Abstract
Background
Drug eluting stent (DES) failure including restenosis and stent thrombosis, or disease progression may result in target vessel revascularization (TVR) but the relative contribution of these mechanisms in the DES era is not well described. We sought to examine the predictors and presentations of patients with clinically driven TVR after DES.
Methods
Patients with all lesions treated with a DES in the Dynamic Registry from 2004 to 2006 were analyzed. Included were 2691 patients with 3401 lesions. Patients with and without incident clinically driven TVR at 2 years were compared according to baseline clinical, procedural, and angiographic characteristics and independent predictors of TVR and target lesion revascularization (TLR) were determined by multivariate analysis.
Results
By 2-years, TVR occurred in 7.2% of patients and TLR in 3.8%, with 71.6% and 82.5% of repeat revascularization events occurring in the first year, respectively. The indication for first TVR was myocardial infarction in 18.6 % (n=34), unstable angina in 42.6 % (n=78), stable coronary disease in 25.7% (n=47) and other/unknown 13.1% (n=24). Disease progression was responsible for 47% of TVR. Among patients with TLR, restenosis was the mechanism in 86.6% and stent thrombosis in 13.4%. Independent predictors of TVR included younger age, diabetes, attempted graft lesion, lesion length >30mm and prior lesion intervention. Independent predictors of TVR and TLR were similar.
Conclusion
The incidence of clinically driven TVR is low in patients treated with DES and nearly half is attributable to disease progression, which along with the low rate of in-stent restenosis explains why the mode of presentation is often an acute coronary syndrome.
Keywords: Predictors, revascularization, restenosis, stent thrombosis
The effectiveness of drug-eluting stents (DES) in reducing in-stent restenosis and target lesion revascularization (TLR) has been demonstrated in many patient and lesion subsets (1–5). The reduction in restenosis has translated into fewer repeat revascularization procedures (6). Although several factors predict restenosis post DES implantation,(7,8) DES failure may be due to several mechanisms including intimal hyperplasia within the stent, proximal or distal edge stenosis, or stent thrombosis. Disease progression of lesions remote from DES implantation also contributes to target vessel revascularization (TVR). Therefore, evaluating clinical and angiographic predictors of TVR may provide incremental value to assessing risk for restenosis alone and is the purpose of our analysis. Additionally, the clinical presentation of patients who experienced TVR after stenting with DES has not been well characterized and this was an additional aim of our study.
Methods
Study design and patient population
The National Heart, Lung, and Blood Institute-sponsored (NHLBI) Dynamic Registry is a prospective observational study of consecutive patients undergoing percutaneous coronary intervention (PCI) at 23 clinical centers in North America during specified time intervals. The study design has been previously described.(9) This analysis includes patients enrolled from 2004 to 2006 that had all lesions treated with DES. Patients were excluded if procedure or lesion forms from the repeat procedure were missing (n=173) or if the patient had no TVR and was referred for coronary bypass surgery (n=92). In these patients, the reason for TVR could not be determined. In total, 2691 patients with 3401 lesions were analyzed.
Data collection and definitions
Demographic, clinical, angiographic, and procedural data during the index hospitalization and 2-year follow-up events were collected by trained research coordinators who used standardized report forms and were guided by a manual of operations. All information was site determined. Two-year follow-up data was available in 2783 (94.8%) of patients that consented to follow-up. Patients without 2 year follow-up that were alive were censored at the time of their last follow up. During follow-up, coronary angiography was obtained as clinically indicated by symptoms or documentation of myocardial ischemia. Planned staged PCI was not considered a repeat PCI. Lesion specific data was collected for repeat PCI to determine target vessel revascularization. TVR was defined as any repeat PCI in the target vessel. TLR was defined as repeat PCI within the index procedure stent or 5mm edge. Only definite stent thrombosis, defined as angiographically confirmed cases, is reported in this study. All stent thrombosis events were independently adjudicated.
Statistical analysis
The proportion of patients that had a TVR by 2 years according to the presence or absence of each baseline characteristics was determined. All variables were analyzed as categorical using chi- square test and Fisher exact test as appropriate. Two-year cumulative incidence rates of TVR and TLR were estimated by the Kaplan–Meier method and tested by the log-rank statistic. Independent predictors of TVR and TLR were determined by logistic regression analysis. Variables previously shown to be associated with TVR or TLR and those biologically relevant were included in the model. The Hosmer-Lemeshow goodness of fit test was performed and the p-value for the TVR logistic regression model was p=0.27 indicating a good fit. We also performed the logistic regression analysis for predictors of TVR and TLR excluding patient with stent thrombosis and found similar results (data not shown).
