Abstract
Study Objectives
Pharyngeal muscle dilators are important in obstructive sleep apnea pathogenesis because the failure of protective reflexes involving these muscles yields pharyngeal collapse. Conflicting results exist in the literature regarding the responsiveness of these muscles during stable non-rapid eye movement sleep. However, variations in posture in previous studies may have influenced these findings. We hypothesized that tongue protruder muscles are maximally responsive to negative pressure pulses during supine sleep, when posterior tongue displacement yields pharyngeal occlusion.
Design
We studied all subjects in the supine and lateral postures during wakefulness and stable non-rapid eye movement sleep by measuring genioglossus and tensor palatini electromyograms during basal breathing and following negative pressure pulses.
Setting
Upper-airway physiology laboratory of Sleep Medicine Division, Brigham and Women’s Hospital.
Subjects/Participants
17 normal subjects.
Measurements and Results
We observed an increase in genioglossal responsiveness to negative pressure pulses in sleep as compared to wakefulness in supine subjects (3.9 percentage of maximum [%max] ± 1.1 vs 4.4 %max ± 1.0) but a decrease in the lateral decubitus position (4.1 %max ± 1.0 vs 1.5 %max ± 0.4), the interaction effect being significant. Despite this augmented reflex, collapsibility, as measured during negative pressure pulses, increased more while subjects were in the supine position as compared with the lateral decubitus position. While the interaction between wake-sleep state and position was also significant for the tensor palatini, the effect was weaker than for genioglossus, although, for tensor palatini, baseline activity was markedly reduced during non-rapid eye movement sleep as compared with wakefulness.
Conclusions
We conclude that body posture does have an important impact on genioglossal responsiveness to negative pressure pulses during non-rapid eye movement sleep. We speculate that this mechanism works to prevent pharyngeal occlusion when the upper airway is most vulnerable to collapse eg, during supine sleep.
Keywords: pharynx, posture, sleep, breathing, genioglossus, lung, tensor palatini, respiration, upper airway, vestibular
INTRODUCTION
THE MECHANISMS GOVERNING PHARYNGEAL PATENCY ARE CLEARLY IMPORTANT, AS THEIR FAILURE LEADS TO PHARYNGEAL COLLAPSE MANIFESTING AS OBSTRUCTIVE SLEEP APNEA (OSA).1–3 OSA is a common disease with important neurocognitive and cardiovascular sequelae.4–6 One of the major factors contributing to the loss of pharyngeal patency during sleep is thought to be the failure of upper-airway reflexes.7,8 One such reflex, the negative pressure reflex (NPR), describes a robust activation of the pharyngeal dilator muscles (primarily the genioglossus muscle) in response to negative pharyngeal pressure.9,10 Most, but not all, data suggest that this reflex is largely attenuated at sleep onset leaving the pharyngeal airway vulnerable to collapse in those anatomically predisposed.
Pharyngeal dilator muscles have been broadly classified into tonic (constant activity throughout the respiratory cycle eg, tensor palatini) and phasic (bursts of activity with inspiration eg, genioglossus). The tensor palatini is thought to stiffen the palate and lower velopharyngeal resistance, whereas the genioglossus is a tongue protruder that prevents pharyngeal collapse when the tongue is moving in a posterior direction.11,12 Multiple factors may modulate the activity of these muscles including cortical (behavioral) activity, the wakefulness drive to breathe, medullary respiratory central pattern generator activity, local mechanoreceptive influences, chemoreceptive reflexes, respiratory premotor inputs, and several different neurochemicals in the brain stem.13–17 However, the role of each of these factors on pharyngeal motor control in sleeping humans remains unclear.
