Abstract
More than 20,000 burn injury victims suffer from smoke inhalation injury in the United States annually. In an ovine model of acute lung injury, gamma-tocopherol (g-T) had a beneficial effect when nebulized into the airway. We hypothesize that g-T scavenges reactive oxygen and nitrogen species (ROS/RNS) resulting from burn and smoke inhalation injury and that these ROS/RNS activate the arginase pathway, leading to increased collagen deposition and decreased pulmonary function. To test this hypothesis, ewes were operatively prepared for chronic study, then they were randomly divided into groups (n=8): uninjured, injured, or injured with nebulization (g-T (950 mg/g) and alpha-T (40 mg/g) from hours 3–48 after the injury. The injury, under deep anesthesia, consisted of a 20% total body surface burn and 36 breaths of cotton smoke; all animals were euthanized after 3 weeks. Treatment increased lung g-T at three weeks post-g-T nebulization compared to injured sheep (1.75 ± 0.62 nmol/g vs 0.45 ± 0.06, p<0.05). The expression of dimethylarginine dimethylaminohydrolase-2, which degrades asymmetrical dimethylarginine, a nitric oxide synthase inhibitor, significantly increases with g-T treatment compared to injured sheep (p<0.05). Arginase activity (0.15 ± 0.02 µM urea/µg protein vs 0.24 ± 0.009, p<0.05), ornithine aminotransferase (11,720 ± 888 vs 13,170 ± 1775), and collagen deposition (0.62 ± 0.12 µM hydroxyproline/µg protein vs 1.02 ± 0.13, p<0.05) significantly decrease with g-T compared to injured animals without g-T. The decreases in arginase and collagen with g-T are associated with significantly increased diffusion capacity (p<0.05) and decreased lung wet-to-dry ratio (p<0.05). Smoke-induced chronic pulmonary dysfunction is mediated through the ROS/asymmetrical dimethylarginine/arginase pathway and ROS scavengers such as g-T may be a potential therapeutic management of burn patients with inhalation injury.
Keywords: Fibrosis, arginine metabolism, wound healing
INTRODUCTION
The combination of burn and smoke inhalation injury greatly increases morbidity and mortality in victims of conflagration 1, 2. Reactive oxygen (ROS) and nitrogen (RNS) species are key players in the pathogenesis of burn and smoke inhalation injury 3. We have previously reported that short-term storages of antioxidants such as alpha-tocopherol in plasma are severely depleted in burned patients, and that the long-term storages of tocopherol in adipose tissue are nearly halved as early as 3 weeks after burn injury 4, 5. Gamma-tocopherol is a highly effective scavenger of ROS and RNS, while alpha-tocopherol scavenges ROS 6, 7. The continuous antioxidant depletion in burned victims is associated with increased mortality 4.
The increase in ROS and RNS created by the burn and smoke inhalation injury up-regulates the inflammatory response that prolong the radical-mediated damage beyond the damage caused by the initial injury 8. We have recently reported that burn and smoke inhalation injury caused a significant increase in collagen deposition in the ovine model after 2 and 3 weeks, which contributes to decreased pulmonary function and deteriorated airway physiology 9. However, an effective and efficient method of attenuating the excess collagen deposition in the lung is unknown.
Our previous studies have shown that the airway nebulization of gamma-tocopherol had several beneficial effects in the ovine model of acute lung injury after 48 and 96 hours, including increased lung antioxidant concentration, increased pulmonary gas exchange, and attenuated airway obstruction 10. We hypothesize that the nebulization of gamma-tocopherol will attenuate the decline in pulmonary function three weeks following burn and smoke inhalation injury. We tested our hypothesis using the well-established ovine model.
MATERIALS AND METHODS
Animal Care and Use
This study was approved by the Animal Care and Use Committee of the University of Texas Medical Branch and conducted in compliance with the guidelines of the National Institutes of Health and of the American Physiology Society for the care and the use of laboratory animals. The studies were completed at UTMB’s Investigative Intensive Care Unit, which is a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All animals in the three study groups survived throughout the experimental time period and were sacrificed under deep anesthesia after 3 weeks.
Animal Model
The acute, 24–96 hr model of this burn and inhalation injury has previously been described in detail 11. The animals in the current 3-week study were prepared in a similar manner. Briefly, 18 adult Merino ewes (body weight, 30–40 kg) were surgically prepared under isoflurane anesthesia with a right femoral artery catheter (Intracath, 16GA, 24IN, Becton Dickinson Vascular Access, Sandy, UT), a thermodilution catheter (Model 131F7, Edwards Lifesciences LLC, Irvine, CA), a left atrial catheter (0.062 in. ID, 0.125 in. OD; Dow Corning, Midland, MI), and a lymphatic catheter (0.025 in. ID, 0.047 in. OD; Dow Corning, Midland, MI). After a 7-day recovery period, sheep were randomly divided into three groups: sham (noninjured, nontreated; n = 6), 3-week injured animals, and 3-week injured animals treated with vitamin E. Then, 3-week injured and vitamin E-treated animals were anesthetized with isoflurane and given flame burn (20% total body surface area (TBSA), third degree) and inhalation injury (36 breaths of cotton smoke, <40°C). The injury was intentionally smaller than the injury used previously by our lab in order to have 100% survival during the study. Sham animals were anesthetized with isoflurane anesthesia and treated in the same manner as the injured sheep but were insufflated with air rather than smoke and were not given a burn injury. After the burn and smoke inhalation injury or the sham procedure, all sheep were awakened and placed on a ventilator with positive end expiratory pressure set to 5 cm H2O and tidal volume maintained at 15 mL/kg. A large tidal volume is required for sheep because their ratio of lung dead space to tidal volume (Vd/Vt) is 0.6 compared to only 0.3 for humans 12. The sheep were ventilated with 100% oxygen for the first 3 hrs after injury for rapid clearance of CO to reduce their carboxyhemoglobin. Following this procedure the fraction of inspired oxygen (FiO2) was adjusted according to blood gas analysis to maintain PaO2 above 80 mm Hg. Respiratory rate was initially set at 30 breaths/minute and thereafter adjusted to keep PaCO2 between 25–35 mm Hg. All sheep were resuscitated with Ringer’s solution using the formula 4 mL/kg/% burned body surface for 24 hrs. The experiment continued for 3 weeks to study long-term collagen deposition and to allow time for the burn wound to heal.
