Abstract
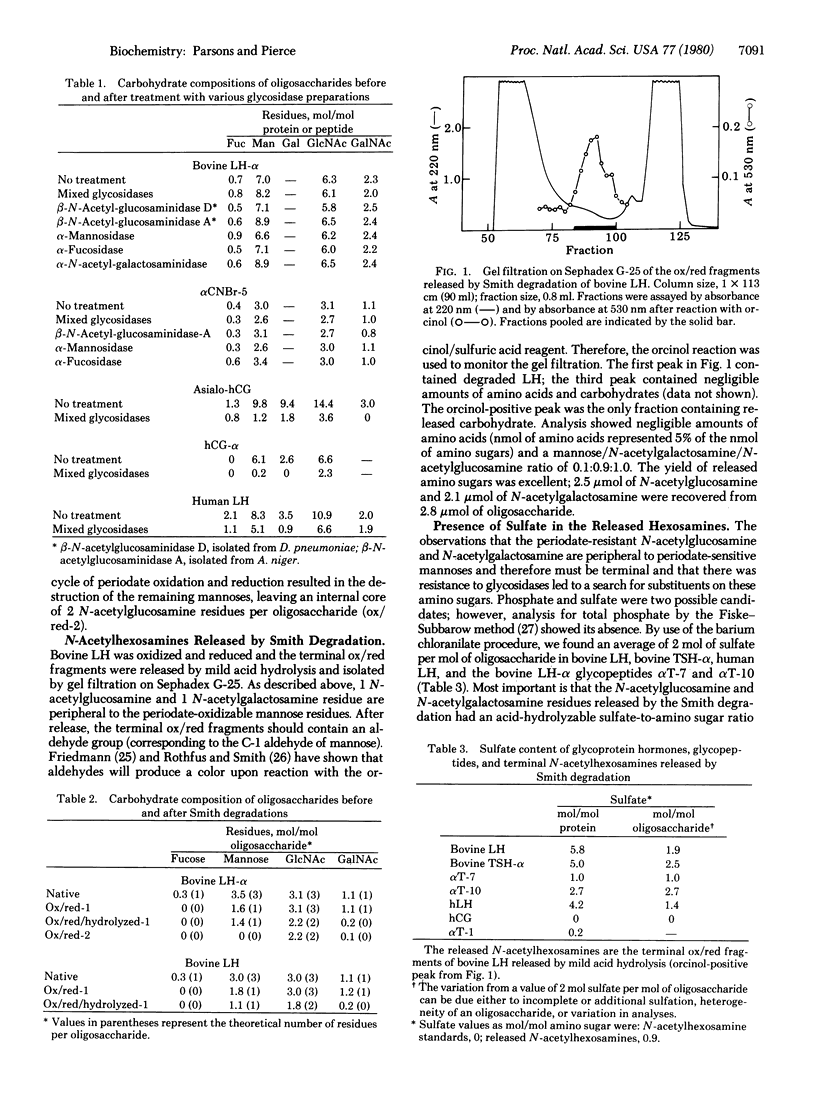
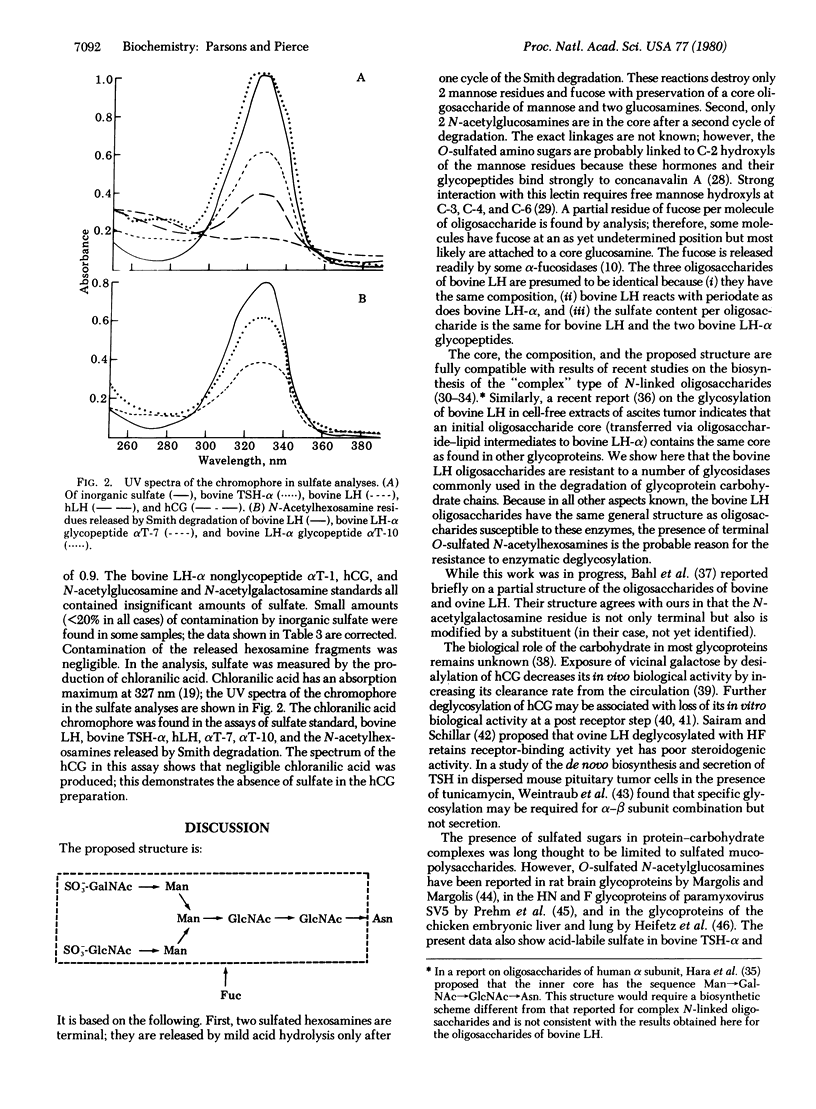
The oligosaccharides of the bovine pituitary gonadotropin lutropin are N-linked to asparagine residues. These carbohydrates are unusual in that, although they contain the mannose, N-acetylglucosamine, and fucose typical of N-linked oligosaccharides, they also contain one residue of N-acetylgalactosamine but insignificant amounts of sialic acid or galactose. These oligosaccharides exhibit complete resistance to several exoglycosidases. This is in contrast to the ready release of peripheral sugars from human chorionic gonadotropin, a placenta hormone which has oligosaccharides of the complex type with terminal sialic acid and galactose residues. Stability of the lutropin hexosamines to periodate oxidation and reduction (Smith degradation) together with other data show that one residue of N-acetylgalactosamine and one of N-acetylglucosamine are peripheral to two periodate-sensitive mannose residues. The insensitivity to periodate of these two terminal amino sugars is found to result from a sulfate group covalently linked to each; sulfation of these hexosamines is also the most probable reason for the resistance to enzymatic deglycosylation. The alpha subunits of bovine thyrotropin and human pituitary lutropin also contain sulfate, in contrast to human chorionic gonadotropin. The results indicate that sulfating enzymes are present in the pituitary and that sulfation of peripheral sialic acids in the placental gonadotropin. The data lead to a partial structure for the oligosaccharides of bovine LH as follows: (formula see text).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979 Apr 10;254(7):2400–2407. [PubMed] [Google Scholar]
- Bellisario R., Carlsen R. B., Bahl O. P. Human chorionic gonadotropin. Linear amino acid sequence of the alpha subunit. J Biol Chem. 1973 Oct 10;248(19):6796–6809. [PubMed] [Google Scholar]
- Bloomfield G. A., Faith M. R., Pierce J. G. Sepharose-linked concanavalin A in the purification and characterization of glycoprotein hormones of the bovine pituitary. Biochim Biophys Acta. 1978 Apr 26;533(2):371–382. doi: 10.1016/0005-2795(78)90383-5. [DOI] [PubMed] [Google Scholar]
- Carlsen R. B., Pierce J. G. Purification and properties of an alpha-L-fucosidase from rat epididymis. J Biol Chem. 1972 Jan 10;247(1):23–32. [PubMed] [Google Scholar]
