| SO | secondary octaval nucleus | ||
| DO | descending octaval nucleus | SOv | secondary octaval nucleus, ventral division |
| DP | dorsal posterior nucleus of the thalamus | ||
| EG | eminentia granularis | SV | saccus vasculosus |
| G | nucleus glomerulosus | Te | midbrain tectum |
| Ha | habenula | Teg | midbrain tegmentum |
| Hd | dorsal periventricular hypothalamus | TeP | midbrain tectum, periventricular layer |
| iaf | internal arcuate fiber tract | TL | torus longitudinalis |
| IL | inferior lobe of the hypothalamus | TPp | periventricular nucleus of posterior tuberculum |
| IP | isthmal paraventricular nucleus | ||
| Is | isthmal nucleus of the midbrain | TS | torus semicircularis |
| ll | lateral lemniscus | TSd | deep layer of the torus semicircularis |
| MED | medial octavolateralis nucleus | Vc | central nucleus of area ventralis of the telencephalon |
| MEDc | medial octavolateralis nucleus, caudal division | ||
| Vd | dorsal nucleus of area ventralis of the telencephalon | ||
| MEDr | medial octavolateralis nucleus, rostral division | ||
| Vg | granule cell layer of the valvula | ||
| MG | magnocelluar octaval nucleus | VL | vagal lobe |
| MLF | medial longitudinal fasciculus | Vm | molecular layer of the valvula |
| nll | nucleus of the lateral lemniscus | VMN | vocal motor nucleus |
| nlv | nucleus lateralis valvulae | VPN | vocal pacemaker nucleus |
| nMLF | nucleus of medial longitudinal fasciculus | VPP | vocal prepacemaker nucleus |
| OE | octavolateralis efferent nucleus | Vs | supracommisural nucleus of area ventralis of the telencephalon |
| OEr | octavolateralis efferent nucleus, rostral division | ||
| VT | ventral tegmental cell group | ||
| OMc | oculo-motor complex | vT | ventral tuberal hypothalamus |
| OT | optic tract | ||
| P | medial pretoral nucleus of the pretectum | III | third ventricle |
| PAG | periaqueductal gray | IV | fourth ventricle |
| PCo | posterior commissure | Vse | trigeminal sensory nucleus |
| Pe | periventricular cell layer of the torus semicircularis | Vmn | trigeminal motor nucleus |
| VII | central tract of the facial nerve | ||
| PGc | nucleus preglomerulosus, caudal division | VIImn | facial motor nucleus |
| PGl | nucleus preglomerulosus, lateral division | Xmn | vagus motor nucleus |
Acknowledgments
Support: These studies were funded by grants from the National Science Foundation (MRI-R2 0959175 to JMK) and the National Institutes of Health, National Institute for Deafness and Communication Disorders (RO1 DC00092 to AHB, NRSA F32 DC06156 to JMK).
Footnotes
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JMK and AHB. Acquisition of data: JMK. Analysis and interpretation of data: JMK and AHB. Drafting of the manuscript: JMK. Critical revision of the manuscript for important intellectual content: JMK and AHB. Obtained funding: JMK and AHB.
Conflict of Interest Statement.
We identify no known or potential conflicts of interest.