Early Excision and Skin Autografting
It is standard clinical practice to surgically remove the dead burned skin (eschar) and cover the wound with a meshed autograph obtained from the contralateral side. The protocol has been previously described in detail 9. Briefly, at 24 hrs post-injury early excision was performed to the muscular fascia to the burned skin under isoflurane anesthesia. The skin was autografted using split-thickness skin (20/1000 inch) that was harvested from the flank of the non-burned skin on the contralaterial side using an electric dermatome (Padgett Electro-Dermatome, Padgett Instruments Inc., KC, USA) and meshed 4:1 using a mesh dermatome (Padgett Mesh-Dermatome, Padgett Instruments Inc., KC, USA). The wound was covered using non-adhering dressing (ADAPTIC, Johnson & Johnson, Skipton, UK) in the graft area and non-adhesive hydrocellular polyurethane dressing (ALLEVYN, Smith & Nephew Medical Limited, Hull, UK) in the donor site. The tie-over dressing was attached using rubber bands and removed 4–6 days after the operation. Operating time was limited to 2 hrs, and ambient temperature was maintained at 30°C to prevent hypothermia. The sham group was exposed to anesthesia with isoflourane for 100 minutes in the operation room for comparison.
Gamma-Tocopherol Nebulization
Sheep in the vitamin E treatment group were nebulized with gamma-tocopherol using a technique that was developed in our laboratory 10. Briefly, the technique uses a novel viscous liquid nebulization nozzle and control that was adapted to a Siemans Servo 300 Ventilator. A solution of 33.3% gamma-tocopherol (d-Gamma Tocopherol 90, Lot #7801, Tama Biochemical, Japan), which contained 950 mg/g of g-T and 40 mg/g of a-T, was mixed (w/w) with 66.6% anhydrous ethanol (95%, Pharmco-AAPER, Louisville, KY). In the nebulization control group, a solution of 33.3% sterile water and 66.6% ethanol (w/w) was substituted for the tocopherol mixture. The solutions were delivered into the 0.203 mm fluid channel nebulizing nozzle with a syringe pump at a rate of 0.071 mL/ hour for 45 hours (3.2 mL total), delivering about 1 gram of tocopherol over the treatment period. The animals were treated with gamma-tocopherol nebulization or water/ethanol 3 hours after the burn and smoke inhalation injury up to 48 hours after the injury. The investigators were masked as to the treatment during the study period. Labels were identified after the termination of the experiment.
Determination of Alpha- and Gamma-Tocopherol
The protocol to determine malondialdehyde, in addition to gamma- and alpha-tocopherol in lung tissue homogenate has previously been described in detail 13. The middle lobe of the right lung was removed at the time of necropsy for measurement of α- and γ-tocopherol concentrations by HPLC. All results were expressed as nmol/g.
Lipid Peroxidation Product
Lung tissue malondialdehyde was measured by a modified version Lykkesfeldt’s method 13. Briefly, lung tissue (~50 mg) was added to 1.5 mL KCl (1%, w:v) and homogenized. A 0.5 mL aliquot of the homogenate was diluted with an equal volume of water, 0.1 mL BHT (butylated hydroxytoluene, 0.06% w:v), and 0.2 mL SDS (8% w:v). To this mixture, 3 mL TBA reagent (thiobarbituric acid (8 g/L) diluted 1:1 with 200 mL/L acetic acid and adjusted to pH 3.5 with NaOH) was added and the reaction mixture was heated at 95°C for 60 minutes. The MDA(TBA)2 adduct was then extracted with 3 mL of n-butanol. Following vortexing and centrifugation, an aliquot of the supernatant was dried under nitrogen, resuspended in methanol and 50 mL injected onto a Shimadzu HPLC reverse phase system including an autosampler and a Beckman 5µ ODS, 4.6×250 mm column. The MDA(TBA)2 adduct was eluted using an isocratic mobile phase consisting of 50% methanol and 50% 25 mM phosphate buffer at pH 6.5 at a flow rate of 1.5 mL/minute. Malondialdehyde was detected by fluorescence at excitation 532 nm and emission 533 nm. Quantitation was done using an external standard of 1, 1, 3, 3-tetraethoxypropane (Sigma, St Louis, MO, USA) prepared using the same method as the samples.
Western Blot Analysis
Dimethylarginine dimethylaminotransferase-2 (DDAH-2) and ornithine aminotransferase (OAT) protein expression in lung tissue was determined using anti-DDAH-2 (C-19) (sc-26071) and anti-OAT (G-20) (sc-55732) antibodies with donkey anti-goat IgG HRP secondary antibody (R&D Systems, Minneapolis, MN, Catalog No. HAF109), as described previously 14. Blots were completed using 20 µg of protein and were quantified by National Institutes of Health IMAGE J (Image and Processing and Analysis in Java) scanning densitometry, and normalized to total actin (I-19) (sc-1616) expression.
Arginase Activity
The protocol to determine arginase activity in lung tissue homogenate has previously been described in detail 15. It is a colorimetric assay that uses α-isonitrosopropiophenone to assess urea formation. Protein levels for each sample were determined using the Protein Quantification Kit (Dojindo Molecular Technologies, Rockville, MD) according to manufacturer’s instructions. All results are expressed as µMol urea/µg protein.
