Abstract
Objective
The purpose of this study is to use updated data and Bayesian methods to evaluate the effectiveness of hyperoxia to reduce surgical site infections (SSIs) and/or mortality in both colorectal and all surgical patients. Because few trials assessed potential harms of hyperoxia, hazards were not included.
Background
Use of hyperoxia to reduce SSIs is controversial. Three recent meta-analyses have had conflicting conclusions.
Methods
A systematic literature search and review were performed. Traditional fixed-effect and random-effects meta-analyses and Bayesian meta-analysis were performed to evaluate SSIs and mortality.
Results
Traditional meta-analysis yielded a relative risk of an SSI with hyperoxia among all surgery patients of 0.84 (95% confidence interval, CI, 0.73–0.97) and 0.84 (95% CI 0.61–1.16) for the fixed-effect and random effects models respectively. The probabilities of any risk reduction in SSIs among all surgery patients were 77%, 81%, and 83% for skeptical, neutral, and enthusiastic priors. Subset analysis of colorectal surgery patients increased the probabilities to 86%, 89%, and 92%. The probabilities of at least a 10% reduction were 57%, 62%, and 68% for all surgical patients and 71%, 75%, and 80% among the colorectal surgery subset.
Conclusions
There is a moderately high probability of a benefit to hyperoxia in reducing SSIs in colorectal surgery patients; however, the magnitude of benefit is relatively small and might not exceed treatment hazards. Further studies should focus on generalizability to other patient populations or on treatment hazards and other outcomes.
Introduction
In addition to appropriate administration of prophylactic antibiotics, other perioperative strategies for preventing surgical site infections (SSIs) have included control of temperature, glucose, and inspired oxygen. Perioperative supplemental oxygen or hyperoxia has been postulated to increase tissue oxygen tension which may lead to an increase in oxidative killing of surgical pathogens and a reduction in SSIs.1–2 In vitro data have suggested that hyperoxia has additional cellular and immunologic effects such as enhancement of intracellular killing by increased production of reactive oxygen species and attenuation of the pro-inflammatory cytokine response.3 However, because of conflicting results from randomized controlled trials (RCTs)4–11 and meta-analyses,12–15 perioperative supplemental oxygen has not been widely recommended or adopted. In fact, a recent RCT evaluating a bundle of interventions in elective colorectal surgery patients, which included hyperoxia, resulted in an increase in SSIs when compared to conventional therapy.16 However, the trial was not designed to evaluate the effect of individual interventions such as hyperoxia alone on SSIs; inclusion of an ineffective intervention and/or presence of an interaction between interventions could have occurred.
There have been at least four published meta-analyses, all of which have had slightly differing conclusions regarding the effectiveness of supplemental oxygen in reducing SSIs. Brar et al concluded that perioperative supplemental oxygen does not reduce SSIs but decreases mortality in colorectal surgery patients.12 Al-Niaimi and Safdar reported that supplemental oxygen reduces SSIs in colorectal surgery patients but cannot be applied more widely to other patient populations due to heterogeneity between studies.13 Chura et al concluded that hyperoxia reduces SSI in colorectal surgery patients and should be considered in other patients undergoing clean-contaminated procedures.15 Qadan et al recommended perioperative supplemental oxygen therapy as a preventive strategy to reduce SSIs without restriction on the patient population.14 The differences in the results of these meta-analyses may be related to the studies included, patients included, and the type of model used (fixed-effect versus random effects). However, despite these differences, the point estimates (expressed as risk ratios, RRs) and 95% confidence intervals (CIs) for the risk ratios for SSIs were very similar between meta-analyses, which suggest differences in the interpretations of the results.
Three meta-analyses used a random effects model to evaluate the relative risk reduction with perioperative oxygen supplementation in colorectal surgery patients.12–13, 15 The point estimates ranged from 0.69 to 0.74, the lower limits of the 95% CI ranged from 0.39–0.43, and the upper limits ranged from 1.10–1.43.12–13, 15 Although none of the results reaches statistical significance as defined by a p-value less than 0.05, none of the confidence intervals exclude the possibility of a clinically significant reduction in SSIs. A p-value greater than 0.05 identified using traditional (frequentist) statistics is often misinterpreted as excluding any effect.17 Use of Bayesian methods to directly assess the probability of benefit may avoid this problem and help clinicians better interpret study findings.17–18
Bayesian methods are already widely accepted in evaluating the results of diagnostic tests and are increasingly being used to design, analyze, and interpret clinical trials.17, 19 Frequentist approaches do not involve consideration of evidence from other relevant studies, leaving this to the reader to do. In contrast Bayesian statistics address the previous evidence as a distribution of hypothesized treatment effects or prior distribution (or prior). Depending on the strength of this evidence, the center of the distribution may be neutral (i.e. centered at no effect), enthusiastic (i.e. shifted towards benefit) or skeptical (i.e. shifted towards harm). Because of the limitations in the quality of the evidence and publication bias or other biases that may exaggerate the apparent benefit of treatment, neutral and skeptical priors are often both used. Using Bayes’ theorem, the prior probability distribution is combined with the data from the study. The result is a distribution of effects or posterior distribution (or posterior) which can be used to estimate the probabilities of any range of effects. Unlike conventional frequentist statistics, Bayesian methods can estimate the likelihood that perioperative oxygen supplementation in surgery patients reduces the risk of SSIs by more than a specific percentage (i.e. 1%, 10% or 25%).17 Diamond and Kaul have devised a system for classifying clinical practice guidelines using Bayesian methods.20
With the publication of additional RCTs including a multi-center trial in laparotomy patients9 and a single center trial in appendectomy patients,11 we have conducted a Bayesian meta-analysis21 to update the estimated treatment effect for SSIs (primary outcome) and mortality (secondary outcome) for all surgical trials and for the subgroup of colorectal surgery. We hypothesized that additional clinical trials are not necessary to demonstrate a reduction in SSIs from perioperative supplemental oxygen therapy.
Methods
Eligibility criteria
Studies were selected if they were randomized trials comparing perioperative supplemental oxygen or hyperoxia to standard oxygen concentrations or normoxia. Only trials that reported SSIs or wound infections as an outcome were included.
