Abstract
Three embryonic tissue sources—the neural ectoderm, the surface ectoderm, and the periocular mesenchyme—contribute to the formation of the mammalian eye. For this reason, the developing eye has presented an invaluable system for studying the interactions among cells and, more recently, genes, in specifying cell fate. This article describes how the eye primordium is specified in the anterior neural plate by four eye field transcription factors and how the optic vesicle becomes regionalized into three distinct tissue types. Specific attention is given to how cross talk between the optic vesicle and surface ectoderm contributes to lens and optic cup formation. This article also describes how signaling networks and cell movements set up axes in the optic cup and establish the multiple cell fates important for vision. How multipotent retinal progenitor cells give rise to the six neuronal and one glial cell type in the mature retina is also explained. Finally, the history and progress of cellular therapeutics for the treatment of degenerative eye disease is outlined. Throughout this article, special attention is given to how disruption of gene function causes ocular malformation in humans. Indeed, the accessibility of the eye has contributed much to our understanding of the basic processes involved in mammalian development.
The eye develops from three embryonic tissues. It is an accessible model for studying genetic interactions and signaling switches that coordinate the development and morphogenesis of diverse cell types.
1. INTRODUCTION
For generations of biologists, the eye has offered an accessible model for investigating the mechanisms that coordinate the development and morphogenesis of diverse cell types. At the beginning of the twentieth century, embryologists studying eye development in amphibians showed the concept of induction for the first time after discovering that tissues of different origins must interact for the lens to develop (Spemann 1901). A century later, with significant advances in molecular technology, the eye has provided a system for studying gene interactions and has revealed that single mutations can lead to congenital diseases. Currently, the ability to profile gene expression in single cells generates a wealth of data on cell-specific transcripts, reveals extensive heterogeneity among retinal progenitor cells, and enables the study of eye development at the systems level (Kim et al. 2008; Roesch et al. 2008; Trimarchi et al. 2008; Byerly and Blackshaw 2009). Of specific interest is that genes that coordinate eye development are highly conserved across species. Therefore, whereas this article focuses principally on mammalian eye development, the underpinning cellular and molecular paradigms also shed light on the genetic interactions and signaling switches that specify the distinct cell types that make up the mature eye in many other animal species.
2. EYE FIELD TRANSCRIPTION FACTORS
Shortly after gastrulation, the eye primordium, or eye field, is specified in the medial anterior neural plate (the eye field) and contains all the progenitors of the neural-derived eye structures (Li et al. 1997; Wilson and Houart 2004; Zaghloul et al. 2005). In mice, the first visible sign of eye field development is the formation of bilateral indentations in the prospective forebrain termed optic sulci or optic pits at embryonic (E) day 8.0 (Fig. 1A) (Adelmann 1929; Li et al. 1997; Wilson and Houart 2004).
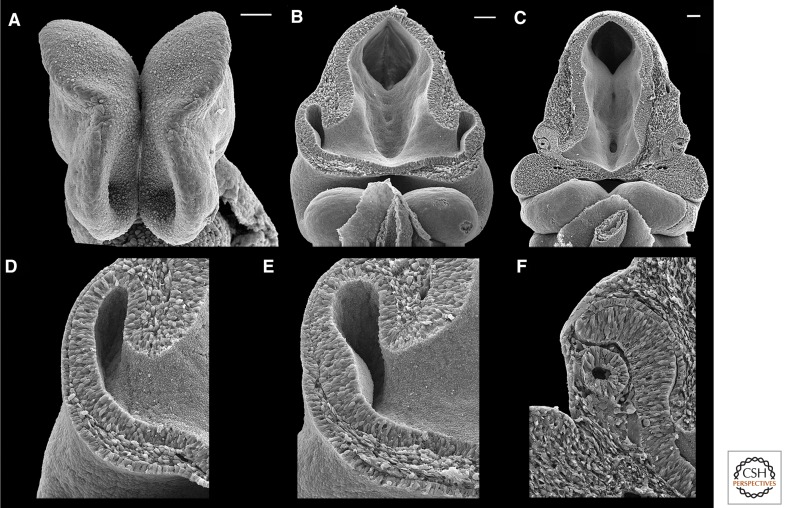
Figure 1.
Anatomy of embryonic mouse forebrain and eyes. (A) Frontal view of embryonic day (E) 8.5 forebrain, just before the eye field splits. The optic sulci are the large pits protruding from the ventral neural ectoderm. (B) Wide and (D) high magnification views of frontal sections of the E9.0 to E9.5 optic vesicle. (E) The coordinated invagination of the distal optic vesicle and the surface ectoderm, where the lens placode has thickened, begins at E9.5. (C) Wide and (F) high magnification views of frontal sections of the E10.5 optic cup and lens vesicle. The retinal pigment epithelium is the thin layer of cells proximal to the neural retina, which is dorsal to the optic stalk. The optic stalk is continuous with the ventral forebrain. The lens vesicle is distal to the neural retina. Dorsal is to the top (A–F), and proximal is to the right (D–F). Scale bars, 50 μm (A); 100 μm (B,C). (Photo from Lee Langer.)
Cells of the eye field express a set of eye field transcription factors (EFTFs) that are highly conserved throughout vertebrates (Moore et al. 2004; Zaghloul et al. 2005; Lee et al. 2006). In mammals, the EFTFs include Pax6, Rax, Six3, and Lhx2. They constitute a regulatory network required for eye development (Zuber et al. 2003). The area in the anterior neural plate where the expression domains of these transcription factors overlap marks the eye field (Zuber et al. 2003; Byerly and Blackshaw 2009). These factors are also involved in forebrain development, thereby complicating the identification of the upstream signaling pathways specifically involved in establishing the eye field (Hagglund et al. 2011).
The molecular mechanisms regulating EFTF expression in mammals are not fully understood. A recent study identified an 11 kb genomic region in the Lhx2 promoter that specifically directs Lhx2 expression to the eye field, thus defining a distinct eye-committed progenitor cell population in the forebrain (Hagglund et al. 2011). The identification of factors controlling the activity of this Lhx2 regulatory element may reveal specific pathways required for eye field specification. In fact, the conditional inactivation of Lhx2 in this cell population has no effect on the activity of the Lhx2 eye field enhancer, suggesting that Lhx2 is not essential for eye field specification (Hagglund et al. 2011). However, the finding that eye development is arrested in mice lacking Lhx2 corroborates a study in which ectopic expression of EFTFs can generate eyes in Xenopus only when endogenous Lhx2 expression is induced (Rasmussen et al. 2001; Cavodeassi et al. 2005; Fuhrmann 2008). This study also showed that Otx2, a transcription factor essential for forebrain development, and Noggin, a BMP antagonist, may potentiate EFTF expression in the anterior neural plate (Zuber et al. 2003). Moreover, in vitro data suggest that OTX2 cooperates with the neural ectoderm transcription factor SOX2 to activate Rax expression, even though Otx2 becomes down-regulated in the Rax expression domain of the early eye field (Andreazzoli et al. 1999; Zuber et al. 2003; Danno et al. 2008). Together, these data support a model of “progressive induction,” which predicts that the anterior forebrain must be specified before eye field formation, where EFTFs then work in a feedback network to specify the eye field (Fig. 2).
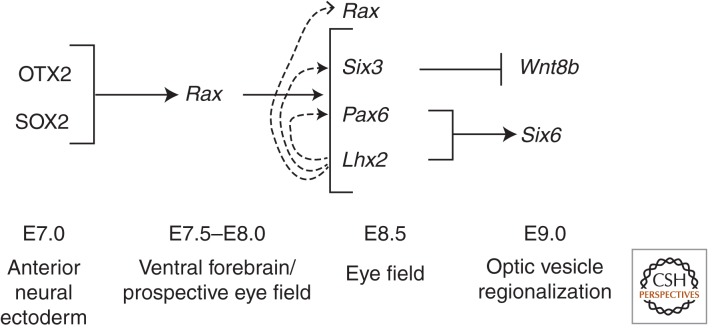
Figure 2.
Network of transcription factors that establish the eye field. The neural ectoderm transcription factors SOX2 and OTX2 activate Rax expression in the prospective eye field, which is located in the ventral forebrain. RAX is required for the up-regulation of the EFTFs Lhx2, Pax6, and Six3 in the eye field. The dashed arrows indicate that LHX2 may coregulate the expression of Rax, Pax6, and Six3 in the eye field, as expression of these genes is delayed in Lhx2−/− embryos (Tetreault et al. 2009). The EFTFs then coordinate the cell-intrinsic and -extrinsic signaling pathways that regionalize the optic vesicle along its axes.
Disruption of these early processes in eye development can lead to ocular malformations in humans. Heterozygous mutations in human SOX2 are most often associated with anophthalmia (absence of eye) and have been reported in 10%–20% of cases of severe bilateral ocular malformation (Fantes et al. 2003; Ragge et al. 2005b). However, in cases in which the SOX2 mutation causes microphthalmia (small eye), the retina remains functional (Fitzpatrick and van Heyningen 2005). Likewise, heterozygous mutations in human OTX2 can cause a range of ocular phenotypes from bilateral anophthalmia to retinal dystrophy (Ragge et al. 2005a). However, in contrast to SOX2 mutations, OTX2 mutations are commonly associated with impaired retinal function, perhaps owing to the role of OTX2 in retinal pigment epithelium (RPE) development (Chase 1944; Hanson et al. 1993; Grindley et al. 1995; Mathers et al. 1997; Wawersik and Maas 2000; Zhang et al. 2000; Tucker et al. 2001; Martinez-Morales et al. 2003; Tabata et al. 2004; Voronina et al. 2004; Ragge et al. 2005a; Medina-Martinez et al. 2009).
2.1. Pax6 and Lhx2
Genetic studies illustrate that many of the EFTFs are also essential for eye development (Wawersik and Maas 2000). An initial genetic demonstration of EFTF function was the identification of haploinsufficiency mutations in the Pax6 locus that cause the mouse small eye (Sey) phenotype (Hogan et al. 1988; Hill et al. 1991). Pax6 is a member of the evolutionarily conserved family of paired domain-containing transcription factors (Walther and Gruss 1991). Humans with mutations in one copy of PAX6 often have aniridia, a severe ocular malformation characterized by abnormal iris development and, more rarely, microphthalmia, corneal cataracts, and macular and foveal hypoplasia (Glaser et al. 1992, 1994; Hever et al. 2006). Mice with one copy of the Sey allele show reduced eye size and variable abnormal development of the retina, iris, lens, and/or cornea (Hill et al. 1991; Hever et al. 2006). Homozygous loss of Pax6 function in humans and mice causes anophthalmia (Hill et al. 1991; Glaser et al. 1994).
Like Pax6Sey/Sey mouse mutants, Lhx2−/− embryos generate optic vesicles but never form optic cups (Porter et al. 1997). Conditional inactivation of Lhx2 in the eye field leads to developmental arrest of the optic vesicle just before optic cup formation, but the expression of Pax6, Rax, and Six3 persists in the optic vesicle (Tetreault et al. 2009; Hagglund et al. 2011). The maintenance of Pax6 in Lhx2−/− mutants, and the maintenance of Lhx2 in Pax6Sey/Sey mutants, suggests that these two EFTFs are independently essential but separately insufficient for proper eye development (Porter et al. 1997). Moreover, Pax6 may cooperate with Lhx2 to induce the expression of Six6, a retinal determinant gene, in the optic vesicle (Tetreault et al. 2009).
