Abstract
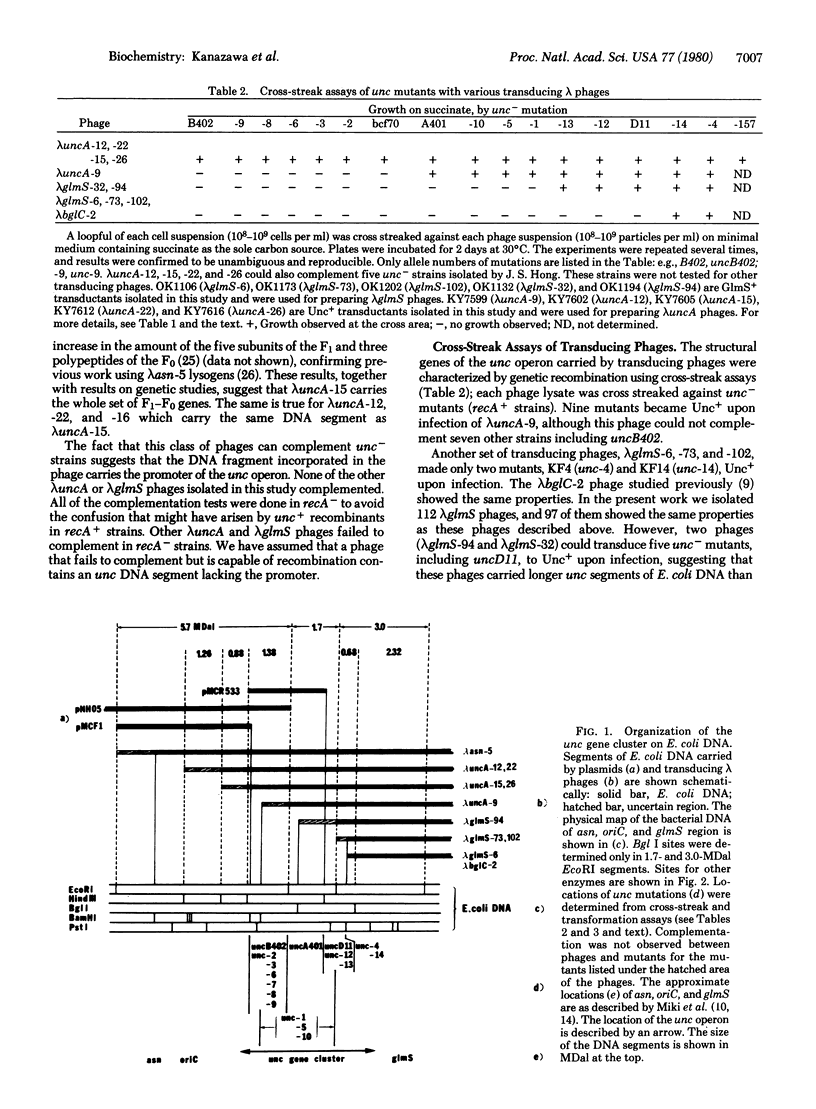
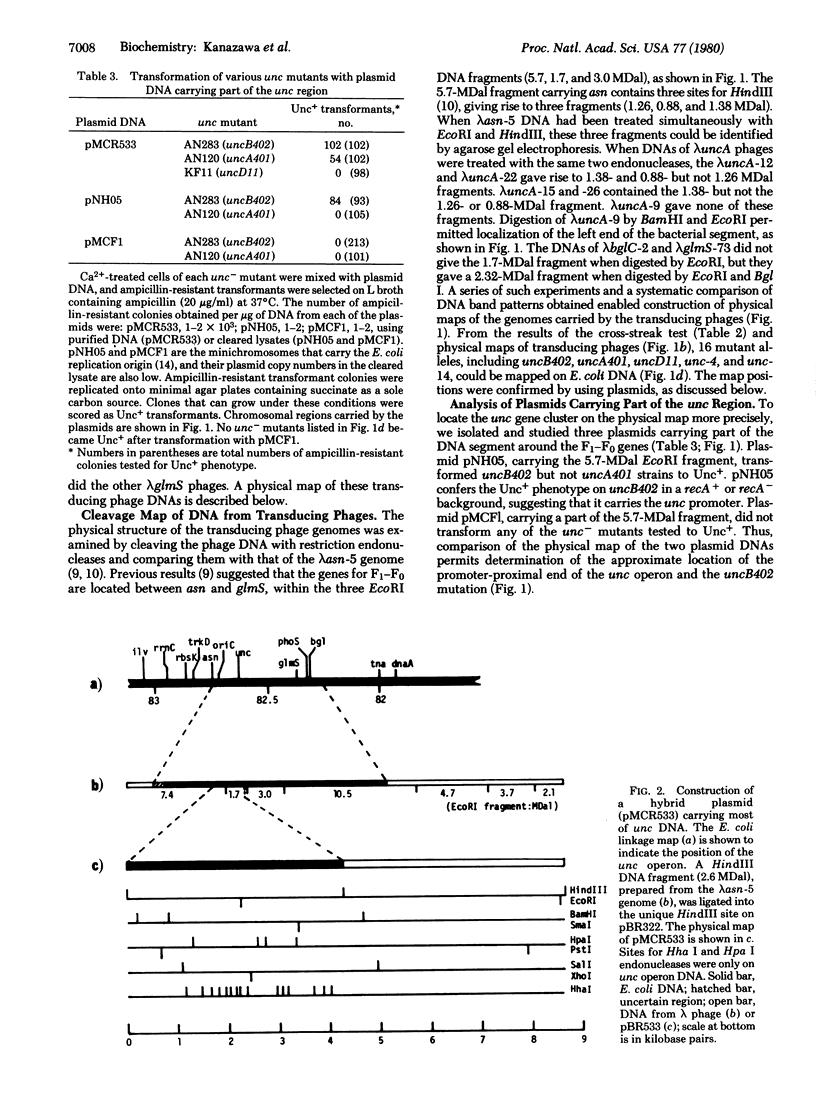
The proton-translocating ATPase (F1-F0) of oxidative phosphorylation (ATP phosphohydrolase, EC 3.6.1.3) is coded for by a set of structural genes comprising the unc operon in Escherichia coli. We have analyzed several new transducing phages and plasmids carrying various lengths of the DNA segments of the unc operon by complementation assay using 14 new unc- mutants and representatives of previously described strains which were made available to us. Transducing phages carrying parts of the unc gene cluster were isolated: lambda uncA-9 and lambda glmS phages converted only some of the unc- mutants to the Unc+, as determined by complementation assays. A new hybrid plasmid (pMCR533) carrying part of the unc operon was constructed by inserting the HindIII fragment of lambda asn-5 DNA (a phage carrying the entire unc operon) into the unique HindIII site of pBR322. This plasmid transformed eight unc- strains to Unc+, including uncB402 and uncA401, but did not complement uncD11 or four other strains. Two minichromosomes which carry the E. coli replication origin were also tested: plasmid pNH05 transformed the uncB402 but not the uncA401 strain to Unc+, whereas plasmid pMCF1 transformed none of the mutants tested. Analysis of the DNAs from these transducing phages and plasmids with restriction endonucleases suggested that all of the structural genes for the F1-F0 complex are localized within a DNA segment of approximately 4.5 megadaltons containing two EcoRI sites. The approximate locations of the unc- mutations were mapped on this DNA segment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B. A., Hammes G. G. Structure of oxidative- and photo-phosphorylation coupling factor complexes. Biochim Biophys Acta. 1979 Jul 3;549(1):31–53. doi: 10.1016/0304-4173(79)90017-x. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Dunn S. D. Identification of the altered subunit in the inactive F1ATPase of an Escherichia coli uncA mutant. Biochem Biophys Res Commun. 1978 May 30;82(2):596–602. doi: 10.1016/0006-291x(78)90916-6. [DOI] [PubMed] [Google Scholar]
- Fayle D. R., Downie J. A., Cox G. B., Gibson F., Radik J. Characterization of the mutant-unc D-gene product in a strain of Escherichia coli K12. An altered beta-subunit of the magnesium ion-stimulated adenosine triphosphatase. Biochem J. 1978 Jun 15;172(3):523–531. doi: 10.1042/bj1720523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Fillingame R. H. Energy-transducing H+-ATPase of Escherichia coli. Purification, reconstitution, and subunit composition. J Biol Chem. 1979 Sep 10;254(17):8230–8236. [PubMed] [Google Scholar]