Search strategy and study selection
Medline, Embase, and the Cochrane Central Register of Controlled Trials were searched in June 2011. Search terms included “oxygen,” “hyperoxia,” “surgery,” “wound,” “infection,” and “randomized controlled trial.” MeSH terms were also used including “oxygen inhalation therapy, “oxygen/administration and dosage.” “hyperoxia,” and “surgical wound infection/prevention and control.” Hand searches were also performed of abstracts from relevant meetings including the Surgical Infection Society and of the reference lists of pertinent review articles and meta-analyses. Clinical trials registries were also searched (ClinicalTrials.gov). Two authors, trained in literature searching techniques, performed the searches independently (LK and SM).
Data collection
Data were extracted from the RCTs regarding SSIs and mortality by two reviewers (LK and SM).
Assessment of risk of bias in individual studies
All RCTs that met the eligibility criteria were assessed for quality by two reviewers and disagreements were resolved by discussion. The Jadad score, which evaluates randomized trials on how randomization was performed, blinding, and reporting of withdrawals and dropouts, was calculated for each trial (LK and SM). Study quality was assessed in regards to randomization, method of allocation concealment, blinding, and use of intent-to-treat analysis, as described in the Cochrane Handbook (LK and KL).22
Statistical analyses
Pooled estimates of the outcomes (SSIs, mortality) were reported as risk ratios (RRs) with 95% confidence intervals (CIs). Both fixed-effect and random-effects models were performed. When there is heterogeneity between studies, fixed-effect and random-effects models differ in the underlying assumption about the effects estimated by the individual studies as well as in the weighting of the studies.23 Fixed-effect models assume that there is a common treatment effect and that each individual study is providing an estimate of that effect. Random-effects models assume that there is a distribution of effects (i.e. that the treatment effect varies based on patient population or other factors) and that the estimated effects from individual studies represent random samples from that distribution. When there is significant heterogeneity between studies, the confidence intervals will be wider with random-effects models.23 Heterogeneity was assessed using the chi-squared statistic (p<0.1) and the I2 statistic. The latter ranges from 0 to 100%; increasing values indicate higher percentages of total variation across studies due to heterogeneity. Although the I2 statistic is a useful tool in assessing heterogeneity across studies, the decisions of whether or not to perform a meta-analysis or other analyses to explore the heterogeneity remain subjective. Publication bias was assessed using a funnel plot. Statistical analyses were performed using RevMan, Version 5.1.
A Bayesian meta-analysis was performed using a hierarchical model to account for between study heterogeneity (see Supplemental Digital Content: Appendix: Bayesian Meta-Analysis).24 All analyses were conducted in WinBUGS. We report posterior medians, 95% credible intervals for RRs and posterior probabilities of treatment effect.
Results
Results of search strategy
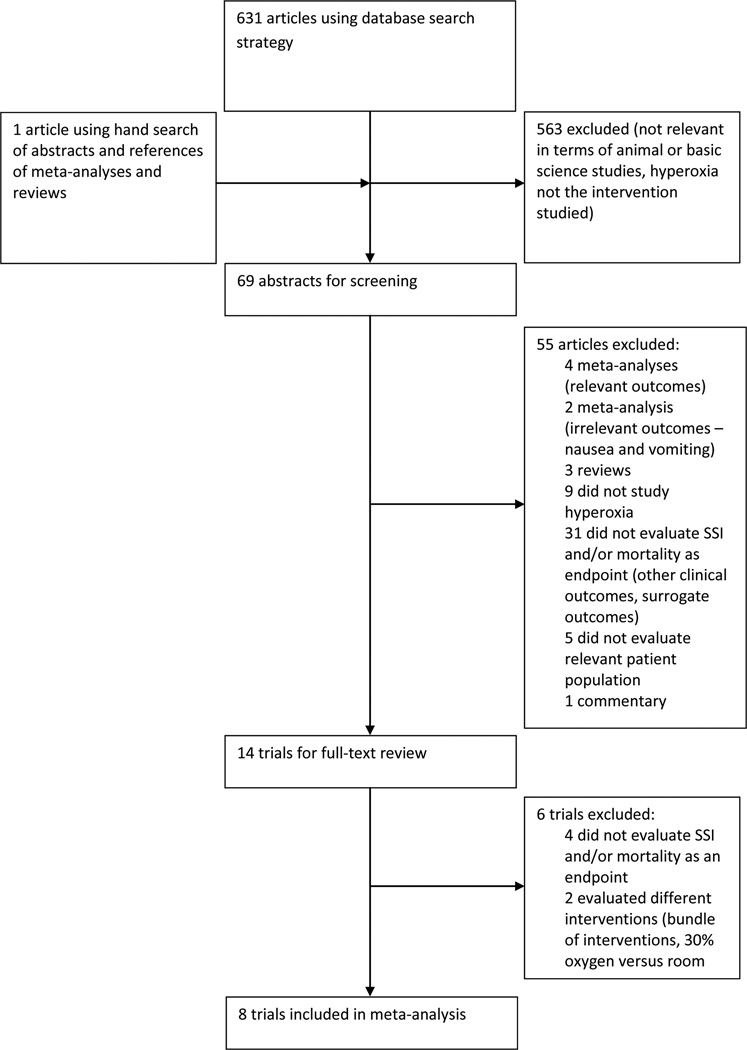
A total of 631 citations were identified in the aforementioned search (Figure 1). Following a manual review of abstracts, a total of eight studies qualified for inclusion in the meta-analysis; the characteristics of each trial are listed in Table 1. These eight studies were randomized controlled trials comparing supplemental perioperative oxygen (FiO2 of 80%) versus standard care (FiO2 of 30 to 35%) on the incidence of SSI. A total of 4778 patients were enrolled in these trials, with 2355 receiving supplemental perioperative oxygen. In 6 of the trials, supplemental oxygen was administered intra-operatively and for two hours postoperatively.4, 6–7, 9–11 The study by Belda continued supplemental oxygen for 6 hours post-operatively.5 The study by Myles et al evaluated SSI as a secondary endpoint in the analysis of 70% nitrous oxide, 30% oxygen versus 80% oxygen, 20% nitrogen given intra-operatively during major surgery lasting at least two hours.8 Patients undergoing abdominal surgery for benign and malignant pathology, on both an acute and elective basis, were included. Three trials included only those patients undergoing elective colorectal surgery,4–5, 7 and three trials recruited patients undergoing any major abdominal surgery.6, 8–9 One trial included only those patients undergoing open appendectomy11 and one trial only evaluated patients undergoing elective Caesarean-section.10 In the studies by Pryor6 and Meyhoff,9 patients were stratified by type of surgery.