2.2. Rax
Like the Sey mouse line, the spontaneous mutant mouse strain eyeless, first discovered in the 1940s, carries a hypomorphic mutation in the Rax locus (Chase 1944; Tucker et al. 2001). Mutations in human RAX are associated with anophthalmia (Hanson et al. 1993; Voronina et al. 2004). The role of RAX has been further elucidated using a mouse model system in which it was shown that Rax−/− mice fail to up-regulate EFTF expression in the presumptive eye field and do not develop optic vesicles (Grindley et al. 1995; Mathers et al. 1997; Zhang et al. 2000). In chimeric mice containing wild-type and Rax−/− cells, the Rax-negative cells segregate together and are never found in eye field-derived tissues. This result suggests that RAX is involved in the sorting of cells to form a distinct eye territory, perhaps through the action of cell-surface molecules (Medina-Martinez et al. 2009). Conversely, overexpression of Rax in mouse embryonic stem cells cocultured with a host retina promotes retinal cell fates (Tabata et al. 2004).
2.3. Six3
Six3 encodes a homeobox-containing transcription factor homologous to the Drosophila sine oculis gene (Oliver et al. 1995). Genetic inactivation of Six3 in presumptive eye tissue has shown that it is essential for eye development in mammals (Marquardt et al. 2001; Liu et al. 2010). Conditional inactivation of Six3 in the eye field abrogates neural retina development, whereas misexpression of Six3 in the midbrain–hindbrain region of mouse embryos causes ectopic optic vesicles (Lagutin et al. 2001; Liu et al. 2010). Mutations in human SIX3 are associated with holoprosencephaly, or a failure of the cerebral hemispheres to separate (described below) (Geng et al. 2008).
Together, these data illustrate the importance of the EFTFs in regulating the network of events that control early eye development, from the specification of the eye field to the morphogenesis and regionalization of the optic vesicle.
3. DIVISION OF THE EYE FIELD
Developmental biologists working in the 1920s observed that both eyes arise from a single eye field that is divided into bilateral hemispheres (Adelmann 1929; Mangold 1931; Li et al. 1997). At least two molecules have been clearly shown to be involved in this morphogenetic process. The first is sonic hedgehog (Shh), which is expressed in the ventral forebrain and prechordal mesoderm (Echelard et al. 1993). Targeted disruption of Shh in mice results in the failure of the eye field to split, resulting in cyclopia and a single Pax6-positive optic vesicle (Chiang et al. 1996). The second player is Six3, which is expressed throughout the anterior neural ectoderm before becoming restricted to the ventral forebrain and eye field (Oliver et al. 1995). In humans, loss-of-function mutations in either SHH or SIX3 result in midline defects that frequently include cyclopia (Belloni et al. 1996; Roessler et al. 1996; Muenke and Cohen 2000). In fact, SIX3was shown to regulate Shh expression in the ventral midline of the rostral diencephalon via an upstream enhancer element (Geng et al. 2008; Jeong et al. 2008).
4. ESTABLISHING BOUNDARIES IN THE OPTIC VESICLE
Ocular development begins with the formation of the optic vesicles. At E8.5–9.0 of mouse development, the walls of the diencephalon evaginate (the optic vesicles) and come into close contact with the surface ectoderm (Fig. 1D) where the lens placode is formed. Each optic vesicle (OV) consists of the retinal stem cells (RSCs) that give rise to all the neuroectoderm-derived cells of the eye. RSC patterning occurs along the dorso–distal/proximal–ventral axis of the OV prior to optic cup formation. Regions along this axis correspond to the presumptive neural retina (NR–distal OV), retinal pigment epithelium (RPE–dorsal/proximal OV), and optic stalk (OS–ventral/proximal OV) (Figs. 3, 4A).
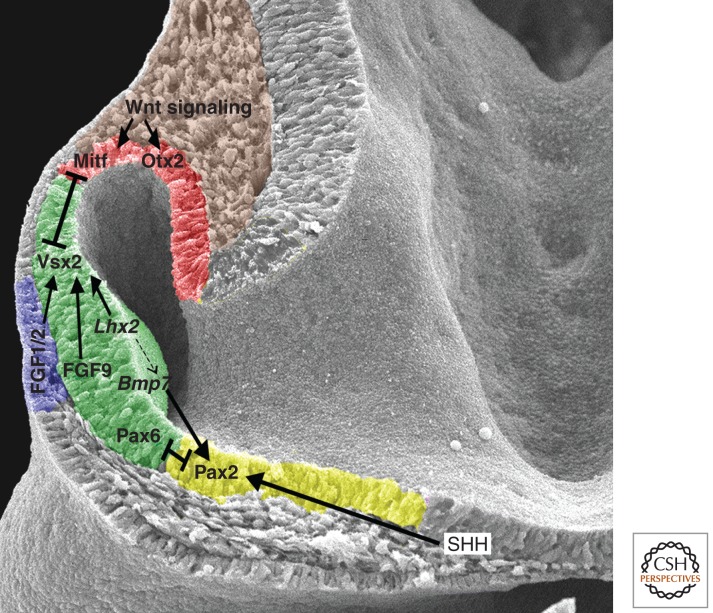
Figure 3.
Signaling networks establish boundaries in the optic vesicle. Dorsal is to the top, and distal is to the left. The optic vesicle is regionalized into prospective RPE (red, dorsal), neural retina (green, central) and optic stalk (yellow, ventral). Extracellular signals organize the optic vesicle in part through the activation of transcription factors that specify the tissue type in which they are expressed. These transcription factors cell-intrinsically regulate optic vesicle organization through mutual repression of one another. The dotted arrow indicates that early Lhx2 expression may be required for Bmp7 expression in the optic vesicle (Yun et al. 2009), but Bmp7 expression is maintained when Lhx2 is ablated specifically in the eye field (Hagglund et al. 2011). The lens placode, which expresses fibroblast growth factor (FGF) ligands important for neural retinal specification, is shown in blue.
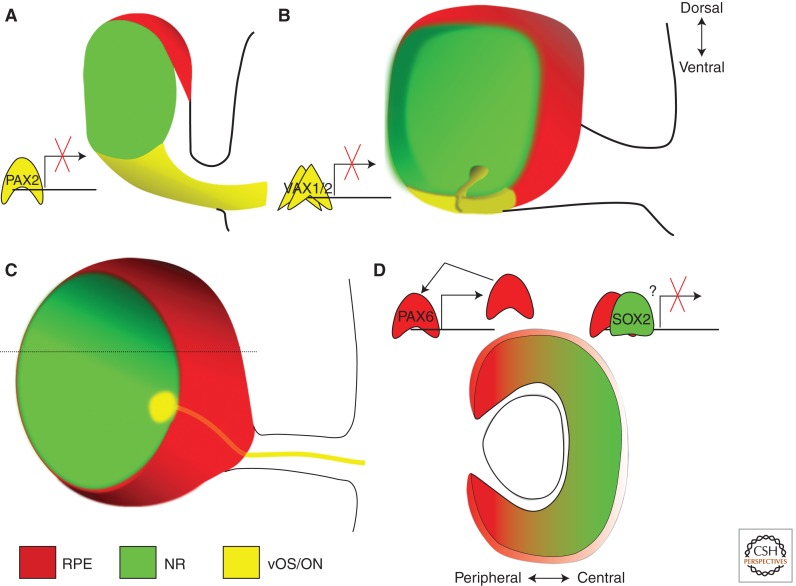
Figure 4.
The regulation of Pax6 expression via its eye-specific enhancer α during the early stages of eye development. (A) At embryonic day (E) 9.5, the optic vesicle is regionalized into at least three presumptive tissues: the retinal pigment epithelium (RPE, red), the central neural retina (NR, green), and the ventral optic stalk (vOS, yellow). The transcription factor PAX2 binds the α enhancer to antagonize Pax6 expression in the ventral optic vesicle. (B) At E10.5, the optic vesicle invaginates centrally, creating two nested cups—the RPE and the NR. The optic vesicle also invaginates ventrally, creating the optic fissure. VAX1 and VAX2 suppress ventral Pax6 expression via the α enhancer. (C) At E11.5, the optic fissure has closed ventrally, leaving a small opening for retinal ganglion cell axons to exit through the optic nerve (ON, yellow). The RPE has completely surrounded the neural retina. (D) Transverse section through the optic cup at the dotted line in C. SOX2 (green) and PAX6 (red) show inversely graded expression patterns, with SOX2 highest in the central optic cup and PAX6 highest in the periphery. The peripheral part of the optic cup, or optic cup margin, gives rise to the epithelia of the ciliary body and iris, whereas the central part gives rise to the neural retina. The optic cup margin highly expresses Pax6 due in part to positive autoregulation via the α enhancer. In the central optic cup, SOX2 may antagonize Pax6 expression through interaction with the α enhancer.
Several cell-intrinsic signaling pathways are involved in patterning the OV. Each compartment of the OV expresses a specific set of transcription factors that are important for the development of the cell type in which they are expressed. The presumptive NR expresses the homeodomain protein Vsx2 (formerly Chx10), the future RPE expresses the basic helix-loop-helix transcription factor Mitf and the prospective OS expresses the paired domain protein Pax2 (Nornes et al. 1990; Hodgkinson et al. 1993; Liu et al. 1994). Many studies have revealed that these transcription factors, in combination with the EFTFs, have cell-intrinsic roles in compartmentalizing the future optic cup, often through reciprocal transcriptional repression of one another (Fig. 3). These studies also suggest that the RSCs of the OV are competent to become NR, RPE, or OS when provided with the appropriate combination of signals.
5. NEURAL RETINA VERSUS RETINAL PIGMENT EPITHELIUM VERSUS OPTIC STALK
Mouse genetic studies have provided evidence for an antagonistic relationship between Vsx2 and Mitf, which serves to establish the boundary between the future NR and RPE (Nguyen and Arnheiter 2000; Horsford et al. 2005). This occurs through the initial expression of Mitf throughout the dorsal OV, but Mitf becomes down-regulated distally upon the expression of Vsx2 (Nguyen and Arnheiter 2000). The function of Mitf in boundary formation is supported by the observation that mice with loss-of-function mutations in Mitf show a conversion from RPE to NR (Bumsted and Barnstable 2000; Nguyen and Arnheiter 2000). A similar RPE-to-NR conversion phenotype occurs in mice that are deficient in both Otx1 and Otx2, transcription factors that are normally expressed in the dorsal OV/presumptive RPE (Martinez-Morales et al. 2001). Conversely, mice with loss-of-function mutations in Vsx2, termed orJ or ocular retardation mice, show ectopic expression of Mitf and Mitf target genes in the NR. Fate-mapping analyses suggest that this phenotype is a direct transdifferentiation of the NR to RPE (Rowan et al. 2004; Horsford et al. 2005).
