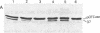Abstract
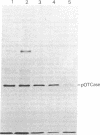
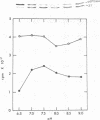
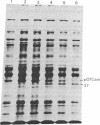
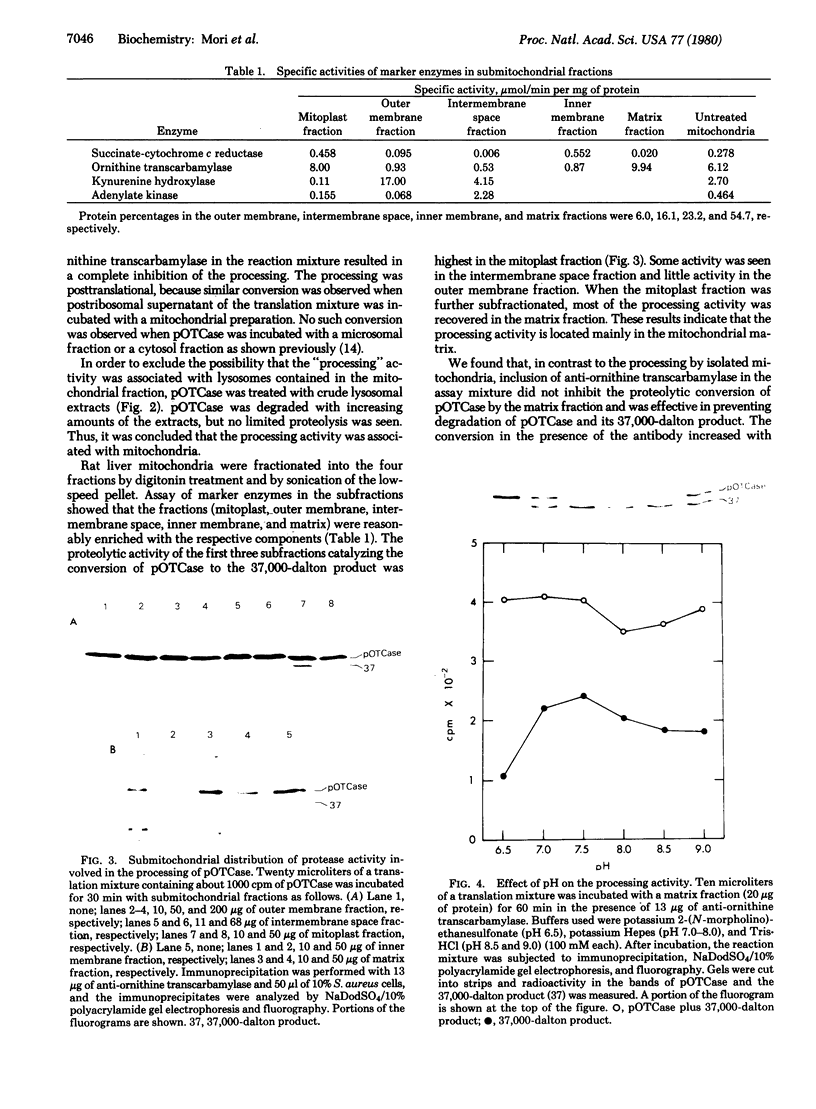
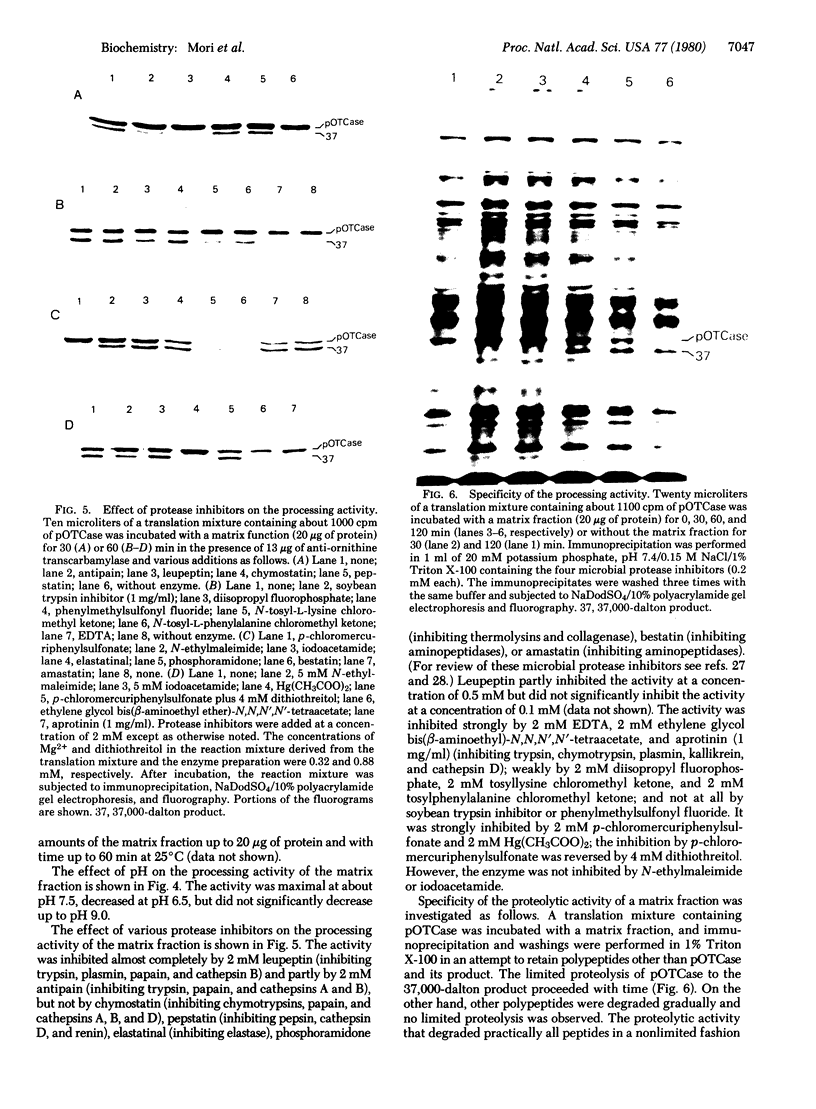
The precursor of rat liver ornithine transcarbamylase (ornithine carbamoyltransferase; carbamoylphosphate:L-ornithine carbamoyltransferase, EC 2.1.3.3) (pre-ornithine transcarbamylase), which was synthesized in a reticulocyte lysate cell-free system, was converted to an apparently mature form of the enzyme by isolated rat liver mitochondria. The proteolytic processing involved two steps: (i) conversion of pre-ornithine transcarbamylase (39,400 daltons) to a product of about 37,000 daltons and (ii) further conversion to the apparently mature form of the enzyme (36,00 daltons). When mitochondria were subfractionated by digitonin treatment followed by sonication of a mitoplast fraction, the proteolytic activity catalyzing the first step was recovered mainly in a matrix fraction. Some activity was found in an intermembrane space fraction. The enzyme activity in the matrix fraction has an optimal pH at about 7.5. The activity was inhibited almost completely by 2 mM leupeptin and partly by 2 mM antipain but not significantly by other microbial protease inhibitors or serine protease inhibitors. It was inhibited strongly by 2 mM EDTA, 2 mM ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetate, 2 mM p-chloromercuriphenylsulfonate, and 2 mM Hg(CH3COO)2 but not by N-ethylmaleimide or iodoacetamide. These results suggest that pre-ornithine transcarbamylase is first transported into the mitochondrial matrix and converted there to the mature form of the enzyme by a novel neutral protease(s).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki Y. Crystallization and characterization of a new protease in mitochondria of bone marrow cells. J Biol Chem. 1978 Mar 25;253(6):2026–2032. [PubMed] [Google Scholar]
- Aoyagi T., Ishizuka M., Takeuchi T., Umezawa H. Enzyme inhibitors in relation to cancer therapy. Jpn J Antibiot. 1977 Dec;30 (Suppl):121–132. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Transport of proteins into mitochondria and chloroplasts. J Cell Biol. 1979 Jun;81(3):461–483. doi: 10.1083/jcb.81.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. A major polypeptide component of rat liver mitochondria: carbamyl phosphate synthetase. J Biol Chem. 1976 Feb 25;251(4):950–961. [PubMed] [Google Scholar]