LITERATURE CITED
- Bajo VM, Moore DR. Descending projections from the auditory cortex to the inferior colliculus in the gerbil, Meriones unguiculatus. J Comp Neurol. 2005;486:101–116. doi: 10.1002/cne.20542. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cerebral Cortex. 2007;17:475–491. doi: 10.1093/cercor/bhj164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bass AH. Shaping brain sexuality. Am Sci. 1996;84:352–363. [Google Scholar]
- Bass AH, Baker R. Sexual dimorphisms in the vocal control system of a teleost fish: morphology of physiologically identified neurons. J Neurobiol. 1990;21:1155–1168. doi: 10.1002/neu.480210802. [DOI] [PubMed] [Google Scholar]
- Bass AH, Baker R. Phenotypic specification of hindbrain rhombomeres and the origins of rhythmic circuits in vertebrates. Brain Behav Evol. 1997;50(suppl 1):3–16. doi: 10.1159/000113351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Bodnar DA, Marchaterre MA. Complementary explanations for existing phenotypes in an acoustic communication system. In: Hauser MD, Konishi M, editors. The Design of Animal Communication. MIT Press; Cambridge, MA: 1999. pp. 493–514. [Google Scholar]
- Bass AH, Bodnar DA, Marchaterre MA. Midbrain acoustic circuitry in a vocalizing fish. J Comp Neurol. 2000;419:505–531. doi: 10.1002/(sici)1096-9861(20000417)419:4<505::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bass AH, Gilland EH, Baker R. Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science. 2008;321:417–421. doi: 10.1126/science.1157632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Lu Z. Neural and behavioral mechanisms of audition. In: Hara T, Zielinski B, editors. Fish Physiology. Vol. 25. Sensory Systems Neurosci. Elsevier Academic Press; San Diego: 2007. pp. 377–410. [Google Scholar]
- Bass AH, Marchaterre MA, Baker R. Vocal-acoustic pathways in a teleost fish. J Neurosci. 1994;14:4025–4039. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog Neurobiol. 2003;69:1–26. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Bodnar DA, Bass AH. Temporal coding of concurrent acoustic signals in auditory midbrain. J Neurosci. 1997;17:7553–7564. doi: 10.1523/JNEUROSCI.17-19-07553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar DA, Bass AH. Midbrain combinatorial code for temporal and spectral information in concurrent acoustic signals. J Neurophysiol. 1999;81:552–563. doi: 10.1152/jn.1999.81.2.552. [DOI] [PubMed] [Google Scholar]
- Brantley RK, Bass AH. Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichthys notatus girard (Teleastei, Batrachoididae). 1994.
- Briganti F, Beani L, Panzica GC. Connections of the dorsomedial part of the nucleus intercollicularis in a male non-songbird, the Grey partridge: a tract-tracing study. Neurosci Lett. 1996;221:61–65. doi: 10.1016/s0304-3940(96)13261-4. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Iqbal AK, Westlund KN, Cliffer KD, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J Comp Neurol. 1995a;351:568–584. doi: 10.1002/cne.903510407. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Iqbal AK, Westlund KN, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. II. Descending projections. J Comp Neurol. 1995b;351:585–601. doi: 10.1002/cne.903510408. [DOI] [PubMed] [Google Scholar]
- Chagnaud BP, Baker R, Bass AH. Vocalization frequency and duration are coded in separate hindbrain nuclei. Nature Commun. 2011;2:346. doi: 10.1038/ncomms1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, May PJ. Premotor circuits controlling eyelid movements in conjunction with vertical saccades in the cat: I. The rostral interstitial nucleus of the medial longitudinal fasciculus. J Comp Neurol. 2002;450:183–202. doi: 10.1002/cne.10313. [DOI] [PubMed] [Google Scholar]
- Demski LS, Gerald JW. Sound production evoked by electrical stimulation of the brain in toadfish (Opsanus beta). Anim Behav. 1972;20:507–513. doi: 10.1016/s0003-3472(72)80015-0. [DOI] [PubMed] [Google Scholar]
- Demski LS, Gerald JW. Sound production and other behavioral effects of midbrain stimulation in free-swimming toadfish, Opsanus beta. Brain Behav Evol. 1974;9:41–59. doi: 10.1159/000123654. [DOI] [PubMed] [Google Scholar]
- Domesick VB. Neuroanatomical organization of dopamine neurons in the ventral tegmental area. Ann NY Acad Sci. 1988;537:10–26. doi: 10.1111/j.1749-6632.1988.tb42094.x. [DOI] [PubMed] [Google Scholar]