A mutually antagonistic relationship in tissue-type specification exists between Pax2 and Pax6. Pax2−/− mice show ventral expansion of the Pax6 expression domain and subsequent expansion of the NR and RPE at the expense of the OS (Schwarz et al. 2000). Conversely, Pax6-deficient mice show a dorsal expansion of the Pax2 expression domain and fail to develop a NR or RPE, only maintaining a Pax2-positive optic stalk. This reciprocally repressive relationship appears to involve a direct molecular interaction, as PAX2 can bind the Pax6 retina-specific enhancer, α (Fig. 4A), and PAX6 can bind the Pax2 OS-specific enhancer (Schwarz et al. 2000). Similarly, humans with PAX2 mutations have optic nerve coloboma caused by the failure of the ventral optic fissure to properly close during development (Torres et al. 1996). Together, these data show that Pax2 and Pax6 establish the boundary between the optic stalk and the NR through mutual repression of one another.
The EFTF Lhx2 appears to act upstream of the above-described genetic interactions, coordinating the events necessary for proper OV regionalization. In Lhx2 loss-of-function mutants, the OV fails to become regionalized, showing ventral expansion of Pax6 but lacking Vsx2, Mitf, and Pax2 (Yun et al. 2009). However, in embryos with specific ablation of Lhx2 in the eye field, Pax2 expression persists in the ventral OV, but Mitf is down-regulated, and Vsx2 is absent (Hagglund et al. 2011).
6. SIGNALING NETWORKS IN THE OPTIC VESICLE
Adding complexity to the system of early eye development is the understanding that these cell-intrinsic transcription factors modulate extrinsic signals to functionally compartmentalize the OV. The extrinsic signals involved in OV patterning include members of the transforming growth factor-β (TGFβ), fibroblast growth factor (FGF), and Wnt families and Sonic hedgehog. Early Lhx2 activity, for instance, is required to transduce the BMP7 signal to activate Pax2 expression in the ventral OV, whereas later in development Lhx2 activity is required to maintain Bmp4 expression in the OV (Yun et al. 2009). Similarly, FGF1 or FGF2 from the surface ectoderm activates Vsx2 in the presumptive NR, which in turn represses Mitf (Nguyen and Arnheiter 2000; Horsford et al. 2005).
FGF9, which is normally expressed in the distal OV, promotes NR fate when ectopically expressed in the presumptive RPE, and mice with targeted deletion of Fgf9 show expansion of the RPE into the NR domain (Zhao et al. 2001). Moreover, OV-specific deletion of the protein phospatase Shp2, which mediates the FGF signaling cascade via sustained activation of Ras, causes a cell-fate conversion from NR to RPE (Cai et al. 2010). Conversely, inactivation of canonical Wnt signaling in the presumptive RPE causes it to transdifferentiate to NR (Westenskow et al. 2009). Lastly, in addition to its role in splitting the eye field, midline-secreted Shh plays an additional role in ventralizing the OV. The OV of Shh mutant mice shows expanded Pax6 expression at the expense of Pax2, whereas Otx2, a presumptive RPE (dorsal) marker, persists in the cyclopic Shh mutant eye (Chiang et al. 1996).
Humans with mutations in genes encoding a subset of these signaling molecules often show ocular malformations. Mutations in human BMP4, for instance, have been described in patients with anophthalmia/microphthalmia, colobomas, and retinal dystrophy (Hayashi et al. 2008). Similarly, mutations in VSX2 are associated with microphthalmia, iris abnormalities, coloboma, and retinal dystrophy (Ferda Percin et al. 2000; Iseri et al. 2010). Although rare in comparison to cyclopia, anopthalmia/microphthalmia and coloboma can result from mutations in human SHH (Bakrania et al. 2010).
7. OPTIC CUP MORPHOGENESIS
After the formation of the OV, a coordinated invagination of the lens placode and the OV form the lens vesicle and the bilayered optic cup (OC) (Fig. 1B,C,E,F). The OV folds into itself creating two nested cups; the distal OV becomes the inner layer of the OC—the presumptive NR—whereas the proximal OV becomes the outer layer of the OC—the presumptive RPE (Figs. 1F, 4B).
An additional invagination occurs in the ventral OV where the optic stalk meets the ventral retina to generate the optic or choroidal fissure (Fig. 4B). The optic fissure provides an exit from the eye for retinal axons and an entrance for the hyaloid artery, which supplies blood to the retina (Saint-Geniez and D’Amore 2004). The OC grows circumferentially until it closes over the optic fissure (Fig. 4C). Cross talk between the presumptive lens and retina may be necessary for the proper invagination of both tissues in vivo (Grindley et al. 1995; Ashery-Padan et al. 2000; Smith et al. 2009; Bassett et al. 2010).
The optic vesicle and lens placode are tightly apposed at the initiation of OV invagination. However, the necessity of the presence of the lens placode for the formation of the two-walled OC remains unclear. One study used three-dimensional culture of mouse embryonic stem cell aggregates to derive hollowed spheres of neuroepithelium containing Rax-positive domains. Many of these regions spontaneously invaginated, in the absence of a lens or ectodermal tissues, to form the OC (Eiraku et al. 2011). This autonomous morphogenetic process appeared to be driven by forces within the retinal anlage, suggesting that, at least in vitro, the OV can form the OC without instruction from other structures (Eiraku et al. 2011).
Conversely, the apposition of the LP and the OV appears to be important for placode invagination. The LP arises from the preplacodal region (PPR), an ectoderm-derived bilateral structure that forms discrete thickenings called placodes, the precursors to various vertebrate sensory structures (Streit 2007). The LP is identified as the group of thickened columnar cells in the head surface ectoderm that arises in response to OV proximity. The LP and OV become physically tethered through cytoplasmic processes, or filopodia, originating from the base of the lens (Chauhan et al. 2009). The LP invaginates to form the lens cup or lens pit, which eventually separates from the surface ectoderm to form the lens vesicle (Graw 2010).
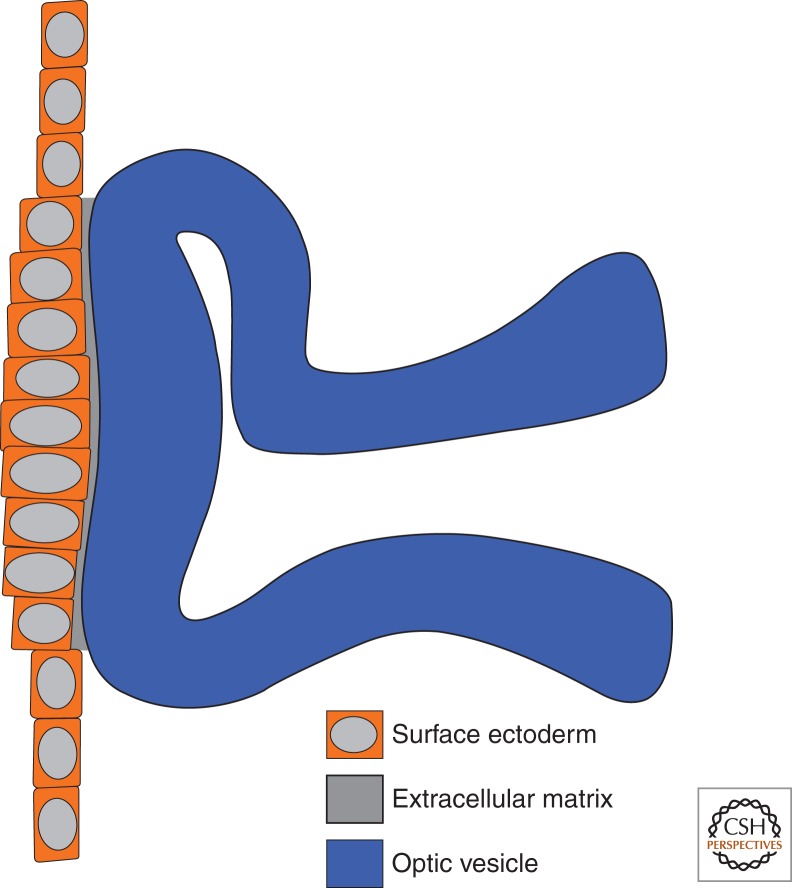
Placode thickening is associated with local changes in cell shape without associated changes in cell volume. LP formation appears to be mediated by adhesion between the OV and the surface ectoderm, where lens precursors continue to proliferate but cannot expand beyond the region of adhesion (Hendrix and Zwaan 1975; Huang et al. 2011). The increase in cell number in this fixed area is sufficient to account for the increase in cell length observed during placode formation (Hendrix and Zwaan 1975). Adhesion between the OV and the surface ectoderm is mediated by the extracellular matrix protein fibronectin1 (fn1) (Huang et al. 2011). Indeed, fn1 expression is lost when Pax6, a master regulator of lens development, is deleted in the surface ectoderm (Fig. 5) (Huang et al. 2011).
Figure 5.
Mechanics of lens placode formation. The optic vesicle contacts the surface ectoderm before lens placode formation. Adhesion between the optic vesicle and the pre-lens ectoderm is mediated by extracellular matrix components. The area of contact between the extracellular matrix and the surface ectoderm restricts expansion of the pre-lens domain. Continued cell division in this area leads to cell crowding and cell elongation resulting in placode formation.
8. RETINOIC ACID SIGNALING AND PERIOCULAR MESENCHYME
Several signaling pathways have been implicated in orchestrating the invagination of the OV in vivo. Of these, one of the best studied is the retinoic acid (RA) signaling pathway (Cvekl and Wang 2009). Mouse transgenic reporter lines have revealed the presence of RA activity in the OV beginning at E8.5, in the lens placode at E8.75, and in the periocular mesenchyme (POM) at E10.25 (Cvekl and Wang 2009). Three retinaldehyde dehydrogenases (Aldh1a1, Aldh1a2, and Aldh1a3), which function to produce RA from vitamin A, are associated with dorsal and ventral OV invagination. Mice deficient in Aldh1a2 fail to initiate OC formation (dorsal invagination) (Mic et al. 2004). Moreover, OVs that receive no RA signaling fail to invaginate ventrally at the boundary of the NR and OS (Molotkov et al. 2006). Humans with mutations in STRA6, which encodes a receptor that mediates the cellular uptake of circulating vitamin A, have ocular defects, including anophthalmia or severe microphthalmia (White et al. 2008).
The POM is a loose collection of cells that gives rise to multiple cell lineages required for normal ocular development. These include the stroma of the iris and cornea, corneal endothelium, trabecular meshwork, Schlemm’s canal, ciliary body muscles, extraocular muscles, sclera, and ocular blood vessels (Gage et al. 2005). The POM is of both neural crest and mesoderm in origin, and each contributes to different cell fates (Gage et al. 2005; Kanakubo et al. 2006). Although the myotubules and myofibers of the extraocular muscles are mesoderm derived, the connective fascia cells arise from the neural crest. The mesoderm gives rise to the endothelial lining of the ocular blood vessels, but the associated smooth muscle cells and pericytes are neural crest-derived, as are the ciliary muscles. The trabecular meshwork and the corneal endothelium and stroma primarily consist of neural crest-derived cells, but a small population of cells in these tissues arises from the mesoderm (Gage et al. 2005).