- Futai M., Sternweis P. C., Heppel L. A. Purification and properties of reconstitutively active and inactive adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2725–2729. doi: 10.1073/pnas.71.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. A mutation affecting a second component of the F0 portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. The uncC424 allele. Biochem J. 1977 Apr 15;164(1):193–198. doi: 10.1042/bj1640193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y. Reconstitution of the energy transformer, gate and channel subunit reassembly, crystalline ATPase and ATP synthesis. Biochim Biophys Acta. 1978 Sep 21;505(1):45–93. doi: 10.1016/0304-4173(78)90008-3. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Horiuchi Y., Takagi M., Ishino Y., Futai M. Coupling factor F1 ATPase with defective beta subunit from a mutant of Escherichia coli. J Biochem. 1980 Sep;88(3):695–703. doi: 10.1093/oxfordjournals.jbchem.a133022. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Miki T., Tamura F., Yura T., Futai M. Specialized transducing phage lambda carrying the genes for coupling factor of oxidative phosphorylation of Escherichia coli: increased synthesis of coupling factor on induction of prophage lambda asn. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1126–1130. doi: 10.1073/pnas.76.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H., Saito S., Futai M. Coupling factor ATPase from Escherichia coli. An uncA mutant (uncA401) with defective alpha subunit. J Biochem. 1978 Dec;84(6):1513–1517. doi: 10.1093/oxfordjournals.jbchem.a132276. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Hiraga S., Nagata T., Yura T. Bacteriophage lambda carrying the Escherichia coli chromosomal region of the replication origin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5099–5103. doi: 10.1073/pnas.75.10.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molholt B., Doskocil J. Increased transformation frequency in E. coli. Biochem Biophys Res Commun. 1978 May 30;82(2):477–483. doi: 10.1016/0006-291x(78)90899-9. [DOI] [PubMed] [Google Scholar]
- Ogura T., Miki T., Hiraga S. Copy-number mutants of the plasmid carrying the replication origin of the Escherichia coli chromosome: evidence for a control region of replication. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3993–3997. doi: 10.1073/pnas.77.7.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W., Graf T., Lukins H. B. The dicyclohexylcarbodiimide-binding protein of the mitochondrial ATPase complex from Neurospora crassa and Saccharomyces cerevisiae. Identification and isolation. Eur J Biochem. 1979 Feb 1;93(3):587–599. doi: 10.1111/j.1432-1033.1979.tb12859.x. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Downie J. A., Cox G. B., Gibson F., Langman L., Fayle D. R. The uncA gene codes for the alpha-subunit of the adenosine triphosphatase of Escherichia coli. Electrophoretic analysis of uncA mutant strains. Biochem J. 1979 Apr 15;180(1):103–109. doi: 10.1042/bj1800103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Carbodiimide-binding protein of H+-translocating ATPase and inhibition of H+ conduction by dicyclohexylcarbodiimide. J Biochem. 1979 Feb;85(2):503–509. doi: 10.1093/oxfordjournals.jbchem.a132357. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