Lung Hydroxyproline Content
The protocol to determine hydroxyproline content in lung tissue supernatant has previously been described in detail 15. Briefly, lung supernatant was incubated with trichloracetic acid, centrifuged, and resuspended in HCl. The pellets were dried and reconstituted with water. The samples were incubated at room temperature with chloramine-T solution, combined with Ehrlich’s solution at 65°C, and optical density was measured at 550 nm.
Masson Trichrome Collagen Stain
The middle, right lobe of the ovine lungs were fixed with 3% paraformaldehyde, processed, embedded in paraffin blocks, serially sectioned at 4 µM, and mounted on Superfrost Plus Slides (VWR, West Chester, PA;). Sections of lung were stained with the Masson Trichrome Stain Kit (Sigma, Catalog No. HT15) to qualitatively assess the degree of collagen deposition.
Lung Wet-to-Dry Weight Ratio Measurement
The bloodless wet weight-to-dry weight ratio of the lung tissues was determined utilizing a modified technique by Pearce et.al. 16, and has been previously described 17.
Diffusing Capacity (DLCO) Measurement
Our lab developed a novel, non-invasive technique to measure diffusing capacity (DLCO). It is based on an open circuit, non-rebreathing carbon monoxide (CO) uptake technique that was previously developed to measure cardiac output during exercise 18. Briefly, the sheep breathes 8–12 breaths of a gas containing 0.3% C18O, 0.7% C2H2, 9% He, 21% O2, and balance N2. Air flow, tidal volume, and inspiratory and expiratory waveforms of CO and helium are then analyzed. The experimental setup consists of the following: screen type pneumotachograph (Celesco Series 381 Flow Transducer), non-rebreathing valve (Hans-Rudolph, St. Louis, MO), inspired port, and two-way switching valve (can alternate sheep’s inspirate between room air and ventilator). The DLCO is calculated after each measurement using mass balance principles 18. The pneumotachograph is connected to a respiratory mass spectrometer (Perkin-Elmer MGA 1100, MA Tech, St Louis, MO) to analyze gas concentrations, and a computer collects flow and gas concentrations (8 ms/sample).
Wound Healing Area (WHA) Calculations
The Wound Healing Area (WHA) percentage was calculated using the following formula: WHA% = 100 * (Total Graft Area – Raw Surface) / Total Graft Area.
Statistical Analysis
All values are expressed as mean ± SEM using GraphPad Prism Software (GraphPad Software, La Jolla, CA, USA). Results were compared between groups using repeated measures (analysis of variance, ANOVA) and the Newman-Keuls multiple comparison tests. A value of p < 0.05 was accepted as statistically significant.
RESULTS
At 3 w, Lung γ-Tocopherol Concentrations Remained Elevated in Vitamin E-treated relative to Injured Sheep
The vitamin E group had significantly higher gamma-tocopherol levels than the injured groups after 3 weeks, even though the nebulization ended after 48 hours (1.75 ± 0.62 nmol/g vs 0.45 ± 0.06, p<0.05, Figure 1A). Alpha-tocopherol levels were slightly higher in the Vitamin E group compared to injured animals without treatment (7.49 ± 0.81 nmol/g vs 6.40 ± 0.62, Figure 1B). Malondialdehyde, a biomarker of lipid peroxidation and highly mutagenic ROS, was significantly decreased after gamma-tocopherol nebulization (Figure 1C). Thus, vitamin E treatment increased lung tocopherols and decreased lipid peroxidation in injured animals, a phenomenon that was apparent 3 weeks following injury.
Figure 1.
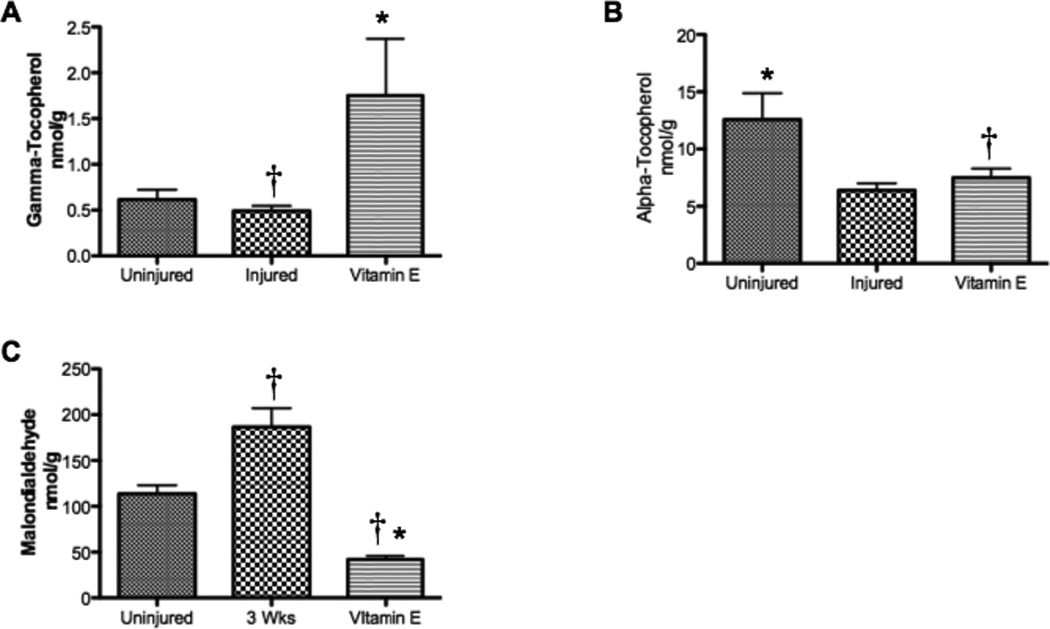
Lung Tocopherol and Malondialdehyde Concentrations after Burn and Smoke Inhalation in Sheep. Injured sheep with or without vitamin E nebulization were compared to uninjured sham animals, but were treated similarly (n=6/group). Lung oxidative stress was evaluated by measuring the concentrations of (A) gamma-tocopherol, an ROS and RNS scavenger, (B) alpha-tocopherol, ROS scavenger, and (C) MDA, a mutagenic ROS. Data are shown as means ± SEM. †p < 0.05 versus uninjured sham animals, *p < 0.05 versus injured animals.
