Abstract
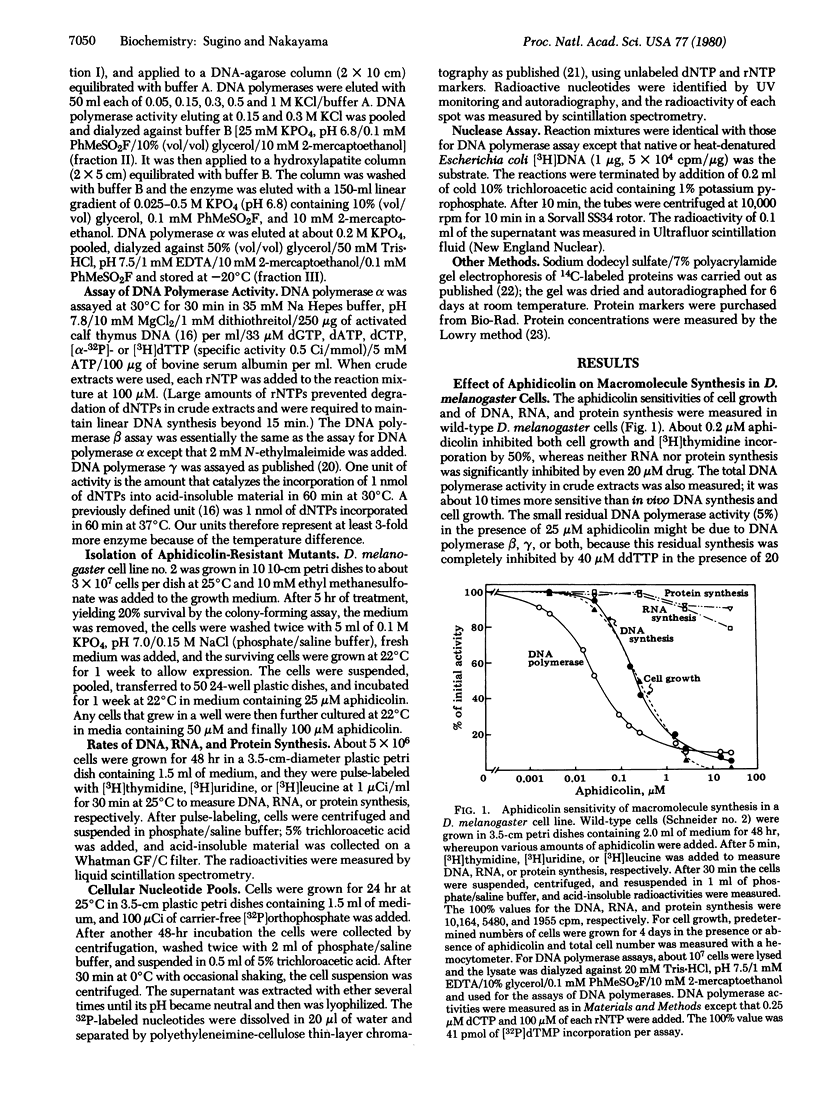
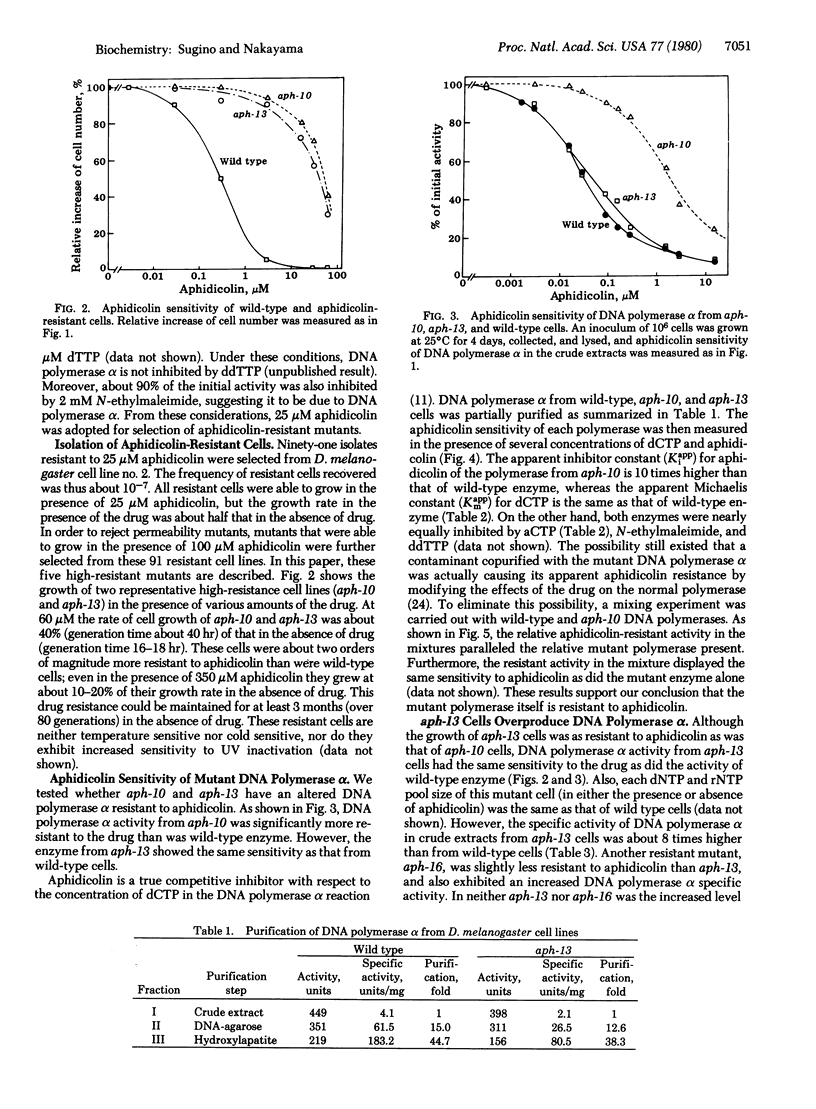
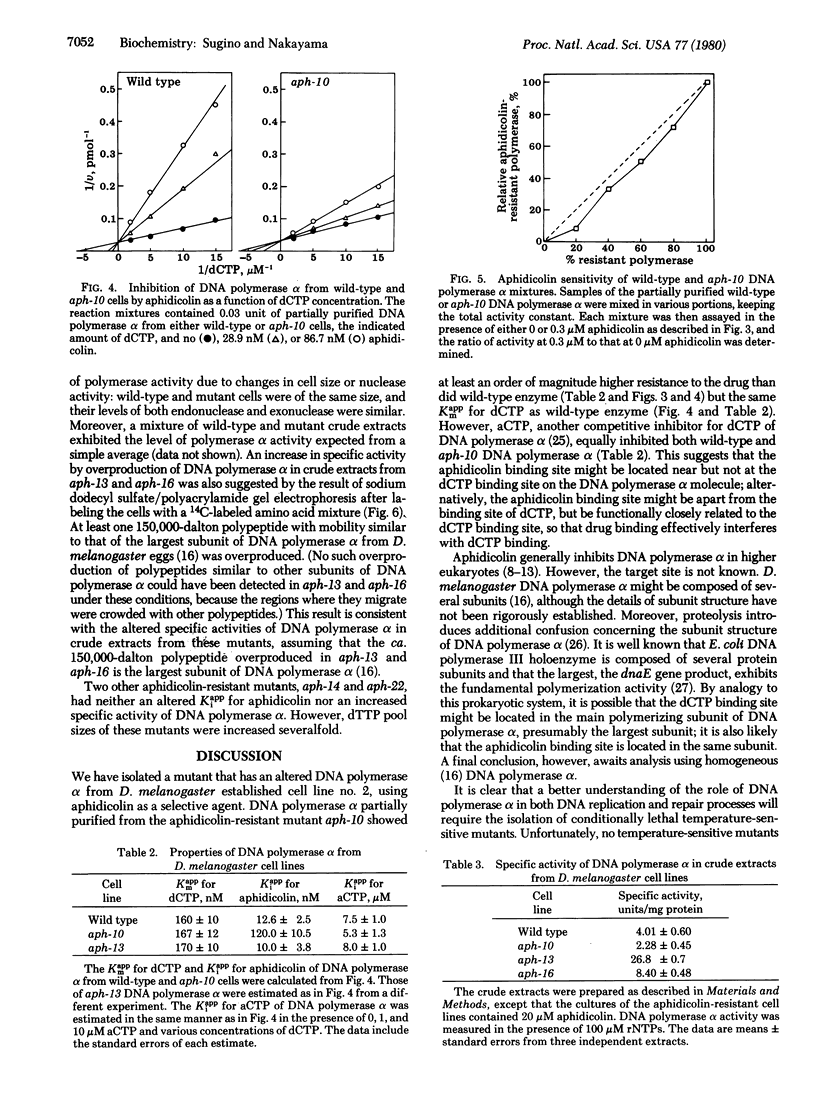
Aphidicolin, a tetracyclic diterpenoid antibiotic, is a specific inhibitor of DNA synthesis in vivo and DNA polymerase (deoxynucleosidetriphosphate:DNA deoxynucleotidyltransferase, EC 2.7.7.7) alpha of eukaryotic organisms. After ethyl methanesulfonate mutagenesis, we have recovered mutants of Drosophila melanogaster Schneider cell line no. 2 that grow at concentrations of aphidicolin that completely inhibit wild-type cells. The DNA polymerase alpha from one of these mutants, aph-10, is much more resistant to inhibition by the drug; the apparent Ki of the wild-type enzyme is 12 nM aphidicolin, whereas the apparent Ki of the aph-10 polymerase is more than 100 nM. (The apparent Km for dCTP is the same for both enzymes.) Another mutant, aph-13, overproduces DNA polymerase alpha at least 8-fold. The DNA polymerase of this mutant has the same apparent Km and Ki for dNTPs and aphidicolin as does wild-type polymerase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arens M., Yamashita T., Padmanabhan R., Tsuruo T., Green M. Adenovirus deoxyribonucleic acid replication. Characterization of the enzyme activities of a soluble replication system. J Biol Chem. 1977 Nov 25;252(22):7947–7954. [PubMed] [Google Scholar]
- Ayusawa D., Iwata K., Kozu T., Ikegami S., Seno T. Increase in dATP pool in aphidicolin-resistant mutants of mouse FM3A cells. Biochem Biophys Res Commun. 1979 Dec 14;91(3):946–954. doi: 10.1016/0006-291x(79)91971-5. [DOI] [PubMed] [Google Scholar]