Results
Baseline characteristics and TVR rates
Baseline clinical characteristics among patients treated with DES and stratified by the need for subsequent TVR are presented in Table 1. Among patients age 65 and younger, 8.1% went on to require TVR compared to 5.2% of those over age 65 (p=0.003). A repeat procedure for TVR occurred more often in patients with prior history of PCI, prior coronary bypass surgery, or diabetes at baseline. With respect to procedural and angiographic characteristics, significant differences in patients treated for TVR were observed based on the indication for PCI and lesion location (Table 2). In addition, TVR was significantly associated with the treatment of lesions with a stent prior to the index PCI compared to those not previously treated with a stent. Furthermore, TVR was associated with the treatment of an index lesion located in an ostial position compared to non ostial location. Among lesions treated with a paclitaxel-eluting stent (PES), 5.6% experienced TVR and 94.4% did not. For sirolimus-eluting stent (SES) treated lesions, 7.4% experienced TVR and 92.6% did not (p=0.05).
Table 1.
Baseline clinical characteristics among patients treated with drug eluting stent(s) stratified by the need for subsequent target vessel revascularization
| Variable | Total number | No TVR % | TVR % | P value |
|---|---|---|---|---|
| Patients | 2691 | 93.2 | 6.8 | |
| Age over 65 | 0.003 | |||
| No | 1463 | 91.9 | 8.1 | |
| Yes | 1228 | 94.8 | 5.2 | |
| Female | 0.11 | |||
| No | 1805 | 93.7 | 6.3 | |
| Yes | 886 | 92.1 | 7.9 | |
| Prior Percutaneous Procedure(s) | <.0001 | |||
| No | 1805 | 94.5 | 5.5 | |
| Yes | 886 | 79.9 | 20.1 | |
| Prior brachytherapy | <.0001 | |||
| No | 2657 | 93.4 | 6.6 | |
| Yes | 34 | 76.5 | 23.5 | |
| Prior stent | <.0001 | |||
| No | 1944 | 94.4 | 5.6 | |
| Yes | 747 | 90.1 | 9.9 | |
| History of in-stent restenosis | 0.002 | |||
| No | 583 | 91.8 | 8.2 | |
| Yes | 165 | 83.6 | 16.4 | |
| Prior coronary bypass | <.0001 | |||
| No | 2193 | 94.0 | 6.0 | |
| Yes | 498 | 70.0 | 30.0 | |
| Prior myocardial infarction | 0.04 | |||
| No | 1984 | 93.8 | 6.3 | |
| Yes | 635 | 91.3 | 8.7 | |
| Diabetes | <.0001 | |||
| No | 1775 | 94.6 | 5.4 | |
| Yes | 913 | 90.5 | 9.5 | |
| History of congestive heart failure | 0.33 | |||
| No | 2386 | 93.4 | 6.6 | |
| Yes | 255 | 91.8 | 8.2 | |
| Hypertension | 0.06 | |||
| No | 588 | 94.9 | 5.1 | |
| Yes | 2079 | 92.7 | 7.3 | |
| Hypercholesterolemia | 0.03 | |||
| No | 559 | 95.2 | 4.8 | |
| Yes | 2059 | 92.6 | 7.4 | |
| Smoking | 0.0003 | |||
| Current | 626 | 93.9 | 6.1 | |
| Never/Former | 1907 | 85.6 | 14.4 | |
| Cerebrovascular disease | 0.83 | |||
| No | 2491 | 93.2 | 6.8 | |
| Yes | 195 | 92.8 | 7.2 | |
| Chronic kidney disease | 0.13 | |||
| No | 2433 | 93.4 | 6.6 | |
| Yes | 253 | 90.9 | 9.1 | |
| Peripheral vascular disease | 0.08 | |||
| No | 2458 | 93.4 | 6.6 | |
| Yes | 228 | 90.4 | 9.6 | |
| Pulmonary disease | 0.69 | |||
| No | 2459 | 93.1 | 6.9 | |
| Yes | 227 | 93.8 | 6.2 |
TVR- target vessel revascularization
Table 2.