Figure 1.
Results of search strategy
Table 1.
Details of included trials.
| Author | Year | Participants | Intervention | Control | Incidence of Surgical Site Infections (intervention vs. control) |
Jadad Score |
|---|---|---|---|---|---|---|
| Greif et al4 | 2000 | Elective colorectal surgery patients | 80% oxygen, 20% nitrogen intra-operatively and for 2 hours post-operatively | 30% oxygen, 70% nitrogen intra-operatively and for 2 hours post-operatively | 5% vs. 11% | 5 |
| Pryor et al6 | 2004 | Patients undergoing major abdominal surgery (general surgery) | 80% oxygen during surgery and 2 hours post-operatively | 35% oxygen during surgery and 2 hours post-operatively | 25% vs. 11% | 5 |
| Belda FJ et al5 | 2005 | Elective colorectal surgery patients | 80% oxygen intra-operatively and for 6 hours post-operatively | 30% oxygen intra-operatively and for 6 hours post-operatively | 15% vs. 24% | 5 |
| Mayzler O et al7 | 2005 | Elective colorectal cancer surgery patients | 80% oxygen, 20% nitrogen intra-operatively and for 2 hours post-operatively | 30% oxygen, 70% nitrogen intra-operatively and for 2 hours post-operatively | 13% vs. 18% | 2 |
| Gardella et al10 | 2008 | Elective C-section patients | 80% oxygen intra-operatively and for 2 hours post-operatively | 30% oxygen intra-operatively and for 2 hours post-operatively | 25% vs. 14% | 5 |
| Myles et al8 | 2007 | Patients undergoing major surgery lasting at least 2 hours | 80% oxygen, 30% nitrogen intra-operatively | 30% oxygen, 70% nitrous oxide intra-operatively | 8% vs. 10% | 5 |
| Meyhoff et al9 | 2009 | Patients undergoing acute or elective laparotomy | 80% oxygen intra-operatively and for 2 hours post-operatively | 30% oxygen intra-operatively and for 2 hours post-operatively | 19% vs. 20% | 5 |
| Bickel et al11 | 2011 | Patients undergoing open appendectomy for acute appendicitis | 80% oxygen intra-operatively and high-flow oxygen (10L/min via nonrebreathing mask) for 2 hours post-operatively | 30% oxygen, 70% nitrogen intra-operatively and oxygen (2L/min via nasal cannula) for 2 hours post-operatively | 6% vs. 14% | 3 |
The criteria for SSI varied between studies, and included predetermined clinical criteria,6–8, 10 Centers for Disease Control criteria,5, 9 positive wound cultures,4 and ASEPSIS (additional treatment, serous discharge, erythema, purulent discharge, separation of deep tissues, isolation of bacteria, and stay in hospital longer than 14 days).11 Post operative follow up ranged between 7 and 30 days; SSI was diagnosed on or before the last day of follow up. In each trial, the wounds were evaluated by a surgical investigator blinded to the treatment allocation. The incidence of SSI in each trial is presented in Table 1.
Ascertainment of bias
Using the Jadad score, two trials7, 11 received a score other than 5, indicating that most trials used an adequate randomization scheme, blinded the patient and outcome assessor(s) adequately, and described dropouts and withdrawals25 (Table 1). All eight trials were stated to be randomized. One trial used a random-number table to generate the allocation sequence,6 and five trials used a computer-generated random number sequence.4–5, 8–10 Two trials assigned treatment arms using sealed envelopes, but neither trial described the exact method of sequence generation for these treatment assignments.7, 11 The methods could have been appropriate randomization techniques but there is not enough information to judge. Six trials performed allocation concealment using sealed envelopes,4–7, 10–11 although only one trial mentioned that the envelopes were opaque.5 Two trials used a telephone or central service to allocate treatment assignment.8–9 Both the sealed envelopes and central randomization service were considered to be adequate methods of allocation concealment. Blinding was evaluated as pertained to the investigators, participants, outcome assessors, and data analysts. All of the studies stated that the outcome assessors were blinded to treatment assignment. Three studies did not specify whether or not the data analysts were blinded.4, 10–11 All but one of the studies described blinding of the investigators and participants.7 All but two of the studies stated that an intention-to-treat analysis was performed;7, 11 one of these two trials included all patients in the analysis but did not explicitly state that there was no loss to follow up or withdrawal of patients.11 Three of the studies using intention-to-treat analysis excluded patients after randomization.5, 8–9
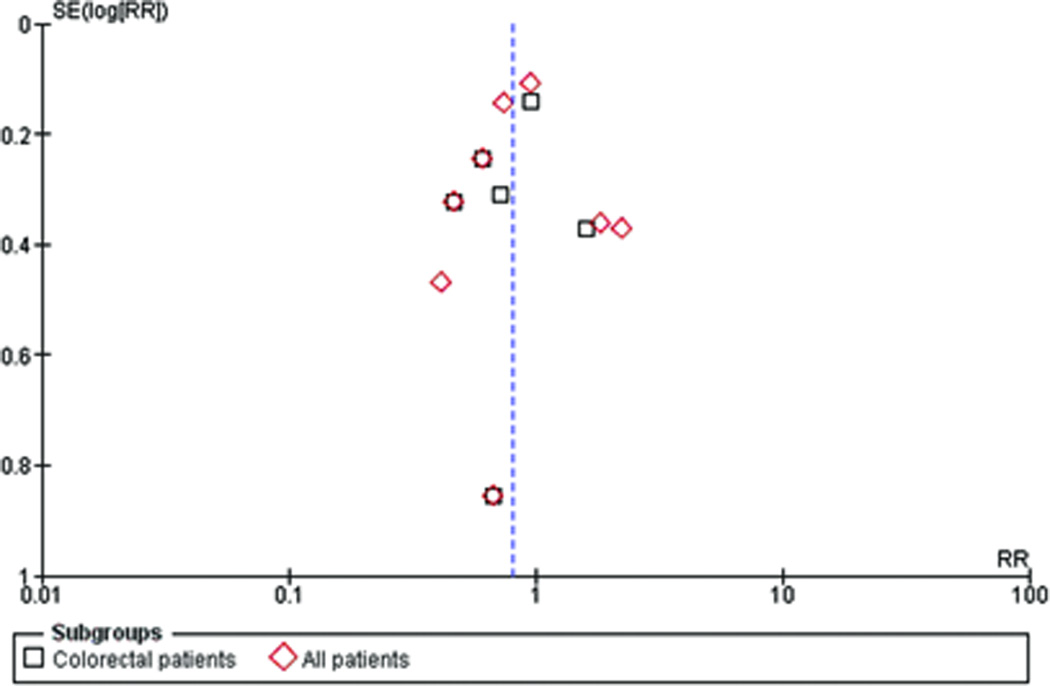
A funnel plot is pictured in Figure 2 for ascertainment of publication bias for the outcome of SSI. The funnel plot is roughly symmetrical and is centered on a relative risk less than 1, favoring hyperoxia. The precision of each trial is similar, with the exception of the Mayzler trial7 which had the lowest sample size and is the bottom point on the funnel plot.