The available literature regarding the responsiveness of pharyngeal muscles during sleep is inconsistent.18–21 During physiologic experiments, the majority of studies have shown minimal responsiveness of these muscles to various mechanoreceptive and chemoreceptive stimuli during non-rapid eye movement (NREM) sleep.7,8,22 On the other hand, when the natural behavior of these muscles is studied, important increases in muscle activity are observed in normal subjects between the onset of sleep and stable NREM sleep and, in patients with OSA, during obstructive apneas.2,23 Both are situations in which intrapharyngeal negative pressure is increasing with the muscles seemingly responding to the incrementing pressure. Why the muscle responds in one situation and not the other is unclear. However, most of the physiologic studies described above were conducted in the lateral decubitus posture, whereas the natural behavior was tested in supine subjects. These apparently discordant data led us to speculate that differences in body position might be important in modulating negative pressure responsiveness. In theory, the NPR should be most active when the airway is most vulnerable to collapse (eg, supine posture) and less active when the airway is relatively protected from collapse (eg, lateral decubitus posture). In particular, the genioglossus muscle may be more sensitive to relevant stimuli when the sleeper is in a body position that facilitates airway obstruction by the tongue (supine position). On the other hand, the tensor palatini, which serves to stiffen the palate, may be equally important in preserving pharyngeal patency in all positions. We therefore elected to test this hypothesis.
Hypothesis
The responsiveness of the genioglossus (in contrast to the tensor palatini) to negative pressure stimulation during stable NREM sleep will be greater in the supine as compared to the lateral decubitus posture.
METHODS
Subjects
Seventeen normal subjects (Table 1) underwent recordings during wakefulness and NREM sleep. Each subject underwent a thorough history and physical examination by an attending physician and was found to be completely healthy and on no medications. All premenopausal women studied were in the follicular phase of the menstrual cycle (by history). Informed consent was obtained from each participant, with the protocol having the prior approval of the Human Subjects Committee of the Brigham and Women’s Hospital.
Table 1.
Subject Demographics
| Subject | Age, y | Sex | BMI, kg/m2 |
|---|---|---|---|
| 1 | 36 | F | 21.7 |
| 2 | 33 | F | 19.9 |
| 3 | 35 | F | 23.2 |
| 4 | 25 | F | 20.1 |
| 5 | 25 | F | 25.6 |
| 6 | 22 | F | 21.2 |
| 7 | 57 | F | 22.6 |
| 8 | 25 | M | 21.0 |
| 9 | 29 | M | 24.4 |
| 10 | 30 | M | 25.7 |
| 11 | 24 | M | 22.0 |
| 12 | 28 | M | 22.9 |
| 13 | 25 | M | 24.0 |
| 14 | 26 | M | 23.7 |
| 15 | 23 | M | 26.1 |
| 16 | 26 | M | 22.8 |
| 17 | 31 | M | 25.9 |
Mean ± SD 29.4 ± 8.2 23.1 ± 2.0 F refers to female; M refers to male.
Instrumentation and Techniques
Using the standard techniques of our laboratory,24–26 the following signals were recorded: wakefulness and sleep with electroencephalography, electrooculography, and submental electromyography (EMG); mask pressure via nasal mask (Healthdyne Technologies, Marietta, GA) and a differential pressure transducer (Validyne Corp); choanal (PCHO) and epiglottic (PEPI) pressures (MPC-500, Millar catheters, Houston, Tex); genioglossus (GGEMG) and tensor palatini (TPEMG) intramuscular EMGs (using paired unipolar electrodes, referenced to a common ground electrode placed on the forehead, quantified as percentage of maximum [%max]); and inspiratory flow with a pneumotachometer (Fleisch, Inc., Lausanne, Switzerland) and a differential pressure transducer (Validyne Corp., Northridge, Calif.).