Expression of Dimethylarginine Dimethylaminotransferase-2 Significantly Increases, while ADMA, Arginase Activity and Ornithine Aminotransferase Decrease after Vitamin E Treatment
DDAH-2 protein, an enzyme that catabolizes ADMA, was measured in homogenate of the lungs removed from the animals at necropsy three weeks after injury. DDAH-2, decreased in injured compared to uninjured animals (p < 0.05; Figure 2A). However, in the vitamin E-treated animals DDAH-2 levels were higher than in the uninjured animals (p < 0.05) and ADMA: arginine ratio decreased (Figure 2B). Three weeks after injury, the arginase activity was significantly increased in the injured compared to uninjured animals, but not in the injured and vitamin E treated animals (Figure 2C).
Figure 2.
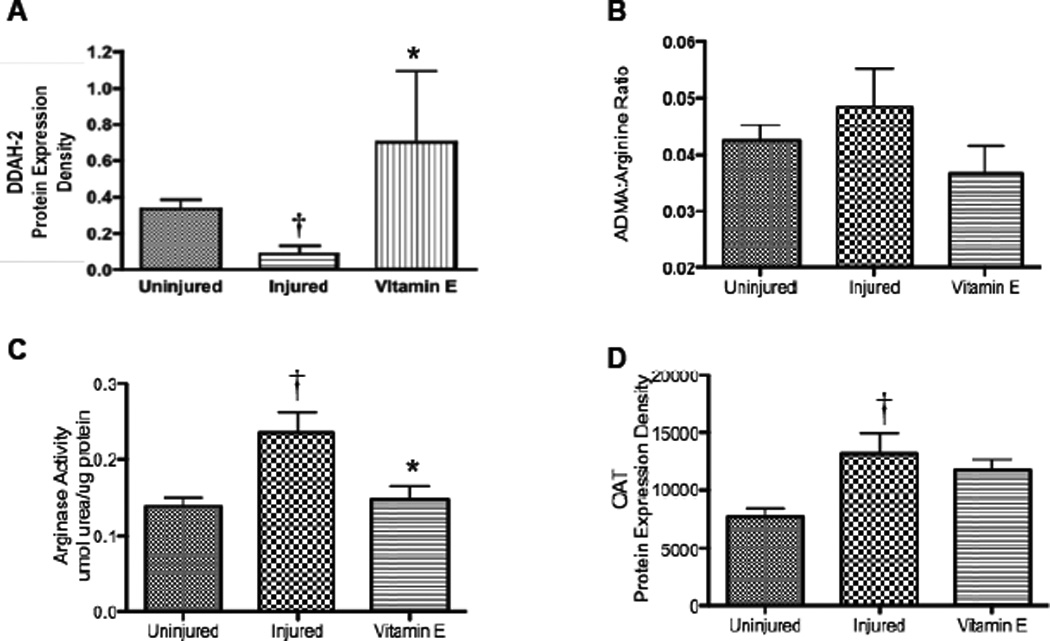
Effect of Burn and Smoke Inhalation in Sheep Treated with and without Vitamin E on Lung DDAH-2 Expression, Arginase Activity, and OAT Expression. Results were compared to uninjured sham animals that were not injured but treated in the same manner. Each group includes 6 animals. Lung homogenate was used to measure (A) DDAH-2 protein expression, (B) ADMA: arginine ratios, (C) arginase activity, and (D) OAT protein expression. Equality of protein loading was confirmed by the expression of B-actin, and each band was quantified by densitometric analysis. Data are shown as means ± SEM. †p < 0.05 versus uninjured sham animals, *p < 0.05 versus injured animals.
The expression of a converting enzyme of ornithine, OAT, was measured by western blot and normalized to B-actin. It showed significant increases after 3 weeks compared to uninjured animals (p < 0.05; Figure 2D). However, the increase was attenuated with the nebulization of Vitamin E (11720 ± 888 vs 13170 ± 1775; Figure 2D). Increased protein expression of ornithine aminotransferase (OAT) was slightly attenuated with the nebulization of Vitamin E to the injured animals (Figure 2D; p < 0.52). OAT is responsible for the conversion of ornithine to proline, which is a precursor for hydroxyproline and collagen deposition in the lung.
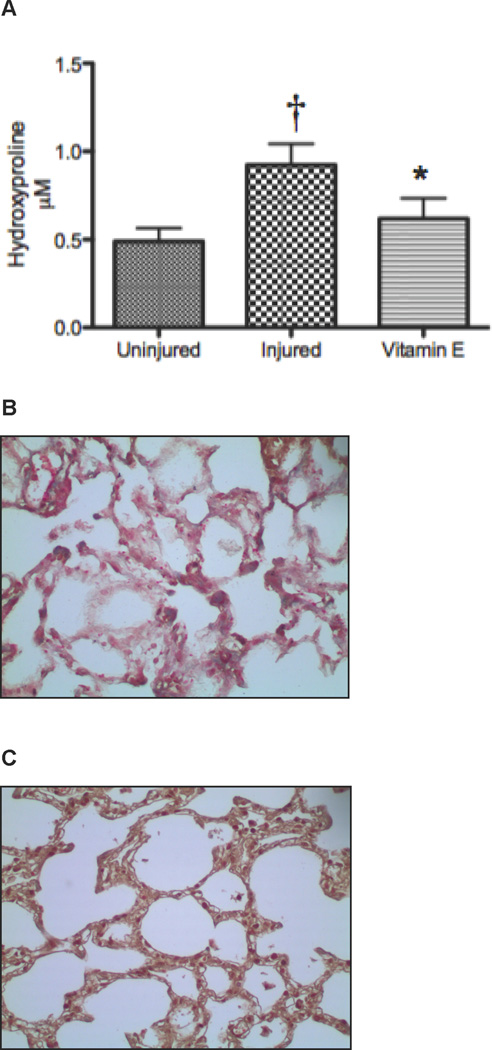
Lung Hydroxyproline and Collagen Decreases after Treatment with Vitamin E compared to Untreated Sheep after Burn and Smoke Inhalation
Lung hydroxyproline contents at 3 weeks were quantified in lung tissue to measure lung collagen deposition. Injured sheep demonstrated increased lung hydroxyproline compared to uninjured sheep, but the increase was attenuated by Vitamin E-treatment (0.62 ± 0.12 µM hydroxyproline/µg protein vs 1.02 ± 0.13, p<0.05, Figure 3A). Direct staining of collagen on lung slices using the Masson Trichrome stain revealed increased deposition of collagen in sheep injured after 3 weeks (Figure 3B) compared to uninjured sheep 9. The intense blue stain representing the deposition of collagen was attenuated by Vitamin E (Figure 3C).