- Clarke S. The polypeptides of rat liver mitochondria: identification of a 36,000 dalton polypeptide as the subunit of ornithine transcarbamylase. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1118–1124. doi: 10.1016/0006-291x(76)90769-5. [DOI] [PubMed] [Google Scholar]
- Conboy J. G., Kalousek F., Rosenberg L. E. In vitro synthesis of a putative precursor of mitochondrial ornithine transcarbamoylase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5724–5727. doi: 10.1073/pnas.76.11.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté C., Solioz M., Schatz G. Biogenesis of the cytochrome bc1 complex of yeast mitochondria. A precursor form of the cytoplasmically made subunit V. J Biol Chem. 1979 Mar 10;254(5):1437–1439. [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Harmey M. A., Neupert W. Biosynthesis of mitochondrial citrate synthase in Neurospora crassa. FEBS Lett. 1979 Dec 15;108(2):385–389. doi: 10.1016/0014-5793(79)80569-4. [DOI] [PubMed] [Google Scholar]
- Hoogenraad N. J., Sutherland T. M., Howlett G. J. Purification of ornithine transcarbamylase from rat liver by affinity chromatography with immobilized transition-state analog. Anal Biochem. 1980 Jan 1;101(1):97–102. doi: 10.1016/0003-2697(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Kalousek F., François B., Rosenberg L. E. Isolation and characterization of ornithine transcarbamylase from normal human liver. J Biol Chem. 1978 Jun 10;253(11):3939–3944. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Jilka R. L., Nietsch E. H. Ornithine transcarbamylase of rat liver. Kinetic, physical, and chemical properties. J Biol Chem. 1979 Oct 25;254(20):10030–10036. [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Schatz G. Transport of proteins across the mitochondrial outer membrane. A precursor form of the cytoplasmically made intermembrane enzyme cytochrome c peroxidase. J Biol Chem. 1979 Aug 25;254(16):7468–7471. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Evidence for an exceptionally reactive arginyl residue at the binding site for carbamyl phosphate in bovine ornithine transcarbamylase. J Biol Chem. 1980 Aug 10;255(15):7301–7305. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylases. Ordering of S-cyano peptides and location of characteristically reactive cysteinyl residues within the sequence. J Biol Chem. 1980 Aug 10;255(15):7287–7290. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. The essential sulfhydryl group of ornithine transcarbamylases. Reaction with anionic, aromatic disulfides and properties of its cyano derivative. J Biol Chem. 1980 Aug 10;255(15):7291–7295. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. The essential sulfhydryl group of ornithine transcarbamylases. pH dependence of the spectra of its 2-mercuri-4-nitrophenol derivative. J Biol Chem. 1980 Aug 10;255(15):7296–7300. [PubMed] [Google Scholar]
- Michel R., Wachter E., Sebald W. Synthesis of a larger precursor for the proteolipid subunit of the mitochondrial ATPase complex of Neurospora crassa in a cell-free wheat germ system. FEBS Lett. 1979 May 15;101(2):373–376. doi: 10.1016/0014-5793(79)81047-9. [DOI] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Cell-free synthesis and processing of a putative precursor for mitochondrial carbamyl phosphate synthetase I of rat liver. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5071–5075. doi: 10.1073/pnas.76.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pierson D. L., Cox S. L., Gilbert B. E. Human ornithine transcarbamylase. Purification and characterization of the enzyme from normal liver and the liver of a Reye's syndrome patient. J Biol Chem. 1977 Sep 25;252(18):6464–6469. [PubMed] [Google Scholar]
- Raymond Y., Shore G. C. The precursor for carbamyl phosphate synthetase is transported to mitochondria via a cytosolic route. J Biol Chem. 1979 Oct 10;254(19):9335–9338. [PubMed] [Google Scholar]
- Schatz G. How mitochondria import proteins from the cytoplasm. FEBS Lett. 1979 Jul 15;103(2):203–211. doi: 10.1016/0014-5793(79)81328-9. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H., Hattori N., Yamamoto T., Kato K. The synthesis of rat liver lysosomes. I. Comparison of the microsomal, golgi, and lysosomal beta-glucuronidases. J Biochem. 1977 Sep;82(3):619–636. doi: 10.1093/oxfordjournals.jbchem.a131737. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Recent advances in bioactive microbial secondary metabolites. Jpn J Antibiot. 1977 Dec;30 (Suppl):138–163. [PubMed] [Google Scholar]
- Yamauchi K., Hayashi N., Kikuchi G. Cell-free synthesis of rat liver delta-aminolevulinate synthase and possible occurrence of processing of the enzyme protein in the course of its translocation from the cytosol into the mitochondrial matrix. FEBS Lett. 1980 Jun 16;115(1):15–18. doi: 10.1016/0014-5793(80)80716-2. [DOI] [PubMed] [Google Scholar]