Tcfap2a (AP-2α) is expressed in the POM and has been shown to be crucial for the correct positioning of the OV adjacent to the lens placode (Bassett et al. 2010). The optic vesicles of mice deficient in Tcfap2a are not positioned correctly in relation to the surface ectoderm and therefore do not receive the correct combinations of extracellular signals, leading to improper regionalization and morphogenesis of the OV/OC in vivo (Bassett et al. 2010). In contrast to the in vitro model, these studies suggest that the positioning of the OV adjacent to the lens placode (LP) in vivo allows the developing OC to receive the correct spatial signals from surrounding tissues for proper regionalization along its axes (Fig. 3).
9. LENS AND CORNEA
In addition to Pax6, several other transcriptional regulators are important for lens specification. Six3 and Sox2, for example, are both essential for proper lens development. Six3 expression precedes that of Pax6 in the presumptive lens ectoderm and activates Pax6 transcription (Liu et al. 2006). Although Sox2 expression in the LP is induced by signals from the OV, it may also be mediated by SIX3 in the surface ectoderm (Furuta and Hogan 1998; Kamachi et al. 1998). Thus, much like the cell-intrinsic regulation of OV patterning, transcription factors involved in lens induction transduce signaling cascades to activate downstream targets. One such signal is BMP4 from the OV, which may activate Sox2 but not Pax6 expression in the LP. Another is BMP7 in the head ectoderm, which may activate Pax6 expression in the LP (Furuta and Hogan 1998; Wawersik et al. 1999).
SOX2 and PAX6 also function in cross- and self-regulatory feedback loops in lens development. Sox2 expression appears to depend on PAX6 only after LP stages (Ashery-Padan et al. 2000; Smith et al. 2009). Moreover, SOX2 cooperates with PAX6 in its own up-regulation in lens precursor cells via the N3 enhancer upstream of the Sox2 coding sequence (Inoue et al. 2007). Likewise, PAX6 and SOX2 synergistically activate Pax6 expression via the head surface ectoderm enhancer element LE9 (Aota et al. 2003). SOX2 and PAX6 may also coregulate other genes important for lens development, such as those encoding lens crystallins (Kamachi et al. 1998, 2001).
The lens vesicle remains transiently attached to the surface ectoderm via the lens stalk. Once detached from the lens, the surface ectoderm proliferates to restore the exterior, eventually giving rise to the corneal epithelium. The corneal endothelium is composed of migrated forebrain and midbrain neural crest-derived mesenchymal cells (Trainor and Tam 1995; Kanakubo et al. 2006). These cells invade the newly formed space between the lens vesicle and the surface ectoderm and condense into a multilayered structure connected by a loose extracellular matrix. The cells between the corneal epithelium and endothelium differentiate into karatocytes and make up the corneal stroma (Cvekl and Tamm 2004). The space between the future cornea and lens, called the anterior chamber, becomes fluid filled. A second group of mesenchymal cells migrates into the angle between the presumptive cornea and peripheral edge of the OC to become the stroma of the iris and ciliary body (described below). Around the same time that mesenchymal cells migrate into the future cornea (E11.5), other POM cells invade the space between the lens and the retina, called the hyaloid, giving rise to the hyaloid vasculature (Gage et al. 2005).
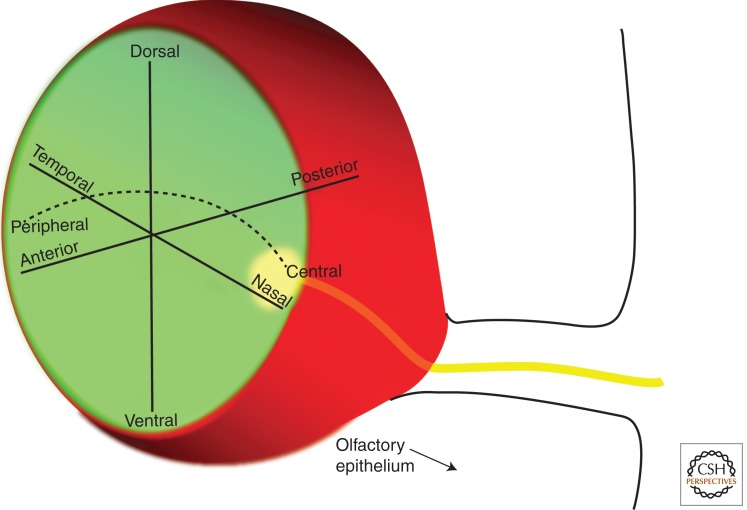
10. AXES IN THE NEURAL RETINA
The inner layer of the OC, which gives rise to the NR, is patterned along its dorsal-ventral (D-V) and nasal-temporal (N-T) axes (Fig. 6). By E10.5, the OS (ventral) has begun to elongate and will eventually give rise to the optic nerve. The axons of retinal ganglion cells from the innermost layer of the NR begin to enter the OS around E11.5 and intersperse with the PAX2-positive OS cells, the glial precursors that will develop into the astrocytes that make up the mature optic nerve (Torres et al. 1996).
Figure 6.
Axes in the optic cup. The optic cup is regionalized along several axes: dorsal–ventral, anterior (cornea)–posterior (RPE), nasal–temporal, and central (central neural retina)–peripheral (ciliary epithelium).
Proper optic nerve placement depends on signals that pattern the OC along the D-V axis. These signals are mediated in part by members of the VAX family of homeodomain transcription factors. At E13.5, Vax1 is expressed in the OS, whereas Vax2 shows a steep ventralhigh-to-dorsallow gradient of expression (Mui et al. 2002, 2005). Both Vax1 and Vax2 are expressed in the OS and ventral retina from E9.5 to E11.5, which may explain why Vax1−/−:Vax2−/− double mutant mice show severe ventral eye defects. The OS of these mice becomes dorsalized, developing into RPE and NR instead of optic nerve (Mui et al. 2005). In vitro and in vivo data suggest that VAX1 and VAX2 cooperatively specify the ventral OC and OS by directly repressing Pax6 expression via the Pax6 retina-specific enhancer α (Mui et al. 2005). Conversely, the T-box transcription factor Tbx5, a BMP4 target, is normally expressed in the dorsal NR (Behesti et al. 2006). Its expression is lost when Pax6 is ablated from the developing NR, further demonstrating Pax6’s role in specifying the dorsal OC (Baumer et al. 2002; Behesti et al. 2006).
N-T patterning of the OC ensures that the axons of retinal ganglion cells will correctly map to their targets in the superior colliculus (SC) and the dorsal lateral geniculate nucleus (dLGN). In mammals, axons from the nasal retina project to the caudal SC, and axons from the temporal retina project to the rostral SC. The earliest transcriptional regulators of N-T identity in the OC are the forkhead transcription factors FOXD1 (BF-2) and FOXG1 (BF-1). Foxg1 is expressed in the nasal retina, but appears to play an additional role in D-V patterning, as Foxg1−/− mutants lose Shh signaling and have dorsalized OVs (Huh et al. 1999). However, Foxd1 functions in N-T patterning to specify the retinal ganglion cells (RGCs) of the temporal retina (Carreres et al. 2011). Indeed, RGCs of Foxd1−/− mutants lose topographic specificity, mapping indiscriminately to the entire extent of the SC (Carreres et al. 2011). Expression of both Foxd1 and Foxg1 is lost in Pax6−/− mutants, demonstrating an additional role for PAX6 in establishing the naso-temporal region of the retina (Baumer et al. 2002).
11. CILIARY BODY AND IRIS
A third axis along which the OC is patterned is the central–peripheral axis (Figs. 4D, 6). The distal tip of the OC, where the presumptive NR meets the RPE, is termed the “ciliary margin” in mice and contains nonneurogenic progenitors that give rise to the epithelia of the ciliary body (CB) and iris (Beebe 1986; Davis-Silberman and Ashery-Padan 2008). The CB epithelia are continuous with the RPE and NR, whereas the iris epithelia are distal to the CB. The outer layer of the ciliary margin gives rise to the pigmented epithelium of the CB and the anterior pigmented layer of the iris, whereas the inner layer gives rise to the nonpigmented epithelium of the CB (herein referred to as the ciliary epithelium or CE) and the posterior pigmented layer of the iris. Nonneurogenic progenitor cells of the OC margin can be identified as early as E12.5 by their specific expression of transcription factors, including Msx1 and Otx1, lack of expression of neuronal markers, and a slower proliferation rate relative to the NR (Beebe 1986; Monaghan et al. 1991; Martinez-Morales et al. 2001; Cho and Cepko 2006; Trimarchi et al. 2009). Indeed, CB development is absent in mice that lack Otx1 (Acampora et al. 1996).
Several cell-intrinsic and -extrinsic pathways have been identified to play a role in specifying the ciliary margin in mammals. CE-specific genes can be induced in embryonic mouse retina when cultured adjacent to an explanted chick lens (Thut et al. 2001). The inductive power of the lens may in part involve BMP signaling, as transgenic expression of the BMP antagonist Noggin in the developing mouse lens abrogates expression of Bmp4 and Bmp7 in the presumptive CE at postnatal stages (Zhao et al. 2002). Without BMP signaling, the CE-specific genes Otx1 and Msx1 fail to be expressed, and NR cells develop in place of the CB after birth (Zhao et al. 2002).
The appearance of the NR at the expense of the CE in the BMP-deficient ciliary margin suggests that progenitor cells at the boundary of the NR and CE remain competent to take on one fate or the other after these tissues have begun to be specified. Transcriptional control of this binary cell-fate decision may be mediated by SOX2 and PAX6 (Fig. 4D). In the early OC, SOX2 and PAX6 show inverse gradients of expression, with SOX2 high in the central OC but low in the periphery where PAX6 is maintained at a high level due in part to its retina-specific enhancer α (Baumer et al. 2002; Matsushima et al. 2011). Specific ablation of SOX2 in OC progenitor cells results in elevated Pax6 expression and cell-fate conversion from NR to CE (Matsushima et al. 2011). This cell-fate conversion can be partially rescued by reducing Pax6 (Matsushima et al. 2011). Indeed, several studies have shown the importance of PAX6 to the development of the ciliary margin. Mice that are haplo-insufficient for Pax6 show reduced ciliary margin size, and humans with mutations in PAX6 have aniridia (no iris) and small ciliary bodies (Hanson et al. 1993; Okamoto et al. 2004; Davis-Silberman et al. 2005). Further evidence suggests that Wnt signaling may play a role in specifying CE fate. Activated Wnt signaling in the peripheral NR induces expression of CE-specific genes (Liu et al. 2007). Moreover, the NR-to-CE cell-fate conversion caused by the loss of SOX2 is associated with centrally expanded Wnt signaling prior to the ectopic expression of CE genes (Matsushima et al. 2011).
12. RETINAL NEUROGENESIS
The mature retina has a laminar organization and is composed of specialized sensory neurons, rods, and cone photoreceptors that form the outer nuclear layer; horizontal, bipolar, and amacrine interneurons that comprise the majority of the inner nuclear layer; and projection neurons, the retina ganglion cells, localized to the ganglion cell layer (Fig. 7). Together they form an elaborate neuronal network that accomplishes the tasks of image detection, processing, and transmission in a way similar to other parts of the central nervous system (CNS). The synaptic connections within the retina are segregated predominantly into two layers: the thin outer plexiform layer and the elaborate inner plexiform layer usually divided into sublamina. The retinal environment is structured and controlled by the specialized radial glia, the Müller glia cells, whose cell bodies are located in the central part of the inner nuclear layer (INL). The precise organization of the retina allows for the identification and characterization of particular cell types through the use of few molecular markers in combination with cell morphology and position within the retina (Haverkamp et al. 2000).
