Abstract
Vacuolar ATPases (V-ATPases) comprise specialized and ubiquitously distributed pumps that acidify intracellular compartments and energize membranes. To gain new insights into the roles of V-ATPases in prostate cancer (PCa) we studied the effects of inhibiting V-ATPase pumps in androgen-dependent (LNCaP) and androgen-independent (C4-2B) cells of a human PCa progression model. Treatment with nanomolar concentrations of the V-ATPase inhibitors bafilomycin A or concanamycin A reduced the in vitro invasion in both cell types by 80%, regardless that V-ATPase was prominent at the plasma membrane of C4-2B cells and only traces were detected in the low-metastatic LNCaP parental cells. In both cell types intracellular V-ATPase was excessive and co-localized with prostate-specific antigen (PSA) in the Golgi compartment. V-ATPase inhibitors reversibly excluded PSA from the Golgi and led to the accumulation of largely dispersed PSA-loaded vesicles of lysosomal composition. Inhibition of acridine orange staining and transferrin receptor recycling suggested defective endosomal and lysosomal acidification. The inhibitors, additionally, interfered with the AR-PSA axis under conditions that reduced invasion. Bafilomycin A significantly reduced steady-state and R1881-induced PSA mRNA expression and secretion in the LNCaP cells which are androgen-dependent, but not in the C4-2B cells which are androgen ablation-resistant. In the C4-2B cells, an increased susceptibility to V-ATPase inhibitors was detected after longer treatments, as proliferation was reduced and reversibility of bafilomycin-induced responses impaired. These findings make V-ATPases attractive targets against early and advanced PCa tumors.
Keywords: Vacuolar ATPase, V-ATPase, prostate cancer, prostate-specific antigen, proton pumps
INTRODUCTION
Vacuolar adenosine triphosphatases (V-ATPases) are protein complexes that use the energy of ATP hydrolysis to pump protons across endosomal and lysosomal membranes 1. The luminal acidic pH generated by V-ATPases facilitates processes such as protein sorting and secretion, receptor recycling, endocytosis, and protein degradation 2–6. Plasmalemmal V-ATPases present in cells specialized for active proton secretion, including cancer cells, pump protons to the extracellular space. They sustain the low pH that activates the extracellular metalloproteases, allowing for tumor cell motility, proliferation, and metastasis 7–10. Accumulated evidence have shown V-ATPase involvement in tumor invasion and multi-drug resistance in breast cancer 11–14, oral squamous cell carcinoma 15, 16, hepatocellular carcinoma 8, melanoma 17, pancreatic cancer 9, and PCa 18. Additional information regarding the functions of V-ATPases in PCa is limited; Iejimalide B, an inhibitor of V-ATPases, induces cell cycle arrest and apoptosis in prostate cancer (PCa) cells 19.
PCa is the second most common cancer in men in the United States. PCa screening is performed by testing the serum levels of the biomarker prostate-specific antigen (PSA), an androgen-dependent 20 serine protease secreted by both healthy and cancerous prostate tissue 21. PSA expression is under control of the androgen receptor (AR). The AR is activated by binding of dihydrotestosterone, which facilitates formation of dimers that are subsequently relocated to the nucleus 22. Nuclear AR is a transcription factor that binds androgen response elements in the promoter region of PSA and other target genes 23. Increased PSA serum levels correlate to an extent with aggressiveness of the cancer and unfavorable outcome for PCa patients 20, 21, 24. Localized (androgen-dependent) PCa is treated by combining androgen ablation and radiation therapies which initially successfully reduces tumor growth and progression. However, androgen ablation-resistant disease eventually develops, where cancer cells have adapted to low androgen levels and have elevated invasive capacity. This poses a major clinical challenge to reduce high lethality rates from metastatic tumors and cancer recurrence after prostatectomy 25.
In this study, the effects of V-ATPase inhibitors on PCa invasion and PSA biology were investigated. We used the LNCaP → C4-2B cell model of PCa progression that mimics the advancing disease from androgen-dependency to androgen ablation-resistance 26. We showed that PSA is down-regulated (reduced mRNA and secretion) by inhibiting V-ATPases in the LNCaP cells regardless of the presence of R1881, suggesting that V-ATPase inhibitors interfered with the AR-PSA axis. Unlike the LNCaP cells, PSA expression and secretion were not altered in the androgen-independent C4-2B cells in spite of a dramatic reduction of the in vitro invasion of both cell types. Both cell types show an extensive distribution of intracellular V-ATPase pumps, but a distinctive distribution at the plasma membrane. Plasma membrane V-ATPases were abundant in the C4-2B cells, which are also more susceptible to V-ATPase inhibitors. Together these findings make V-ATPase pumps attractive targets against early and advanced PCa tumors. Combined with other therapies, V-ATPase inhibitors could help prevent transformation into the castration-resistant phenotype.
MATERIALS AND METHODS
Cell culture
LNCaP and PC-3 cells (both from ATCC, Manassas, VA, USA) and C4-2B cells (kind gift from Prof. Dr. George N. Thalmann) were cultured in T-medium (DMEM, Sigma Aldrich, St. Louis, MO, USA; 20% F12 nutrient mixture, 5 µg/mL insulin, 25 µg/mL adenine hydrochloride, 10 µg/mL transferrin, 0.25 µg/mL biotin, 15 pg/mL trijodothyronine, 100 U/mL penicillin/streptomycin) supplemented with 10% FBS. Cells were maintained at 37°C and 5% CO2 in a humidified atmosphere. Media was routinely changed every 2–3 days, and cells passaged at 80–90% confluency.
Antibody generation
The polyclonal antibody to V-ATPase was developed against the peptide N-I162KHKIMLPPRNRGT175-C of the subunit V1A by BioGenes (Berlin, Germany). Single peptides were used for the immunization of 2 rabbits over 35 days. The animals were intramuscularly immunized using BioGenes' adjuvant mixed 2:1 with the V1A antigen. Parts of the sera were affinity purified against the peptide that was used for immunization. Antibodies were tested for specificity by performing BLAST alignment searches and by Western blotting and immunocytochemistry experiments.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 10 min at RT and cell membranes permeabilized with 0.02% TritonX-100 in PBS. Cells were blocked with 5% GS-PBS for 30 min at RT. Incubation with primary antibodies was performed at 1:100 dilution in 5% GS-PBS for 1 h at RT (LAMP-1, LAMP-2, clathrin, Na+K+-ATPase, Giantin antibodies: Abcam, Cambridge, MA, USA; AR and transferrin receptor/TR antibodies: Invitrogen). Cells were washed with PBS and incubated for 30 min with the secondary fluorescent antibodies (AF488 and AF546, Invitrogen; 1:500 in 5% GS-PBS). Cells were washed with PBS and mounted onto microscope slides in mounting media. Primary and secondary antibody controls were included for all immunostaining experiments. For acridine orange staining, cells were incubated for 30 min at 37°C with 1 µM acridine orange diluted in cell culture medium and fixed with paraformaldehyde as described above. Slides were analyzed with the Zeiss LSM510 confocal system.Line profiles of fluorescent intensity were obtained using ZEN 2009 Light Edition © Carl Zeiss MicroImaging software.