Figure 2.
Funnel plot
Primary outcome – SSIs
Traditional analysis
When all RCTs were combined, supplemental oxygen resulted in a statistically significant reduction in SSIs using a fixed-effect model (RR 0.84, 95% CI 0.73–0.97, p=0.02) (Figure 3a). A random-effects model yielded a similar point estimate but a wider confidence interval (RR 0.84, 95% CI 0.61–1.16, p=0.29) (Figure 3b). When only colorectal patients were included in the meta-analysis, supplemental oxygen resulted in a statistically significant reduction in SSIs using a fixed-effect model (RR 0.80, 95% CI 0.66–0.98, p=0.03) (Figure 3a) while a random-effects model yielded an estimate of RR 0.78 (95% CI 0.57–1.07, p=0.12) (Figure 3b). The similarity of the fixed-effect and random-effects models suggests that there is little impact of small studies on the estimates of treatment effect.23 There was substantial heterogeneity when studies of all patients were included (I2 = 68%), with slightly less heterogeneity when only the subgroup of colorectal patients were used (I2 = 45%). Excluding the Mayzler trial, which had the lowest Jadad score did not significantly change the estimates of treatment effect.
Figure 3.
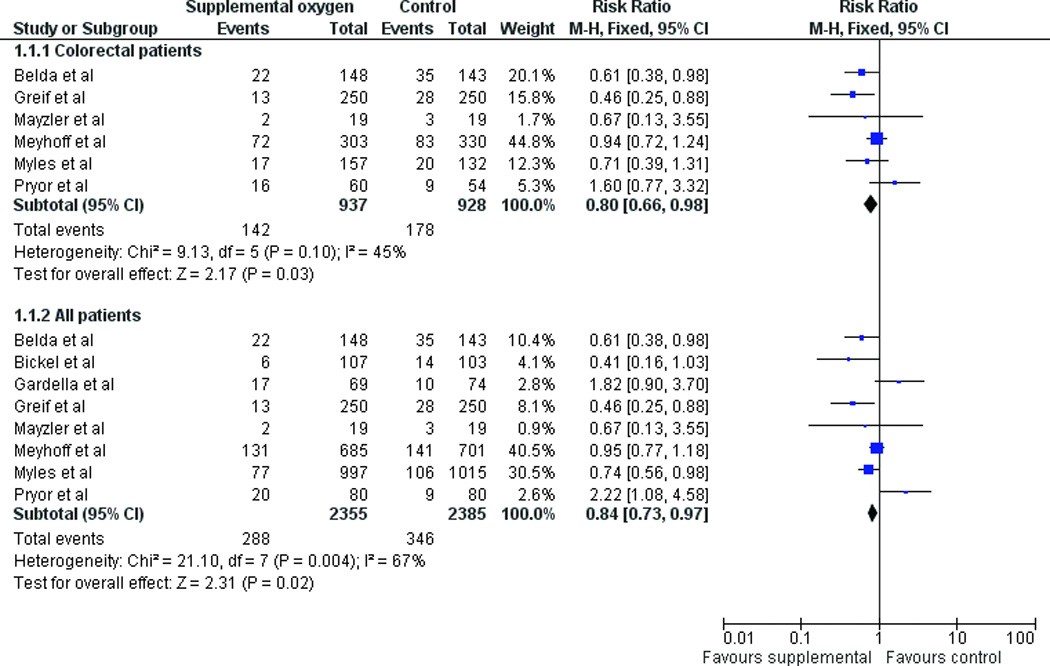
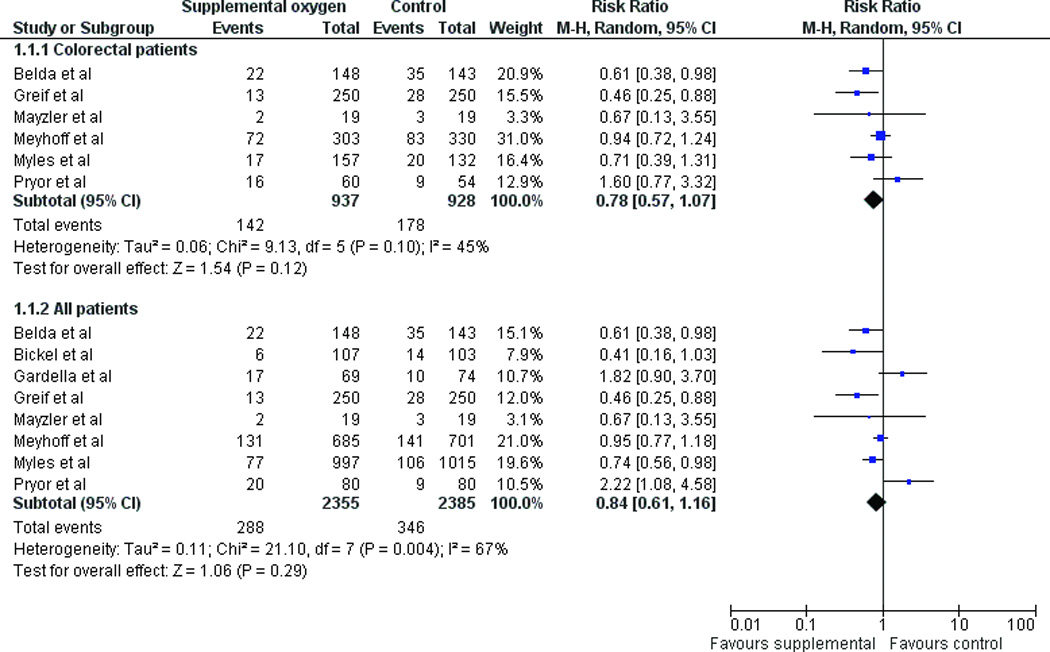
(a) Fixed effect model of hyperoxia vs. normoxia on surgical site infections (SSIs) for colorectal surgery patients only and for all patients (b) Random effects model of hyperoxia vs. normoxia on SSIs.
