Abstract
Aims
To study whether HbA1c, and its relationship with fasting plasma glucose, was significantly different among Chinese, Malays and Indians in Singapore.
Methods
A sample of 3895 individuals without known diabetes underwent detailed interview and health examination, including anthropometric and biochemical evaluation, between 2004 and 2007. Pearson’s correlation, analysis of variance and multiple linear regression analyses were used to examine the influence of ethnicity on HbA1c.
Results
As fasting plasma glucose increased, HbA1c increased more in Malays and Indians compared with Chinese after adjustment for age, gender, waist circumference, serum cholesterol, serum triglyceride and homeostasis model assessment of insulin resistance (P-interaction < 0.001). This translates to an HbA1c difference of 1.1 mmol/mol (0.1%, Indians vs. Chinese), and 0.9 mmol/mol (0.08%, Malays vs. Chinese) at fasting plasma glucose 5.6 mmol/l (the American Diabetes Association criterion for impaired fasting glycaemia); and 2.1 mmol/mol (0.19%, Indians vs. Chinese) and 2.6 mmol/mol (0.24%, Malays vs. Chinese) at fasting plasma glucose 7.0 mmol/l, the diagnostic criterion for diabetes mellitus.
Conclusions
Using HbA1c in place of fasting plasma glucose will reclassify different proportions of the population in different ethnic groups. This may have implications in interpretation of HbA1c results across ethnic groups and the use of HbA1c for diagnosing diabetes mellitus.
Keywords: diabetes mellitus, diagnosis, ethnicity, glycated haemoglobin
Introduction
Glycated haemoglobin (HbA1c), the fraction of haemoglobin non-enzymatically linked to glucose (glycated) at the valine terminal of the β-chain, is widely used as an indicator of glycaemic status in the management of diabetes mellitus. The level of HbA1c is proportional to the blood glucose levels and reflects the glycaemic status over the preceding 3 months (the average lifespan of erythrocytes) [1,2]. Data from large randomized controlled trials comparing intensive and conventional glycaemic control have been used to define therapeutic targets for HbA1c that guide diabetes management [3–6].
An International Expert Committee, the American Diabetes Association and the World Health Organization have now recommended the use of HbA1c as a diagnostic tool for diabetes mellitus [7–9]. The measurement of HbA1c was favoured as it exhibited less intra-individual variability, greater analytical stability and also obviated the need for fasting. In addition, HbA1c assays are also being standardized worldwide. It has also been demonstrated that HbA1c performs comparably with fasting and 2-h postprandial glucose measures in identifying individuals with and without retinopathy [7–11]. The criteria recommended by the American Diabetes Association are HbA1c ≥ 39 mmol/mol (5.7%) for increased risk for diabetes and ≥ 48 mmol/mol (6.5%) for diabetes.
Several studies have demonstrated that individuals may exhibit different HbA1c at the same glucose level [12–14]. In particular, some ethnic groups appear to have higher HbA1c levels compared with others at the same levels of plasma glucose [14–17]. The use of HbA1c to diagnose diabetes has been shown to under- or overestimate the prevalence of diabetes in comparison with glucose-based criteria in different populations or ethnic groups [17–20]. This indicates that the relationship between HbA1c and blood glucose may not be similar across ethnic groups and populations.
Delineating ethnic differences in the relationship between plasma glucose and HbA1c is especially important in Asian populations, given that the prevalence of cases of diabetes in this region is projected to more than double by 2030 [21,22].
In this study, we examined the relationship between fasting plasma glucose and HbA1c among the three major ethnic groups, Chinese, Malay and Indians living in Singapore, whose prevalence rates for diabetes mellitus are relatively high at 7.1, 11 and 15.3%, respectively [23].
Patients and methods
Study population
Individuals who had participated previously in one of four population-based cross-sectional studies were invited to be part of this study (Singapore Prospective Study). The four studies include: the Thyroid and Heart Study 1982–1984 [24], the National Health Survey 1992 [25], the National University of Singapore Heart Study 1993–1995 [26] and the National Health Survey 1998 [27] and have been described previously [28]. Briefly, all studies were a random sample of individuals from the Singapore population, with disproportionate sampling stratified by ethnicity to increase the number of the minority ethnic groups (Malays and Asian Indians). Subjects deceased at time of follow-up (as shown by data-linkage to Registry of Births and Deaths) were excluded (n = 517). Also excluded were six subjects who had emigrated and 85 subjects who had errors in the records for the identity card number.