Plasma membrane isolation
Plasma membrane fractions were obtained by Percoll density gradient centrifugation as described before 27.
RNA isolation and cDNA synthesis
RNA was isolated from cells grown in multiwell plates with the RNeasy Mini kit (Qiagen, Germantown, MD, USA), following the manufacturer’s instructions using the QIAshredder (Qiagen) to homogenize cell lysates. Complimentary DNA was synthesized from 2,000 ng RNA with the RETROscript® cDNA kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions.
Quantitative realtime PCR (qRT-PCR)
Specific primers were designed against V-ATPase subunits, PSA, and TATA-binding protein (TBP) as an internal standard; primer sequences are listed in Table S1. The SYBR Green I Mastermix (Roche, Indianapolis, IN, USA) was used for the following PCR conditions: denaturation at 95°C for 10 min, followed by 40 cycles of: 30 s at 95°C, 30 s at 65°C, and 20 s at 72°C. qRT-PCR was performed on the Roche LightCycler480 instrument.
Western blotting
Whole cell lysates were obtained by incubating cells for 10 min on ice with lysis buffer (25 mM Tris, 8 mM MgCl2, 1 mM DTT, 15% glycerol, 1% TritonX-100, protease inhibitor cocktail). Insoluble cell debris was removed by centrifugation of lysates at 13,000 rpm for 10 min at 4°C. The protein concentration was determined by bicinchoninic acid assay (Pierce, Rockford, IL, USA) against a BSA standard curve. 80 µg total protein were analyzed by SDS-PAGE and immunoblotted with primary antibodies overnight, followed by incubation and detection with secondary HRP-conjugated antibodies (Invitrogen). Densitometry quantification of western blot bands was performed using ImageJ software.
Cell treatments and PSA secretion
Cells were incubated for 24 h with culture media containing 5% charcoal-treated FBS to deplete cells of androgens and then incubated for 24 h or 48 h with 10 nM bafilomycin A (Baf.A), 10 nM R1881, or both, in T-media supplemented with 1% charcoal-treated FBS. RNA isolation, RT-PCR, Western blotting and immunostaining were then performed as described above. PSA secreted into the cell culture media was quantified using an ELISA kallikrein 3/PSA kit (R&D Biosystems) according to the manufacturer’s instructions.
Invasion and proliferation assays
Invasion Matrigel invasion assays were performed according to the manufacture’s instructions (BD Biosciences, San Jose, CA, USA). LNCaP and C4-2B cells were seeded for ~40 hours before treatment. Cells in invasion chambers were treated for 24 h with 10 nM Baf.A, then excess cells were removed with a cotton swab from the top of the chamber, invaded cells were fixed in methanol for 2 min and stained with Toluidine Blue for 2 min. Inserts were excised, mounted in immersion oil, and counted under the microscope. Proliferation. LNCaP and C4-2B cells were treated with 10 nM Baf.A or Conc.A (24 h or 48 h) and cell proliferation measured with the MTT cell proliferation assay kit (ATCC) by reading absorbance at 570 nm.
Statistical analysis
Student’s t-tests were performed to determine statistically significant differences in V-ATPase subunit mRNA expression levels between cell lines, and in mRNA and secretion levels after treatments, using GraphPad Prism 4 software.
RESULTS
Two PCa epithelial cell models with different invasiveness potential were compared to study the effects of V-ATPase inhibitors on PCa cell invasion and PSA biology. We measured the in vitro invasion of the poorly invasive, LNCaP, and highly invasive, C4-2B, human PCa cells treated with bafilomycin A (Baf.A) and Concanamycin A (Conc.A), two highly potent (IC50 1–10 nM) and specific V-ATPase inhibitors 28, 29. V-ATPase inhibitors are known to reduce the in vitro invasion of highly invasive human breast cancer cells, which express V-ATPases at the cell surface, but no the invasion of the poorly invasive cell lines, which do not express plasmmalemal V-ATPases 11.
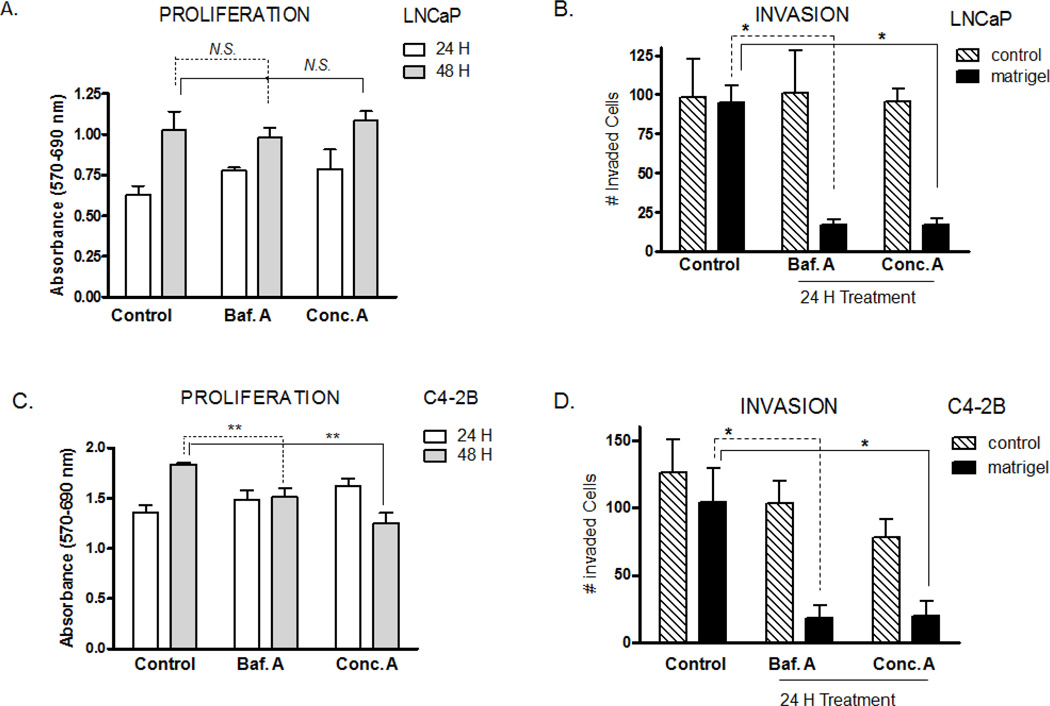
The V-ATPase inhibitors prevented in vitro invasion of both human PCa cells, LNCaP and C4-2B, by about 80 % (Fig. 1B,D) under conditions where proliferation was not compromised (10 nM Baf.A or Conc.A for 24 hours) (Fig. 1A,C). However, after 48 hours of treatment, proliferation of the highly invasive C4-2B cells was significantly reduced (Fig. 1A,C). Suppression of the in vitro invasion of both PCa cells could be explained if the poorly invasive and highly invasive cells express V-ATPases at the cell surface, as the hallmark of V-ATPase pumps in cancer biology is their specialized role at the plasma membrane where V-ATPases facilitate extracellular acidification and invasion 5, 9, 11, 12, 30, 31.