Baseline procedural and angiographic characteristics among patients treated with drug eluting stent(s) stratified by the need for subsequent target vessel revascularization
| Variable | Total number | No TVR % | TVR % | P value |
|---|---|---|---|---|
| Patients | 2691 | 93.2 | 6.8 | |
| Primary reason for PCI | 0.04 | |||
| Acute myocardial infarction | 754 | 95.1 | 4.9 | |
| Unstable angina | 920 | 91.3 | 8.7 | |
| Stable angina | 571 | 92.5 | 7.5 | |
| Asymptomatic CAD | 361 | 94.7 | 5.3 | |
| Number of Lesions Attempted | 0.44 | |||
| 1 | 2095 | 93.6 | 6.4 | |
| 2 | 498 | 92.2 | 7.8 | |
| 3 | 83 | 90.4 | 9.6 | |
| 4 | 14 | 85.7 | 14.3 | |
| 5 | 1 | 100.0 | 0.0 | |
| Ejection fraction | 0.09 | |||
| Less than 45 | 448 | 95.1 | 4.9 | |
| At least 45 | 1551 | 92.8 | 7.2 | |
| Lesions | 3401 | 92.8 | 7.2 | |
| Lesion location | <.0001 | |||
| Right coronary | 1060 | 93.5 | 6.5 | |
| Left main | 43 | 95.3 | 4.7 | |
| Left anterior descending | 1267 | 93.7 | 6.3 | |
| Left circumflex | 813 | 93.2 | 6.8 | |
| Saphenous vein graft | 218 | 82.6 | 17.4 | |
| Lesion previously stented | <.0001 | |||
| No | 3180 | 93.5 | 6.5 | |
| Yes | 216 | 83.3 | 16.7 | |
| Reference vessel size | 0.46 | |||
| Diameter less than 2.5 mm | 132 | 90.2 | 9.8 | |
| Diameter between 2.5 mm and 3 mm | 1926 | 92.9 | 7.1 | |
| Diameter more than 3 mm | 1211 | 93.1 | 6.9 | |
| Total Occlusion | 0.16 | |||
| No | 3093 | 92.6 | 7.4 | |
| Yes | 308 | 94.8 | 5.2 | |
| Calcified | 0.88 | |||
| No | 2347 | 92.8 | 7.2 | |
| Yes | 1000 | 92.7 | 7.3 | |
| Bifurcation | 0.66 | |||
| No | 3054 | 92.9 | 7.1 | |
| Yes | 334 | 92.2 | 7.8 | |
| Ostial lesion | 0.003 | |||
| No | 3124 | 93.2 | 6.8 | |
| Yes | 277 | 88.4 | 11.6 | |
| Paclitaxel-eluting stent | 0.09 | |||
| No | 1802 | 92.6 | 7.4 | |
| Yes | 889 | 94.4 | 5.6 | |
| Sirolimus-eluting stent | 0.05 | |||
| No | 824 | 94.7 | 5.3 | |
| Yes | 1867 | 92.6 | 7.4 |
PCI-percutaneous coronary intervention, TVR- target vessel revascularization
Clinical presentation and mechanism of target vessel revascularization
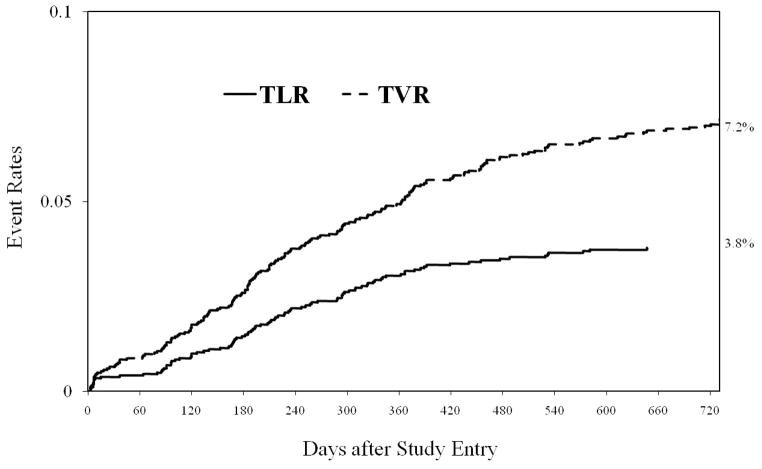
The incidence of clinically driven TVR occurred in 7.2% of patients (n=183) and 7.2% of lesions (n=245) at 2 years, with 71.6% of TVR occurring within the first year. The incidence rate of TLR was 3.8% at 2 years with 82.5% of TLR occurring within the first year. The timing of TVR and TLR is presented in Figure 1.. The indication for first TVR was myocardial infarction in 18.6 % (n=34), unstable angina in 42.6 % (n=78), stable coronary disease in 25.7% (n=47) and arrhythmia/unknown 13.1% (n=24). Disease progression was responsible for 47% of TVR and the remaining 53% was related to the index lesion. Among patients with target lesion revascularization, restenosis and stent thrombosis accounted for 86.6% (n=84) and 13.4% (n=13), respectively. Stent thrombosis occurred early (0–30 days) in 61.5% of cases and late (31 days to 1 year) in 38.5%. No very late (> 1 year) stent thrombosis was observed.