Subjects were contacted to obtain an appointment for investigators to administer the questionnaire at the subject’s home. Three home visits were made on three different occasions, including one weekend day and one weekday, before a subject was deemed non-contactable. In total, 2673 subjects were non-contactable and, of the remaining subjects, 30 (0.3%) refused to participate. All subjects were invited to attend a health examination for additional tests and collection of biological specimens.
A total of 10 633 individuals were invited; 7742 subjects (response rate 74.1%) completed the health questionnaire, of which 5157 (66.6% of those who completed the questionnaire or 49.4% of all eligible subjects) also attended the health examination. Ethics approval was obtained from two Institutional Review Boards (National University of Singapore and Singapore General Hospital). Informed consent was obtained before commencement of the study.
Data collection
Data on demographic and lifestyle (alcohol intake, smoking) factors, as well as medical history (including physician-diagnosed hypertension, diabetes mellitus and hyperlipidaemia) were collected using interviewer-administered questionnaires. For the health examination, participants were examined in the morning following a 10-h overnight fast. Venous blood was drawn and collected in plain and fluoride oxalate tubes and stored at 4 °C for a maximum of 4 h prior to processing.
All biochemical analyses on blood were carried out at the National University Hospital Referral Laboratory, which is accredited by the College of American Pathologists. Serum total cholesterol, triglyceride and HDL cholesterol levels were measured using an automated autoanalyser (ADVIA 2400; Bayer Diagnostics, Tarrytown, NY, USA). LDL cholesterol levels were calculated using the Friedewald formula.
Plasma glucose was also assayed using enzymatic methods (ADVIA 2400; Bayer Diagnostics) using blood collected in fluoride oxalate tubes. Plasma creatinine was measured by enzymatic methods (reaction of Tanganelli) and implemented on an ADVIA 2400 chemistry system. The intra- and interday variability for total cholesterol, triglycerides, HDL cholesterol, plasma glucose and creatinine was 0.80–1.57, 0.93–1.15, 0.00–3.85, 1.27–3.4, 0.56–0.65, 1.18–2.00, 0.00–0.93, 1.68–1.83, 2.50–6.60 and 5.60–7.20%, respectively.
HbA1c was measured using high-pressure liquid chromatography on a Biorad Variant II analyser (Bio-Rad Laboratories, Hercules, CA, USA), an assay that was accredited by the National Glycoprotein Standardization Program with controls traceable to the Diabetes Control and Complications Trial. The intra- and interday coefficients of variability for HbA1c were 0.0–2.0 and 0.85–1.54%, respectively.
Height was measured without shoes using a wall-mounted stadiometer. Weight was measured in light clothing using the same digital scale (SECA, model 782 2321009; Vogel & Halke, Hamberg, Germany). Participants were instructed to remove any objects such as keys and mobile phone before measurement. Two readings of blood pressure were taken from participants after 5 min of resting using an automated blood pressure monitor (Dinamap Pro100V2; Criticon, Norderstedt, Germany). A third reading was performed if the difference between two readings of systolic blood pressure was greater than 10 mmHg or of diastolic blood pressure was greater than 5 mmHg. Mean values of the closest two readings were calculated. The inter- and intra-observer coefficient of variation for systolic blood pressure was 0.51–10.2 and 0-2.5%, whilst it was 0.41–7.5 and 0–2.5% for diastolic blood pressure. Waist circumference was measured midway between the lower rib margin and the iliac crest and hip circumference was measured at the widest point over the greater trochanters. The waist–hip ratio was calculated by dividing waist circumference (in cm) by hip circumference (in cm).
Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as [fasting insulin (μU/ml) × fasting glucose (mmol/l)]/22.5.
Known diabetes was defined as history of diabetes and/or currently taking anti-diabetic agents.