Bayesian analysis
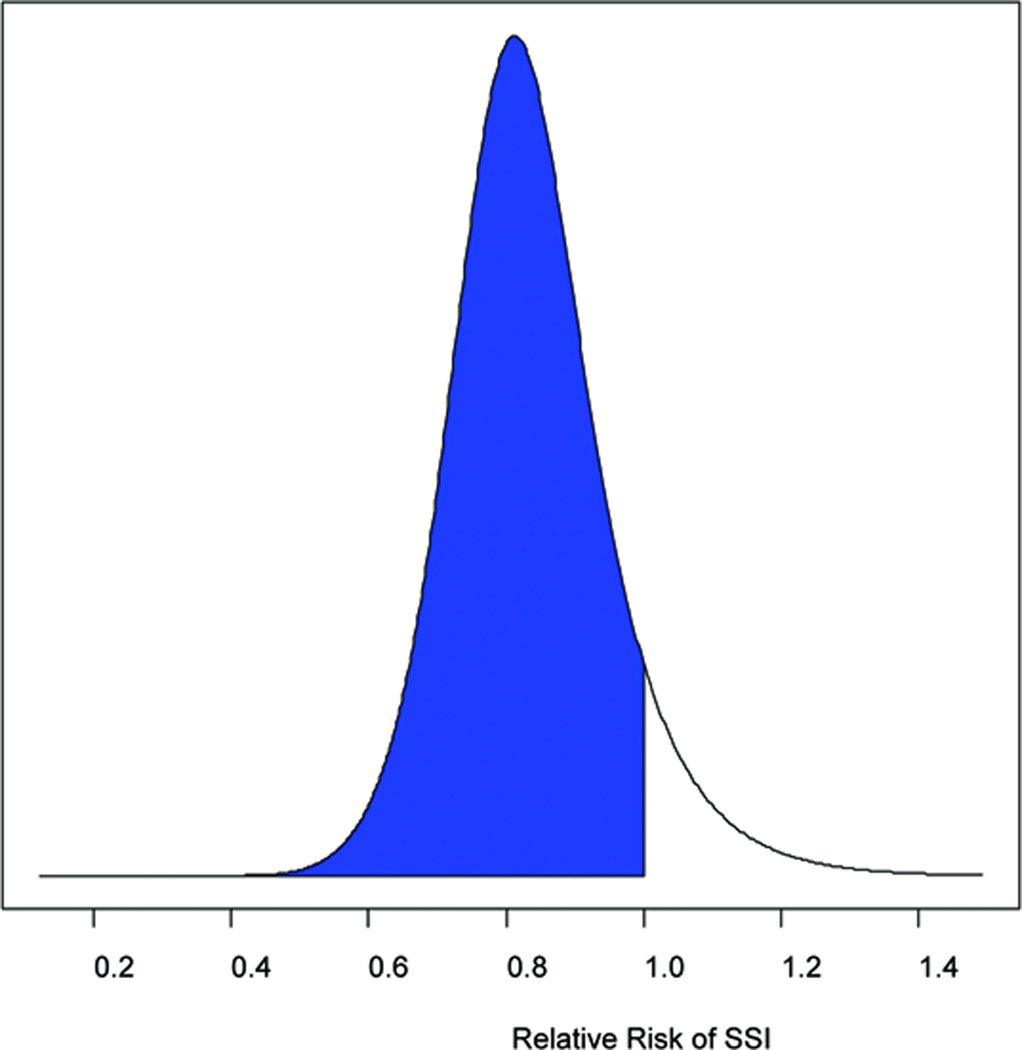
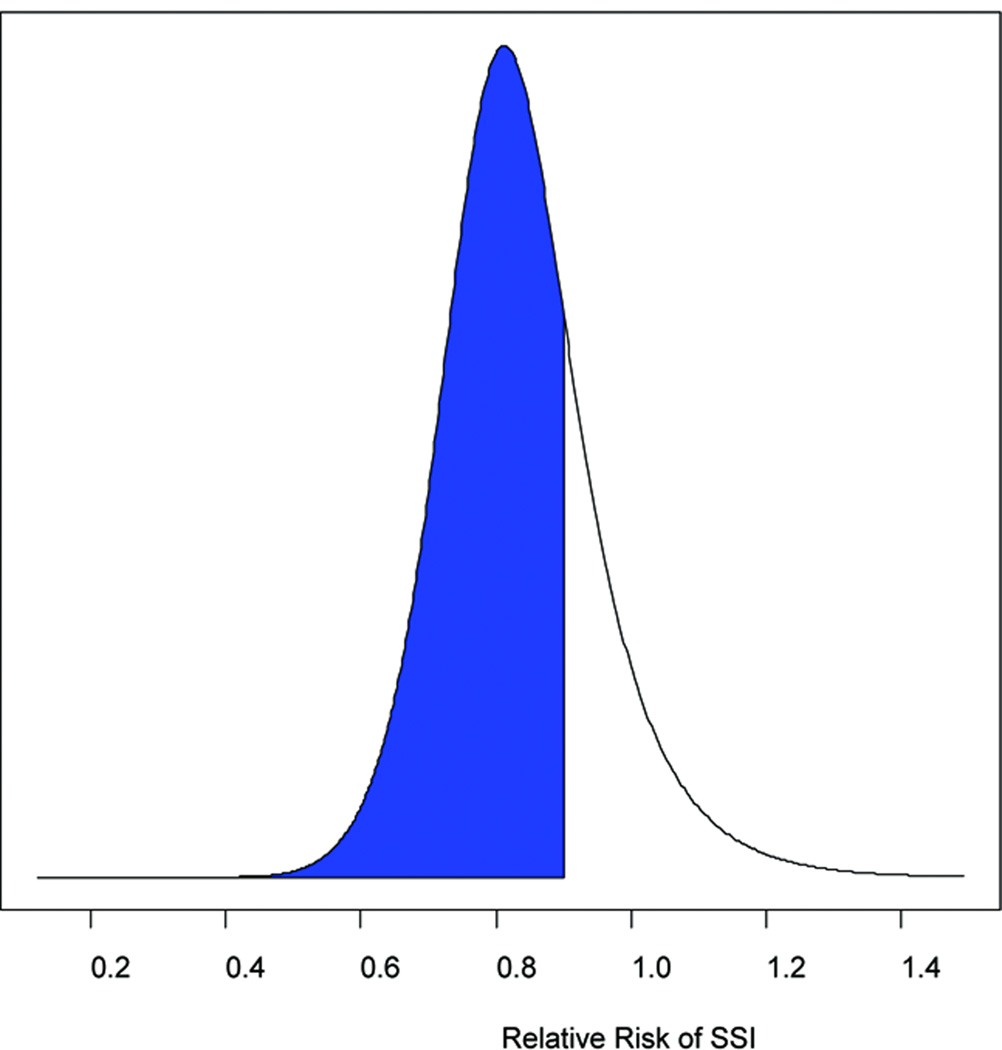
The RR and 95% credible intervals (CrI) were calculated for SSI reduction in all abdominal surgery patients and in colorectal surgery patients only using skeptical, neutral, and enthusiastic priors (Table 2). When all surgery patients were considered, the probability of any reduction in SSIs with hyperoxia ranged from 77 to 85%. If a treatment effect of a 10% or 20% reduction in SSIs was considered, the probabilities decreased significantly to 57–68% and 31–41% respectively. For colorectal surgery patients only, the probability of any reduction in SSIs with hyperoxia ranged from 86 to 92% depending upon whether a skeptical or enthusiastic prior was used (Figure 4a). Exclusion of the Mayzler trial did not change the probabilities significantly.
Table 2.
Median relative risks (RRs) and 95% credible intervals (CrIs) representing the posterior distributions using skeptical, neutral, and enthusiastic prior distributions to evaluate the probabilities of benefit of hyperoxia in colorectal surgery patients and all abdominal surgery patients.
| RR | CrI | P(RR<1) | P(RR<0.9) | P(RR<0.8) | ||
|---|---|---|---|---|---|---|
| Colorectal surgery patients | ||||||
| Skeptical prior | 0.82 | 0.59–1.27 | 86% | 71% | 43% | |
| Neutral prior | 0.80 | 0.57–1.19 | 89% | 75% | 49% | |
| Enthusiastic prior | 0.78 | 0.55–1.15 | 92% | 80% | 55% | |
| All patients | ||||||
| Skeptical prior | 0.87 | 0.61–1.33 | 77% | 57% | 31% | |
| Neutral prior | 0.85 | 0.59–1.28 | 81% | 62% | 36% | |
| Enthusiastic prior | 0.83 | 0.57–1.22 | 85% | 68% | 42% | |
Figure 4.
(a) The probability of any benefit from hyperoxia on SSIs (RR<1, represented by the shaded area) using a neutral prior distribution. (b) The probability of at least a 10% reduction in SSIs from hyperoxia (RR<0.9, represented by the shaded area) using a neutral prior.
Secondary outcome – mortality
Traditional analysis
Five of the trials evaluated mortality. All of the studies had a point estimate suggesting a reduction in mortality with hyperoxia, except for the Meyhoff study which had the largest number of participants and a point estimate suggesting a significant increase in mortality. Three of the trials had no deaths in one group (Figure 4b). A meta-analysis was not performed since there were only a small number of studies with rare binary events and not enough information to estimate the between study variability.26
Bayesian analysis
The probabilities of any reduction in mortality with hyperoxia with skeptical, neutral, and enthusiastic prior distributions were 59%, 70%, and 75% respectively.
Discussion