Figure 1.
Kaplan–Meier curves for target vessel revascularization (TVR) and target lesion revascularization (TLR).
Predictors of target vessel and target lesion revascularization
Several independent clinical and angiographic predictors of TVR and TLR were identified (Table 3). Attempted saphenous vein graft lesion was associated with the greatest risk of TVR. Diabetes mellitus, younger age, lesion length >30mm and prior lesion intervention were also predictors of subsequent TVR after DES placement. The predictors of TVR and TLR were similar. Stent type, SES vs. other (PES or PES and SES) and PES vs. other (SES or SES and PES), was not independently associated with TVR or TLR. There was also no independent effect of indication of index PCI.
Table 3.
Adjusted odds ratio model for target vessel and target lesion revascularization
| Target Vessel Revascularization | Target Lesion Revascularization | |||||
|---|---|---|---|---|---|---|
| Event | OR | 95% C.I. | P-value | OR | 95% C.I. | P-value |
| Age | 10.97 | 0.96, 0.98 | <.0001 | 0.97 | 0.95, 0.99 | 0.001 |
| Diabetes mellitus | 1.72 | 1.25, 2.35 | 0.0008 | 2.03 | 1.33, 3.10 | 0.0009 |
| History of ISR | 1.61 | 0.95, 2.73 | 0.08 | 1.47 | 0.74, 2.94 | 0.27 |
| Indication for PCI | ||||||
| Unstable angina | 1.50 | 0.92, 2.44 | 0.11 | 1.87 | 0.92, 3.80 | 0.08 |
| Stable angina | 1.39 | 0.82, 2.38 | 0.22 | 1.72 | 0.80, 3.70 | 0.16 |
| Acute MI | 0.87 | 0.50, 1.50 | 0.61 | 1.02 | 0.46, 2.25 | 0.96 |
| Attempted graft lesion | 2.80 | 1.75, 4.47 | <.0001 | 3.09 | 1.72, 5.54 | 0.0002 |
| Attempted ostial lesion | 1.49 | 0.96, 2.32 | 10.08 | 1.29 | 0.71, 2.34 | 0.41 |
| Long lesion (>30 mm) | 1.54 | 1.02, 2.34 | 0.04 | 1.59 | 0.93, 2.73 | 0.09 |
| Previously lesion PCI | 1.79 | 1.12, 2.87 | 0.02 | 1.86 | 1.00, 3.43 | 0.05 |
| SES (vs. other DES) | 1.08 | 10.43, 2.70 | 0.87 | 0.69 | 0.25, 1.91 | 0.48 |
| PES (vs. other DES) | 0.79 | 0.30, 2.05 | 0.62 | 0.43 | 0.15, 1.27 | 0.13 |
ISR- in-stent restenosis, PCI-percutenous coronary intervention, MI-myocardial infarction, SES- sirolimus eluting stent, PES- paclitaxel eluting stent, DES- drug eluting stent
Discussion
In this study we evaluated the incidence and predictors of TVR and TLR after DES implantation in a large cohort of unselected patients with 2-year follow up. Our study demonstrates a post DES-implantation TVR rate of 7.2% at 2 years with the highest rate in the first year and 53% of revascularizations related to the index lesion. In-stent restenosis was the mechanism for TLR in the majority of patients. Although stent thrombosis in the study population was low at 0.5%, among patients that required TVR after DES, stent thrombosis accounted for 7.1%. We also report on the clinical presentation at the time of TVR. The relatively low rates of in-stent restenosis combined with a high rate of disease progression and proportion of patients with stent thrombosis explain why approximately 60% of patients in our study requiring TVR presented with an acute coronary syndrome.
