Abstract
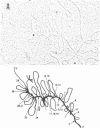
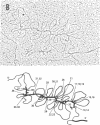
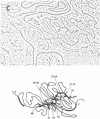
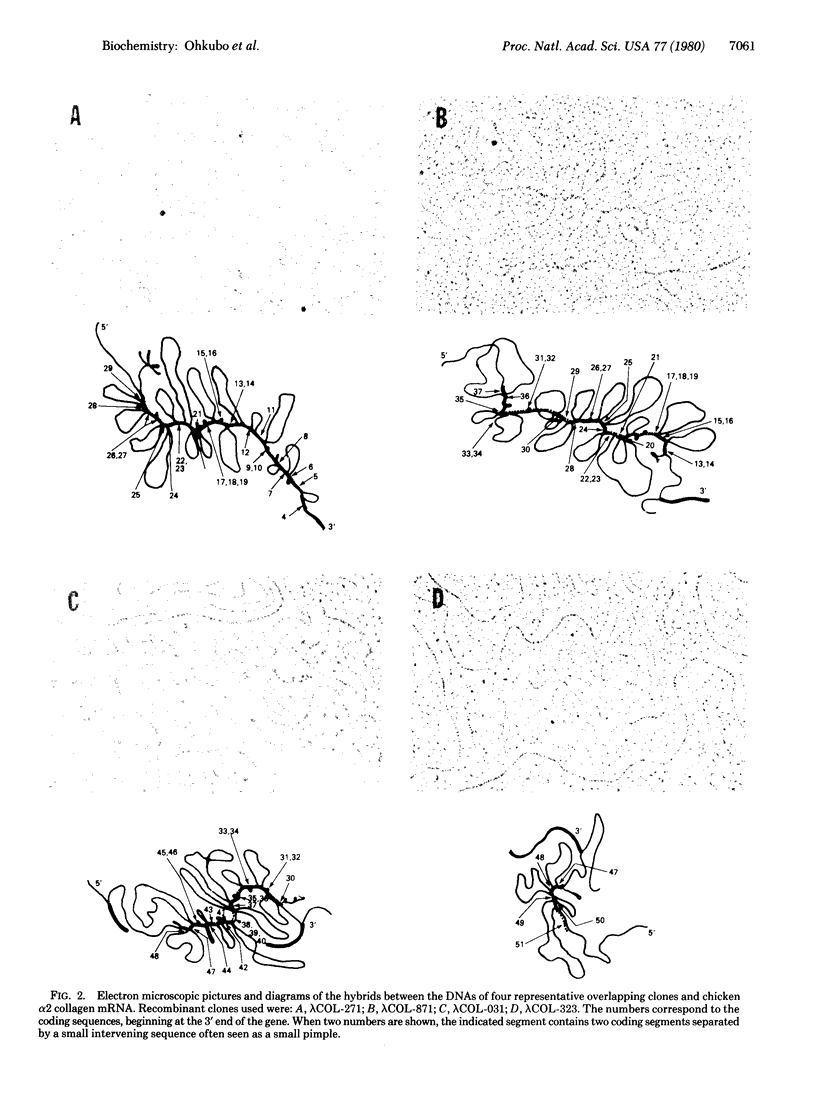
A series of overlapping recombinant clones, which cover the alpha 2 (type I) collagen gene, have been isolated by stepwise screening of two libraries of chicken genomic DNA fragments. The first genomic clone was isolated by using a cloned cDNA containing alpha 2 collagen DNA sequences as hybridization probe. The other clones were obtained by a sequence of screenings using defined fragments of the successive genomic clones as hybridization probes. Several types of experiments indicated that the DNA of these clones are truly overlapping and span 55 kilobase pairs of contiguous DNA sequences in the chicken genome. Sequence analysis of small DNA segments of some of these clones confirm that they contain coding sequences which specify alpha 2 collagen. Electron microscopic analysis of hybrids between type I alpha 2 collagen mRNA and the overlapping genomic clones indicates that the chicken alpha 2 collagen gene has a length of at least 37 kilobases, about 7.4 times longer than the corresponding translatable cytoplasmic mRNA. The coding information for alpha 2 collagen is distributed in more than 50 coding sequences which are interrupted by intervening sequences of various sizes. The structure of the gene implies that the conversion of precursor RNA to mature mRNA for alpha 2 collagen includes at least 50 splicing events.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Alwine J. C., de Crombrugghe B., Pastan I. Use of recombinant plasmids to characterize collagen RNAs in normal and transformed chick embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):4935–4938. [PubMed] [Google Scholar]
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvedimento V. E., Vogeli G., Yamada Y., Maizel J. V., Jr, Pastan I., de Crombrugghe B. Correlation between splicing sites within an intron and their sequence complementarity with U1 RNA. Cell. 1980 Oct;21(3):689–696. doi: 10.1016/0092-8674(80)90432-8. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Boyd C. D., Tolstoshev P., Schafer M. P., Trapnell B. C., Coon H. C., Kretschmer P. J., Nienhuis A. W., Crystal R. G. Isolation and characterization of a 15-kilobase genomic sequence coding for part of the Pro alpha 2 chain of sheep type I collagen. J Biol Chem. 1980 Apr 10;255(7):3212–3220. [PubMed] [Google Scholar]