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Brakel C. L., Blumenthal A. B. Multiple forms of Drosophila embryo DNA polymerase: evidence for proteolytic conversion. Biochemistry. 1977 Jul 12;16(14):3137–3143. doi: 10.1021/bi00633a016. [DOI] [PubMed] [Google Scholar]
- Bucknall R. A., Moores H., Simms R., Hesp B. Antiviral effects of aphidicolin, a new antibiotic produced by Cephalosporium aphidicola. Antimicrob Agents Chemother. 1973 Sep;4(3):294–298. doi: 10.1128/aac.4.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R. The mechanism of action of inhibitors of DNA synthesis. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- Hanaoka F., Kato H., Ikegami S., Oashi M., Yamada M. Aphidicolin does inhibit repair replication in HeLa cells. Biochem Biophys Res Commun. 1979 Mar 30;87(2):575–580. doi: 10.1016/0006-291x(79)91833-3. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Knopf K. W., Yamada M., Weissbach A. HeLa cell DNA polymerase gamma: further purification and properties of the enzyme. Biochemistry. 1976 Oct 5;15(20):4540–4548. doi: 10.1021/bi00665a032. [DOI] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Yasuda H., Ikegami S., Ohashi M., Yamada M. Aphidicolin resistant mutant of which DNA polymerase alpha is induced by this drug. Biochem Biophys Res Commun. 1979 Dec 14;91(3):939–945. doi: 10.1016/0006-291x(79)91970-3. [DOI] [PubMed] [Google Scholar]
- Oguro M., Suzuki-Hori C., Nagano H., Mano Y., Ikegami S. The mode of inhibitory action by aphidicolin on eukaryotic DNA polymerase alpha. Eur J Biochem. 1979 Jul;97(2):603–607. doi: 10.1111/j.1432-1033.1979.tb13149.x. [DOI] [PubMed] [Google Scholar]