The use of hyperoxia to prevent SSIs remains controversial. A consensus statement on perioperative care after colorectal surgery by the Enhanced Recovery After Surgery Group does not mention hyperoxia,27 while a recent review of non-pharmacologic mechanisms for reducing SSIs in colorectal surgery concluded that hyperoxia has a beneficial, although limited effect, in reducing SSIs.28 The latter review based their recommendation on an updated meta-analysis of the same RCTs included in this study and obtained a similar estimate of treatment effect (reported as an odds ratio rather than a risk ratio). On the other hand, the Guidelines for Implementation of Enhanced Recovery Protocols published by the Association of Surgeons of Great Britain and Ireland in 2009 recommends high inspired oxygen concentrations (80%) during anesthesia and for at least 6 hours post-operatively.29 The recommendation is based on the results of the Qadan meta-analysis evaluating the effects of hyperoxia on SSIs but also on a meta-analysis citing a reduction in post-operative nausea and vomiting.30 The Bayesian meta-analysis in this paper provides a complementary interpretation of the results of traditional analytic methods.17
Both the frequentist and Bayesian meta-analyses support the use of hyperoxia to reduce SSIs, with Bayesian methods assigning a higher probability of benefit in colorectal patients than in all patients. The results of this study are similar to all four prior meta-analyses.12–14 Although a real difference in outcome for colorectal surgery patients versus other types of surgical patients cannot be excluded, there is no obvious biological rationale for such a difference. The subset analysis of colorectal patients differed from the analysis of all patients largely due to the latter’s inclusion of the Pryor trial, where the treatment effect favored normoxia in colorectal patients, which increased when all patients were included, and the Gardella trial which only enrolled Caesarean section patients. However, the Pryor trial has been previously criticized for methodological flaws such as imbalances between groups in potential effect modifiers such as obesity (24% versus 11% of patients with body mass index > 30 kg/m2 in the hyperoxia group).14 Both the Pryor and Gardella trials were stopped early. The Pryor trial was stopped early due to harm (in this case, more SSIs in the normoxia group) and the Gardella trial was stopped early due to futility. Although pre-determined stopping rules were used in both cases, there are multiple reasons that stopping a trial early should be avoided such as implausibility of a large treatment effect (benefit or harm) and imprecision in the estimate of treatment effect.31 In fact, systematic reviews have shown that stopping randomized trials early for benefit resulted in overestimation of treatment effect sizes.32–34
This updated meta-analysis supports previous studies suggesting that the benefit from hyperoxia is likely modest, given that the probability of a 10% or greater relative risk reduction in SSIs in colorectal surgery patients was about 75%, but the probability of more than 20% reduction was only about 50% (using a neutral point of view). Several of the trials were powered to detect much larger treatment effects such as the Meyhoff trial (33%)9 and the Pryor trial (40%).6 Meta-analysis allows a more precise estimate of treatment effect due to increased sample size, and Bayesian meta-analysis offers the additional advantage of being able to quantify the probability of benefit above specific thresholds.