Subjects’ lips were taped shut, and they were instructed to breathe exclusively through the nose. Further, they were carefully monitored to ensure that the mouth remained closed. Mask leak was detected by CO2 sampling, as previously described.28
Each negative pressure pulse (NPP) was rapid and generated − 8 to −12 cmH2O pressure at the choanae, with a goal of −10 cmH2O. All recordings were signal averaged to generate a representative stimulus (PCHO) and response (GGEMG or TPEMG) for analysis. Collapsibility was quantified as the pressure difference between the choanae and the epiglottis during the NPP.27 In theory, a perfectly rigid airway would transmit all of the pressure from the choanae to the epiglottis during a NPP applied nasally. On the other hand, a highly collapsible airway would transmit essentially none of the pressure from the choanae to the epiglottis during a nasally applied pulse. This technique ignores the resistive pressure drop related to airflow, which is small during a rapid pulse that induces airway occlusion. We have previously validated this technique and shown that the spectrum of pharyngeal collapsibilities can be quantified as a function of this pressure difference (PCHO minus PEPI). To equate stimulus magnitude, we normalized this measure of collapsibility for the level of applied negative pressure:
, as previously reported.27
As the genioglossus is a phasic muscle (bursts with inspiration), 3 levels of activity are reported during basal breathing: tonic (during expiration), peak phasic (peak inspiration), and phasic (peak phasic minus tonic). The tonic activity is the expiratory EMG activity and is thought to be critical in preventing pharyngeal collapse at end expiration, while the phasic activity represents respiratory premotor plus NPR-mediated neural input to the genioglossus muscle. The peak phasic activity represents the aggregate of all inputs to genioglossus activation and is likely to be important in preventing collapse at midinspiration. As the tensor palatini is a tonic muscle, 1 measure, indicating average level of activity throughout the respiratory cycle, is reported. During the NPPs, both muscles show an increase in activity that we characterize as a “ΔGG or ΔTP” and as a percentage increase in GGEMG or TPEMG. These values characterize the magnitude of the neural reflex response.
Study Protocol
Subjects reported in the evening and then were fully instrumented. During wakefulness, basal breathing (5 minutes) followed by NPPs in the lateral decubitus and supine postures were recorded (20 to 30 pulses in each position). Subjects were then allowed to fall asleep, and the basal breathing and NPPs were again quantified during stable NREM sleep. During sleep, once the recording was completed in 1 posture, the subject was awoken, the posture was changed, and the recording resumed once stable NREM sleep was achieved. The order of the postures both awake and asleep was randomly assigned.
Each variable, both from the baseline and NPP response data, were analyzed in a 2 × 2 analysis of variance (ANOVA) with repeated measures on each factor, the 2 factors being Body Position (supine and lateral) and Sleep-Wake State (awake and asleep). Thus the 2 × 2 ANOVAs provided testing of significance for state effects (ie, wake vs sleep), posture effects (ie, supine vs lateral), and their interaction (ie, state/posture). Nonnormally distributed variables were log transformed for analysis. For 4 subjects, missing data points occurred during sleep, requiring us to censor the data points and to reduce the degrees of freedom accordingly. For analysis purposes, missing values were interpolated using the cross-product of the subject’s wake values and the group trend during sleep. This statistical approach to missing data points has been validated and accepted in the statistical literature.29 However, we did also run paired t tests on the values obtained during sleep with very similar results. Only the ANOVA results are presented in the manuscript. Results are presented as mean ± SD, with P < .05 being considered statistically significant.
RESULTS
Baseline Measurements
Ventilation and airflow were lower during baseline sleep, compared to baseline wakefulness (see Table 2). The lower ventilation was associated with higher airway resistance in the nasal and pharyngeal airways. However, sleep-wake state did not affect respiratory timing. While body position had somewhat less of an effect, airflow, nasal negative pressure, and duty cycle were significantly greater in the lateral position, while PMASK was less negative (data not shown). Only ventilation showed a significant interaction between sleep-wake state and body position, with the sleep-related reduction in ventilation being greater in the supine position. Thus, in general, ventilation and airway patency were the same in the 2 body positions but were reduced by sleep.
Table 2.