Statistical analysis
Of the 5157 individuals who completed both the study questionnaire and the health examination, those with known diabetes mellitus (n = 325), without measured HbA1c values (n = 932) and without recorded ethnicity (n = 2) were excluded. The analysis reported in this paper is based on data from 3895 subjects without previous diagnosis of diabetes and with measured HbA1c values. Descriptive analysis was used to study the baseline characteristics. Variables with non-linear distributions were log-transformed to ensure linearity, as needed. Analysis of covariance was used to obtain adjusted means. The step-down Bonferroni method was used to adjust for multiple comparisons. Pearson’s correlation and one-way analysis of variance (anova) were used to identify continuous and categorical variables significantly influencing HbA1c. Multiple linear regression was used to further clarify the effect of these variables on HbA1c. Fasting plasma glucose was centred to the mean for regression analysis. Two dummy variables were created and used to study the interaction between ethnicity and fasting plasma glucose in relation to HbA1c, by incorporating the cross-product term in the model.
All unweighted statistical analysis was conducted using Predictive Analytics SoftWare (PASW) Statistics (version 18; IBM, Chicago, IL, USA). Weighted analysis to test the robustness of interactions was conducted using R (version 2; R Foundation for Statistical Computing, Vienna, Austria). All statistical tests used were two-sided, with P < 0.05 being considered as significant. All values are mean (standard error) unless otherwise specified.
Results
Baseline characteristics of the population studied are shown in Table 1. The mean age was 49 (12) years, with almost equal gender distribution. The majority were of Chinese ethnicity (2697, 69.2%), followed by Malays (633, 16.3%) and Indians (565, 14.5%). The mean HbA1c was 40 ± 9.1 mmol/mol (5.8% ± 0.83), with mean fasting plasma glucose and insulin values of 4.94 mmol/l (± 1.16) and 7.85 mU/l (± 6.31).
Table 1.
Participant characteristics by ethnic group in the Singapore Prospective Study Program, 2004–2007
| Variable | All | Chinese (n = 2697, 69.2%) | Malay (n = 633, 16.3%) | Indian (n = 565, 14.5%) | P |
|---|---|---|---|---|---|
| Age | 49 (0.2) | 49 (0.2) | 49 (0.5) | 51 (0.5) | 0.005 |
| Male | 1853 (47.6) | 1263 (46.8) | 312 (49.4) | 276 (48.8) | 0.411 |
| BMI (kg/m2)‡ | 23.7 (0.1) | 22.8 (0.1) | 25.8 (0.2)*** | 25.8 (0.2)*** | < 0.001 |
| Waist–hip ratio‡ | 0.85 (0.001) | 0.84 (0.001) | 0.85 (0.003)* | 0.87 (0.003)***††† | < 0.001 |
| Waist circumference (cm)‡ | 83.6 (0.2) | 81.5 (0.2) | 86.8 (0.4)*** | 89.9 (0.4)***††† | < 0.001 |
| Systolic blood pressure (mmHg)‡a | 131 (0.3) | 130 (0.3) | 136 (0.7)*** | 131 (0.7)††† | < 0.001 |
| Diastolic blood pressure (mmHg)‡ | 78 (0.2) | 77 (0.2) | 79 (0.4)*** | 79 (0.4)** | < 0.001 |
| HbA1c (mmol/mol) | 40 (0.1) | 40 (0.2) | 42 (0.3) | 43 (0.3) | < 0.001 |
| HbA1c (%)‡ | 5.84 (0.01) | 5.77 (0.02) | 5.95 (0.03)*** | 6.06 (0.03)***† | |
| Fasting plasma glucose (mmol/l)‡ | 4.94 (0.02) | 4.84 (0.02) | 5.10 (0.04)*** | 5.24 (0.05)*** | < 0.001 |
| Insulin (mU/l)‡ | 7.85 (0.1) | 7.03 (0.12) | 8.24 (0.24)*** | 11.32 (0.26)***††† | < 0.001 |
| HOMA-IR‡ | 1.79 (0.03) | 1.56 (0.03) | 1.90 (0.07)*** | 2.72 (0.07)***††† | < 0.001 |
| Cholesterol (mmol/l)‡ | 5.27 (0.02) | 5.22 (0.02) | 5.53 (0.04)*** | 5.24 (0.04)††† | < 0.001 |
| Triglycerides (mmol/l)‡ | 1.35 (0.01) | 1.31 (0.02) | 1.47 (0.03)*** | 1.41 (0.03)* | < 0.001 |
| HDL cholesterol (mmol/l)‡ | 1.44 (0.01) | 1.49 (0.01) | 1.39 (0.01)*** | 1.25 (0.01)***††† | < 0.001 |
| LDL cholesterol (mmol/l)‡ | 3.22 (0.01) | 3.14 (0.02) | 3.47 (0.03)*** | 3.35 (0.03)***† | < 0.001 |
| Creatinine (μmol/l)‡ | 79.97 (0.31) | 78.99 (0.29) | 81.77 (0.59)*** | 82.65 (0.62)*** | < 0.001 |
HOMA-IR, homeostasis model assessment of insulin resistance.