The DES failure rate we observed is similar to that reported in the United States EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) Registry, which enrolled a similar patient population as the Dynamic Registry. Stolker et al (8) reported an incidence of TLR at 1 year of 4.2% and a slightly lower rate of 3.6% when mechanical complications and stent thrombosis events were excluded.
Very little has been written on the clinical presentation of patients requiring repeat revascularization in the DES era. In the BMS era, a single center study showed that of the in-stent restenosis episodes, 9.5% presented as acute MI (7.3% as non-ST-segment elevation MI and 2.2% as ST-segment elevation MI), 26.4% as unstable angina and 64.1% as exertional angina (10). Compared to bare metal stents, patients treated with DES have a 43% to 56% lower risk of TVR within one-year depending on the complexity of the index PCI (6,11). Therefore, the clinical presentation of DES patients with TVR may differ from bare metal stent patients. A small study described the clinical presentation of 39 patients with DES in-stent restenosis and found a mean time to clinical detection of 13 ± 10 months. The indication for angiography was stable angina in 77%, unstable angina in 5%, myocardial infarction in 10% and silent ischemia in 8%. Our study adds to the literature by reporting on a larger population of patients and including several mechanisms for TVR including non-TLR events.
We observed a high rate of TVR due to disease progression. In low risk patients included in randomized trials of DES and bare metal stents, late non-TVR events occurred in 25% of patients.(12) In another study involving unselected patients undergoing complete revascularization that had both clinical and stress myocardial perfusion imaging follow-up, remote MI and repeat revascularization accounted for 37% of events and 23% of patients without clinical events had new abnormal perfusion.(13) Our rate may be higher due to the presence of untreated moderate lesions at the time of PCI, but this was not evaluated in the present study. A prior study, however, found a non-target PCI rate of 5.8% at one year and that patients with multivessel disease and moderate stenosis during the index PCI were more likely to require non-target lesion PCI during one-year follow-up.(14) These results emphasize the need for aggressive secondary prevention and monitoring for disease progression.
With respect to predictors of TVR and TLR, we found that the strongest independent predictors included attempted graft lesion, diabetes, and prior lesion intervention. Additional factors included younger age and lesion length >30mm. In the EVENT registry, 6 predictors of DES TLR were identified including age less than 60, prior PCI, unprotected left main PCI, saphenous vein graft PCI, stent diameter less than or equal to 2.5 mm and stent length greater than or equal to 40 mm. Several of these overlap with our findings, however, individual factors likely vary between studies due to difference in the complexity of the patients and lesions treated (15). For example, only 1% of patients in our registry had unprotected left main PCI therefore we were underpowered to assess the impact of this variable on TVR. The overlap in predictive factors in the two registries does suggest the importance of these factors for both TLR and TVR. This is understandable as many of the variables identified for TLR or restenosis also predict stent thrombosis or disease progression including diabetes mellitus and saphenous vein graft PCI (1,16,17). In addition, several predictors of TLR and/or TVR in DES are similar to those reported with BMS including long lesion length, diabetes mellitus, prior PCI, and small minimal lumen diameter (18–20).
In our study population, DES type was not an independent predictor of TVR. This is discordant from a prior study that found that SES was associated with a lower risk of TLR than PES.(7) The difference however may be related to the fact that the median reference vessel size in our study was relatively large at 3.0 mm and DES type is more important in small vessels (21). Also, no routine angiographic follow-up was obtained in our study. In a large registry study with clinically driven follow-up, no difference in target vessel revascularization was observed between SES and PES treated patients; consistent with our findings.(22)
The limitations of our study include a lack of data regarding the clinical presentation at the time of TVR in all patients and use of first generation DES. Additionally, the exclusion of patients that had revascularization with CABG and those in whom data regarding TVR was missing may have affected the results. The influence of DES type on risk of TVR is limited by lack of randomization to SES or PES. In addition, angiographic characteristics were site determined and certain risk factors for stent thrombosis and disease progression are unknown such as compliance and duration of dual antiplatelet therapy and statin therapy. Our findings do, however, represent a large cohort of unselected patients in the DES era with clinically driven TVR.