Figure 1. Effect of V-ATPase inhibitors on cell proliferation and in vitro invasion of PCa cells.
Baf.A and Conc.A prevents invasion of LNCaP and C4-2B cells. Poorly invasive LNCaP (A, B) and highly invasive C4-2B (C, D) cells were treated with Baf.A or Conc.A (10 nM) for 24 hours or 48 hours. Proliferation (A, C) was measured with the MTT cell proliferation assay and invasion (B, D) with a Matrigel invasion assay. N.S., not significant; *, p<0.05; **, p<0.01; significance levels relative to untreated control.
We made an antibody against the V-ATPase subunit V1A to study V-ATPase cellular distribution. Subunit V1A does not have isoforms and is therefore a component of every V-ATPase complex assembled in the cell. Western blots of whole cell lysates revealed one major band that migrated at about 70-kDa, the molecular mass of subunit V1A (Fig. 2A) 2, 7. Treatment with the peptide antigen competed labeling, validating the antibody’s specificity for subunit V1A. Plasma membrane fractions isolated from the poorly invasive LNCaP cells had only trace amounts of V-ATPase subunit V1A, and V1A was not detectable at the plasma membrane by immunofluorescence in these cells (Fig. 2C). Immunoblots showed V1A present at the plasma membrane of the highly invasive C4-2B cells (Fig. 2B) but at lower levels than the plasma membrane protein Na+K+-ATPase. Thus, like other cancer cells, expression of V-ATPase pumps at the plasma membrane correlates with an increased invasive phenotype in PCa cells.
Figure 2. Cellular distribution of V-ATPase in PCa cells.
(A) Peptide antibody specificity for subunit V1A. Western blot of whole cell lysates from the LNCaP cells were probed with anti-V1A antibody in the presence and absence of its peptide antigen (10 µM). The same membrane was probed with β-actin (loading control). Antibody (6 ug) and peptide (60ug) were incubated 24 hours at 4°C (wells 4 &5) or 2 hours at RT (wells 5 &6). Lanes 1, 3, and 5 (20 µg protein); Lanes 2, 4, and 6 (50 µg protein). (B) Plasma membrane fractions from LNCaP and C4-2B cells. The same gel was proved with the plasma membrane protein Na+K+-ATPase. WCL=whole cell lysate; PM=plasma membrane. (C) V-ATPase is present in endosomal and lysosomal membranes of LNCaP cells. Cells were co-immunostained with antibodies against the V-ATPase subunit V1A and the indicated marker proteins of the endocytic recycling pathway (transferring receptor, clathrin), secretory pathway (clathrin), lysosomes (LAMP-1), and plasma membrane (Na,K-ATPase). Proteins were labeled with AlexaFluor secondary antibodies, and co-localization analyzed using confocal microscopy determining a line profile of fluorescent intensity. (D) V-ATPase and PSA co-localize in the Golgi compartment of PCa cells. LNCaP and C-4-2B cells were co-immunostained with anti-V1A, Golgi protein giantin, and PSA as described above. Scale bar =10 µm.
Suppression of invasion after treating LNCaP cells with Baf.A and Conc.A considering that have elfin amounts of plasmalemmal V-ATPases (Fig. 2B) suggests novel roles for intracellular V-ATPases that can influence the invasive phenotype of androgen-dependent PCa cells. Co-immunostaining with the anti-V1A antibody and antibodies to subcellular markers showed the intracellular V-ATPase pumps distributed throughout the endomembrane system of LNCaP (Fig. 2C) and C4-2B cells (Fig. S1). V-ATPase signal was predominant in the Golgi compartment (Fig. 2D); the Golgi tether protein giantin completely co-localized with V-ATPase in both cell lines. PSA was abundant in the Golgi compartment and notably, we showed that PSA completely to co-localize with Golgi V-ATPase (Fig. 2D). V-ATPase in the Golgi and peri-Golgi regions was positive also for endosomal proteins such as clathrin and tranferrin receptor, and partly overlapped with the lysosomal marker LAMP-1 (Fig. 2C).