The updated meta-analysis cannot make any conclusions about the effects of perioperative hyperoxia on mortality. There were only a small number of trials evaluating mortality as an endpoint and the outcome was rare. Furthermore, hyperoxia has been demonstrated to increase mortality in other patient populations such as depressed newborns35 and patients resuscitated after cardiac arrest.36 Given these findings, care should be given in extrapolating the results of this meta-analysis to other patient populations without further evaluation, such as emergency surgery patients who present in shock. The other potential clinical harms of hyperoxia that have been postulated include increased pulmonary complications,9, impairment of glucose regulation,37 and increased systemic vascular resistance resulting in a decreased cardiac output,38–39 The Meyhoff trial included pulmonary complications – specifically atelectasis, pneumonia, and respiratory failure – as secondary outcomes. There were no statistically significant differences in any of these outcomes, although again, clinically important differences cannot be excluded.
Bayesian posterior probabilities can be used to inform clinical decision making as well as formal guidelines. Diamond and Kaul have suggested a Bayesian schema for evidentiary classification of clinical practice guidelines which are based on probabilities for best-case and worst-case scenarios.20 Using this schema, the “preponderance of evidence” supports the use of peri-operative hyperoxia and would rate a recommendation for its use as having 3 of 5 stars for all patients. Using Bayesian probabilities, the strength of the recommendation can be determined across a spectrum of thresholds of clinical benefit. For example, if the clinician will only implement the intervention if it results in at least a 10% reduction in SSIs, the strength of the recommendation can be recalculated and altered accordingly.
The limitations of meta-analyses, both traditional and Bayesian, include the questions of appropriateness of combining heterogeneous studies. Other meta-analyses have performed sensitivity analyses such as excluding studies that did not include colorectal surgery patients and studies that did not use nitrous oxide to evaluate the source of the heterogeneity.14 Specific to Bayesian methods, a criticism is the subjectivity used in determining the prior probability distributions.19 However, when the data are strong, the influence of the prior distributions is minimal,40 as can be seen by the consistency of estimates of treatment effect for reduction of SSIs.
In conclusion, both traditional and Bayesian meta-analysis provides support for perioperative hyperoxia to reduce SSIs in colorectal surgery patients as well as surgical patients. Bayesian methods can complement traditional frequentist approaches to synthesizing and interpreting data, particularly when the interpretation is controversial. Further studies on peri-operative hyperoxia should focus on other patient populations and on better identifying and quantifying harms.
Supplementary Material
Acknowledgments
Grant support: Three authors are supported by grants from the NIH. Dr. Kao is supported by K23RR020020-03; Dr. Tyson is supported by 5 U10 HD021373-26, 5KL2 RR024149-04, and 5 UL1RR024148-04; and Dr. Pedroza is supported by KL2 RR024149.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Digital Content
Supplemental Digital Content 1: Appendix: Bayesian Meta-Analysis
References
- 1.Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997–1004. doi: 10.1001/archsurg.1997.01430330063010. discussion 5. [DOI] [PubMed] [Google Scholar]
- 2.Allen DB, Maguire JJ, Mahdavian M, et al. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132:991–996. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 3.Qadan M, Battista C, Gardner SA, Anderson G, Akca O, Polk HC., Jr Oxygen and surgical site infection: a study of underlying immunologic mechanisms. Anesthesiology. 2010;113:369–377. doi: 10.1097/ALN.0b013e3181e19d1d. [DOI] [PubMed] [Google Scholar]
- 4.Greif R, Akca O, Horn EP, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342:161–167. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 5.Belda FJ, Aguilera L, Garcia de la Asuncion J, et al. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. Jama. 2005;294:2035–2042. doi: 10.1001/jama.294.16.2035. [DOI] [PubMed] [Google Scholar]
- 6.Pryor KO, Fahey TJ, 3rd, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population: a randomized controlled trial. Jama. 2004;291:79–87. doi: 10.1001/jama.291.1.79. [DOI] [PubMed] [Google Scholar]
- 7.Mayzler O, Weksler N, Domchik S, Klein M, Mizrahi S, Gurman GM. Does supplemental perioperative oxygen administration reduce the incidence of wound infection in elective colorectal surgery? Minerva Anestesiol. 2005;71:21–25. [PubMed] [Google Scholar]
- 8.Myles PS, Leslie K, Chan MT, et al. Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology. 2007;107:221–231. doi: 10.1097/01.anes.0000270723.30772.da. [DOI] [PubMed] [Google Scholar]
- 9.Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. Jama. 2009;302:1543–1550. doi: 10.1001/jama.2009.1452. [DOI] [PubMed] [Google Scholar]
- 10.Gardella C, Goltra LB, Laschansky E, et al. High-concentration supplemental perioperative oxygen to reduce the incidence of postcesarean surgical site infection: a randomized controlled trial. Obstet Gynecol. 2008;112:545–552. doi: 10.1097/AOG.0b013e318182340c. [DOI] [PubMed] [Google Scholar]