P < 0.05,
P < 0.01,
P < 0.001 (Chinese as reference);
P < 0.05,
††P < 0.01,
P < 0.001 (Malay as reference) by anova using step-down Bonferroni correction for multiple comparisons.
Means adjusted for age and gender.
All values are means (± se) unless otherwise specified.
There were significant differences between the three ethnic groups in the variables measured. Both Malays and Indians had higher mean BMI and serum creatinine than the Chinese (P < 0.001), adjusted for age and gender. Indians had higher age- and gender-adjusted mean waist circumference and waist–hip ratio compared with the other ethnic groups (P < 0.001). Malays had higher mean systolic blood pressure and serum cholesterol compared with Chinese and Indians (P < 0.001). Indians had the highest mean fasting insulin levels (P < 0.001), followed by the Malay (P < 0.001), compared with the Chinese. There were significant differences (P < 0.05) in unadjusted HbA1c between the three ethnic groups, with Indians having the highest mean HbA1c (43 mmol/mol, 95% CI 42–44; 6.09%, 95% CI 5.99–6.18), followed by Malays (42 mmol/mol, 95% CI 41–43; 5.96%, 95% CI 5.87–6.05) and Chinese (39 mmol/mol, 95% CI 39–40; 5.76%, 95% CI 5.74–5.79). The distribution of HbA1c was shifted towards the right in Indians and Malays, compared with the Chinese across the range of HbA1c values, suggesting that these differences were not just attributable to a higher proportion of undiscovered diabetes mellitus in the Indians and Malays (Fig. 1).
FIGURE 1.
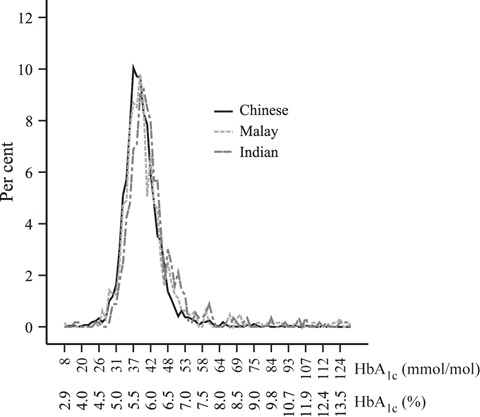
Distribution of HbA1c by ethnic group in the Singapore Prospective Study Program, 2004–2007
On univariate analysis using Pearson’s correlation, fasting plasma glucose (r = 0.79, P < 0.001), age (r = 0.208, P < 0.001), BMI (log-transformed; r = 0.21, P < 0.001), waist circumference (r = 0.23, P < 0.001), waist–hip ratio (r = 0.18, P < 0.001), systolic blood pressure (r = 0.19, P < 0.001), diastolic blood pressure (r = 0.15, P < 0.001), total cholesterol (r = 0.13, P < 0.001), serum triglyceride (log-transformed; r = 0.20, P < 0.001), fasting insulin (r = 0.17, P < 0.001) and HOMA-IR (log-transformed; r = 0.34, P < 0.001) were significantly associated with HbA1c. Age, gender, ethnicity, waist circumference, fasting plasma glucose, total cholesterol, triglycerides and HOMA-IR continued to be significantly associated with HbA1c on stepwise multiple linear regression, although the magnitudes of the main effects were small for all variables except fasting plasma glucose (Table 2). Fasting plasma glucose showed the strongest association with HbA1c, explaining 62.4% of the variance for HbA1c. In addition, a statistically significant interaction between fasting plasma glucose and ethnicity was noted (P-interactions < 0.001). As fasting plasma glucose increased, HbA1c increased more in Malays and Indians compared with Chinese. Subsequently, we conducted robust analysis to reduce the effect of outliers, which gave similar results for the interaction between ethnicity and fasting plasma glucose (data not shown). Estimation of the mean HbA1c values over a range of fasting plasma glucose (4–7.5 mmol/l) showed that Malays and Indians appear to have lower HbA1c at low fasting plasma glucose values and higher HbA1c at higher values of fasting plasma glucose, compared with the Chinese, with crossover at fasting plasma glucose 5.0 mmol/l (Fig. 2). This translates to a difference of 1.1 mmol/mol (95% CI 0.5–1.6; 0.1%, 95% CI 0.05–0.15; Indians vs. Chinese) and 0.9 mmol/mol (95% CI 0.3–1.4; 0.08%, 95% CI 0.03–0.13; Malays vs. Chinese) at fasting plasma glucose 5.6 mmol/l, the American Diabetes Association criterion for impaired fasting glycaemia; and a difference of 2.1 mmol/mol (95% CI 1.2–3.0; 0.19%, 95% CI 0.11–0.27; Indians vs. Chinese) and 2.6 mmol/mol (95% CI 1.7–3.5; 0.24%, 95% CI 0.16–0.32; Malays vs. Chinese) at fasting plasma glucose 7.0 mmol/l, the diagnostic criterion for diabetes mellitus.