- Ohashi M., Taguchi T., Ikegami S. Aphidicolin: a specific inhibitor of DNA polymerases in the cytosol of rat liver. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1084–1090. doi: 10.1016/0006-291x(78)90298-x. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Mazza G., Focher F., Spadari S. Lack of mutagenicity and metabolic inactivation of aphidicolin by rat liver microsomes. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1094–1103. doi: 10.1016/0006-291x(80)90601-4. [DOI] [PubMed] [Google Scholar]
- Schaller H., Nüsslein C., Bonhoeffer F. J., Kurz C., Nietzschmann I. Affinity chromatography of DNA-binding enzymes on single-stranded DNA-agarose columns. Eur J Biochem. 1972 Apr 24;26(4):474–481. doi: 10.1111/j.1432-1033.1972.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Kaufman R. J., Alt F. W., Kellems R. F. Gene amplification and drug resistance in cultured murine cells. Science. 1978 Dec 8;202(4372):1051–1055. doi: 10.1126/science.715457. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Sheinin R., Humbert J. Some aspects of eukaryotic DNA replication. Annu Rev Biochem. 1978;47:277–316. doi: 10.1146/annurev.bi.47.070178.001425. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Weissbach A. Eukaryotic DNA polymerases. Annu Rev Biochem. 1977;46:25–47. doi: 10.1146/annurev.bi.46.070177.000325. [DOI] [PubMed] [Google Scholar]
- Wist E., Prydz H. The effect of aphidicolin on DNA synthesis in isolated HeLa cell nuclei. Nucleic Acids Res. 1979 Apr;6(4):1583–1590. doi: 10.1093/nar/6.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Yamada M., Masaki S. Inhibition of DNA polymerase-alpha and -beta of calf thymus by 1-beta-D-arabinofuranosylcytosine-5'-triphosphate. Biochim Biophys Acta. 1977 Jul 15;477(2):144–150. doi: 10.1016/0005-2787(77)90230-1. [DOI] [PubMed] [Google Scholar]