- Dubbeldam JL, den Boer-Visser AM. The central mesencephalic grey in birds: Nucleus intercollicularis and substantia grisea centralis. Brain Res Bull. 2002;57:349–352. doi: 10.1016/s0361-9230(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Brocard F, Antri M, Fenelon K, Gariepy JF, Smetana R, Menard A, Le Ray D, Di Prisco GV, Pearlstein E, Sirota MG, Derjean D, St-Pierre M, Zielinski B, Auclair F, Veilleux D. Initiation of locomotion in lampreys. Brain Res Rev. 2008;57:172–182. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Dujardin E, Jurgens U. Afferents of vocalization-controlling periaqueductal regions in the squirrel monkey. Brain Res. 2005;1034:114–131. doi: 10.1016/j.brainres.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Dusterhoft F, Hausler U, Jurgens U. Neuronal activity in the periaqueductal gray and bordering structures during vocal communication in the squirrel monkey. Neurosci. 2004;123:53–60. doi: 10.1016/j.neuroscience.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Echteler SM. Connections of the auditory midbrain in a teleost fish, Cyprinus carpio. J Comp Neurol. 1984;230:536–551. doi: 10.1002/cne.902300405. [DOI] [PubMed] [Google Scholar]
- Edds-Walton PL, Fay RR, Highstein SM. Dendritic arbors and central projections of physiologically characterized auditory fibers from the saccule of the toadfish, Opsanus tau. J Comp Neurol. 1999;411:212–238. [PubMed] [Google Scholar]
- Fine ML. Sounds evoked by brain stimulation in the oyster toadfish Opsanus tau. Exp Brain Res. 1979;35:197–212. doi: 10.1007/BF00236611. [DOI] [PubMed] [Google Scholar]
- Fine ML, Perini MA. Sound production evoked by electrical stimulation of the forebrain in the oyster toadfish. J Comp Physiol A. 1994;174:173–185. doi: 10.1007/BF00193784. [DOI] [PubMed] [Google Scholar]
- Finger TE, Tong SL. Central organization of eighth nerve and mechanosensory lateral line systems in the brainstem of ictalurid catfish. J Comp Neurol. 1984;229:129–151. doi: 10.1002/cne.902290110. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Marchaterre M, Deitcher DL, Bass AH. Distribution of androgen receptor mRNA expression in vocal, auditory, and neuroendocrine circuits in a teleost fish. J Comp Neurol. 2010;518:493–512. doi: 10.1002/cne.22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Bass AH. Neural and hormonal mechanisms of reproductive-related arousal in fishes. Horm Behav. 2011;59:616–629. doi: 10.1016/j.yhbeh.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahtan E, O'Malley DM. Visually guided injection of identified reticulospinal neurons in zebrafish: a survey of spinal arborization patterns. J Comp Neurol. 2003;459:186–200. doi: 10.1002/cne.10621. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000a;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Rhythmic midbrain-evoked vocalization is inhibited by vasoactive intestinal polypeptide in the teleost Porichthys notatus. Brain Res. 2000b;865:107–111. doi: 10.1016/s0006-8993(00)02232-0. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J Comp Neurol. 2000c;422:363–379. doi: 10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannig S, Jurgens U. Projections of the ventrolateral pontine vocalization area in the squirrel monkey. Exp Brain Res. 2006;169:92–105. doi: 10.1007/s00221-005-0128-5. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Kitch R, Carey J, Baker R. Anatomical organization of the brainstem octavolateralis area of the oyster toadfish, Opsanus tau. J Comp Neurol. 1992;319:501–518. doi: 10.1002/cne.903190404. [DOI] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Integration of sensory and motor processing underlying social behaviour in tungara frogs. Proc Royal Soc B. 2007;274:641–649. doi: 10.1098/rspb.2006.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Sexually dimorphic sensory gating drives behavioral differences in tungara frogs. J Exp Biol. 2010;213:3463–3472. doi: 10.1242/jeb.043992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- Holstege G. The emotional motor system in relation to the supraspinal control of micturition and mating behavior. Behav Brain Res. 1998;92:103–109. doi: 10.1016/s0166-4328(97)00182-4. [DOI] [PubMed] [Google Scholar]
- Ikenaga T, Yoshida M, Uematsu K. Efferent connections of the cerebellum of the goldfish, Carassius auratus. Brain Behav Evol. 2002;60:36–51. doi: 10.1159/000064120. [DOI] [PubMed] [Google Scholar]
- Ikenaga T, Yoshida M, Uematsu K. Morphology and immunohistochemistry of efferent neurons of the goldfish corpus cerebelli. J Comp Neurol. 2005;487:300–311. doi: 10.1002/cne.20553. [DOI] [PubMed] [Google Scholar]
