Abstract
cAMP was the first second messenger to be identified. Its three main effectors are PKA (which phosphorylates numerous metabolic enzymes), EPAC (a guanine-nucleotide-exchange factor), and cyclic-nucleotide-gated ion channels.
Cyclic adenosine 3′,5′-monophosphate (cAMP) was the first second messenger to be identified and plays fundamental roles in cellular responses to many hormones and neurotransmitters (Sutherland and Rall 1958). The intracellular levels of cAMP are regulated by the balance between the activities of two enzymes (see Fig. 1): adenylyl cyclase (AC) and cyclic nucleotide phosphodiesterase (PDE). Different isoforms of these enzymes are encoded by a large number of genes, which differ in their expression patterns and mechanisms of regulation, generating cell-type and stimulus-specific responses (McKnight 1991).
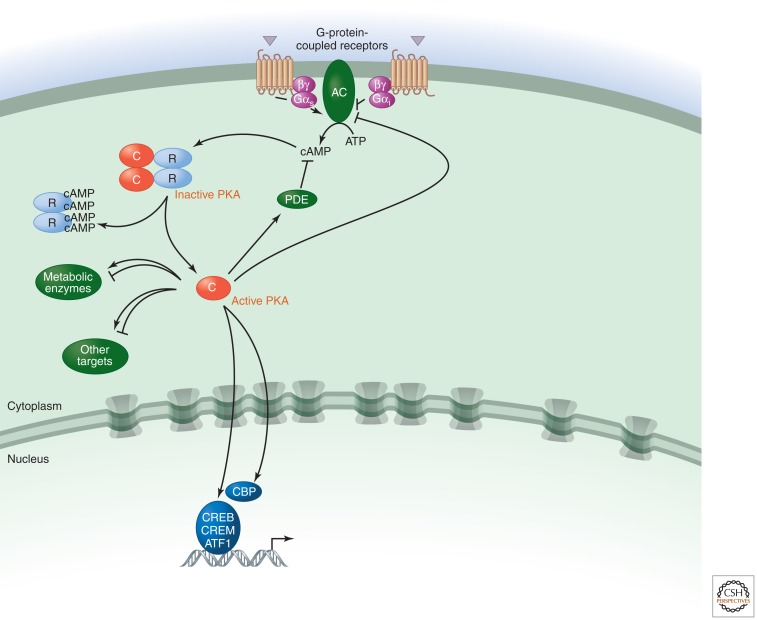
Figure 1.
PKA regulation.
Most ACs (soluble bicarbonate-regulated ACs are the exception) are activated downstream from G-protein-coupled receptors (GPCRs) such as the β adrenoceptor by interactions with the α subunit of the Gs protein (αs). αs is released from heterotrimeric αβγ G-protein complexes following binding of agonist ligands to GPCRs (e.g., epinephrine in the case of β adrenoceptors) and binds to and activates AC. The βγ subunits can also stimulate some AC isoforms. cAMP generated as a consequence of AC activation can activate several effectors, the most well studied of which is cAMP-dependent protein kinase (PKA) (Pierce et al. 2002).
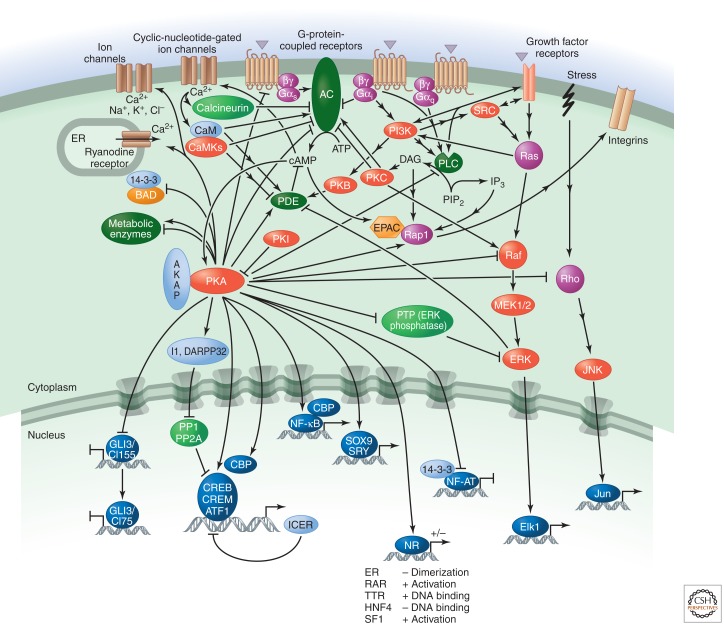
Alternatively, AC activity can be inhibited by ligands that stimulate GPCRs coupled to Gi and/or cAMP can be degraded by PDEs. Indeed both ACs and PDEs are regulated positively and negatively by numerous other signaling pathways (see Fig. 2), such as calcium signaling (through calmodulin [CaM], CamKII, CamKIV, and calcineurin [also know as PP2B]), subunits of other G proteins (e.g., αi, αo, and αq proteins, and the βγ subunits in some cases), inositol lipids (by PKC), and receptor tyrosine kinases (through the ERK MAP kinase and PKB) (Yoshimasa et al. 1987; Bruce et al. 2003; Goraya and Cooper 2005). Crosstalk with other pathways provides further modulation of the signal strength and cell-type specificity, and feedforward signaling by PKA itself stimulates PDE4.
Figure 2.
The cAMP/PKA pathway.