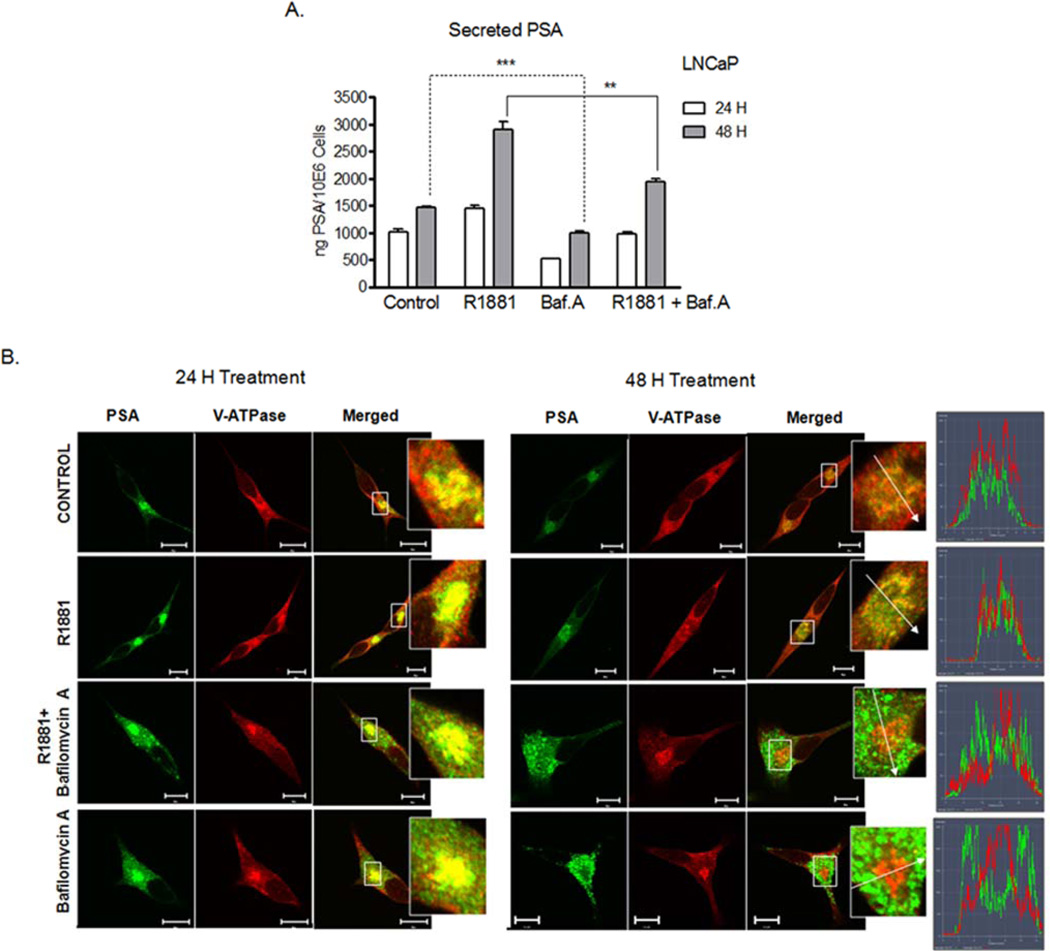
V-ATPase function is necessary for protein trafficking across endocytic and secretory pathways 6, 32; thus, we anticipated PSA trafficking from the Golgi and its secretion to be V-ATPase dependent. Because PSA expression and secretion are induced by androgen 22, we measured PSA secretion from the androgen-dependent LNCaP cells after 24 and 48 hours exposure to R1881, a dihydrotestosterone analogue, which stimulates androgen receptor-mediated PSA secretion; and after exposure to Baf.A to block V-ATPase function. As expected, secretion of PSA was stimulated by a saturating concentration of R1881 (10 nM) (Fig. 3A), and both basal and R1881-induced PSA secretion were decreased in the presence of Baf.A.
Figure 3. Effect of V-ATPase inhibitors on R1881-induced PSA secretion and cellular distribution.
(A) Baf.A treatment reduces PSA secretion. The LNCaP cells were incubated with 10 nM Baf.A in the presence and absence of 10 nM R1881 (24 or 48 hours) or with 0.01% DMSO (Control). The PSA secreted was quantified by ELISA and normalized to cell number. (B) Baf.A treatment disturbs PSA recruitment in the Golgi. The LNCaP cells treated as described in A were immunostained with antibodies to V1A and PSA and analyzed as described for Figure 2C. ***, p<0.001; **, p<0.01; significance levels relative to controls.
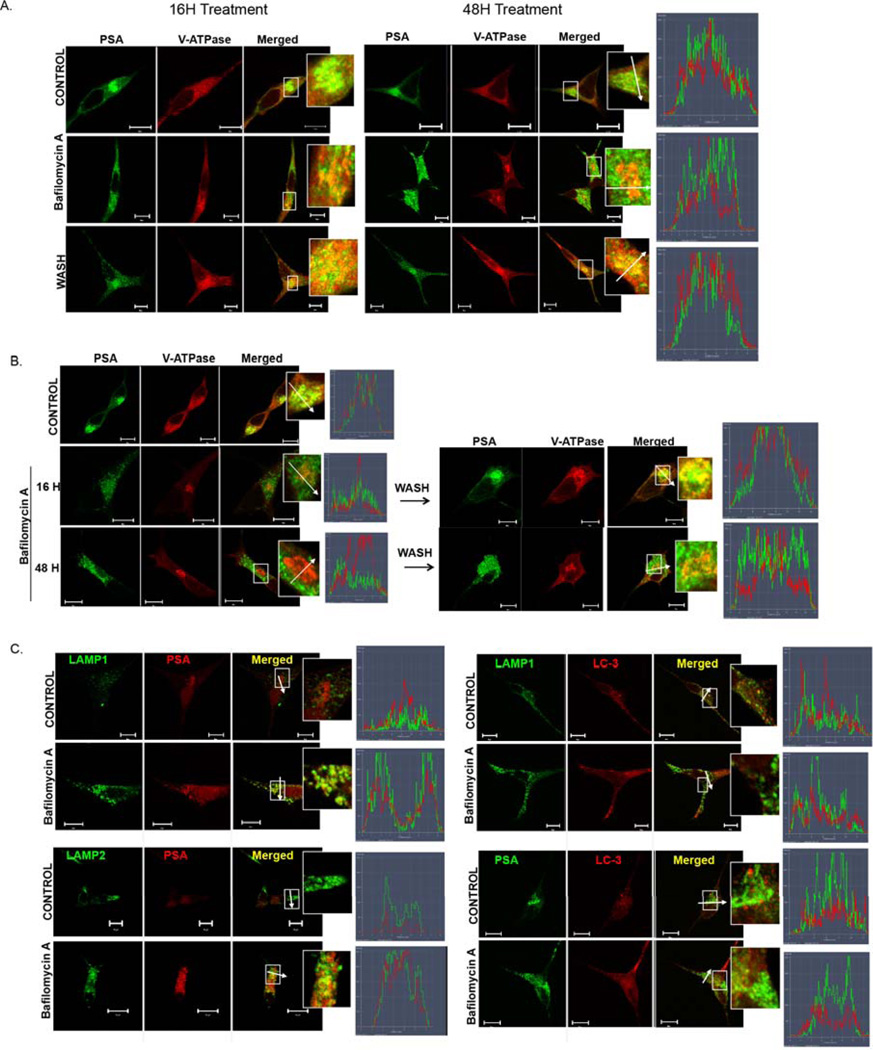
In addition to suppressing PSA secretion, V-ATPase inhibitors disturbed PSA recruitment in the Golgi compartment. Treatment with Baf.A (Figs. 3B, 4A,B) or Conc.A (Fig. S2) for 16 hours and up to 48 hours excluded PSA from the Golgi. PSA became dispersed in discrete vesicles throughout the cell, whereas V-ATPase remained in the Golgi (Fig.S3). This response was observed in the LNCaP and C2-4B cells alike (Fig. 4A,B), and therefore was independent of the invasive potential of the PCa cells. Removal of Baf.A from the media restored compartmentalization of PSA with V-ATPase in the Golgi of the LNCaP cells (Fig. 4A), in line with the notion that PSA recruitment into the Golgi required active intracellular V-ATPases. In the highly invasive C4-2B cells, reversibility was detected at 16 hours (Fig. 4B), but was impaired after 48 hours incubation with the V-ATPase inhibitor. Susceptibility of the C4-2B cells to Baf.A and Conc.A treatments was also evident in the proliferation assays after 48 hours (Fig. 1B).