- Ikenaga T, Yoshida M, Uematsu K. Cerebellar efferent neurons in fish. Cerebell. 2006;5:268–274. doi: 10.1080/14734220600930588. [DOI] [PubMed] [Google Scholar]
- Jordan LJ. Initiation of locomotion in mammals. Ann NY Acad Sci. 1998;860:83–93. doi: 10.1111/j.1749-6632.1998.tb09040.x. [DOI] [PubMed] [Google Scholar]
- Jurgens U. The role of the periaqueductal grey in vocal behaviour. Behav Brain Res. 1994;62:107–117. doi: 10.1016/0166-4328(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Jurgens U. The neural control of vocalization in mammals: a review. J Voice. 2009;23:1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS One. 2011;6:e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelberger JM, Land BR, Bass AH. Midbrain periaqueductal gray and vocal patterning in a teleost fish. J Neurophysiol. 2006;96:71–85. doi: 10.1152/jn.00067.2006. [DOI] [PubMed] [Google Scholar]
- Klop EM, Mouton LJ, Holstege G. Periparabigeminal and adjoining mesencephalic tegmental field projections to the dorsolateral periaqueductal grey in cat – a possible role for oculomotor input in the defensive system. Eur J Neurosci. 2006;23:2145–2157. doi: 10.1111/j.1460-9568.2006.04740.x. [DOI] [PubMed] [Google Scholar]
- Kozloski J, Crawford JD. Functional neuroanatomy of auditory pathways in the sound-producing fish Pollimyrus. J Comp Neurol. 1998;401:227–252. [PubMed] [Google Scholar]
- Luque MA, Torres-Torrelo J, Carrascal L, Torres B, Herrero L. GABAergic projections to the oculomotor nucleus in the goldfish (Carassius auratus). Front Neuroanat. 2011;5:1–7. doi: 10.3389/fnana.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma PM. Catecholaminergic systems in the zebrafish. IV. Organization and projection pattern of dopaminergic neurons in the diencephalon. J Comp Neurol. 2003;460:13–37. doi: 10.1002/cne.10544. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Forebrain projections to the periaqueductal gray in the monkey, with observations in the cat and rat. J Comp Neurol. 1982;206:146–158. doi: 10.1002/cne.902060205. [DOI] [PubMed] [Google Scholar]
- McCormick CA. Anatomy of the central auditory pathways of fish and amphibians. In: Popper A, Fay RR, editors. Comparative hearing: fish and amphibians. Springer; New York: 1999. pp. 155–217. [Google Scholar]
- Metzner W. Anatomical basis for audio-vocal integration in echolocating horseshoe bats. J Comp Neurol. 1996;368:252–269. doi: 10.1002/(SICI)1096-9861(19960429)368:2<252::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J Comp Neurol. 2006;494:903–943. doi: 10.1002/cne.20853. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Oka Y, Satou M, Ueda K. Descending pathways to the spinal cord in the Hime salmon (landlocked red salmon, Oncorhynchus nerka). J Comp Neurol. 1986;254:91–103. doi: 10.1002/cne.902540108. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Periaqueductal gray afferents synapse onto dopamine and GABA neurons in the rat ventral tegmental area. J Neurosci Res. 2010;88:981–991. doi: 10.1002/jnr.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper F, Jurgens U. Neuronal activity in the inferior colliculus and bordering structures during vocalization in the squirrel monkey. Brain Res. 2003;979:153–164. doi: 10.1016/s0006-8993(03)02897-x. [DOI] [PubMed] [Google Scholar]
- Rao PDP, Jadhao AG, Sharma SC. Topographic organization of descending projection neurons to the spinal cord of the goldfish, Carassius auratus. Brain Res. 1993;620:211–220. doi: 10.1016/0006-8993(93)90158-j. [DOI] [PubMed] [Google Scholar]
- Rink E, Wulliman MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: A WGA-HRP and PHA-L study. J Comp Neurol. 1992;315:1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- Sankrithi NS, O'Malley DM. Activation of a multisensory, multifunctional nucleus in the zebrafish midbrain during diverse locomotor behaviors. Neurosci. 2010;166:970–993. doi: 10.1016/j.neuroscience.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Schuller G, Fischer S, Schweizer H. Significance of the paralemniscal tegmental area for audio-motor control in the moustached bat, Pteronotus p. parnellii: The afferent and efferent connections of the paralemniscal area. Eur J Neurosci. 1997;9:342–355. doi: 10.1111/j.1460-9568.1997.tb01404.x. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Representations of motivational drives in mesial cortex, medial thalamus, hypothalamus and midbrain. Brain Res Bull. 2003;61:25–49. doi: 10.1016/s0361-9230(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Benaur M. GABA injected into the anterior dorsal tegmentum (ADT) of the midbrain blocks stepping initiated by stimulation of the hypothalamus. Brain Res. 1997;766:271–275. doi: 10.1016/s0006-8993(97)00734-8. [DOI] [PubMed] [Google Scholar]