- Channing C. P., Sakai C. N., Bahl O. P. Role of the carbohydrate residues of human chorionic gonadotropin in binding and stimulation of adenosine 3',5'-monophosphate accumulation by porcine granulosa cells. Endocrinology. 1978 Aug;103(2):341–348. doi: 10.1210/endo-103-2-341. [DOI] [PubMed] [Google Scholar]
- Chapman A., Li E., Kornfeld S. The biosynthesis of the major lipid-linked oligosaccharide of Chinese hamster ovary cells occurs by the ordered addition of mannose residues. J Biol Chem. 1979 Oct 25;254(20):10243–10249. [PubMed] [Google Scholar]
- Cornell J. S., Pierce J. G. The subunits of human pituitary thyroid-stimulating hormone. Isolation, properties, and composition. J Biol Chem. 1973 Jun 25;248(12):4327–4333. [PubMed] [Google Scholar]
- Endo Y., Yamashita K., Tachibana Y., Tojo S., Kobata A. Structures of the asparagine-linked sugar chains of human chorionic gonadotropin. J Biochem. 1979 Mar;85(3):669–679. [PubMed] [Google Scholar]
- Friedmann R. Characterization of sugar components of protein. Biochem J. 1949;44(1):117–126. [PMC free article] [PubMed] [Google Scholar]
- Hara K., Rathnam P., Saxena B. B. Structure of the carbohydrate moieties of alpha subunits of human follitropin, lutropin, and thyrotropin. J Biol Chem. 1978 Mar 10;253(5):1582–1591. [PubMed] [Google Scholar]
- Heifetz A., Kinsey W. H., Lennarz W. J. Synthesis of a novel class of sulfated glycoproteins in embryonic liver and lung. J Biol Chem. 1980 May 25;255(10):4528–4534. [PubMed] [Google Scholar]
- Howard S. M., Pierce J. G. The tryptic glycopeptides of bovine thyrotropin. Their composition and similarities to those of luteinizing hormone. J Biol Chem. 1969 Dec 10;244(23):6468–6476. [PubMed] [Google Scholar]
- Kessler M. J., Mise T., Ghai R. D., Bahl O. P. Structure and location of the O-glycosidic carbohydrate units of human chorionic gonadotropin. J Biol Chem. 1979 Aug 25;254(16):7909–7914. [PubMed] [Google Scholar]
- Kessler M. J., Reddy M. S., Shah R. H., Bahl O. P. Structures of N-glycosidic carbohydrate units of human chorionic gonadotropin. J Biol Chem. 1979 Aug 25;254(16):7901–7908. [PubMed] [Google Scholar]
- Kim J. H., Shome B., Liao T. H., Pierce J. G. Analysis of neutral sugars by gas-liquid chromatography of alditol acetates: application to thyrotropic hormone and other glycoproteins. Anal Biochem. 1967 Aug;20(2):258–274. doi: 10.1016/0003-2697(67)90031-0. [DOI] [PubMed] [Google Scholar]