Figure 4. Formation of lysosomal PSA-loaded vesicles by treatment with inhibitors of V-ATPase pumps.
(A) PSA vesicle formation is reversible in the LNCaP cells (16 – 48 hour), (B) but not in the C4-2B cells (48 hours) treated with Baf.A. The LNCaP (A, C) and C4-2B (B) cells were incubated with 10 nM Baf.A (16, 24, or 48 hours) or with 0.01% DMSO (Control). The V-ATPase inhibitor was removed by incubation in normal growth media for an additional 24 hours (Wash) to restore PSA compartmentalization with V-ATPase in the Golgi. (C) PSA-containing vesicles have lysosomal composition. The LNCaP cells were treated with 10 nM Baf.A (24 hours) or with 0.01% DMSO (Control). (A-C) Cells were immunostained with antibodies to V1A, PSA, LAMP1, LAMP2, or LC-3 and analyzed as described for Figure 2C. Scale bar =10 µm.
The dispersion of PSA was detected after administrating Baf.A alone or in combination with R1881 (Fig. 3B). R1881 alone did not disturb PSA compartmentalization in the Golgi regardless that R1881 enhanced PSA secretion, indicating that Baf.A-induced formation of PSA-containing vesicles differed from normal PSA secretory events. Treatment with V-ATPase inhibitors likely targeted PSA for lysosomal degradation via autophagy-independent processes, because: 1) vesicles containing PSA were positive for the lysosomal markers LAMP1 and LAMP2 (Fig. 4C); 2) the autophagic LC-3-positive vesicles were observed as distinct punctae (Fig. 4C); 3) the ratio LC-3I/LC3II was unchanged, as assessed by Western blots (not shown); and 4) PSA-containing vesicles did not form under conditions that block autophagy (100 nM Baf.A, 2 hours) (Fig. S4) 33.
The fact that V1A is absent in the lysosomal PSA-containing vesicles suggests that the organelles were not acidified and degradation events impaired. Accordingly, V-ATPase inhibitors prevented vesicle staining with acridine-orange, therefore supporting the idea that endosomal and lysosomal acidification are defective (Fig. 5A). In addition, transferrin receptor recycling to the cell surface was blocked, as indicated by its retention in the Golgi with V-ATPase pumps in the Baf.A-treated cells (Fig. 5B), suggesting that cell-surface transport is disrupted upon V-ATPase inhibition. Finally, total intracellular PSA was significantly greater when R1881 was added in the presence of Baf. A (Fig. 5C), suggesting that PSA is not degraded when V-ATPase function is inhibited.
Figure 5. Effect of V-ATPase inhibitors on endosomal acification and trafficking.
(A) V-ATPase inhibitors disturb endosomal and lysosomal acidification. Cells were treated with 10 mnM Baf.A or Conc.A for 24 h, incubated with 1 µM acridine orange, and analyzed by confocal microscopy. (B) Transferrin Receptor recycling to the plasma membrane is reduced by Baf. A. LNCaP treated with 10 nM Baf.A or 0.01% DMSO (Control) for 24 hours were analyzed as described for Figure 2C. (C) Baf.A treatment cause intracellular accumulation of PSA. Cells were treated with R1881 and/or Baf.A as described for Figure 3A, whole cell lysates analyzed by immunoblots using antibodies against PSA and β-actin (loading control), and relative PSA protein levels quantified with Image J software (n=3–4). Scale bar =10 µm. **, p<0.01; significance levels relative to controls.
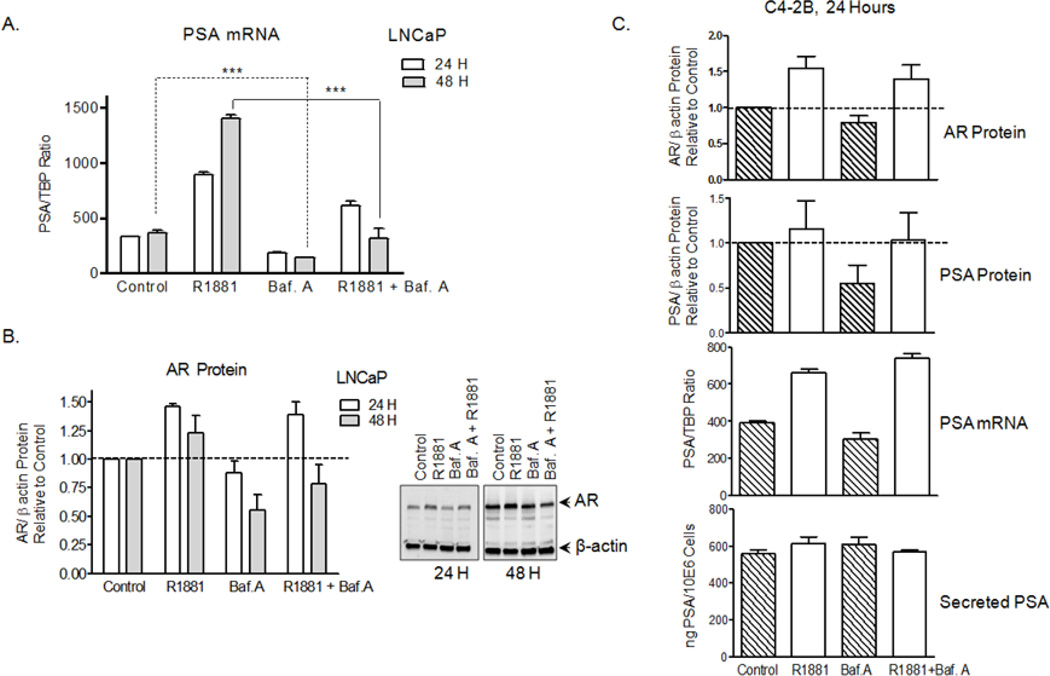
We measured PSA transcripts levels by qRT-PCR and determined that basal PSA was down-regulated at the transcriptional level in the poorly invasive androgen-dependent LNCaP cells treated with Baf.A. PSA mRNA decreased by 1.8- and 2.6-fold at 24 and 48 hours incubations with Baf.A (Fig. 6A). In addition to lowering PSA at steady-state, Baf.A dramatically suppressed R1881-induction of PSA, suggesting that V-ATPase inhibition interferes with AR transcriptional activity. A more modest decreased of AR protein was observed in the LNCaP cells after treatment with Baf.A for 48 hours in the presence (induced state) and absence (steady state) of R1881 (Fig.6B). PSA secretion, PSA expression, and AR protein were not reduced in the C4-2B cells by Baf.A after 24 hours (Fig. 6C), when invasion significantly declined (Fig. 1D). These results attribute increasingly important roles to plasma membrane-associated V-ATPase pumps in the highly invasive PCa cells, whereas intracellular V-ATPase pumps appear to be primarily responsible for defective PSA expression and secretion and reduction of invasion triggered by Baf.A and Conc.A in the LNCaP cells.