- Sirota MG, Di Prisco GV, Dubuc R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur J Neurosci. 2000;12:4081–4092. doi: 10.1046/j.1460-9568.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Straka H, Beck JC, Pastor AM, Baker R. Morphology and physiology of the cerebellar vestibulolateral lobe pathways linked to oculomotor function in the goldfish. J Neurophysiol. 2006;96:1963–1980. doi: 10.1152/jn.00334.2006. [DOI] [PubMed] [Google Scholar]
- Sukikara MH, Mota-Ortiz SR, Baldo MV, Felicio LF, Canteras NS. A role for the periaqueductal gray in switching adaptive behavioral responses. J Neurosci. 2006;26:2583–2589. doi: 10.1523/JNEUROSCI.4279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Lu Z. Octavolateral projections and organization in the medulla of a teleost fish, the sleeper goby (Dormitator latifrons). J Comp Neurol. 2005;481:96–117. doi: 10.1002/cne.20363. [DOI] [PubMed] [Google Scholar]
- Torres B, Pastor AM, Cabrera B, Salas C, Delgado-Garcia JM. Afferents to the oculomotor nucleus in the goldfish (Carassius auratus) as revealed by retrograde labeling with horseradish peroxidase. J Comp Neurol. 1992;324:449–461. doi: 10.1002/cne.903240311. [DOI] [PubMed] [Google Scholar]
- Torres B, Fernandez S, Rogriguez F, Salas C. Distribution of neurons projecting to the trochlear nucleus in goldfish (Carassius auratus). Brain Behav Evol. 1995;45:272–285. doi: 10.1159/000113556. [DOI] [PubMed] [Google Scholar]
- Uematsu K, Todo T. Identification of the midbrain locomotor nuclei and their descending pathways in the teleost carp, Cyprinus carpio. Brain Res. 1997;773:1–7. doi: 10.1016/s0006-8993(97)00619-7. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VGJM, Terasawa E, Ralston HJ, Holstege G. Monosynaptic projections from the lateral periaqueductal gray to the nucleus retroambiguus in the rhesus monkey: implications for vocalization and reproductive behavior. J Comp Neurol. 2000;424:251–268. doi: 10.1002/1096-9861(20000821)424:2<251::aid-cne5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Varga T, Palkovits M, Usdin TB, Dobolyi A. The medial paralemniscal nucleus and its afferent neuronal connections in rat. J Comp Neurol. 2008;511:221–237. doi: 10.1002/cne.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelli A, Repici M, Garbossa D, Grimaldi A. Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Res Bull. 2000;51:11–28. doi: 10.1016/s0361-9230(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Weeg MS, Bass AH. Central lateral line pathways in a vocalizing fish. J Comp Neurol. 2000;418:41–64. [PubMed] [Google Scholar]
- Weeg MS, Land BR, Bass AH. Vocal pathways modulate efferent neurons to the inner ear and lateral line. J Neurosci. 2005;25:5967–5974. doi: 10.1523/JNEUROSCI.0019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM, Li D, Eagleton C. Projections of the dorsomedial nucleus of the intercollicular complex (DM) in relation to respiratory-vocal nuclei in the brainstem of pigeon (Columba livia) and zebra finch (Taeniopygia guttata). J Comp Neurol. 1997;377:392–413. doi: 10.1002/(sici)1096-9861(19970120)377:3<392::aid-cne7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Northcutt RG. Connections of the corpus cerebelli in the green sunfish and the common goldfish: a comparison of perciform and cypriniform teleosts. Brain Behav Evol. 1988;32:293–316. doi: 10.1159/000116558. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Vernier P. The evolution of dopamine systems in chordates. Front Neuroanat. 2011;5:1–20. doi: 10.3389/fnana.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Ito H. Fiber connections of the anterior preglomerular nucleus in cyprinids with notes on telencephalic connections of the preglomerular complex. J Comp Neurol. 2005;491:212–233. doi: 10.1002/cne.20681. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Ito H. Visual, lateral line, and auditory ascending pathways to the dorsal telencephalic area through the rostrolateral region of the lateral preglomerular nucleus in cyprinids. J Comp Neurol. 2008;508:615–647. doi: 10.1002/cne.21717. [DOI] [PubMed] [Google Scholar]