Table 2.
Multivariate regression model with HbA1c as dependent variable; the Singapore Prospective Study Program, 2004–2007
| 95% CI | |||||
|---|---|---|---|---|---|
| Variables in model | β-coefficient | Standard error | P | Lower | Upper |
| Intercept | 5.078 | 0.092 | < 0.001 | 4.942 | 5.324 |
| Age | 0.005 | 0.001 | < 0.001 | 0.003 | 0.006 |
| Female | 0.085 | 0.018 | < 0.001 | 0.049 | 0.12 |
| Indian | 0.055 | 0.025 | 0.024 | 0.007 | 0.103 |
| Malay | 0.005 | 0.023 | 0.813 | −0.039 | 0.05 |
| Fasting plasma glucose (centred) | 0.493 | 0.012 | < 0.001 | 0.469 | 0.517 |
| Waist circumference | 0.004 | 0.001 | < 0.001 | 0.002 | 0.005 |
| Serum triglyceride (log) | 0.14 | 0.043 | 0.001 | 0.056 | 0.224 |
| Serum cholesterol | 0.033 | 0.009 | < 0.001 | 0.015 | 0.052 |
| HOMA-IR (log) | -0.072 | 0.036 | 0.043 | −0.142 | −0.002 |
| Indian × fasting plasma glucose (centred) | 0.066 | 0.017 | < 0.001 | 0.033 | 0.1 |
| Malay × fasting plasma glucose (centred) | 0.133 | 0.017 | < 0.001 | 0.079 | 0.147 |
R2 = 0.632.
HOMA–IR, homeostasis model assessment of insulin resistance.
FIGURE 2.
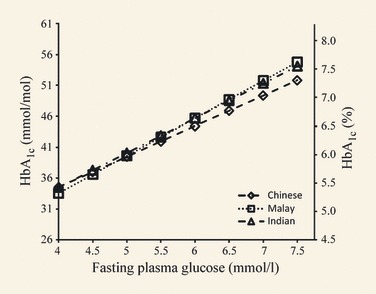
Estimated mean HbA1c at different fasting plasma glucose values, the Singapore Prospective Study Program, 2004–2007. Mean HbA1c estimated after adjusting for age, gender, ethnicity, fasting plasma glucose–ethnicity interaction, waist circumference, serum cholesterol, triglyceride and homeostasis model assessment of insulin resistance (HOMA-IR) at each fasting plasma glucose level.
Discussion
In this study, we have shown that blood glucose is the major determinant of HbA1c in Chinese, Malays and Indians living in Singapore. This is in line with previous studies [1,29,30]. The novelty of our study is the demonstration that the relationship between fasting plasma glucose and HbA1c differs between the three major ethnic groups living in Singapore. This is particularly relevant as these ethnic groups are closely related to the majority of the population living in Asia, where we anticipate a large increase in the prevalence of diabetes in the next several decades. HbA1c values were lower in Malays and Indians compared with Chinese at lower fasting plasma glucose levels, with crossover at fasting plasma glucose of around 5 mmol/l. At fasting plasma glucose levels above 5 mmol/l, HbA1c values were higher in Malays and Indians compared with Chinese, with greater differences at higher fasting plasma glucose concentrations. To illustrate the relevance of these findings to clinical practice, we estimated that ethnicity contributed to a difference in HbA1c of 2.1–2.6 mmol/mol (0.19–0.24%) at the fasting plasma glucose cut-off of 7.0 mmol/l, the current level for the diagnosis of diabetes mellitus. If a single HbA1c cut-off is used to diagnose diabetes (and pre-diabetes) across these three ethnic groups, it will reclassify more Indians and Malays as having these conditions than Chinese, compared with fasting plasma glucose-based criteria. Although the effect of ethnicity in HbA1c seems small, it may be significant if HbA1c is widely accepted and applied as a diagnostic tool in our population. To provide some context, it is useful to note that 4% of the population has an HbA1c between 48 and 50 mmol/mol (6.5–6.7%).