Figure 3.
Lung Hydroxyproline and Collagen Deposition after Burn and Smoke Inhalation in Sheep with and without Vitamin E. Results were compared to uninjured sham animals that were not injured but treated in the same manner. Each group includes 6 animals. (A) The quantitative assessment of collagen used a hydroxyproline colorimetric assay in lung homogenate supernatant. The qualitative assessment of collagen deposition was illustrated by the Masson’s Trichrome stain for collagen deposition on 4-µm paraffin sections. Blue color indicates collagen deposition on representative sections from (B) injured animals (original magnification: 20X), (C) injured animals treated with Vitamin E. Data are shown as means ± SEM. †p < 0.05 versus uninjured sham animals, *p < 0.05 versus injured animals.
Lung Bloodless Wet-to-Dry Ratio Significantly Decreases, while Lung DLCO Significantly Increases with Vitamin E compared to Untreated Sheep
The nebulization of Vitamin E was also responsible for a wide variety of physiological changes in the ovine model after burn and inhalation injury. The bloodless wet-to-dry ratio in the lung, which is an indicator of lung water and edema, significantly increased after the burn and inhalation injury but was significantly decreased with Vitamin E compared to injured animals (p < 0.05; Figure 4A). DLCO was measured in the lung and was significantly decreased after injury compared to uninjured animals (p < 0.05; Figure 4B). DLCO significantly increased compared to injured animals with Vitamin E, but it was still significantly lower than uninjured animals (p < 0.05; Figure 4B). The PaO2:FiO2 ratio in sheep treated and untreated with Vitamin E was also determined (p < 0.05; Figure 5). Results were compared to uninjured sham animals that were not injured but treated in the same manner.
Figure 4.
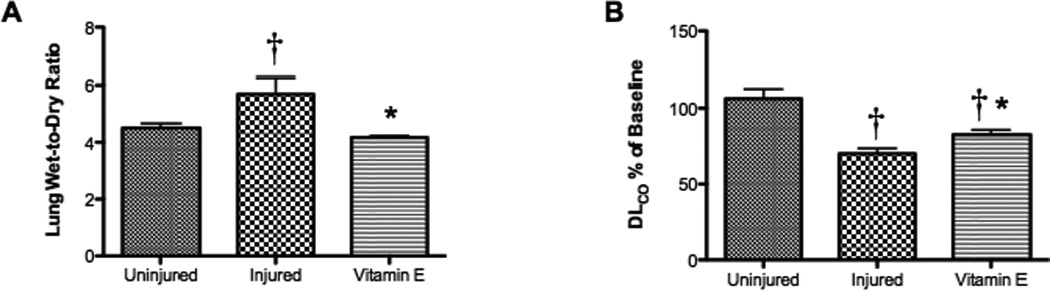
Effect of Burn and Smoke Inhalation in Sheep Treated and Untreated with Vitamin E on Lung Wet-to-Dry Ratio and DLCO. Results were compared to uninjured sham animals that were not injured but treated in the same manner. Each group includes 6 animals. (A) The right lobe of the lung was used to measure the bloodless wet-to-dry ratio, and (B) the DLCO was measured through a carbon monoxide technique. Data are shown as means ± SEM. †p < 0.05 versus uninjured sham animals, *p < 0.05 versus injured animals.
Figure 5.