- Kobata A. Use of endo- and exoglycosidases for structural studies of glycoconjugates. Anal Biochem. 1979 Nov 15;100(1):1–14. doi: 10.1016/0003-2697(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Li E., Tabas I. The synthesis of complex-type oligosaccharides. II. Characterization of the processing intermediates in the synthesis of the complex oligosaccharide units of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7771–7778. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li E., Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. I. Structure of the lipid-linked oligosaccharide precursor of the complex-type oligosaccharides of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7762–7770. [PubMed] [Google Scholar]
- Liao T. H., Hennen G., Howard S. M., Shome B., Pierce J. G. Bovine thyrotropin. Countercurrent distribution and a comparison with the isolated subunits of luteinizing hormone. J Biol Chem. 1969 Dec 10;244(23):6458–6467. [PubMed] [Google Scholar]
- Liao T. H., Pierce J. G. The presence of a common type of subunit in bovine thyroid-stimulating and luteinizing hormones. J Biol Chem. 1970 Jul 10;245(13):3275–3281. [PubMed] [Google Scholar]
- Liu W. K., Ward D. N. The purification and chemistry of pituitary glycoprotein hormones. Pharmacol Ther B. 1975;1(3):545–570. doi: 10.1016/0306-039x(75)90053-7. [DOI] [PubMed] [Google Scholar]
- Margolis R. K., Margolis R. U. Sulfated glycopeptides from rat brain glycoproteins. Biochemistry. 1970 Oct 27;9(22):4389–4396. doi: 10.1021/bi00824a020. [DOI] [PubMed] [Google Scholar]
- Morgan F. J., Birken S., Canfield R. E. The amino acid sequence of human chorionic gonadotropin. The alpha subunit and beta subunit. J Biol Chem. 1975 Jul 10;250(13):5247–5258. [PubMed] [Google Scholar]
- Moyle W. R., Bahl O. P., März L. Role of carbohydrate of human chorionic gonadotropin in the mechanism of hormone action. J Biol Chem. 1975 Dec 10;250(23):9163–9169. [PubMed] [Google Scholar]
- Pierce J. G. Eli Lilly lecture. The subunits of pituitary thyrotropin--their relationship to other glycoprotein hormones. Endocrinology. 1971 Dec;89(6):1331–1344. doi: 10.1210/endo-89-6-1331. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Liao T. H., Carlsen R. B., Reimo T. Comparisons between the alpha chain of bovine thyrotropin and the CI chain of luteinizing hormone. Compositions of tryptic peptides, cyanogen bromide fragments, and carbohydrate moieties. J Biol Chem. 1971 Feb 25;246(4):866–872. [PubMed] [Google Scholar]