Figure 6. V-ATPase inhibition interfere with the AR-PSA axis in LNCaP, but not in the C4-2B cells.
(A) Baf.A treatment reduces PSA mRNA expression in LNCaP cells. Cells were treated with 10 nM Baf.A and/or R1881 and PSA mRNA was quantified by qRT-PCR using specific primers (Table S1) and expressed as a ratio relative to the internal control TATA-binding protein (TBP) (B) Baf.A treatment lower AR protein levels in the LNCaP cells. Immunoblots of whole cell lysates were analyzed using antibodies to the AR and β-actin (loading control) and relative AR protein quantified as described for Figure 5C. (C) PSA secretion, PSA expression (mRNA, protein), and AR protein levels are not altered by Baf.A in the C4-2B cells. Cells were treated with Baf.A and analyzed as described above for B (AR protein), A (PSA mRNA), and for Figures 5C (PSA protein) and 2A (Secreted PSA). Relative protein levels were quantified with Image J software (n=3–4). ***, p<0.001; significance levels relative to controls.
DISCUSSION
This study revealed important new roles for V-ATPase pumps in PCa cells. Pharmacological inactivation of V-ATPase interferes with the AR-PSA axis. It reduces PSA secretion and PSA mRNA expression in the androgen-dependent LNCaP cells. Together with a dramatic reduction (by 80%) of the in vitro invasion of LNCaP (poorly invasive) and C4-2B (highly invasive) cells, these findings make V-ATPase pumps attractive new targets against early and advanced PCa tumors. In this study, we used Baf.A at its IC50 concentration (10 nM) to avoid any secondary and unspecific effects 34 and reproduced our studies using Conc.A, another V-ATPase inhibitor for which V-ATPase is the only known target, arguing against a general toxic effect independent of V-ATPase inhibition.
The link of plasma membrane-associated V-ATPases and invasion of high-metastatic cells has been broadly disseminated 5, 9, 11–13. Our findings indicate that intracellular V-ATPases also contribute to the invasive phenotype of PCa cells because the low-metastatic androgen-dependent LNCaP cells have only traces of V-ATPase pumps at the cell surface and excessive intracellular V-ATPase pumps. It has been shown that Golgi associated intracellular V-ATPases are necessary for Golgi functions 35 and that V-ATPases in endosomal and lysosomal compartments support protein trafficking and degradation in all eukaryotic cells 2, 4, 5, 6. In this context, PCa cells are not different. V-ATPase inhibitors decrease PSA secretion and recycling of the transferrin receptor to the plasma membrane. They also lead to accumulation of PSA-loaded vesicles of lysosomal nature (LAMP-1 and LAMP-2 positive), which could be degradative or secretory lysosomes. Since the catalytic subunit of the V-ATPase complex (V1A) is absent from the PSA-loaded vesicles, we expected luminal acidification to be impaired. A defective endosomal and lysosomal acidification can help explain the membrane trafficking defects; and it is supported by absence of acridine orange staining in Baf.A and Con.A treated cells.
The scope of V-ATPase in PCa physiology extends beyond the boundaries of the organelles where they are present. V-ATPase inhibitors suppress androgen responses such as R1881 induction of PSA mRNA expression in the androgen-dependent LNCaP cells, but not in the C4-2B cells that have developed androgen resistance. One explanation to how V-ATPase inhibitors reduce PSA mRNA is that by excluding PSA from the Golgi and redistributing it into the lysosomal vesicles, V-ATPase inhibitors activate a negative feed-back loop and PSA expression is decreased. Time course analysis of PSA mRNA expression levels (not shown) support this model. PSA vesicle formation is detected at least at 16 hours treatment and precedes PSA mRNA down-regulation, which is detected only after 24 hours. It has been shown that V-ATPase inhibition lowers the cytosolic pH 11. An aberrant cytosolic pH can interfere with the AR-PSA axis, and we will expect other cellular processes to be defective too. Any cytosolic pH defect will be more drastic in the C4-2B cells where the plasma membrane-associated V-ATPase pumps are more abundant.
The isogenic nature of the LNCaP and C4-2B cell lines and their resemblance to androgen-ablation resistant progressive disease 26 allow us to compare cellular events associated with V-ATPase function in progressively invasive PCa cells. Our results revealed an increasing importance of V-ATPase in the metastatic C4-2B PCa cells. The C4-2B cells express significantly more V-ATPase pumps at the cell surface and many Vo subunit isoforms are upregulated (Table S2) relative to LNCaP (up to 9-fold) and to non-cancerous (up to 60-fold) prostate cells. Additionally, V-ATPase inhibitors suppress proliferation in the C4-2B cells (48 hours incubation) and Baf.A-induced responses become irreversible over time.