There are three main effectors of cAMP: PKA, the guanine-nucleotide-exchange factor (GEF) EPAC and cyclic-nucleotide-gated ion channels. Protein kinase (PKA), the best-understood target, is a symmetrical complex of two regulatory (R) subunits and two catalytic (C) subunits (there are several isoforms of both subunits). It is activated by the binding of cAMP to two sites on each of the R subunits, which causes their dissociation from the C subunits (Taylor et al. 1992). The catalytic activity of the C subunit is decreased by a protein kinase inhibitor (PKI), which can also act as a chaperone and promote nuclear export of the C subunit, thereby decreasing nuclear functions of PKA. PKA-anchoring proteins (AKAPs) provide specificity in cAMP signal transduction by placing PKA close to specific effectors and substrates. They can also target it to particular subcellular locations and anchor it to ACs (for immediate local activation of PKA) or PDEs (to create local negative feedback loops for signal termination) (Wong and Scott 2004).
A large number of cytosolic and nuclear proteins have been identified as substrates for PKA (Tasken et al. 1997). PKA phosphorylates numerous metabolic enzymes, including glycogen synthase and phosphorylase kinase, which inhibits glycogen synthesis and promotes glycogen breakdown, respectively, and acetyl CoA carboxylase, which inhibits lipid synthesis. PKA also regulates other signaling pathways. For example, it phosphorylates and thereby inactivates phospholipase C (PLC) β2. In contrast, it activates MAP kinases; in this case, PKA promotes phosphorylation and dissociation of an inhibitory tyrosine phosphatase (PTP). PKA also decreases the activities of Raf and Rho and modulates ion channel permeability. In addition, it regulates the expression and activity of various ACs and PDEs.
Regulation of transcription by PKA is mainly achieved by direct phosphorylation of the transcription factors cAMP-response element-binding protein (CREB), cAMP-responsive modulator (CREM), and ATF1. Phosphorylation is a crucial event because it allows these proteins to interact with the transcriptional coactivators CREB-binding protein (CBP) and p300 when bound to cAMP-response elements (CREs) in target genes (Mayr and Montminy 2001). The CREM gene also encodes the powerful repressor ICER, which negatively feeds back on cAMP-induced transcription (Sassone-Corsi 1995). Note, however, that the picture is more complex, because CREB, CREM, and ATF1 can all be phosphorylated by many different kinases, and PKA can also influence the activity of other transcription factors, including some nuclear receptors.
In addition to the negative regulation by signals that inhibit AC or stimulate PDE activity, the action of PKA is counterbalanced by specific protein phosphatases, including PP1 and PP2A. PKA in turn can negatively regulate phosphatase activity by phosphorylating and activating specific PP1 inhibitors, such as I1 and DARPP32. PKA-promoted phosphorylation can also increase the activity of PP2A as part of a negative feedback mechanism.
Another important effector for cAMP is EPAC, a GEF that promotes activation of certain small GTPases (e.g., Rap1). A major function of Rap1 is to increase cell adhesion via integrin receptors (how this occurs is unclear) (Bos 2003).
Finally, cAMP can bind to and modulate the function of a family of cyclic-nucleotide-gated ion channels. These are relatively nonselective cation channels that conduct calcium. Calcium stimulates CaM and CaM-dependent kinases and, in turn, modulates cAMP production by regulating the activity of ACs and PDEs (Zaccolo and Pozzan 2003). The channels are also permeable to sodium and potassium, which can alter the membrane potential in electrically active cells.
Acknowledgments
Figure 2 adapted from Fimia and Sassone-Corsi (2001).
Footnotes
Editors: Lewis Cantley, Tony Hunter, Richard Sever, and Jeremy Thorner
Additional Perspectives on Signal Transduction available at www.cshperspectives.org
REFERENCES
- Bos JL 2003. Epac: A new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4: 733–738 [DOI] [PubMed] [Google Scholar]
- Bruce JI, Straub SV, Yule DI 2003. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium 34: 431–444 [DOI] [PubMed] [Google Scholar]
- Fimia GM, Sassone-Corsi P 2001. Cyclic AMP signaling. J Cell Sci 114: 1971–1972 [DOI] [PubMed] [Google Scholar]
- Goraya TA, Cooper DMF 2005. Ca2+-calmodulin-dependent phosphodiesterase (PDE1): Current perspectives. Cell Signal 17: 789–797 [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M 2001. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2: 599–609 [DOI] [PubMed] [Google Scholar]
- McKnight GS 1991. Cyclic AMP second messenger systems. Curr Opin Cell Biol 3: 213–217 [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ 2002. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650 [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P 1995. Transcription factors responsive to cAMP. Annu Rev Cell Dev Biol 11: 355–377 [DOI] [PubMed] [Google Scholar]
- Sutherland EW, Rall TW 1958. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem 232: 1077–1091 [PubMed] [Google Scholar]
- Tasken K, Skalhegg BS, Tasken KA, Solberg R, Knutsen HK, Levy FO, Sandberg M, Orstavik S, Larsen T, Johansen AK, et al. 1997. Structure, function and regulation of human cAMP-dependent protein kinases. Adv Second Messenger Phosphoprotein Res 31: 191–203 [DOI] [PubMed] [Google Scholar]
- Taylor SS, Knighton DR, Zheng J, Ten Eyck LF, Sowadski JM 1992. Structural framework for the protein kinase family. Annu Rev Cell Biol 8: 429–462 [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD 2004. AKAP signaling complexes: Focal points in space and time. Nat Rev Mol Cell Biol 5: 959–970 [DOI] [PubMed] [Google Scholar]
- Yoshimasa T, Sibley DR, Bouvier M, Lefkowitz RJ, Caron MG 1987. Cross-talk between cellular signaling pathways suggested by phorbol-ester induced adenylate cyclase phosphorylation. Nature 327: 67–70 [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T 2003. cAMP and Ca2+ interplay: A matter of oscillation patterns. Trends Neurosci 26: 53–55 [DOI] [PubMed] [Google Scholar]