Mean Values During Baseline Breathing for 4 Conditions
| Parameter | Supine | Lateral | F statistic | ||||
|---|---|---|---|---|---|---|---|
| Awake | Sleep | Awake | Sleep | Position | State | Interaction | |
| Peak GGEMG,%max | 14.2±12.4 | 19.7±18.7 | 9.2±9.6 | 10.0±13.5 | 12.8** | 3.97 | 3.89 |
| Phasic.GGEMG,%max | 5.0±4.3 | 7.6±6.2 | 3.5±3.5 | 3.3±3.5 | 14.6** | 6.51*† | 5.43*† |
| Tonic GGEMG,%max | 9.2±8.3 | 12.1±13.2 | 5.7±6.4 | 6.6±10.2 | 11.2** | 2.06 | 1.58 |
| Average TPEMG | 4.4±4.5 | 1.9±1.1 | 2.7±1.9 | 1.6±0.9 | 3.19 | 7.52* | 1.84 |
| TI/TTOT | 41.7±3.4 | 39.6±3.8 | 38.2±2.5 | 38.0±2.8 | 31.6*** | 2.08 | 2.58 |
| VT, mL | 518±119 | 448±85.6 | 490±109 | 470±71.5 | 0.07 | 11.1** | 7.51* |
| VE, L/min | 7.8±1.21 | 6.3±1.07 | 7.4±1.43 | 6.7±1.04 | 0.00 | 33.2*** | 7.56* |
| Peak flow, L/s | 0.4±0.5 | 0.3±0.1 | 0.4±0.1 | 0.4±0.1 | 10.2** | 49.4*** | 4.97* |
| RNAS, cmH2O · L−1· s−1 | 0.91±0.59 | 1.46±1.08 | 1.07±0.39 | 1.50±0.99 | 0.52 | 5.88* | 0.55 |
| RPHA, cmH2O· L−1· s−1 | 2.0±3.0 | 4.4±5.8 | 1.3±0.7 | 3.9±4.8 | 0.48 | 4.81* | 0.02 |
| RSUP, cmH2O· L−1· s−1 | 3.0±3.0 | 5.9±5.8 | 2.4±0.8 | 5.4±4.9 | 0.34 | 8.69** | 0.00 |
Data are presented as mean ± SD.
P < .05
P < .01
P < .001
Not significant in transformed analysis
GGEMG refers to genioglossal electromyogram; %max, percentage of maximum; TPEMG, tensor palatini electromyogram; RNAS, nasal resistance; RPHA, pharyngeal resistance; RSUP, supraglottic resistance; VT, tidal volume; VE, minute ventilation; TI/TTOT, duty cycle
Baseline peak phasic, phasic, and tonic GGEMG activity were influenced by body position, with the muscle being more active in the supine position both awake and during NREM sleep. Further, there was a tendency for this difference to be greater during sleep than during wakefulness, the interaction effect being significant for the phasic component of the muscle (see Table 2). Finally, baseline TPEMG fell markedly during sleep but was not influenced by body position.
Responses to NPP
The effect of the NPP stimulus on the upper airway was complex (Table 3, Figure 1). The pharyngeal airway was more collapsible in response to the negative pressure stimulus when subjects were in the supine as compared to the lateral position and during sleep as compared to wakefulness. There was also a significant interaction between sleep-wake state and position, with sleep having a greater effect on collapsibility in the supine position. Further, the main effects remained significant when collapsibility was normalized for stimulus strength (collapsibility as a percentage of applied PCHO), although the interaction effect was no longer significant.
Table 3.
Mean Responses to Negative Pulse Pressure for 4 Conditions
| Parameter | Supine | Lateral | F statistic | ||||
|---|---|---|---|---|---|---|---|
| Awake | Sleep | Awake | Sleep | Position | State | Interaction | |
| Δ GGEMG, %max | 3.9±1.10 | 4.4±0.97 | 4.1±1.05 | 1.5±0.36 | 5.30* | 2.96† | 9.98** |
| GGEMG, %increase | 38.7±7.3 | 38.8±9.8 | 65.7±18.4 | 30.0±8.2 | 1.46 | 9.23** | 4.95* |
| Δ TPEMG, %max | 3.5±1.5 | 2.4±0.7 | 4.8±1.7 | 1.3±0.4 | 0.01 | 4.15 | 5.45* |
| TPEMG, %increase | 89.5±32.0 | 100.2±27.1 | 108.6±35.1 | 58.7±15.6 | 0.36 | 1.12 | 2.82† |
| Collapsibility,cmH2O | 3.5 ± 0.5 | 5.6 ± 0.5 | 2.9 ± 0.5 | 4.0 ± 0.6 | 16.9** | 15.5** | 5.40* |
| Collapsibility, PCHO, % | 37.5±5.4 | 56.8±4.8 | 30.6±5.0 | 46.4±6.5 | 9.78** | 25.5*** | 0.59 |
Data are presented as mean ± SD.
ΔGGEMG refers to change in genioglossal electromyogram; %max, percentage of maximum; ΔTPEMG, change in tensor palatini electromyogram; PCHO, choanal pressure.