Future work will focus on identifying the V-ATPase subunit isoforms directly involved in PCa invasion, and suppression of PSA expression and secretion to selectively target prostate tumors in vivo. Baf.A and Conc.A do not discriminate between V-ATPase isoforms 28, 29, although the V-ATPase complex comprises multiple subunit isoforms which combine to yield tissue- and organelle-specific V-ATPase pumps 6, 7. Our attempts at siRNA-mediated knockdown V1A resulted in lower mRNA expression (up to 70%) but stable V1A protein expression (not shown). Obviously, knockdown of subunit isoforms of large multisubunit complexes such as V-ATPase is not trivial; targeting of multiple subunits simultaneously may be necessary to reach inactivation levels comparable to the V-ATPase inhibitors.
The AR in LNCaP cells carries the mutation T877A in the ligand-binding domain 36, 37 which is equally distributed in those patients who have received hydroxyflutamide or bicalutamide therapy 38. This clinically relevant mutation prevents a blockade of receptor function by most anti-androgen therapies in LNCaP cells 36, 37. Therefore, V-ATPase inhibitors may result in important clinical benefits for patients expressing anti-androgen resistant mutations such as T877A. In the long-term, responsiveness of tumor cells to V-ATPase inhibitors might be used to control and prevent prostate tumor metastases. V-ATPase inhibitors may also help distinguish androgen-dependent and -independent tumor cells, as PSA secretion and mRNA expression are reduced only in the androgen-dependent cells (LNCaP cells). Combined with other therapies, V-ATPase inhibitors could additionally help prevent transformation into the castration-resistant phenotype.
Supplementary Material
Novelty and relation to the state of the field.
Down-regulation of PSA mRNA by V-ATPase proton pump inhibitors is novel. Reduction of the in vitro invasion of LNCaP and C4-2B cells alike by V-ATPase inhibitors, regardless that V-ATPase is prominent at the plasma membrane of C4-2B cells and only traces are detected in the low-metastatic LNCaP parental cells, is a new concept in the V-ATPase field. These findings make V-ATPase attractive new targets against early and advanced PCa tumors. They could pave the way for the investigation of new cellular processes that intertwine V-ATPase and intracellular pH homeostasis with the AR-PSA axis.
ACKNOWLEDGEMENTS
This publication was made possible by the Grant P20RR016480 from the INBRE Program of the National Center for Research Resources, a component of NIH to KJP; and in part, by NIH R01GM086495 (KJP) and UNM Cancer Center Post-Doctoral Fellow Matching Funds (VM and KJP). Images were generated in the UNM & Cancer Center Fluorescence Microscopy Shared Resource, funded as detailed on: http://hsc.unm.edu/crtc/microscopy/Facility.html. We acknowledge Rebecca Lee and Genevieve Phillips for professional assistance with confocal imaging, and Anna Jones for technical assistance with immunohistochemistry.
Abbreviations
- AR
androgen receptor
- Baf.A
bafilomycin A
- Conc.A
concanamycin A
- LAMP-1
lysosomal-associated membrane protein 1
- LAMP-2
lysosomal-associated membrane protein 2
- PCa
prostate cancer
- PSA
prostate-specific antigen
- TBP
TATA-binding protein
- V-ATPase
vacuolar adenosine triphosphatase
Footnotes
No potential conflicts of interest were disclosed.
References
- 1.Cipriano DJ, Wang Y, Bond S, Hinton A, Jefferies KC, Qi J, Forgac M. Structure and regulation of the vacuolar ATPases. Biochim Biophys Acta. 2008;1777:599–604. doi: 10.1016/j.bbabio.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kane PM. The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev. 2006;70:177–191. doi: 10.1128/MMBR.70.1.177-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breton S, Brown D. New insights into the regulation of V-ATPase-dependent proton secretion. Am J Physiol Renal Physiol. 2007;292:F1–F10. doi: 10.1152/ajprenal.00340.2006. [DOI] [PubMed] [Google Scholar]
- 4.Brown D, Breton S. H(+)V-ATPase-dependent luminal acidification in the kidney collecting duct and the epididymis/vas deferens: vesicle recycling and transcytotic pathways. J Exp Biol. 2000;203:137–145. doi: 10.1242/jeb.203.1.137. [DOI] [PubMed] [Google Scholar]
- 5.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 6.Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol. 2008;20:415–426. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toei M, Saum R, Forgac M. Regulation and isoform function of the V-ATPases. Biochemistry. 2010;49:4715–4723. doi: 10.1021/bi100397s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X, Qin W, Li J, Tan N, Pan D, Zhang H, Xie L, Yao G, Shu H, Yao M, Wan D, Gu J, et al. The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res. 2005;65:6843–6849. doi: 10.1158/0008-5472.CAN-04-3822. [DOI] [PubMed] [Google Scholar]
- 9.Chung C, Mader CC, Schmitz JC, Atladottir J, Fitchev P, Cornwell ML, Koleske AJ, Crawford SE, Gorelick F. The vacuolar-ATPase modulates matrix metalloproteinase isoforms in human pancreatic cancer. Lab Invest. 2011;91:732–743. doi: 10.1038/labinvest.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geho DH, Bandle RW, Clair T, Liotta LA. Physiological mechanisms of tumor-cell invasion and migration. Physiology (Bethesda) 2005;20:194–200. doi: 10.1152/physiol.00009.2005. [DOI] [PubMed] [Google Scholar]
- 11.Hinton A, Sennoune SR, Bond S, Fang M, Reuveni M, Sahagian GG, Jay D, Martinez-Zaguilan R, Forgac M. Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem. 2009;284:16400–16408. doi: 10.1074/jbc.M901201200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Zaguilan R, Raghunand N, Lynch RM, Bellamy W, Martinez GM, Rojas B, Smith D, Dalton WS, Gillies RJ. pH and drug resistance. I. Functional expression of plasmalemmal V-type H+-ATPase in drug-resistant human breast carcinoma cell lines. Biochem Pharmacol. 1999;57:1037–1046. doi: 10.1016/s0006-2952(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 13.Sennoune SR, Bakunts K, Martinez GM, Chua-Tuan JL, Kebir Y, Attaya MN, Martinez-Zaguilan R. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol. 2004;286:C1443–C1452. doi: 10.1152/ajpcell.00407.2003. [DOI] [PubMed] [Google Scholar]