In conclusion, DES TVR as reported by our study is reasonably low at 2-year follow up. Nonetheless, given the expansive use of DES in clinical practice, the absolute magnitude of DES TVR is not insignificant. The finding that nearly half of TVR is non-TLR suggests that careful attention to modifying risk for disease progression and the need for revascularization is warranted. Our study shows that a considerable proportion of patients will present with an acute coronary syndrome and that the predictors of DES TVR overlap with BMS. It is necessary to gain a complete understanding of the full spectrum of causative processes leading to TVR. This will guide both new developments in clinical practice as well as DES designs with potential for further reduction in DES TVR.
Acknowledgments
Funding Source: The Dynamic Registry is funded by grant HL-33292 from the National Heart, Lung and Blood Institutes of Health, Bethesda, MD.
Footnotes
The authors report no financial relationships or conflicts of interest regarding the content herein
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brilakis ES, Lichtenwalter C, Abdel-karim A-rR, de Lemos JA, Obel O, Addo T, Roesle M, Haagen D, Rangan BV, Saeed B, et al. Continued benefit from paclitaxel-eluting compared with bare-metal stent implantation in saphenous vein graft lesions during long-term follow-up of the SOS (Stenting of Saphenous Vein Grafts) trial. JACC: Cardiovasc Interven. 2011;4(2):176–82. doi: 10.1016/j.jcin.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Holmes DR, Jr, Teirstein P, Satler L, Sketch M, O’Malley J, Popma JJ, Kuntz RE, Fitzgerald PJ, Wang H, Caramanica E, et al. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. Jama. 2006;295(11):1264–73. doi: 10.1001/jama.295.11.1264. [DOI] [PubMed] [Google Scholar]
- 3.Sabate M, Jimenez-Quevedo P, Angiolillo DJ, Gomez-Hospital JA, Alfonso F, Hernandez-Antolin R, Goicolea J, Banuelos C, Escaned J, Moreno R, et al. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trial. Circulation. 2005;112(14):2175–83. doi: 10.1161/CIRCULATIONAHA.105.562421. [DOI] [PubMed] [Google Scholar]
- 4.Schofer J, Schluter M, Gershlick AH, Wijns W, Garcia E, Schampaert E, Breithardt G, Investigators ES. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS) Lancet. 2003;362(9390):1093–9. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- 5.Suttorp MJ, Laarman GJ, Rahel BM, Kelder JC, Bosschaert MA, Kiemeneij F, Ten Berg JM, Bal ET, Rensing BJ, Eefting FD, et al. Primary Stenting of Totally Occluded Native Coronary Arteries II (PRISON II): a randomized comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions. Circulation. 2006;114(9):921–8. doi: 10.1161/CIRCULATIONAHA.106.613588. [DOI] [PubMed] [Google Scholar]
- 6.Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, Hannan E, Harjai K, Jensen LO, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119(25):3198–206. doi: 10.1161/CIRCULATIONAHA.108.826479. [DOI] [PubMed] [Google Scholar]
- 7.Kastrati A, Dibra A, Mehilli J, Mayer S, Pinieck S, Pache J, Dirschinger J, Schomig A. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation. 2006;113(19):2293–300. doi: 10.1161/CIRCULATIONAHA.105.601823. [DOI] [PubMed] [Google Scholar]
- 8.Stolker JM, Kennedy KF, Lindsey JB, Marso SP, Pencina MJ, Cutlip DE, Mauri L, Kleiman NS, Cohen DJ, Investigators E. Predicting restenosis of drug-eluting stents placed in real-world clinical practice: derivation and validation of a risk model from the EVENT registry. Circ Cardiovasc Interven. 2010;3(4):327–34. doi: 10.1161/CIRCINTERVENTIONS.110.946939. [DOI] [PubMed] [Google Scholar]
- 9.Williams DO, Holubkov R, Yeh W, Bourassa MG, Al-Bassam M, Block PC, Coady P, Cohen H, Cowley M, Dorros G, et al. Percutaneous coronary intervention in the current era compared with 1985–1986: the National Heart, Lung, and Blood Institute Registries. Circulation. 2000;102(24):2945–51. doi: 10.1161/01.cir.102.24.2945. [DOI] [PubMed] [Google Scholar]