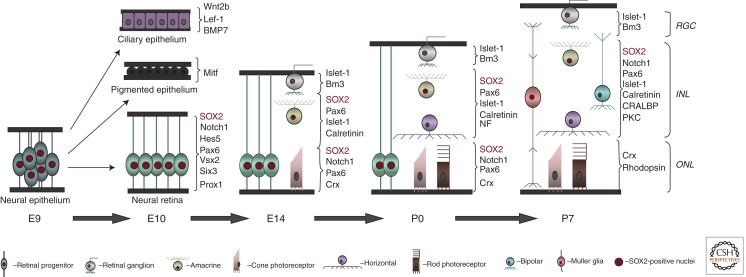
Figure 7.
Temporal progression of retinogenesis in the mouse. During development of the optic cup, neural epithelium of the optic vesicle gives rise to pigmented and ciliary epithelia, as well as neural retinal progenitor cells. The embryonic wave of neurogenesis in the retina is characterized by production of early-born retinal ganglion cells, horizontal interneurons, cone photoreceptors, and amacrine interneurons. Postnatal retinal progenitor cells predominantly give rise to rod photoreceptors, bipolar interneurons, and Müller glia. In the adult neural retina, neuronal and glial cell types are organized into three distinct cellular layers. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
Throughout neurogenesis retinal progenitor cells undergo symmetric and asymmetric cell divisions, giving rise to postmitotic neurons as well as progenitor cells. The cell bodies of postmitotic neurons are generally translocated to their final laminar positions, which are either embedded in the neuroblast (outer) layer of the ventricular zone (photoreceptors and horizontal cells) or concentrate within the vitreal (inner) ganglion cell layer (ganglion and amacrine cells). The seven major retinal cell types are generated in a sequential yet overlapping order with RGCs differentiating first and Müller glia last (Fig. 7). A wave of early neuronal differentiation in the retina is characterized by the production of early-born retinal neurons: ganglion cells, horizontal interneurons, cone photoreceptors, and amacrine interneurons. A temporal switch in differentiation potential of retinal neural progenitor cells leads to the production of late-born rod photoreceptors, bipolar interneurons, and Müller glia. Cell lineage analyses have shown that vertebrate retinal neural progenitor cells (NPCs) remain multipotent and at consecutive developmental stages, at each cell division, their progenies can assume several different cell fates. Despite their persisting multipotency and common proliferative behavior, retinal NPCs at each time point preferentially develop into one or more cell types and have limited potential for self-renewal (Cepko et al. 1996). Heterochronic transplantations have shown that early and late retinal progenitor cells have distinct differentiation capacities when placed in similar environments (Watanabe and Raff 1990; Morrow et al. 1998; Belliveau and Cepko 1999; Belliveau et al. 2000). In addition, molecular marker analyses have shown that proliferating progenitor cells are heterogeneous with regard to gene-expression profiles (Jasoni and Reh 1996; Alexiades and Cepko 1997; Yang 2004).
The intrinsic components that define properties of retinal progenitor cell competence, including transcription factors (Fig. 7), cell-surface receptors, and intracellular signaling components, undergo progressive changes as development proceeds (Cepko et al. 1996). It has been suggested that observed chronological cell birth sequence might be a reflection of conserved molecular events that underlie dynamic changes in NPC competence (Cepko et al. 1996). Moreover, dynamically changing cell-extrinsic cues are imposed on uncommitted and differentiating progenitor cells (Yang 2004). The onset of cellular differentiation in the NR appears to depend on signaling from the midline and the OS (Masai et al. 2000), and both Shh and FGF have been implicated (Yang 2004). bHLH transcriptional activators are known to promote neuronal fate and inhibit glial fate in the brain (Bertrand et al. 2002). In the developing mouse retina, five neuron-promoting bHLH genes have been identified: Math5, Ngn2, Math3, NeuroD, and Mash1. It has been proposed that bHLH and homeodomain transcription factors provide combinatorial influence on retinal cell-fate specification. Mammalian retinal progenitors that express one or more bHLH genes become biased to particular neuronal cell fates (Hatakeyama and Kageyama 2004).
13. MÜLLER GLIAL CELLS
During postnatal stages of development in the mouse, as neurogenesis is diminishing, cell division in the remaining progenitor cells results in two daughter cells that acquire neuronal or glial fate. Although RGCs, horizontal cells, cone photoreceptors, and a subset of amacrine cells (first wave of retinogenesis E11–E18) are born predominantly during embryonic stages of retinal development, rod photoreceptors, bipolar interneurons, and Müller glial cells are produced postnatally (second wave of retinogenesis P0–P7 (Fig. 7). Thus, Müller glial cells and retinal neurons are derived from a common progenitor that is multipotent at all stages of retinal histogenesis.
Müller glial cells constitute the principal glial cell population in the retina, and thus, their primary role is maintaining retinal homeostasis. Spanning the entire retinal thickness, Müller glial cells define retinal boundaries by forming intercellular junctions and a neuronal scaffold, and they play a decisive role in establishing the retinal laminar pattern and polarity (Willbold et al. 1997; Bringmann et al. 2006). Under normal homeostatic conditions in the postnatal retina, Müller glial cells act to ensure healthy retinal function by maintaining retinal architecture and providing trophic support for neurons. Consistent with this, loss of Müller glial cells in the postnatal retina results in severe lamination defects and eventual retinal degeneration (Rich et al. 1995; Dubois-Dauphin et al. 2000). In several organisms including mouse and rat, Müller glial cells can re-enter the cell cycle in response to injury and give rise to retinal neurons, indicating a regenerative capacity (Fischer and Bongini 2001; Haruta et al. 2004; Hitchcock and Raymond 2004) In addition, isolated Müller glial cells can be induced to form neurospheres, consisting of multipotent neural progenitors, which can provide a source of retinal neurons for transplantation studies (Engelhardt et al. 2004; Das et al. 2006). Therefore, Müller glial cells are considered the key target cell for retinal regenerative studies. However, it remains unclear whether the cellular and molecular mechanisms that maintain neural progenitor identity throughout embryogenesis act similarly to maintain the postnatal neurogenic capacity of Müller glial cells (Jadhav et al. 2009). The key to harnessing the neurogenic potential of Müller glial cells is to identify the transcription factors that enable their dedifferentiation, proliferation, and neurogenesis. Several pathways that regulate embryonic neural progenitor cell identity have been implicated in this process (Jadhav et al. 2009). One of the key regulators of both neural progenitor identity and gliogenesis is NOTCH1 (Jadhav et al. 2009). Components of the NOTCH1 signaling pathway, including its transcriptional downstream regulators HES1/5, not only serve as specific markers of Müller glial cells during postnatal development but also play a crucial role in establishing Müller glial cell identity. Furthermore, HES1 can act as a safeguard against irreversible cell-cycle exit during quiescence, thereby possibly preventing premature senescence and inappropriate differentiation of a neural stem cell (Sang et al. 2008; Sang and Coller 2009).
14. CONCLUDING REMARKS
The mature eye provides a valuable model for cellular replacement and regenerative therapies. It is surgically accessible and nonessential for life, and failed tissue grafts can be ablated or removed with relative ease. Moreover, advanced imaging technologies for evaluating the structure and function of the adult eye, and the wealth of basic knowledge of eye field and retinal development, together have enhanced the field of regenerative medicine for the treatment of currently incurable eye disease.
At present, the only viable option for treating loss of retinal cells in the adult eye is cellular replacement therapy. Emerging technologies have shown promise for improving the vision of patients suffering from diseases affecting the pigmented (RPE) and photoreceptor layers of the retina. The RPE serves as a barrier between the outer retina, where the photoreceptors reside, and the choriocapillaris, the network of capillaries that provides nutrients to the retina. Because the RPE actively maintains the homeostasis of the outer retina, diseases of the RPE can lead to secondary photoreceptor cell loss. The apposition of the RPE and photoreceptor layers creates the surgically accessible subretinal space where cells can be readily transplanted to treat retinal cell loss. Two diseases that affect these layers and may benefit from transplants to the subretinal space include age-related macular degeneration (AMD) and retinitis pigmentosa (RP).
Retinal transplants have been studied for their therapeutic potential since the 1950s when it was shown that fetal rat retina is viable for months after being transplanted to the anterior chamber of the maternal eye (Royo and Quay 1959). Subsequent studies have shown that full thickness sheets of fetal retina transplanted intraocularly into the adult rodent eye survive, differentiate into retinal cell types, form synapses with host tissue, and perhaps improve vision (del Cerro et al. 1991). The first indication that similar results could be obtained in humans was the temporary visual improvement of patients with RP after microaggregate suspensions of human fetal NR were injected into the subretinal space (Humayun et al. 2000). It was subsequently shown that injections of intact human fetal retinal sheets with attached RPE could survive and improve the vision of patients with RP or AMD (Radtke et al. 2002, 2008).
Immune reactivity, insufficient donor material, and persistent difficulty establishing graft-host connectivity present challenges to the use of fetal retinal transplants as a common treatment for degenerative eye disease. An alternative to fetal tissue is the use of healthy autologous RPE transplanted from the peripheral retina to the damaged central retina of patients with AMD (Binder et al. 2002, 2004; Falkner-Radler et al. 2011). However, the surgical morbidity associated with this therapy, the volume of patients in need of RPE transplants, and the genetic defects of autologous RPE cells isolated from patients with AMD highlight a need for alternative therapies to treat degenerative eye disease (MacLaren et al. 2007).
Stem cell biology has rapidly advanced the regenerative medicine field. The ability to generate large quantities of multipotent cells, which can then be directed to become any retinal cell type in culture, greatly expands the potential for new cell replacement therapeutics. Retinal progenitor cells (RPCs) are considered to be the active regenerating component of fetal retinal transplants. In 2004, Young and colleagues showed that RPCs from mouse neonates can self-renew in culture, develop into photoreceptors, and improve the visual light response after being injected into the subretinal space of adult mice suffering from retinal degeneration (Klassen et al. 2004). Another study suggested that immature rod precursor cells, as opposed to multipotent RPCs, preferentially integrate into the host retina, form synaptic connections, and improve visual function in mouse models of retinal degeneration (MacLaren et al. 2006).
This landmark finding that donor cells must be differentiated to incorporate into the host retina anticipated future studies that used embryonic stem cells to generate photoreceptor precursors for transplantation. Indeed, both mouse and human embryonic stem cells (ESCs) can be directed to form retinal neurons or RPE in culture, and these differentiated cells improve vision when injected into mouse models of retinal degeneration (Kawasaki et al. 2002; Haruta et al. 2004; Ikeda et al. 2005; Osakada et al. 2008; Lamba et al. 2009; Lu et al. 2009). However, as with fetal retinal transplants, issues of donor–host compatibility and ethics of tissue isolation have spurred investigation into the utility of adult stem cells and induced pluripotent stem cells for cellular replacement therapy.