Several previous studies have described ethnic differences in HbA1c, but only a few have examined for an interaction between ethnicity and glucose in determining HbA1c. Herman et al. showed differences in HbA1c in subjects with impaired glucose tolerance of white, Hispanic, Asian, Indian American and African American ethnicities. An HbA1c difference of 0.15–0.4%, a magnitude that is similar to that found in our study, persisted across ethnic groups after adjusting for age, gender, systolic and diastolic blood pressures, BMI, glucose and insulin levels [14]. In another study, Ziemer et al. demonstrated that African Americans had higher HbA1c levels compared with white Caucasians across a range of glucose levels. Similar to our study, they found that these differences increased with an increase in glucose levels [15]. However, neither of these studies formally tested for an interaction between ethnicity and glucose in determining HbA1c. Jorgensen et al. reported that Greenland Inuit had significantly higher levels of HbA1c than Danes at any level of glycaemia. However, they did not find that ethnicity modified the association between plasma glucose and HbA1c in their analysis [17]. In another study of white and African American subjects with diabetes, Bleyer et al. reported an interaction between ethnicity and random serum glucose for HbA1c. However, this study was limited to individuals with established diabetes [16]. Although we excluded individuals with known diabetes in our study, fasting plasma glucose values ranged from 2.7 to 21.5 mmol/l. Thus, we were able to demonstrate an interaction between ethnicity and glucose levels in relation to HbA1c across a larger range of glycaemia than has been studied previously.
We have a few hypotheses that may explain this differential relationship between HbA1c and fasting plasma glucose across ethnic groups. Firstly, there could be differences in the daily glycaemic exposure (such as postprandial glucose excursions) amongst the three groups at the same fasting plasma glucose. This reflects the failure of fasting plasma glucose to accurately represent an individual’s glycaemic exposure, a finding confirmed by the A1c-Derived Average Glucose (ADAG) Study [30].
Secondly, factors independent of glycaemia may influence HbA1c levels to different extents in separate ethnic groups. Although HbA1c and blood glucose levels are highly correlated, glucose exposure only explains a portion of the variability in HbA1c. In the A1c-Derived Average Glucose study, the average estimated glucose obtained from continuous glucose monitoring explained only 53% of the variation in HbA1c [30]. This suggests that HbA1c may be determined by non-glycaemic factors. Glucose-independent factors such as red cell turnover and iron deficiency are known to affect HbA1c concentration. Cohen et al. have demonstrated that variation in mean erythrocyte age, even in haematologically normal individuals, can significantly affect the time available for glycation and therefore the HbA1c concentration [31]. Iron-deficiency anaemia has been associated with higher HbA1c levels, with reductions after iron replacement therapy [32]. A recent meta-analysis of 23 genome-wide association studies identified ten genomic loci associated with HbA1c. Of these, seven were unrelated to glycaemic pathways, and influenced HbA1c via their effects on iron status and red cell indices [33]. These studies highlight the contributions of non-glycaemic factors to HbA1c levels and may potentially explain the observed ethnic differences in HbA1c.
The strengths of this study include a large sample size comprising individuals without a known diagnosis of diabetes mellitus, allowing the results to be extrapolated to the general population. By examining Chinese, Malay and Indian ethnic groups, this study also provides important information for other countries in Asia where these ethnic groups predominate. This is especially relevant as Asia is expected to witness a doubling in the numbers of people with diabetes by 2030 [21]. The ranges of fasting plasma glucose and HbA1c values obtained were wide, which allowed exploration of the relationship between ethnicity, fasting plasma glucose and HbA1c across the normal, impaired glucose and diabetic spectrum of values.