- 11.Bickel A, Gurevits M, Vamos R, Ivry S, Eitan A. Perioperative hyperoxygenation and wound site infection following surgery for acute appendicitis: a randomized, prospective, controlled trial. Arch Surg. 2011;146:464–470. doi: 10.1001/archsurg.2011.65. [DOI] [PubMed] [Google Scholar]
- 12.Brar MS, Brar SS, Dixon E. Perioperative Supplemental Oxygen in Colorectal Patients: A Meta-Analysis. J Surg Res. 2009 doi: 10.1016/j.jss.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Al-Niaimi A, Safdar N. Supplemental perioperative oxygen for reducing surgical site infection: a meta-analysis. J Eval Clin Pract. 2009;15:360–365. doi: 10.1111/j.1365-2753.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 14.Qadan M, Akca O, Mahid SS, Hornung CA, Polk HC., Jr Perioperative supplemental oxygen therapy and surgical site infection: a meta-analysis of randomized controlled trials. Arch Surg. 2009;144:359–366. doi: 10.1001/archsurg.2009.1. discussion 66-7. [DOI] [PubMed] [Google Scholar]
- 15.Chura JC, Boyd A, Argenta PA. Surgical site infections and supplemental perioperative oxygen in colorectal surgery patients: a systematic review. Surg Infect (Larchmt) 2007;8:455–461. doi: 10.1089/sur.2006.034. [DOI] [PubMed] [Google Scholar]
- 16.Anthony T, Murray BW, Sum-Ping JT, et al. Evaluating an Evidence-Based Bundle for Preventing Surgical Site Infection: A Randomized Trial. Arch Surg. 2010 doi: 10.1001/archsurg.2010.249. [DOI] [PubMed] [Google Scholar]
- 17.Wijeysundera DN, Austin PC, Hux JE, Beattie WS, Laupacis A. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol. 2009;62:13–21. e5. doi: 10.1016/j.jclinepi.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Speigelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health Care Evaluation. Chicester: Wiley; 2004. [Google Scholar]
- 19.Berry DA. Bayesian clinical trials. Nat Rev Drug Discov. 2006;5:27–36. doi: 10.1038/nrd1927. [DOI] [PubMed] [Google Scholar]
- 20.Diamond GA, Kaul S. Bayesian classification of clinical practice guidelines. Arch Intern Med. 2009;169:1431–1435. doi: 10.1001/archinternmed.2009.235. [DOI] [PubMed] [Google Scholar]
- 21.Berry SM, Ishak KJ, Luce BR, Berry DA. Bayesian meta-analyses for comparative effectiveness and informing coverage decisions. Med Care. 2010;48:S137–S144. doi: 10.1097/MLR.0b013e3181e24563. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] The Cochrane Collaboration; 2009. [Google Scholar]
- 23.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] The Cochrane Collaboration; 2008. [Google Scholar]
- 24.Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med. 1995;14:2685–2699. doi: 10.1002/sim.4780142408. [DOI] [PubMed] [Google Scholar]
- 25.Jadad A. Randomised controlled trials: a user's guide. London: BMJ Books; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961–969. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 28.Murray BW, Huerta S, Dineen S, Anthony T. Surgical site infection in colorectal surgery: a review of the nonpharmacologic tools of prevention. J Am Coll Surg. 2010;211:812–822. doi: 10.1016/j.jamcollsurg.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Kahn S, Gatt M, Horgan A, Anderson I, MacFie J. Guidelines for implementation of enhanced recovery protocols. Issues in Professional Practice. 2009 [Google Scholar]
- 30.Orhan-Sungur M, Kranke P, Sessler D, Apfel CC. Does supplemental oxygen reduce postoperative nausea and vomiting? A meta-analysis of randomized controlled trials. Anesthesia and analgesia. 2008;106:1733–1738. doi: 10.1213/ane.0b013e3181731c5a. [DOI] [PubMed] [Google Scholar]
- 31.Pocock SJ. When to stop a clinical trial. BMJ (Clinical research ed. 1992;305:235–240. doi: 10.1136/bmj.305.6847.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassler D, Briel M, Montori VM, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. Jama. 2010;303:1180–1187. doi: 10.1001/jama.2010.310. [DOI] [PubMed] [Google Scholar]
- 33.Bassler D, Ferreira-Gonzalez I, Briel M, et al. Systematic reviewers neglect bias that results from trials stopped early for benefit. J Clin Epidemiol. 2007;60:869–873. doi: 10.1016/j.jclinepi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Montori VM, Devereaux PJ, Adhikari NK, et al. Randomized trials stopped early for benefit: a systematic review. Jama. 2005;294:2203–2209. doi: 10.1001/jama.294.17.2203. [DOI] [PubMed] [Google Scholar]
- 35.Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology. 2008;94:176–182. doi: 10.1159/000143397. [DOI] [PubMed] [Google Scholar]
- 36.Kilgannon JH, Jones AE, Parrillo JE, et al. Relationship Between Supranormal Oxygen Tension and Outcome After Resuscitation From Cardiac Arrest. Circulation. 2011;123:2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 37.Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA, Joyner MJ. Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol. 2010;588:4593–4601. doi: 10.1113/jphysiol.2010.197491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson KJ, Harten JM, Booth MG, et al. The cardiovascular effects of normobaric hyperoxia in patients with heart rate fixed by permanent pacemaker. Anaesthesia. 2010;65:167–171. doi: 10.1111/j.1365-2044.2009.06195.x. [DOI] [PubMed] [Google Scholar]
- 39.Harten JM, Anderson KJ, Angerson WJ, Booth MG, Kinsella J. The effect of normobaric hyperoxia on cardiac index in healthy awake volunteers. Anaesthesia. 2003;58:885–888. doi: 10.1046/j.1365-2044.2003.03333.x. [DOI] [PubMed] [Google Scholar]
- 40.Christensen R. Testing Fisher, Neyman, Pearson, and Bayes. American Statistician. 2005;59:121–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.