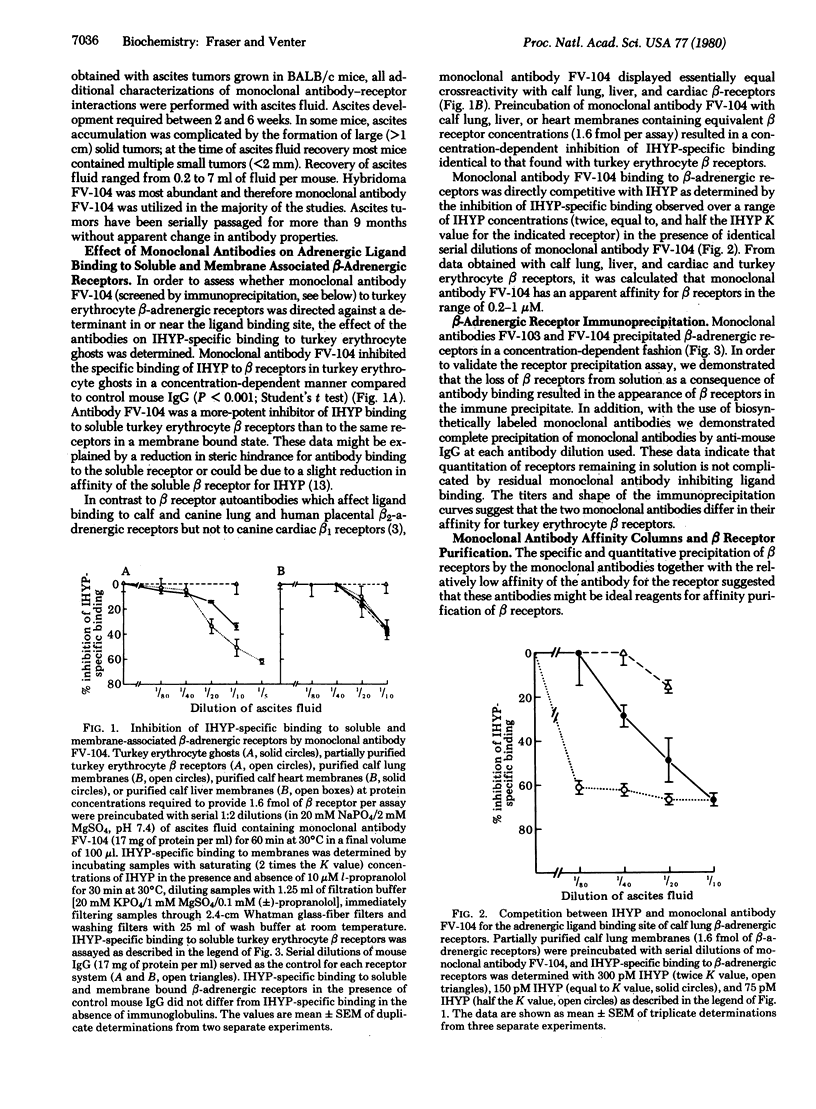
Abstract
We have developed four hybridomas that produce monoclonal antibodies to the turkey erythrocyte beta 1-adrenergic receptor and one hybridoma that produces a monoclonal antibody to the calf lung beta 2 receptor. Splenic lymphocytes from BALB/c mice immunized with partially purified turkey erythrocyte beta 1 receptors or calf lung beta 2 receptors were used with the mouse myeloma line SP2/O-Ag14 to yield hybridoma cultures producing beta receptor monoclonal antibodies of the IgG class. The anti-turkey erythrocyte beta receptor antibodies precipitated partially purified beta receptors and inhibited adrenergic ligand binding. In contrast to autoantibodies to beta 2-adrenergic receptors [Venter, J. C., Fraser, C. M. & Harrison, L. C. (1980) Science 207, 1361-1363] which do not crossreact with cardiac beta 1 receptors, monoclonal antibody FV-104 directed against the adrenergic ligand binding site of turkey erythrocyte beta receptors crossreacted equally with calf liver and lung beta 2 receptors as well as calf heart beta 1 receptors. These data suggest that some molecular homology exists between beta-adrenergic receptors of substantially diverse pharmacological classes. We utilized the monoclonal antibodies in the final stage of turkey erythrocyte beta 1 receptor purification. Turkey erythrocyte beta receptors eluted from FV-104 monoclonal antibody affinity columns with Na-DodSO4 appeared as three components of Mr 70,000, 31,000, and 22,000 on NaDodSO4/polyacrylamide gels. Iodination of material eluted from immunoaffinity columns with propranolol demonstrated the existence of only a single component (Mr, 70,000), indicating that the turkey erythrocyte beta 1 receptor can be purified to homogeneity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Heinrich J., Pilch P. F., Czech M. P. Purification of the adipocyte insulin receptor by immunoaffinity chromatography. J Biol Chem. 1980 Feb 25;255(4):1732–1737. [PubMed] [Google Scholar]
- Jeffery D. R., Charlton R. R., Venter J. C. Reconstitution of turkey erythrocyte beta-adrenergic receptors into human erythrocyte acceptor membranes. Demonstration of guanine nucleotide regulation of agonist affinity. J Biol Chem. 1980 Jun 10;255(11):5015–5018. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lang U., Kahn C. R., Harrison L. C. Subunit structure of the insulin receptor of the human lymphocyte. Biochemistry. 1980 Jan 8;19(1):64–70. doi: 10.1021/bi00542a010. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Galfre G., Secher D. S., Springer T. Monoclonal antibodies and cell surface antigens. Cell Biol Int Rep. 1979 Jan;3(1):1–16. doi: 10.1016/0309-1651(79)90063-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pearson T., Anderson L. Analytical techniques for cell fractions. XXVIII. Dissection of complex antigenic mixtures using monoclonal antibodies and two-dimensional gel electrophoresis. Anal Biochem. 1980 Jan 15;101(2):377–386. doi: 10.1016/0003-2697(80)90203-1. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Strauss W. L., Ghai G., Fraser C. M., Venter J. C. Detergent solubilization of mammalian cardiac and hepatic beta-adrenergic receptors. Arch Biochem Biophys. 1979 Sep;196(2):566–573. doi: 10.1016/0003-9861(79)90309-6. [DOI] [PubMed] [Google Scholar]
- Venter J. C., Fraser C. M., Harrison L. C. Autoantibodies to beta 2-adrenergic receptors: a possible cause of adrenergic hyporesponsiveness in allergic rhinitis and asthma. Science. 1980 Mar 21;207(4437):1361–1363. doi: 10.1126/science.6153472. [DOI] [PubMed] [Google Scholar]