Potential adult stem cells have been identified in the ciliary margin of humans and mice, but their ability to self-renew and give rise to all of the retinal cell types remains unclear (Tropepe et al. 2000; Coles et al. 2004; Cicero et al. 2009). An alternative is the reprogramming of adult somatic cells to pluripotency using retroviral transfection of four embryonic transcription factors, Oct4, Sox2, KLF4, and c-Myc (Takahashi and Yamanaka 2006). Like ESCs, these induced pluripotent stem (iPS) cells can be directed to produce RPE, RPCs, and retinal neurons in culture, and have shown therapeutic potential in mouse models of retinal degeneration (Buchholz et al. 2009; Carr et al. 2009; Meyer et al. 2009; Lamba et al. 2010; Tucker et al. 2011). One barrier to stem cell therapy for humans is the potential for cells to dedifferentiate in the subretinal space, giving rise to teratomas. However, successive rounds of depletion of undifferentiated cells pretransplantation have been shown to greatly decrease this risk (Tucker et al. 2011). Another challenge is the potential need to replace the damaged Bruch’s membrane, the basement membrane of the RPE, to provide a scaffold for injected cells (Lee and Maclaren 2011).
In addition to cellular replacement therapy, viral delivery of genes to the subretinal space has shown therapeutic potential for a number of diseases. Leber congenital amourosis (LCA) is a group of rare hereditary retinal dystrophies caused by a mutation in one of more than 14 genes. Gene therapy has been used to treat human patients with a specific form of LCA caused by a mutation in RPE65. After receiving subretinal injections of AAV carrying the human RPE65 gene, LCA patients reported improvements in visual sensitivity (Hauswirth et al. 2008). Similarly, viral delivery of the light-activated chloride pump halorhodopsin to cone photoreceptor cell bodies can restore light sensitivity in mouse models of RP (Busskamp et al. 2010).
Advances in cellular replacement therapy for the treatment of degenerative eye disease have depended on a deep understanding of the basic science of eye field and retinal development. Indeed, protocols for generating retinal neurons and RPE from pluripotent cells have taken into account what is known about the signaling pathways involved in establishing the eye field—BMP and Wnt signaling inhibition and IGF-1 signaling—and generating specific retinal cell types—Notch pathway inhibition for photoreceptors, for example (Jadhav et al. 2006; Tucker et al. 2011). Knowledge of the timing and location of tissue-specific gene expression in vivo has allowed for the identification and enrichment of specific cell types differentiated from human ESCs (hESCs) and induced pluripotent stem cells (iPSCs) (Vaajasaari et al. 2011). The intersection of basic and clinical science recently materialized in the use of hESC-derived RPE to treat dry AMD and Stargardt’s macular dystrophy in humans. The preliminary results of this phase I/II clinical trial established the safety and tolerability of transplantation of hESC-RPE into the subretinal space (Schwartz et al. 2012). These cells did not appear to hyperproliferate, grow abnormally, or cause intraocular inflammation. Moreover, no vision was lost, and there was even some evidence to suggest that vision was slightly improved (Schwartz et al. 2012). The ability to characterize differentiated RPE using basic science principles, including morphology, gene expression, and functional assays, was critical to this therapeutic use of hESC-RPE.
The eye has served as an invaluable model for understanding the mechanisms that coordinate human embryogenesis. Developmental genes can be identified readily through ocular phenotypes, because the eye is not essential for the survival of the organism. Continued characterization of previously unidentified eye development genes and persistent dialogue between basic scientists and clinicians will serve as a paradigm for understanding fundamental processes in human development and treating degenerative disease.
Footnotes
Editors: Patrick P.L. Tam, W. James Nelson, and Janet Rossant
Additional Perspectives on Mammalian Development available at www.cshperspectives.org
REFERENCES
- Acampora D, Mazan S, Avantaggiato V, Barone P, Tuorto F, Lallemand Y, Brulet P, Simeone A 1996. Epilepsy and brain abnormalities in mice lacking the Otx1 gene. Nat Genet 14: 218–222 [DOI] [PubMed] [Google Scholar]
- Adelmann HB 1929. Experimental studies on the development of the eye. I. The effect of removal of median and lateral areas of the anterior end of the urodelan neural plate on the development of the eyes (Triton teniatus and Amblystoma punctatum). J Exp Zool 54: 249–317 [Google Scholar]
- Alexiades MR, Cepko CL 1997. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development 124: 1119–1131 [DOI] [PubMed] [Google Scholar]
- Andreazzoli M, Gestri G, Angeloni D, Menna E, Barsacchi G 1999. Role of Xrx1 in Xenopus eye and anterior brain development. Development 126: 2451–2460 [DOI] [PubMed] [Google Scholar]
- Aota S, Nakajima N, Sakamoto R, Watanabe S, Ibaraki N, Okazaki K 2003. Pax6 autoregulation mediated by direct interaction of Pax6 protein with the head surface ectoderm-specific enhancer of the mouse Pax6 gene. Dev Biol 257: 1–13 [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P 2000. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev 14: 2701–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakrania P, Ugur Iseri SA, Wyatt AW, Bunyan DJ, Lam WW, Salt A, Ramsay J, Robinson DO, Ragge NK 2010. Sonic hedgehog mutations are an uncommon cause of developmental eye anomalies. Am J Med Genet A 152A: 1310–1313 [DOI] [PubMed] [Google Scholar]
- Bassett EA, Williams T, Zacharias AL, Gage PJ, Fuhrmann S, West-Mays JA 2010. AP-2α knockout mice exhibit optic cup patterning defects and failure of optic stalk morphogenesis. Hum Mol Genet 19: 1791–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer N, Marquardt T, Stoykova A, Ashery-Padan R, Chowdhury K, Gruss P 2002. Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development 129: 4535–4545 [DOI] [PubMed] [Google Scholar]
- Beebe DC 1986. Development of the ciliary body: A brief review. Trans Ophthalmol Soc UK 105: 123–130 [PubMed] [Google Scholar]
- Behesti H, Holt JK, Sowden JC 2006. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev Biol 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau MJ, Cepko CL 1999. Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development 126: 555–566 [DOI] [PubMed] [Google Scholar]
- Belliveau MJ, Young TL, Cepko CL 2000. Late retinal progenitor cells show intrinsic limitations in the production of cell types and the kinetics of opsin synthesis. J Neurosci 20: 2247–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, et al. 1996. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet 14: 353–356 [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F 2002. Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3: 517–530 [DOI] [PubMed] [Google Scholar]
- Binder S, Stolba U, Krebs I, Kellner L, Jahn C, Feichtinger H, Povelka M, Frohner U, Kruger A, Hilgers RD, et al. 2002. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: A pilot study. Am J Ophthalmol 133: 215–225 [DOI] [PubMed] [Google Scholar]
- Binder S, Krebs I, Hilgers RD, Abri A, Stolba U, Assadoulina A, Kellner L, Stanzel BV, Jahn C, Feichtinger H 2004. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: A prospective trial. Invest Ophthalmol Vis Sci 45: 4151–4160 [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A 2006. Muller cells in the healthy and diseased retina. Prog Retin Eye Res 25: 397–424 [DOI] [PubMed] [Google Scholar]
- Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, Clegg DO 2009. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells 27: 2427–2434 [DOI] [PubMed] [Google Scholar]
- Bumsted KM, Barnstable CJ 2000. Dorsal retinal pigment epithelium differentiates as neural retina in the microphthalmia (mi/mi) mouse. Invest Ophthalmol Vis Sci 41: 903–908 [PubMed] [Google Scholar]
- Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC, Cabuy E, Forster V, Seeliger M, et al. 2010. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 329: 413–417 [DOI] [PubMed] [Google Scholar]
- Byerly MS, Blackshaw S 2009. Vertebrate retina and hypothalamus development. Wiley Interdiscip Rev Syst Biol Med 1: 380–389 [DOI] [PubMed] [Google Scholar]
- Cai Z, Feng GS, Zhang X 2010. Temporal requirement of the protein tyrosine phosphatase Shp2 in establishing the neuronal fate in early retinal development. J Neurosci 30: 4110–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AJ, Vugler A, Lawrence J, Chen LL, Ahmado A, Chen FK, Semo M, Gias C, da Cruz L, Moore HD, et al. 2009. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis 15: 283–295 [PMC free article] [PubMed] [Google Scholar]
- Carreres MI, Escalante A, Murillo B, Chauvin G, Gaspar P, Vegar C, Herrera E 2011. Transcription factor Foxd1 is required for the specification of the temporal retina in mammals. J Neurosci 31: 5673–5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavodeassi F, Carreira-Barbosa F, Young RM, Concha ML, Allende ML, Houart C, Tada M, Wilson SW 2005. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/β-catenin pathway. Neuron 47: 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D 1996. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci 93: 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HB 1944. Studies on an anophthalmic strain of mice. IV. A second major gene for anophthalmia. Genetics 29: 264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BK, Disanza A, Choi SY, Faber SC, Lou M, Beggs HE, Scita G, Zheng Y, Lang RA 2009. Cdc42- and IRSp53-dependent contractile filopodia tether presumptive lens and retina to coordinate epithelial invagination. Development 136: 3657–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA 1996. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383: 407–413 [DOI] [PubMed] [Google Scholar]
- Cho SH, Cepko CL 2006. Wnt2b/β-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development 133: 3167–3177 [DOI] [PubMed] [Google Scholar]
- Cicero SA, Johnson D, Reyntjens S, Frase S, Connell S, Chow LM, Baker SJ, Sorrentino BP, Dyer MA 2009. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci 106: 6685–6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles BL, Angenieux B, Inoue T, Del Rio-Tsonis K, Spence JR, McInnes RR, Arsenijevic Y, van der Kooy D 2004. Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci 101: 15772–15777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER 2004. Anterior eye development and ocular mesenchyme: New insights from mouse models and human diseases. Bioessays 26: 374–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Wang WL 2009. Retinoic acid signaling in mammalian eye development. Exp Eye Res 89: 280–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danno H, Michiue T, Hitachi K, Yukita A, Ishiura S, Asashima M 2008. Molecular links among the causative genes for ocular malformation: Otx2 and Sox2 coregulate Rax expression. Proc Natl Acad Sci 105: 5408–5413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I 2006. Neural stem cell properties of Muller glia in the mammalian retina: Regulation by Notch and Wnt signaling. Dev Biol 299: 283–302 [DOI] [PubMed] [Google Scholar]
- Davis-Silberman N, Ashery-Padan R 2008. Iris development in vertebrates; genetic and molecular considerations. Brain Res 1192: 17–28 [DOI] [PubMed] [Google Scholar]
- Davis-Silberman N, Kalich T, Oron-Karni V, Marquardt T, Kroeber M, Tamm ER, Ashery-Padan R 2005. Genetic dissection of Pax6 dosage requirements in the developing mouse eye. Hum Mol Genet 14: 2265–2276 [DOI] [PubMed] [Google Scholar]