- Delclos K. B., Blumberg P. M. Decrease in collagen production in normal and Rous sarcoma virus-transformed chick embryo fibroblasts induced by phorbol myristate acetate. Cancer Res. 1979 May;39(5):1667–1672. [PubMed] [Google Scholar]
- Dixit S. N., Seyer J. M., Kang A. H. Covalent structure of collagen: isolation of chymotryptic peptides and amino acid sequence of the amino-terminal region of alpha2-CB3 from chick skin. Eur J Biochem. 1977 Feb 15;73(1):213–221. doi: 10.1111/j.1432-1033.1977.tb11309.x. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Eyre D. R. Collagen: molecular diversity in the body's protein scaffold. Science. 1980 Mar 21;207(4437):1315–1322. doi: 10.1126/science.7355290. [DOI] [PubMed] [Google Scholar]
- Fietzek P. P., Rexrodt F. W. The covalent structure of collagen. The amino-acid sequence of alpha2-CB4 from calf-skin collagen. Eur J Biochem. 1975 Nov 1;59(1):113–118. doi: 10.1111/j.1432-1033.1975.tb02431.x. [DOI] [PubMed] [Google Scholar]
- Howard B. H., Adams S. L., Sobel M. E., Pastan I., de Crombrugghe B. Decreased levels of collagen mRNA in rous sarcoma virus-transformed chick embryo fibroblasts. J Biol Chem. 1978 Aug 25;253(16):5869–5874. [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Boedtker H. Construction and characterization of pro alpha 1 collagen complementary deoxyribonucleic acid clones. Biochemistry. 1979 Jul 10;18(14):3146–3152. doi: 10.2196/47873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Crkvenjakov R., Boedtker H., Doty P. Construction and characterization of a 2.5-kilobase procollagen clone. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5417–5421. doi: 10.1073/pnas.75.11.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Sobel M. E., Yamamoto T., Adams S. L., DiLauro R., Avvedimento V. E., de Crombrugghe B., Pastan I. Construction of a recombinant bacterial plasmid containing a chick pro-alpha2 collagen gene sequence. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5846–5850. doi: 10.1073/pnas.75.12.5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeli G., Avvedimento E. V., Sullivan M., Maizel J. V., Jr, Lozano G., Adams S. L., Pastan I., de Crombrugghe B. Isolation and characterization of genomic DNA coding for alpha 2 type I collagen. Nucleic Acids Res. 1980 Apr 25;8(8):1823–1837. doi: 10.1093/nar/8.8.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Sobel M. E., Adams S. L., Avvedimento V. E., DiLauro R., Pastan I., de Crombrugghe B., Showalter A., Pesciotta D., Fietzek P. Construction of a recombinant bacterial plasmid containing pro-alpha 1(I) collagen DNA sequences. J Biol Chem. 1980 Mar 25;255(6):2612–2615. [PubMed] [Google Scholar]