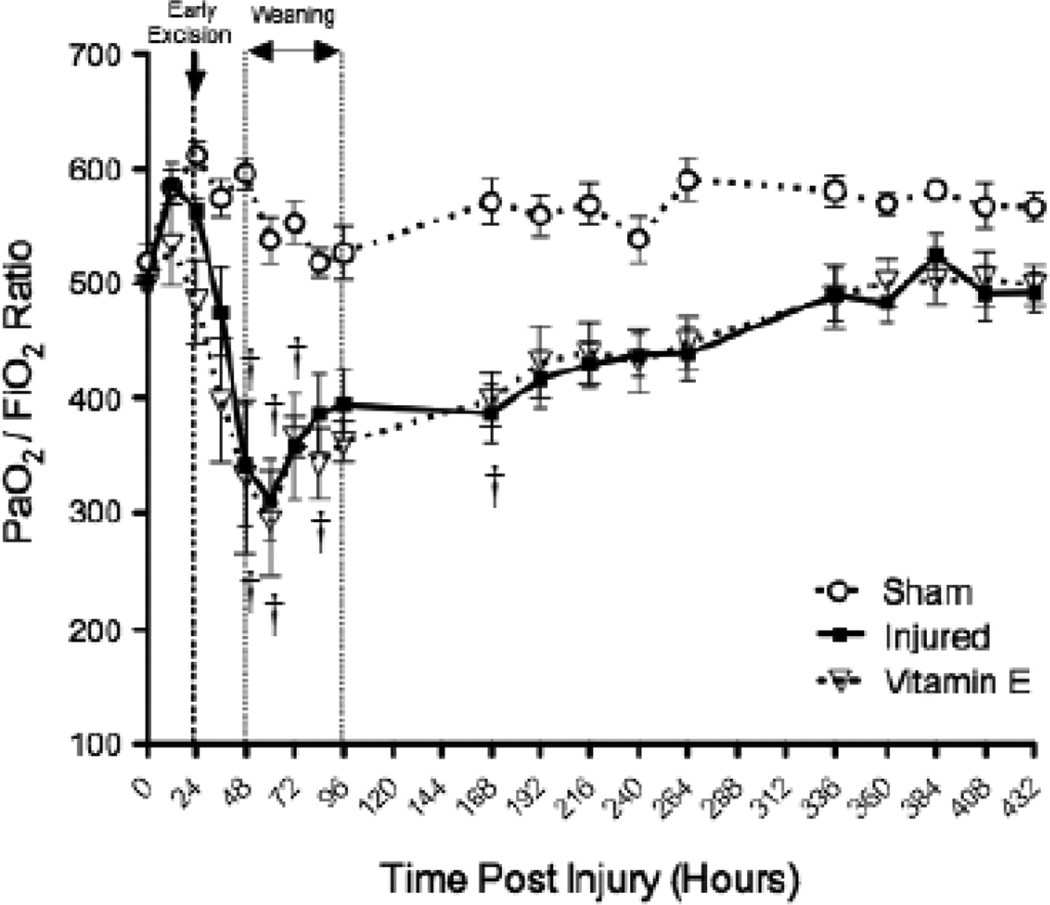
PaO2:FiO2 Ratio in Sheep Treated and Untreated with Vitamin E. Results were compared to uninjured sham animals that were not injured but treated in the same manner. Each group includes 6 animals. †p < 0.05 versus uninjured sham animals.
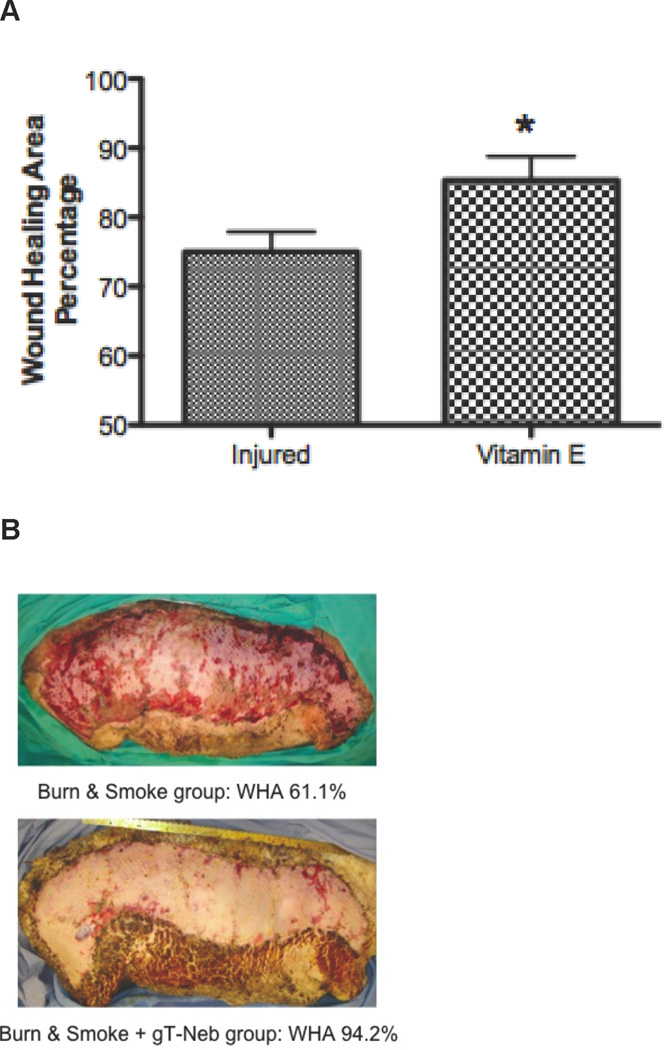
Wound Healing Area (WHA) Significantly Increases with Vitamin E Treatment after Burn and Smoke Inhalation compared to Untreated Sheep
An unexpected result was to see that the surviving graft area, which is an indicator of wound healing, was significantly expanded in the Vitamin E group compared to injured animals (Figure 6). The percentage of the WHA was calculated by equation. Figure 6A shows the quantification of the WHA (p < 0.05), while Figure 6B illustrates the statistical differences of the wounds.
Figure 6.
Wound Healing Area in Sheep after Burn and Smoke Inhalation in with and without Vitamin E. Results were compared to uninjured sham animals that were not injured but treated in the same manner. Each group includes 6 animals. (A) The WHA was determined by calculations, and images of the wound were taken of the (B) injured animals without Vitamin E treatment (Top) and with treatment (Bottom). Data are shown as means ± SEM. *p < 0.05 versus injured animals.
DISCUSSION
We have previously reported that thermally injured patients are deficient in tocopherol 5. We have also previously reported that changes in collagen deposition in our ovine model were the result of oxidation from ROS and RNS 19. ROS and RNS may be scavenged by tocopherol nebulized into the airway. Consequently, we hypothesized that nebulization of tocopherol into the airway would reduce collagen formation in the lung. We have previously nebulized uninjured sheep with flaxseed oil, injured sheep with saline, injured sheep with flaxseed oil, and injured sheep with flaxseed oil and gamma-tocopherol 10. In our current study, we have established a clinically relevant ovine model burn and smoke inhalation after 3 weeks with and without the nebulization of vitamin E. The vitamin E group was nebulized with gamma-tocopherol in ethanol from 3 to 48 hours post-injury.