- del Cerro M, Ison JR, Bowen GP, Lazar E, del Cerro C 1991. Intraretinal grafting restores visual function in light-blinded rats. Neuroreport 2: 529–532 [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Poitry-Yamate C, de Bilbao F, Julliard AK, Jourdan F, Donati G 2000. Early postnatal Muller cell death leads to retinal but not optic nerve degeneration in NSE-Hu-Bcl-2 transgenic mice. Neuroscience 95: 9–21 [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP 1993. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75: 1417–1430 [DOI] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y 2011. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472: 51–56 [DOI] [PubMed] [Google Scholar]
- Engelhardt M, Wachs FP, Couillard-Despres S, Aigner L 2004. The neurogenic competence of progenitors from the postnatal rat retina in vitro. Exp Eye Res 78: 1025–1036 [DOI] [PubMed] [Google Scholar]
- Falkner-Radler CI, Krebs I, Glittenberg C, Povazay B, Drexler W, Graf A, Binder S 2011. Human retinal pigment epithelium (RPE) transplantation: Outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. Br J Ophthalmol 95: 370–375 [DOI] [PubMed] [Google Scholar]
- Fantes J, Ragge NK, Lynch SA, McGill NI, Collin JR, Howard-Peebles PN, Hayward C, Vivian AJ, Williamson K, van Heyningen V, et al. 2003. Mutations in SOX2 cause anophthalmia. Nat Genet 33: 461–463 [DOI] [PubMed] [Google Scholar]
- Ferda Percin E, Ploder LA, Yu JJ, Arici K, Horsford DJ, Rutherford A, Bapat B, Cox DW, Duncan AM, Kalnins VI, et al. 2000. Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat Genet 25: 397–401 [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Bongini R 2001. Turning Muller glia into neural progenitors in the retina. Mol Neurobiol 42: 199–209 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DR, van Heyningen V 2005. Developmental eye disorders. Curr Opin Genet Dev 15: 348–353 [DOI] [PubMed] [Google Scholar]
- Fuhrmann S 2008. Wnt signaling in eye organogenesis. Organogenesis 4: 60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL 1998. BMP4 is essential for lens induction in the mouse embryo. Genes Dev 12: 3764–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T 2005. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci 46: 4200–4208 [DOI] [PubMed] [Google Scholar]
- Geng X, Speirs C, Lagutin O, Inbal A, Liu W, Solnica-Krezel L, Jeong Y, Epstein DJ, Oliver G 2008. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev Cell 15: 236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Walton DS, Maas RL 1992. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet 2: 232–239 [DOI] [PubMed] [Google Scholar]
- Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL 1994. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet 7: 463–471 [DOI] [PubMed] [Google Scholar]
- Graw J 2010. Eye development. Curr Top Dev Biol 90: 343–386 [DOI] [PubMed] [Google Scholar]
- Grindley JC, Davidson DR, Hill RE 1995. The role of Pax-6 in eye and nasal development. Development 121: 1433–1442 [DOI] [PubMed] [Google Scholar]
- Hagglund AC, Dahl L, Carlsson L 2011. Lhx2 is required for patterning and expansion of a distinct progenitor cell population committed to eye development. PLoS ONE 6: e23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson IM, Seawright A, Hardman K, Hodgson S, Zaletayev D, Fekete G, van Heyningen V 1993. PAX6 mutations in aniridia. Hum Mol Genet 2: 915–20 [DOI] [PubMed] [Google Scholar]
- Haruta M, Sasai Y, Kawasaki H, Amemiya K, Ooto S, Kitada M, Suemori H, Nakatsuji N, Ide C, Honda Y, et al. 2004. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol Vis Sci 45: 1020–1025 [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Kageyama R 2004. Retinal cell fate determination and bHLH factors. Semin Cell Dev Biol 15: 83–89 [DOI] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, et al. 2008. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum Gene Ther 19: 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wassle H 2000. The cone pedicle, a complex synapse in the retina. Neuron 27: 85–95 [DOI] [PubMed] [Google Scholar]
- Hayashi S, Okamoto N, Makita Y, Hata A, Imoto I, Inazawa J 2008. Heterozygous deletion at 14q22.1-q22.3 including the BMP4 gene in a patient with psychomotor retardation, congenital corneal opacity and feet polysyndactyly. Am J Med Genet A 146A: 2905–2910 [DOI] [PubMed] [Google Scholar]
- Hendrix RW, Zwaan J 1975. The matrix of the optic vesicle-presumptive lens interface during induction of the lens in the chicken embryo. J Embryol Exp Morphol 33: 1023–1049 [PubMed] [Google Scholar]
- Hever AM, Williamson KA, van Heyningen V 2006. Developmental malformations of the eye: The role of PAX6, SOX2 and OTX2. Clin Genet 69: 459–470 [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V 1991. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 354: 522–525 [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Raymond PA 2004. The teleost retina as a model for developmental and regeneration biology. Zebrafish 1: 257–271 [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Moore KJ, Nakayama A, Steingrimsson E, Copeland NG, Jenkins NA, Arnheiter H 1993. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 74: 395–404 [DOI] [PubMed] [Google Scholar]
- Hogan BL, Hirst EM, Horsburgh G, Hetherington CM 1988. Small eye (Sey): A mouse model for the genetic analysis of craniofacial abnormalities. Development 103: 115–119 [DOI] [PubMed] [Google Scholar]
- Horsford DJ, Nguyen MT, Sellar GC, Kothary R, Arnheiter H, McInnes RR 2005. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development 132: 177–187 [DOI] [PubMed] [Google Scholar]
- Huang J, Rajagopal R, Liu Y, Dattilo LK, Shaham O, Ashery-Padan R, Beebe DC 2011. The mechanism of lens placode formation: A case of matrix-mediated morphogenesis. Dev Biol 355: 32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh S, Hatini V, Marcus RC, Li SC, Lai E 1999. Dorsal-ventral patterning defects in the eye of BF-1-deficient mice associated with a restricted loss of shh expression. Dev Biol 211: 53–63 [DOI] [PubMed] [Google Scholar]
- Humayun MS, de Juan E Jr, del Cerro M, Dagnelie G, Radner W, Sadda SR, del Cerro C 2000. Human neural retinal transplantation. Invest Ophthalmol Vis Sci 41: 3100–3106 [PubMed] [Google Scholar]
- Ikeda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, Kamiya D, Honda Y, Sasai N, Yoshimura N, et al. 2005. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci 102: 11331–11336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Kamachi Y, Matsunami H, Imada K, Uchikawa M, Kondoh H 2007. PAX6 and SOX2-dependent regulation of the Sox2 enhancer N-3 involved in embryonic visual system development. Genes Cells 12: 1049–1061 [DOI] [PubMed] [Google Scholar]
- Iseri SU, Wyatt AW, Nurnberg G, Kluck C, Nurnberg P, Holder GE, Blair E, Salt A, Ragge NK 2010. Use of genome-wide SNP homozygosity mapping in small pedigrees to identify new mutations in VSX2 causing recessive microphthalmia and a semidominant inner retinal dystrophy. Hum Genet 128: 51–60 [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Mason HA, Cepko CL 2006. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development 133: 913–923 [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Roesch K, Cepko CL 2009. Development and neurogenic potential of Muller glial cells in the vertebrate retina. Prog Retin Eye Res 28: 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasoni CL, Reh TA 1996. Temporal and spatial pattern of MASH-1 expression in the developing rat retina demonstrates progenitor cell heterogeneity. J Comp Neurol 369: 319–327 [DOI] [PubMed] [Google Scholar]
- Jeong Y, Leskow FC, El-Jaick K, Roessler E, Muenke M, Yocum A, Dubourg C, Li X, Geng X, Oliver G, et al. 2008. Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat Genet 40: 1348–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H 1998. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 125: 2521–2532 [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H 2001. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev 15: 1272–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakubo S, Nomura T, Yamamura K, Miyazaki J, Tamai M, Osumi N 2006. Abnormal migration and distribution of neural crest cells in Pax6 heterozygous mutant eye, a model for human eye diseases. Genes Cells 11: 919–933 [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Suemori H, Mizuseki K, Watanabe K, Urano F, Ichinose H, Haruta M, Takahashi M, Yoshikawa K, Nishikawa S, et al. 2002. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc Natl Acad Sci 99: 1580–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Ross SE, Trimarchi JM, Aach J, Greenberg ME, Cepko CL 2008. Identification of molecular markers of bipolar cells in the murine retina. J Comp Neurol 507: 1795–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P, Young MJ 2004. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci 45: 4167–4173 [DOI] [PubMed] [Google Scholar]
- Lagutin O, Zhu CC, Furuta Y, Rowitch DH, McMahon AP, Oliver G 2001. Six3 promotes the formation of ectopic optic vesicle-like structures in mouse embryos. Dev Dyn 221: 342–349 [DOI] [PubMed] [Google Scholar]
- Lamba DA, Gust J, Reh TA 2009. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 4: 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA 2010. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS ONE 5: e8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Maclaren RE 2011. Sources of retinal pigment epithelium (RPE) for replacement therapy. Br J Ophthalmol 95: 445–449 [DOI] [PubMed] [Google Scholar]
- Lee HS, Bong YS, Moore KB, Soria K, Moody SA, Daar IO 2006. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat Cell Biol 8: 55–63 [DOI] [PubMed] [Google Scholar]
- Li H, Tierney C, Wen L, Wu JY, Rao Y 1997. A single morphogenetic field gives rise to two retina primordia under the influence of the prechordal plate. Development 124: 603–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu IS, Chen JD, Ploder L, Vidgen D, van der Kooy D, Kalnins VI, McInnes RR 1994. Developmental expression of a novel murine homeobox gene (Chx10): Evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron 13: 377–393 [DOI] [PubMed] [Google Scholar]
- Liu W, Lagutin OV, Mende M, Streit A, Oliver G 2006. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J 25: 5383–5395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Xu S, Wang Y, Mazerolle C, Thurig S, Coles BL, Ren JC, Taketo MM, van der Kooy D, Wallace VA 2007. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol 308: 54–67 [DOI] [PubMed] [Google Scholar]
- Liu W, Lagutin O, Swindell E, Jamrich M, Oliver G 2010. Neuroretina specification in mouse embryos requires Six3-mediated suppression of Wnt8b in the anterior neural plate. J Clin Invest 120: 3568–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R 2009. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 27: 2126–2135 [DOI] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR 2006. Retinal repair by transplantation of photoreceptor precursors. Nature 444: 203–207 [DOI] [PubMed] [Google Scholar]
- MacLaren RE, Uppal GS, Balaggan KS, Tufail A, Munro PM, Milliken AB, Ali RR, Rubin GS, Aylward GW, da Cruz L 2007. Autologous transplantation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Ophthalmology 114: 561–570 [DOI] [PubMed] [Google Scholar]
- Mangold O 1931. Das determination problem. III. Das wirbeltierague in der entwicklung und regeneration. Ergeb Biol 7: 193–403 [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P 2001. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105: 43–55 [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Signore M, Acampora D, Simeone A, Bovolenta P 2001. Otx genes are required for tissue specification in the developing eye. Development 128: 2019–2030 [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Dolez V, Rodrigo I, Zaccarini R, Leconte L, Bovolenta P, Saule S 2003. OTX2 activates the molecular network underlying retina pigment epithelium differentiation. J Biol Chem 278: 21721–21731 [DOI] [PubMed] [Google Scholar]