- 10.Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006;151(6):1260–4. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Abbott JD, Voss MR, Nakamura M, Cohen HA, Selzer F, Kip KE, Vlachos HA, Wilensky RL, Williams DO, et al. National Heart L. Unrestricted use of drug-eluting stents compared with bare-metal stents in routine clinical practice: findings from the National Heart, Lung, and Blood Institute Dynamic Registry. J Am Coll Cardiol. 2007;50(21):2029–36. doi: 10.1016/j.jacc.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 12.Chacko R, Mulhearn M, Novack V, Novack L, Mauri L, Cohen SA, Moses J, Leon MB, Cutlip DE. Impact of target lesion and nontarget lesion cardiac events on 5-year clinical outcomes after sirolimus-eluting or bare-metal stenting. JACC: Cardiovasc Interv. 2009;2(6):498–503. doi: 10.1016/j.jcin.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Zellweger MJ, Kaiser C, Jeger R, Brunner-La Rocca H-P, Buser P, Bader F, Mueller-Brand J, Pfisterer M. Coronary artery disease progression late after successful stent implantation. J Am Coll Cardiol. 2012;59(9):793–9. doi: 10.1016/j.jacc.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Glaser R, Selzer F, Faxon DP, Laskey WK, Cohen HA, Slater J, Detre KM, Wilensky RL. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005;111(2):143–9. doi: 10.1161/01.CIR.0000150335.01285.12. [DOI] [PubMed] [Google Scholar]
- 15.Lemos PA, Hoye A, Goedhart D, Arampatzis CA, Saia F, van der Giessen WJ, McFadden E, Sianos G, Smits PC, Hofma SH, et al. Clinical, angiographic, and procedural predictors of angiographic restenosis after sirolimus-eluting stent implantation in complex patients: an evaluation from the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) study. Circulation. 2004;109(11):1366–70. doi: 10.1161/01.CIR.0000121358.26097.06. [DOI] [PubMed] [Google Scholar]
- 16.Joyal D, Filion KB, Eisenberg MJ. Effectiveness and safety of drug-eluting stents in vein grafts: a meta-analysis. Am Heart J. 2010;159(2):159–169.e4. doi: 10.1016/j.ahj.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. Jama. 2005;293(17):2126–30. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 18.Singh M, Gersh BJ, McClelland RL, Ho KKL, Willerson JT, Penny WF, Holmes DR., Jr Clinical and angiographic predictors of restenosis after percutaneous coronary intervention: insights from the Prevention of Restenosis With Tranilast and Its Outcomes (PRESTO) trial. Circulation. 2004;109(22):2727–31. doi: 10.1161/01.CIR.0000131898.18849.65. [DOI] [PubMed] [Google Scholar]
- 19.Kastrati A, Schomig A, Elezi S, Schuhlen H, Dirschinger J, Hadamitzky M, Wehinger A, Hausleiter J, Walter H, Neumann FJ. Predictive factors of restenosis after coronary stent placement. J Am Coll Cardiol. 1997;30(6):1428–36. doi: 10.1016/s0735-1097(97)00334-3. [DOI] [PubMed] [Google Scholar]
- 20.Cutlip DE, Chauhan MS, Baim DS, Ho KK, Popma JJ, Carrozza JP, Cohen DJ, Kuntz RE. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol. 2002;40(12):2082–9. doi: 10.1016/s0735-1097(02)02597-4. [DOI] [PubMed] [Google Scholar]
- 21.Mehilli J, Dibra A, Kastrati A, Pache J, Dirschinger J, Schomig A. Intracoronary Drug-Eluting Stenting to Abrogate Restenosis in Small Arteries Study I. Randomized trial of paclitaxel- and sirolimus-eluting stents in small coronary vessels. Eur Heart J. 2006;27(3):260–6. doi: 10.1093/eurheartj/ehi721. [DOI] [PubMed] [Google Scholar]
- 22.Williams DO, Abbott JD, Kip KE, Investigators DE. Outcomes of 6906 patients undergoing percutaneous coronary intervention in the era of drug-eluting stents: report of the DEScover Registry. Circulation. 2006;114(20):2154–62. doi: 10.1161/CIRCULATIONAHA.106.667915. [DOI] [PubMed] [Google Scholar]