Abstract
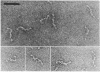
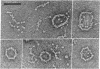
Monomeric C1s (Mr, 85,000; s20,w, 4.3S), a subcomponent of first component of complement (C1), the dimer (Mr, 170,000; s20,w, 6.7 S) of C1r, another subcomponent, and the tetrameric complex (C1r,C1s)2 (Mr, 340,000; s20,w, 8.7 S) are elongated molecules. Hydrodynamic equivalents of cylindrical shape have a diameter of 3.3 nm and lengths of 20 nm for C1s, 36 nm for (C1r)2, and 64 nm for (C1r,C1s)2. In electron micrographs the C1r,C1s complex appears as a chain composed of six to eight globular domains with a contour length of 51 nm. A structure is proposed in which (C1r)2 forms a core to which C1s protomers are associated at both ends. The C1 complex (s20,w, 16.3 S) reconstituted from C1q, C1r, and C1s dissociates under the conditions used for electron microscopy. Some features of the C1 complex are revealed in the dissociation products.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimeh S. N., Bing D. H., Painter R. H. A simple method for the isolation of the subcomponents of the first component of complement by affinity chromatography. J Immunol. 1974 Jul;113(1):225–234. [PubMed] [Google Scholar]
- Bloomfield V., Van Holde K. E., Dalton W. O. Frictional coefficients of multisubunit structures. II. Application to proteins and viruses. Biopolymers. 1967 Feb;5(2):149–159. doi: 10.1002/bip.1967.360050203. [DOI] [PubMed] [Google Scholar]
- Brodsky-Doyle B., Leonard K. R., Reid K. B. Circular-dichroism and electron-microscopy studies of human subcomponent C1q before and after limited proteolysis by pepsin. Biochem J. 1976 Nov;159(2):279–286. doi: 10.1042/bj1590279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Ziccardi R. J. Reconstitution of C1 in native proenzyme form and its use in a quantitative C1 activation test. J Immunol. 1977 Nov;119(5):1664–1667. [PubMed] [Google Scholar]
- Gigli I., Porter R. R., Sim R. B. The unactivated form of the first component of human complement, C1. Biochem J. 1976 Sep 1;157(3):541–548. doi: 10.1042/bj1570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobel H. R., Villiger W., Isliker H. Chemical analysis and electron microscopy studies of human C1q prepared by different methods. Eur J Immunol. 1975 Jan;5(1):78–82. doi: 10.1002/eji.1830050119. [DOI] [PubMed] [Google Scholar]
- LEPOW I. H., NAFF G. B., TODD E. W., PENSKY J., HINZ C. F. Chromatographic resolution of the first component of human complement into three activities. J Exp Med. 1963 Jun 1;117:983–1008. doi: 10.1084/jem.117.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberman R. Use of uranyl formate as a negative stain. J Mol Biol. 1965 Sep;13(2):606–606. doi: 10.1016/s0022-2836(65)80124-3. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. Isolation of a thermolabile serum protein which precipitates gamma-globulin aggregates and participates in immune hemolysis. Proc Soc Exp Biol Med. 1961 Feb;106:291–295. doi: 10.3181/00379727-106-26313. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Takahashi K., Koyama J. Isolation of a complex of the subcomponents of the activated first component of complement, Clr--Cls, from ACD-human plasma. FEBS Lett. 1974 May 1;41(2):280–282. doi: 10.1016/0014-5793(74)81229-9. [DOI] [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. Activation of the complement system by antibody-antigen complexes: the classical pathway. Adv Protein Chem. 1979;33:1–71. doi: 10.1016/s0065-3233(08)60458-1. [DOI] [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. The biochemistry of complement. Nature. 1978 Oct 26;275(5682):699–704. doi: 10.1038/275699a0. [DOI] [PubMed] [Google Scholar]
- Shelton E., Yonemasu K., Stroud R. M. Ultrastructure of the human complement component, Clq (negative staining-glutamine synthetase-biologically active Clq). Proc Natl Acad Sci U S A. 1972 Jan;69(1):65–68. doi: 10.1073/pnas.69.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Porter R. R., Reid K. B., Gigli I. The structure and enzymic activities of the C1r and C1s subcomponents of C1, the first component of human serum complement. Biochem J. 1977 May 1;163(2):219–227. doi: 10.1042/bj1630219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Nagasawa S., Koyama J. A gross structure of an activated form of a subunit of the first component of human complement. Clr. FEBS Lett. 1975 Jul 15;55(1):156–160. doi: 10.1016/0014-5793(75)80982-3. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nagasawa S., Koyama J. The NH-2-terminal sequences of a subunit of the first component of human complement, C1s, and its activated form, C1s. FEBS Lett. 1975 Feb 15;50(3):330–333. doi: 10.1016/0014-5793(75)80521-7. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Schulthess T., Engel J., Jaton J. C. Antigen-independent activation of the first component of complement C1 by chemically crosslinked rabbit IgG-oligomers. FEBS Lett. 1980 Apr 7;112(2):152–154. doi: 10.1016/0014-5793(80)80168-2. [DOI] [PubMed] [Google Scholar]
- Valet G., Cooper N. R. Isolation and characterization of the proenzyme form of the C1s subunit of the first complement component. J Immunol. 1974 Jan;112(1):339–350. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Physicochemical and functional characterization of the C1r subunit of the first complement component. J Immunol. 1976 Feb;116(2):496–503. [PubMed] [Google Scholar]