- 14.You H, Jin J, Shu H, Yu B, De Milito A, Lozupone F, Deng Y, Tang N, Yao G, Fais S, Gu J, Qin W. Small interfering RNA targeting the subunit ATP6L of proton pump V-ATPase overcomes chemoresistance of breast cancer cells. Cancer Lett. 2009;280:110–119. doi: 10.1016/j.canlet.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Otero-Rey EM, Somoza-Martin M, Barros-Angueira F, Garcia-Garcia A. Intracellular pH regulation in oral squamous cell carcinoma is mediated by increased V-ATPase activity via over-expression of the ATP6V1C1 gene. Oral Oncol. 2008;44:193–199. doi: 10.1016/j.oraloncology.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Sayans M, Garcia-Garcia A, Reboiras-Lopez MD, Gandara-Vila P. Role of V-ATPases in solid tumors: importance of the subunit C (Review) Int J Oncol. 2009;34:1513–1520. doi: 10.3892/ijo_00000280. [DOI] [PubMed] [Google Scholar]
- 17.Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y, Yasui N, Yoneda T. The a3 Isoform Vacuolar Type H+-ATPase Promotes Distant Metastasis in the Mouse B16 Melanoma Cells. Mol Cancer Res. 2011;9:845–855. doi: 10.1158/1541-7786.MCR-10-0449. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, You J, Pei F. Silencing of a novel tumor metastasis suppressor gene LASS2/TMSG1 promotes invasion of prostate cancer cell in vitro through increase of vacuolar ATPase activity. J Cell Biochem. 2012;113:2356–2363. doi: 10.1002/jcb.24106. [DOI] [PubMed] [Google Scholar]
- 19.Wang WL, McHenry P, Jeffrey R, Schweitzer D, Helquist P, Tenniswood M. Effects of Iejimalide B, a marine macrolide, on growth and apoptosis in prostate cancer cell lines. J Cell Biochem. 2008;105:998–1007. doi: 10.1002/jcb.21898. [DOI] [PubMed] [Google Scholar]
- 20.Lilja H. Biology of prostate-specific antigen. Urology. 2003;62:27–33. doi: 10.1016/s0090-4295(03)00775-1. [DOI] [PubMed] [Google Scholar]
- 21.Lilja H, Stenman UH. Successful separation between benign prostatic hyperplasia and prostate cancer by measurement of free and complexed PSA. Cancer Treat Res. 1996;88:93–101. doi: 10.1007/978-1-4615-6343-3_5. [DOI] [PubMed] [Google Scholar]
- 22.Brinkmann AO, Blok LJ, de Ruiter PE, Doesburg P, Steketee K, Berrevoets CA, Trapman J. Mechanisms of androgen receptor activation and function. J Steroid Biochem Mol Biol. 1999;69:307–313. doi: 10.1016/s0960-0760(99)00049-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Coetzee GA. Prostate specific antigen gene regulation by androgen receptor. J Cell Biochem. 2004;93:233–241. doi: 10.1002/jcb.20228. [DOI] [PubMed] [Google Scholar]
- 24.Denmeade SR, Isaacs JT. The role of prostate-specific antigen in the clinical evaluation of prostatic disease. BJU Int. 2004;93(Suppl 1):10–15. doi: 10.1111/j.1464-410x.2003.04634.x. [DOI] [PubMed] [Google Scholar]
- 25.Coffey DS. Prostate cancer. An overview of an increasing dilemma. Cancer. 1993;71:880–886. doi: 10.1002/1097-0142(19930201)71:3+<880::aid-cncr2820711403>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, Pathak S, Chung LW. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000 Jul 1;44(2):91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. 44. [DOI] [PubMed] [Google Scholar]
- 27.Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dröse S, Bindseil KU, Bowman EJ, Siebers A, Zeeck A, Altendorf K. Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases. Biochemistry. 1993;32:3902–3906. doi: 10.1021/bi00066a008. [DOI] [PubMed] [Google Scholar]
- 29.Bowman BJ, Bowman EJ. Mutations in subunit C of the vacuolar ATPase confer resistance to bafilomycin and identify a conserved antibiotic binding site. J Biol Chem. 2002;277:3965–3972. doi: 10.1074/jbc.M109756200. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F, Rey JM, Garcia-Garcia A. V-ATPase inhibitors and implication in cancer treatment. Cancer Treat Rev. 2009;35:707–713. doi: 10.1016/j.ctrv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Kubota S, Seyama Y. Overexpression of vacuolar ATPase 16-kDa subunit in 10T1/2 fibroblasts enhances invasion with concomitant induction of matrix metalloproteinase-2. Biochem Biophys Res Commun. 2000;278:390–394. doi: 10.1006/bbrc.2000.3802. [DOI] [PubMed] [Google Scholar]
- 32.Sobota JA, Back N, Eipper BA, Mains RE. Inhibitors of the V0 subunit of the vacuolar H+-ATPase prevent segregation of lysosomal- and secretory-pathway proteins. J Cell Sci. 2009;122:3542–3553. doi: 10.1242/jcs.034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 34.Teplova VV, Tonshin AA, Grigoriev PA, Saris NE, Salkinoja-Salonen MS. Bafilomycin A1 is a potassium ionophore that impairs mitochondrial functions. J Bioenerg Biomembr. 2007;39:321–329. doi: 10.1007/s10863-007-9095-9. [DOI] [PubMed] [Google Scholar]
- 35.Huang C, Chang A. pH-dependent cargo sorting from the Golgi. J Biol Chem. 2011;286:10058–10065. doi: 10.1074/jbc.M110.197889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GG, Jenster G, Trapman J, Brinkmann AO, Mulder E. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41:665–669. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 37.Veldscholte J, Berrevoets CA, Brinkmann AO, Grootegoed JA, Mulder E. Anti-androgens and the mutated androgen receptor of LNCaP cells: differential effects on binding affinity, heat-shock protein interaction, and transcription activation. Biochemistry. 1992;31:2393–2399. doi: 10.1021/bi00123a026. [DOI] [PubMed] [Google Scholar]
- 38.Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, Stadler W, Hayes DF, Kantoff PW, Vogelzang NJ, Small EJ. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673–2678. doi: 10.1200/JCO.2003.11.102. [DOI] [PubMed] [Google Scholar]
- 39.Denmeade SR, Sokoll LJ, Dalrymple S, Rosen DM, Gady AM, Bruzek D, Ricklis RM, Isaacs JT. Dissociation between androgen responsiveness for malignant growth vs. expression of prostate specific differentiation markers PSA, hK2, and PSMA in human prostate cancer models. Prostate. 2003;54:249–257. doi: 10.1002/pros.10199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.