Nitric oxide synthase converts arginine to n-Ω-hydroxyl arginine (NOHA), which is converted to citrulline and NO. Arginase converts arginine to urea and ornithine, and ornithine is converted to hydroxyproline, which is the building block of collagen 20, 21. NOHA is an inhibitor of arginase. Thus, when NOS is active, arginase activity and therefore collagen formation are inhibited. We have previously reported that NOS is increased following burn and smoke inhalation injury 22. As NOS is increased, there is a corresponding increase in peroxynitrite, which is indexed by 3-nitrotyrosine formation. More recently, we have reported that the increase in NOS activity is short-lived 9 because of the long-term significant increase in asymmetric dimethylarginine (ADMA), which is an analog of arginine and an endogenous inhibitor of all three isoforms of NOS 23, 24. ADMA is normally not active under most conditions because it is rapidly metabolized by dimethylarginine dimethylaminohydrolase-2 (DDAH-2), and the activity of DDAH-2 is highly sensitive to oxidation 25. Consequently, as oxidants such as peroxynitrite are formed following injury, DDAH-2 is inhibited and ADMA increases. As ADMA increases, NOS is inhibited because NOHA is not formed while arginase activity increases, which results in increased collagen deposition and impaired lung function 9.
In the present study, we confirmed these findings and we show that the administration of tocopherol prevented the increase in oxidants. Tocopherol prevented the fall in DDAH, the rise in ADMA, and the subsequent deposition of collagen and lung pathology. Our working model suggests that the combined effects of increased arginase activity and increased oxidative stress result in altered pulmonary structure, and that the scavenging of oxidants can attenuate the injury.
Nitric oxide is an important factor in regulating vascular tone and reducing the interaction of white blood cells and platelets with the endothelium 26. An interesting finding was that an antioxidant such as gamma-tocopherol was associated with an increased production of NO because ADMA did not rise to inhibit NOS. In previous studies, we have reported the beneficial effects of NOS inhibitors in burn and smoke inhalation. Neuronal NOS and inducible NOS inhibitors such as ZK 234238 and BBS-2 have significantly improved pulmonary gas exchange following burn and smoke inhalation injury in the ovine model 27, 28. This may be because they only prevented the formation of peroxynitrite, whereas a decrease in ADMA through vitamin E decreases RNS formation. Despite the association with increased NO in vitamin E-treated animals, the sheep showed an improved pulmonary physiology after 3 weeks.
Previous reports have determined that burn patients were intolerant to exercise. Although some of this intolerance may be related to impaired temperature regulation 29, burn patients have been reported to have hyaline membranes at autopsy. An 8-year follow-up of burn survivors reported high instances of restrictive lung disease that could have been the result of deposition collagen 30. Although we were not able to demonstrate marked changes in PaO2/FiO2 in our ovine model three weeks after injury (Figure 5), the sheep did show deposition of collagen and reduced diffusion capacity. These changes may have been more profound with exercise.
The inhalation injury by smoke or toxic gases alone increased mortality by 20% or more in burned patients 31. The addition of the smoke injury significantly increases the severity of edema and positive fluid balance in burned patients compared to the burn injury alone 32. Future studies will determine the effects of the inhalation injury alone to the burn injury alone. The results may provide insight to the prioritization of therapy for patients that suffer from both burn and smoke inhalation.
In summary, we present evidence that the scavenging of oxidative stress by gamma-tocopherol nebulization increased DDAH-2 expression, decreased arginase activity, hydroxyproline precursors, and hydroxyproline, and attenuated the burn and smoke inhalation injury. It also reduced lung water content and edema, and increased lung diffusion capacity and skin wound healing area. These finding are important because the efficient management of burn and smoke inhalation injury by gamma-tocopherol administration into the lungs immediately post-injury may prevent the long-term collagen deposition in the lung.
ACKNOWLEDGEMENTS
The authors would like to thank the staff of the Investigational Intensive Care Unit at the University of Texas Medical Branch for their valuable assistance, especially C. Moncebaiz, J. Jinkins, T. Walker, and C. Hallum. The authors also thank Y. Larson, H. Ito, S. Asmussen and Y. Zhu for laboratory support.
This work was supported by Grant GM66312 from the National Institute of General Medical Science, and Grants 8541 and 8450 from the Shrine Hospitals for Children (SHC).