- Prehm P., Scheid A., Choppin P. W. The carbohydrate structure of the glycoproteins of the paramyxovirus SV5 grown in bovine kidney cells. J Biol Chem. 1979 Oct 10;254(19):9669–9677. [PubMed] [Google Scholar]
- ROTHFUS J. A., SMITH E. L. Glycopeptides. IV. The periodate oxidation of glycopeptides from human gamma-globulin. J Biol Chem. 1963 Apr;238:1402–1410. [PubMed] [Google Scholar]
- SPENCER B. The ultramicro determination of inorganic sulphte. Biochem J. 1960 Jun;75:435–440. doi: 10.1042/bj0750435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam M. R., Schiller P. W. Receptor binding, biological, and immunological properties of chemically deglycosylated pituitary lutropin. Arch Biochem Biophys. 1979 Oct 1;197(1):294–301. doi: 10.1016/0003-9861(79)90248-0. [DOI] [PubMed] [Google Scholar]
- Shome B., Brown D. M., Howard S. M., Pierce J. G. Bovine, human and porcine thyrotropins: molecular weights, amino- and carboxyl-terminal studies. Arch Biochem Biophys. 1968 Aug;126(2):456–468. doi: 10.1016/0003-9861(68)90430-x. [DOI] [PubMed] [Google Scholar]
- Shome B., Parlow A. F., Ramirez V. D., Elrick H., Pierce J. G. Human and porcine thyrotropins: a comparison of electrophoretic and immunological properties with the bovine hormone. Arch Biochem Biophys. 1968 Aug;126(2):444–455. doi: 10.1016/0003-9861(68)90429-3. [DOI] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Van Hall E. V., Vaitukaitis J. L., Ross G. T., Hickman J. W., Ashwell G. Immunological and biological activity of HCG following progressive desialylation. Endocrinology. 1971 Feb;88(2):456–464. doi: 10.1210/endo-88-2-456. [DOI] [PubMed] [Google Scholar]
- WARD D. N., COFEY J. A. ANALYSIS OF THE ACETYL GROUPS IN OVINE LUTEINIZING HORMONE BY GAS CHROMATOGRAPHY. Biochemistry. 1964 Oct;3:1575–1577. doi: 10.1021/bi00898a032. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Weintraub B. D., Stannard B. S., Linnekin D., Marshall M. Relationship of glycosylation to de novo thyroid-stimulating hormone biosynthesis and secretion by mouse pituitary tumor cells. J Biol Chem. 1980 Jun 25;255(12):5715–5723. [PubMed] [Google Scholar]