- Masai I, Stemple DL, Okamoto H, Wilson SW 2000. Midline signals regulate retinal neurogenesis in zebrafish. Neuron 27: 251–263 [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M 1997. The Rx homeobox gene is essential for vertebrate eye development. Nature 387: 603–607 [DOI] [PubMed] [Google Scholar]
- Matsushima D, Heavner W, Pevny LH 2011. Combinatorial regulation of optic cup progenitor cell fate by SOX2 and PAX6. Development 138: 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Martinez O, Amaya-Manzanares F, Liu C, Mendoza M, Shah R, Zhang L, Behringer RR, Mahon KA, Jamrich M 2009. Cell-autonomous requirement for rx function in the mammalian retina and posterior pituitary. PLoS ONE 4: e4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM 2009. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci 106: 16698–16703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Molotkova N, Duester G 2004. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev Dyn 231: 270–277 [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G 2006. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133: 1901–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan AP, Davidson DR, Sime C, Graham E, Baldock R, Bhattacharya SS, Hill RE 1991. The Msh-like homeobox genes define domains in the developing vertebrate eye. Development 112: 1053–1061 [DOI] [PubMed] [Google Scholar]
- Moore KB, Mood K, Daar IO, Moody SA 2004. Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev Cell 6: 55–67 [DOI] [PubMed] [Google Scholar]
- Morrow EM, Belliveau MJ, Cepko CL 1998. Two phases of rod photoreceptor differentiation during rat retinal development. J Neurosci 18: 3738–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenke M, Cohen MM Jr 2000. Genetic approaches to understanding brain development: Holoprosencephaly as a model. Ment Retard Dev Disabil Res Rev 6: 15–21 [DOI] [PubMed] [Google Scholar]
- Mui SH, Hindges R, O’Leary DD, Lemke G, Bertuzzi S 2002. The homeodomain protein Vax2 patterns the dorsoventral and nasotemporal axes of the eye. Development 129: 797–804 [DOI] [PubMed] [Google Scholar]
- Mui SH, Kim JW, Lemke G, Bertuzzi S 2005. Vax genes ventralize the embryonic eye. Genes Dev 19: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Arnheiter H 2000. Signaling and transcriptional regulation in early mammalian eye development: A link between FGF and MITF. Development 127: 3581–3591 [DOI] [PubMed] [Google Scholar]
- Nornes HO, Dressler GR, Knapik EW, Deutsch U, Gruss P 1990. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development 109: 797–809 [DOI] [PubMed] [Google Scholar]
- Okamoto F, Nakano S, Okamoto C, Hommura S, Oshika T 2004. Ultrasound biomicroscopic findings in aniridia. Am J Ophthalmol 137: 858–862 [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P 1995. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 121: 4045–4055 [DOI] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M 2008. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 26: 215–224 [DOI] [PubMed] [Google Scholar]
- Porter FD, Drago J, Xu Y, Cheema SS, Wassif C, Huang SP, Lee E, Grinberg A, Massalas JS, Bodine D, et al. 1997. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development 124: 2935–2944 [DOI] [PubMed] [Google Scholar]
- Radtke ND, Seiler MJ, Aramant RB, Petry HM, Pidwell DJ 2002. Transplantation of intact sheets of fetal neural retina with its retinal pigment epithelium in retinitis pigmentosa patients. Am J Ophthalmol 133: 544–550 [DOI] [PubMed] [Google Scholar]
- Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ 2008. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol 146: 172–182 [DOI] [PubMed] [Google Scholar]
- Ragge NK, Brown AG, Poloschek CM, Lorenz B, Henderson RA, Clarke MP, Russell-Eggitt I, Fielder A, Gerrelli D, Martinez-Barbera JP, et al. 2005a. Heterozygous mutations of OTX2 cause severe ocular malformations. Am J Hum Genet 76: 1008–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragge NK, Lorenz B, Schneider A, Bushby K, de Sanctis L, de Sanctis U, Salt A, Collin JR, Vivian AJ, Free SL, et al. 2005b. SOX2 anophthalmia syndrome. Am J Med Genet A 135: 1–7 (discussion 8) [DOI] [PubMed] [Google Scholar]
- Rasmussen JT, Deardorff MA, Tan C, Rao MS, Klein PS, Vetter ML 2001. Regulation of eye development by frizzled signaling in Xenopus. Proc Natl Acad Sci 98: 3861–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich KA, Figueroa SL, Zhan Y, Blanks JC 1995. Effects of Muller cell disruption on mouse photoreceptor cell development. Exp Eye Res 61: 235–248 [DOI] [PubMed] [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL 2008. The transcriptome of retinal Muller glial cells. J Comp Neurol 509: 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M 1996. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet 14: 357–360 [DOI] [PubMed] [Google Scholar]
- Rowan S, Chen CM, Young TL, Fisher DE, Cepko CL 2004. Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development 131: 5139–5152 [DOI] [PubMed] [Google Scholar]
- Royo PE, Quay WB 1959. Retinal transplantation from fetal to maternal mammalian eye. Growth 23: 313–336 [PubMed] [Google Scholar]
- Saint-Geniez M, D’Amore PA 2004. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol 48: 1045–1058 [DOI] [PubMed] [Google Scholar]
- Sang L, Coller HA 2009. Fear of commitment: Hes1 protects quiescent fibroblasts from irreversible cellular fates. Cell Cycle 8: 2161–2167 [DOI] [PubMed] [Google Scholar]
- Sang L, Roberts JM, Coller HA 2008. Hijacking HES1: How tumors co-opt the anti-differentiation strategies of quiescent cells. Trends Mol Med 16: 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R 2012. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 379: 713–720 [DOI] [PubMed] [Google Scholar]
- Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P 2000. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development 127: 4325–4334 [DOI] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Radice G, Ashery-Padan R, Lang RA 2009. Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development 136: 2977–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spemann H 1901. Uber correlationen in der entwicklung des auges. Verhand Anat Ges 15: 61–79 [Google Scholar]
- Streit A 2007. The preplacodal region: An ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol 51: 447–461 [DOI] [PubMed] [Google Scholar]
- Tabata Y, Ouchi Y, Kamiya H, Manabe T, Arai K, Watanabe S 2004. Specification of the retinal fate of mouse embryonic stem cells by ectopic expression of Rx/rax, a homeobox gene. Mol Cell Biol 24: 4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Tetreault N, Champagne MP, Bernier G 2009. The LIM homeobox transcription factor Lhx2 is required to specify the retina field and synergistically cooperates with Pax6 for Six6 trans-activation. Dev Biol 327: 541–550 [DOI] [PubMed] [Google Scholar]
- Thut CJ, Rountree RB, Hwa M, Kingsley DM 2001. A large-scale in situ screen provides molecular evidence for the induction of eye anterior segment structures by the developing lens. Dev Biol 231: 63–76 [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Gruss P 1996. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development 122: 3381–3391 [DOI] [PubMed] [Google Scholar]
- Trainor PA, Tam PP 1995. Cranial paraxial mesoderm and neural crest cells of the mouse embryo: Co-distribution in the craniofacial mesenchyme but distinct segregation in branchial arches. Development 121: 2569–2582 [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Stadler MB, Cepko CL 2008. Individual retinal progenitor cells display extensive heterogeneity of gene expression. PLoS ONE 3: e1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Cho SH, Cepko CL 2009. Identification of genes expressed preferentially in the developing peripheral margin of the optic cup. Dev Dyn 238: 2327–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D 2000. Retinal stem cells in the adult mammalian eye. Science 287: 2032–2036 [DOI] [PubMed] [Google Scholar]
- Tucker P, Laemle L, Munson A, Kanekar S, Oliver ER, Brown N, Schlecht H, Vetter M, Glaser T 2001. The eyeless mouse mutation (ey1) removes an alternative start codon from the Rx/rax homeobox gene. Genesis 31: 43–53 [DOI] [PubMed] [Google Scholar]
- Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, Redenti S, Daley GQ, Young MJ 2011. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS ONE 6: e18992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaajasaari H, Ilmarinen T, Juuti-Uusitalo K, Rajala K, Onnela N, Narkilahti S, Suuronen R, Hyttinen J, Uusitalo H, Skottman H 2011. Toward the defined and xeno-free differentiation of functional human pluripotent stem cell-derived retinal pigment epithelial cells. Mol Vis 17: 558–575 [PMC free article] [PubMed] [Google Scholar]
- Voronina VA, Kozhemyakina EA, O’Kernick CM, Kahn ND, Wenger SL, Linberg JV, Schneider AS, Mathers PH 2004. Mutations in the human RAX homeobox gene in a patient with anophthalmia and sclerocornea. Hum Mol Genet 13: 315–322 [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P 1991. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development 113: 1435–1449 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Raff MC 1990. Rod photoreceptor development in vitro: Intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron 4: 461–467 [DOI] [PubMed] [Google Scholar]
- Wawersik S, Maas RL 2000. Vertebrate eye development as modeled in Drosophila. Hum Mol Genet 9: 917–925 [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R 1999. BMP7 acts in murine lens placode development. Dev Biol 207: 176–188 [DOI] [PubMed] [Google Scholar]
- Westenskow P, Piccolo S, Fuhrmann S 2009. β-Catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression. Development 136: 2505–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Lu T, Metlapally R, Katowitz J, Kherani F, Wang TY, Tran-Viet KN, Young TL 2008. Identification of STRA6 and SKI sequence variants in patients with anophthalmia/microphthalmia. Mol Vis 14: 2458–2465 [PMC free article] [PubMed] [Google Scholar]
- Willbold E, Berger J, Reinicke M, Wolburg H 1997. On the role of Muller glia cells in histogenesis: Only retinal spheroids, but not tectal, telencephalic and cerebellar spheroids develop histotypical patterns. J Hirnforsch 38: 383–396 [PubMed] [Google Scholar]
- Wilson SW, Houart C 2004. Early steps in the development of the forebrain. Dev Cell 6: 167–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ 2004. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol 15: 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S, Saijoh Y, Hirokawa KE, Kopinke D, Murtaugh LC, Monuki ES, Levine EM 2009. Lhx2 links the intrinsic and extrinsic factors that control optic cup formation. Development 136: 3895–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul NA, Yan B, Moody SA 2005. Step-wise specification of retinal stem cells during normal embryogenesis. Biol Cell 97: 321–337 [DOI] [PubMed] [Google Scholar]
- Zhang L, Mathers PH, Jamrich M 2000. Function of Rx, but not Pax6, is essential for the formation of retinal progenitor cells in mice. Genesis 28: 135–142 [PubMed] [Google Scholar]
- Zhao S, Hung FC, Colvin JS, White A, Dai W, Lovicu FJ, Ornitz DM, Overbeek PA 2001. Patterning the optic neuroepithelium by FGF signaling and Ras activation. Development 128: 5051–5060 [DOI] [PubMed] [Google Scholar]
- Zhao S, Chen Q, Hung FC, Overbeek PA 2002. BMP signaling is required for development of the ciliary body. Development 129: 4435–4442 [DOI] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA 2003. Specification of the vertebrate eye by a network of eye field transcription factors. Development 130: 5155–5167 [DOI] [PubMed] [Google Scholar]