This study also has several limitations. Firstly, we did not capture the full range of glucose variability. Only a single point measurement of fasting plasma glucose was used, whereas the HbA1c represents the average level of glycaemia over several months. Failure to capture the intra-individual variability of fasting plasma glucose may have led us to underestimate the proportion of variance in HbA1c explained by fasting plasma glucose. Nevertheless, only one or two glucose measurements taken over a relatively short time are used to diagnose diabetes and, as such, we believe that our findings remain clinically important. We also failed to fully capture the non-fasting blood glucose. This has an impact on our ability to fully appreciate the relationship between blood glucose and HbA1c between ethnic groups. However, as most guidelines now recommend fasting plasma glucose as the diagnostic test of choice for the diagnosis of diabetes, our findings remain relevant to the diagnosis of diabetes. Thirdly, although our findings suggest that the use of HbA1c for diagnosis will result in reclassification of different numbers of individuals in different ethnic groups, it remains unclear if this reclassification does or does not result in the appropriate identification of persons at higher risk of diabetes-associated complications. As a consequence, we cannot interpret these data as suggesting that ethnic-specific cut-offs are required for the diagnosis of diabetes. Long-term prospective data will be required to draw such a conclusion. Finally, other non-glycaemic factors known to influence HbA1c were not studied. Our study, however, captures the variation that may be observed in the usual clinical setting where such detailed profiling will be rarely undertaken. Given that most clinicians will not have access to information on these parameters for individual patients presenting to them for glycaemic testing, knowledge and awareness that ethnicity can introduce this magnitude of difference in HbA1c levels may be important in decision making.
In summary, we have shown that Malays and Indians have a higher HbA1c than Chinese at the same fasting plasma glucose level. In addition, as fasting plasma glucose increases, HbA1c increases more rapidly in Malays and Indians than in Chinese. The use of HbA1c, in place of fasting plasma glucose, will reclassify different proportions of the population in different ethnic groups. Further studies are required to understand the glycaemic and non-glycaemic effects of ethnicity on HbA1c and diabetes-associated complications, prior to determining whether ethnic specific cut-offs for HbA1c are appropriate for the diagnosis of diabetes mellitus.
Acknowledgments
This work was supported by grants from the National Medical Research Council, Singapore (grant nos 0838/2004, IRG07nov013 and NMRC/0863/2004); the Biomedical Research Council (grant nos 03/1/27/18/216 and 08/1/35/19/550) and the Diabetes Research Fund, National University of Singapore.
Competing interests
Nothing to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Additional characteristics of participants in the study.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than for missing material) should be directed to the corresponding author for the article.
References
- 1.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 2.Gabbay KH, Hasty K, Breslow JL, Ellison RC, Bunn HF, Gallop PM. Glycosylated hemoglobins and long-term blood glucose control in diabetes mellitus. J Clin Endocrinol Metab. 1977;44:859–864. doi: 10.1210/jcem-44-5-859. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33:983–990. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 6.IDF. Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. Brussels: International Diabetes Federation; 2005. [Google Scholar]
- 7.The International Expert Committee. International Expert Committee Report on the role of the A1C Assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;33:S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Abbreviated Report of a WHO Consultation. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 10.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YJ, Gregg EW, Geiss LS, Imperatore G, Williams DE, Zhang X, et al. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the US population: implications for diabetes diagnostic thresholds. Diabetes Care. 2009;32:2027–2032. doi: 10.2337/dc09-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohlfing C, Wiedmeyer HM, Little R, Grotz VL, Tennill A, England J, et al. Biological variation of glycohemoglobin. Clin Chem. 2002;48:1116–1118. [PubMed] [Google Scholar]