DISCLOSURES: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Saffle JR, Davis B, Williams P. Recent outcomes in the treatment of burn injury in the United States: a report from the American Burn Association Patient Registry. J Burn Care Rehabil. 1995 May-Jun;16((3 Pt 1)):219–232. doi: 10.1097/00004630-199505000-00002. discussion 288-219. [DOI] [PubMed] [Google Scholar]
- 2.Barrow RE, Spies M, Barrow LN, Herndon DN. Influence of demographics and inhalation injury on burn mortality in children. Burns. 2004 Feb;30(1):72–77. doi: 10.1016/j.burns.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Traber DL, Hawkins HK, Enkhbaatar P, et al. The role of the bronchial circulation in the acute lung injury resulting from burn and smoke inhalation. Pulm Pharmacol Ther. 2007;20(2):163–166. doi: 10.1016/j.pupt.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TT, Cox CS, Traber DL, et al. Free radical activity and loss of plasma antioxidants, vitamin E, sulfhydryl groups in patients with burns: the 1993 Moyer Award. J Burn Care Rehabil. 1993;14:602–609. doi: 10.1097/00004630-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Traber MG, Leonard SW, Traber DL, et al. alpha-Tocopherol adipose tissue stores are depleted after burn injury in pediatric patients. Am. J. Clin. Nutr. 2010 Dec;92(6):1378–1384. doi: 10.3945/ajcn.2010.30017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kagan VE. Tocopherol stabilizes membrane against phospholipase A, free fatty acids, and lysophospholipids. Ann N.Y. Acad. Sci. 1989;570:121–135. doi: 10.1111/j.1749-6632.1989.tb14913.x. [DOI] [PubMed] [Google Scholar]
- 7.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. Journal of Lipid Research. 1996;37:893–901. [PubMed] [Google Scholar]
- 8.Greenhalgh DG, Saffle JR, Holmes JHt, et al. American Burn Association Consensus Conference to Define Sepsis and Infection in Burns. J Burn Care Res. 2008 Nov-Dec;28(6):776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 9.Sousse LE, Yamamoto Y, Enkhbaatar P, et al. Acute Lung Injury-Induced Collagen Deposition is Associated with Elevated Asymmetric Dimethylarginine and Arginase Activity. Shock. 2010 Oct 7;35(3):282–288. doi: 10.1097/SHK.0b013e3181fddd82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamahata A, Enkhbaatar P, Kraft ER, et al. gamma-Tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury. Free Radic Biol Med. 2008 May 3;45(4):425–433. doi: 10.1016/j.freeradbiomed.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soejima K, Traber LD, Schmalstieg FC, et al. Role of nitric oxide in vascular permeability after combined burns and smoke inhalation injury. Am. J. Respir. Crit. Care Med. 2001;163:745–752. doi: 10.1164/ajrccm.163.3.9912052. [DOI] [PubMed] [Google Scholar]
- 12.Vidal Melo MF, Harris RS, Layfield D, Musch G, Venegas JG. Changes in regional ventilation after autologous blood clot pulmonary embolism. Anesthesiology. 2002 Sep;97(3):671–681. doi: 10.1097/00000542-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Lykkesfeldt J. Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: comparison with ultraviolet-visible spectrophotometry. Clin. Chem. 2001 Sep;47(9):1725–1727. [PubMed] [Google Scholar]
- 14.Gillett AM, Wallace MJ, Gillespie MT, Hooper SB. Increased expansion of the lung stimulates calmodulin 2 expression in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2002 Mar;282((3)):L440–L447. doi: 10.1152/ajplung.00202.2001. [DOI] [PubMed] [Google Scholar]
- 15.Wells SM, Buford MC, Migliaccio CT, Holian A. Elevated Asymmetric Dimethylarginine Alters Lung Function and Induces Collagen Deposition in Mice. Am. J. Respir. Cell Mol. Biol. 2008 Aug 14; doi: 10.1165/rcmb.2008-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce ML, Yamashita J, Beazell J. Measurement of pulmonary edema. Circ Res. 1965;16:482–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- 17.Prien T, Traber LD, Herndon DN, Stothert JC, Jr, Lübbesmeyer HJ, Traber DL. Pulmonary edema with smoke inhalation, undetected by indicator-dilution technique. J Appl Physiol. 1987;63:907–911. doi: 10.1152/jappl.1987.63.3.907. [DOI] [PubMed] [Google Scholar]
- 18.Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol. 2000 May;88(5):1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- 19.Sousse LE, Yamamoto Y, Enkhbaatar P, et al. Acute Lung Injury-Induced Collagen Deposition is Associated with Elevated Asymmetric Dimethylarginine and Arginase Activity. Shock. 2011 Mar;35(3):282–288. doi: 10.1097/SHK.0b013e3181fddd82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moncada S, Palmer RM, Higgs EA. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension. 1988;12:365–372. doi: 10.1161/01.hyp.12.4.365. [DOI] [PubMed] [Google Scholar]
- 21.Murakami K, Enkhbaatar P, Yu YM, et al. L-Arginine Attenuates Acute Lung Injury after Smoke Inhalation and Burn Injury in Sheep. Shock. 2007 Oct;28(4):477–483. doi: 10.1097/shk.0b013e31804a59bd. [DOI] [PubMed] [Google Scholar]
- 22.Soejima K, Schmalstieg FC, Traber LD, Szabo C, Salzman A, Traber DL. Role of nitric oxide in myocardial dysfunction after combined burn and smoke inhalation injury. Burns. 2001;27:809–815. doi: 10.1016/s0305-4179(01)00051-1. [DOI] [PubMed] [Google Scholar]
- 23.Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. Journal of Cardiovascular Pharmacology. 1992;20(Suppl 12):S60–S62. doi: 10.1097/00005344-199204002-00018. [DOI] [PubMed] [Google Scholar]
- 24.Ueda S, Kato S, Matsuoka H, et al. Regulation of cytokine-induced nitric oxide synthesis by asymmetric dimethylarginine: role of dimethylarginine dimethylaminohydrolase. Circ Res. 2003 Feb 7;92(2):226–233. doi: 10.1161/01.res.0000052990.68216.ef. [DOI] [PubMed] [Google Scholar]
- 25.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol. 2004 Jun;24(6):1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 26.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006 Jan;147(Suppl 1):S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enkhbaatar P, Connelly R, Wang J, et al. Inhibition of neuronal nitric oxide synthase in ovine model of acute lung injury*. Crit Care Med. 2009 Nov 28;37(1):208–214. doi: 10.1097/CCM.0b013e318193226a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enkhbaatar P, Murakami K, Shimoda K, et al. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med. 2003 Apr 1;167(7):1021–1026. doi: 10.1164/rccm.200209-1031PP. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RD, Knapp C, Priano LL, Traber DL. Thermoregulatory failure of the burn scar. Journal of Trauma. 1971;11:518–521. doi: 10.1097/00005373-197106000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Mlcak R, Desai MH, Robinson E, Nichols R, Herndon DN. Lung function following thermal injury in children--an 8-year follow up. Burns. 1998;24:213–216. doi: 10.1016/s0305-4179(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 31.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205:82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demling R, Lalonde C, Youn YK, Picard L. Effect of graded increases in smoke inhalation injury on the early systemic response to a body burn. Crit Care Med. 1995 Jan;23(1):171–178. doi: 10.1097/00003246-199501000-00027. [DOI] [PubMed] [Google Scholar]