- 13.Cohen RM, Smith EP. Frequency of HbA1c discordance in estimating blood glucose control. Curr Opin Clin Nutr Metab Care. 2008;11:512–517. doi: 10.1097/MCO.0b013e32830467bd. [DOI] [PubMed] [Google Scholar]
- 14.Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the diabetes prevention program. Diabetes Care. 2007;30:2453–2457. doi: 10.2337/dc06-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, et al. Glucose-independent, black–white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152:770–777. doi: 10.7326/0003-4819-152-12-201006150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bleyer AJ, Hire D, Russell GB, Xu J, Divers J, Shihabi Z, et al. Ethnic variation in the correlation between random serum glucose concentration and glycated haemoglobin. Diabet Med. 2009;26:128–133. doi: 10.1111/j.1464-5491.2008.02646.x. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen ME, Bjerregaard P, Borch-Johnsen K, Witte D. New diagnostic criteria for diabetes: is the change from glucose to HbA1c possible in all populations? J Clin Endocrinol Metab. 2010;95:E333–E336. doi: 10.1210/jc.2010-0710. [DOI] [PubMed] [Google Scholar]
- 18.Christensen DL, Witte DR, Kaduka L, Jorgensen ME, Borch-Johnsen K, Mohan V, et al. Moving to an A1C-based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care. 2010;33:580–582. doi: 10.2337/dc09-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohan V, Vijayachandrika V, Gokulakrishnan K, Anjana RM, Ganesan A, Weber MB, et al. A1C cut points to define various glucose intolerance groups in Asian Indians. Diabetes Care. 2010;33:515–519. doi: 10.2337/dc09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Pang Z, Gao W, Wang S, Zhang L, Ning F, et al. Performance of an A1C and fasting capillary blood glucose test for screening newly diagnosed diabetes and pre-diabetes defined by an oral glucose tolerance test in Qingdao, China. Diabetes Care. 2010;33:545–550. doi: 10.2337/dc09-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 22.IDF. IDF Diabetes Atlas. 4th edn. Brussels: International Diabetes Federation; 2009. [PubMed] [Google Scholar]
- 23.Ministry of Health. N ational Health Survey 2004 Singapore. Singapore: Ministry of Health; 2004. pp. 5–10. [Google Scholar]
- 24.Hughes K, Yeo PP, Lun KC, Thai AC, Wang KW, Cheah JS. Obesity and body mass indices in Chinese, Malays and Indians in Singapore. Ann Acad Med Singapore. 1990;19:333–338. [PubMed] [Google Scholar]
- 25.Tan CE, Emmanuel SC, Tan BY, Jacob E. Prevalence of diabetes and ethnic differences in cardiovascular risk factors. The 1992 Singapore National Health Survey. Diabetes Care. 1999;22:241–247. doi: 10.2337/diacare.22.2.241. [DOI] [PubMed] [Google Scholar]
- 26.Hughes K, Aw TC, Kuperan P, Choo M. Central obesity, insulin resistance, syndrome X, lipoprotein(a), and cardiovascular risk in Indians, Malays, and Chinese in Singapore. J Epidemiol Community Health. 1997;51:394–399. doi: 10.1136/jech.51.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutter J, Tan BY, Chew SK. Levels of cardiovascular disease risk factors in Singapore following a national intervention programme. Bull World Health Organ. 2001;79:908–915. [PMC free article] [PubMed] [Google Scholar]
- 28.Nang EE, Khoo CM, Tai ES, Lim SC, Tavintharan S, Wong TY, et al. Is there a clear threshold for fasting plasma glucose that differentiates between those with and without neuropathy and chronic kidney disease?: the Singapore Prospective Study Program. Am J Epidemiol. 2009;169:1454–1462. doi: 10.1093/aje/kwp076. [DOI] [PubMed] [Google Scholar]
- 29.Koenig RJ, Peterson CM, Kilo C, Cerami A, Williamson JR. Hemoglobin AIc as an indicator of the degree of glucose intolerance in diabetes. Diabetes. 1976;25:230–232. doi: 10.2337/diab.25.3.230. [DOI] [PubMed] [Google Scholar]
- 30.Borg R, Kuenen JC, Carstensen B, Zheng H, Nathan DM, Heine RJ, et al. Associations between features of glucose exposure and A1C: the A1C-Derived Average Glucose (ADAG) study. Diabetes. 2010;59:1585–1590. doi: 10.2337/db09-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, Ciraolo PJ, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;11:4284–4291. doi: 10.1182/blood-2008-04-154112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coban E, Ozdogan M, Timuragaoglu A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in non-diabetic patients. Acta Haematol. 2004;112:126–128. doi: 10.1159/000079722. [DOI] [PubMed] [Google Scholar]
- 33.Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, et al. Common variants at 10 genomic loci influence hemoglobin A(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59:3229–3239. doi: 10.2337/db10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