P < .05
P < .01
P < .001
Significant in transformed analysis
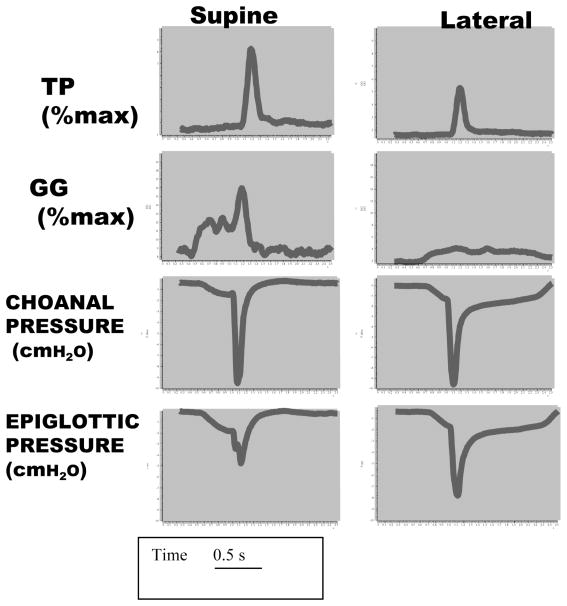
Figure 1.
Example of raw data showing the negative pressure reflex. The figure shows data from an individual illustrating a pulse of negative pressure delivered during early inspiration in stable non-rapid eye movement sleep. The choanal pressure reflects the magnitude of the stimulus, while the epiglottic pressure reflects the extent to which the pressure pulse is transmitted through the pharynx (measure of collapsibility). The reflex activity of the genioglossus (GG) and tensor palatini (TP) is greater in the supine posture than in the lateral decubitus position. As well, the pharynx is more collapsible supine since less of the choanal pressure is transmitted in this position as compared with the lateral decubitus.
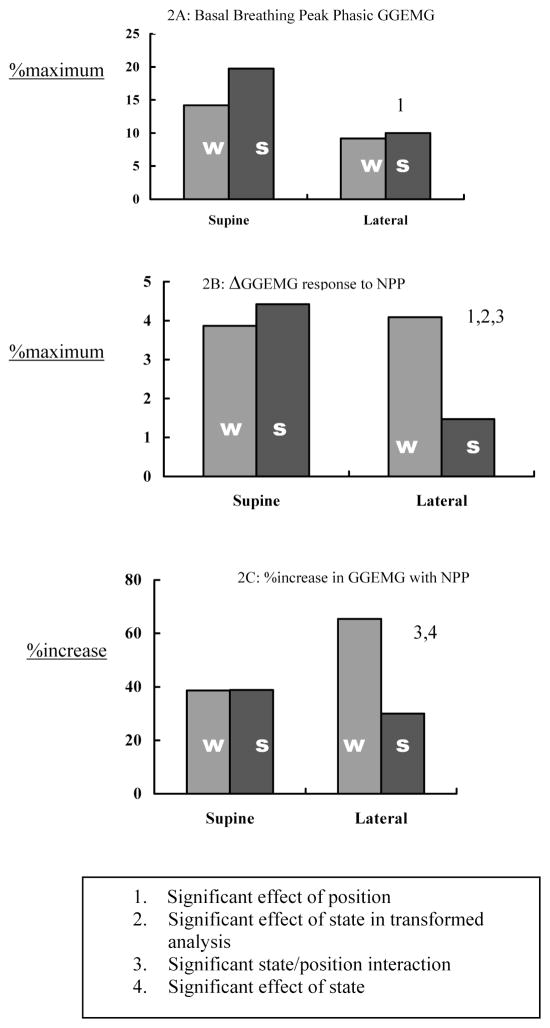
Administration of the NPP stimulus produced a transient increase in activity for both GGEMG activity (ΔGGEMG) and TPEMG activity (ΔTPEMG). We did not observe inhibition of either muscle in response to the NPP stimulus. For the GGEMG, the response was of the same magnitude in the 2 body positions when subjects were awake. However, during sleep, the response increased in the supine, and decreased in the lateral position, resulting in significant interaction and position effects (see Table 3 and Figure 2, Panel B). In anticipation of the possibility that the GGEMG response during sleep might be a function of a variable baseline level, ΔGGEMG was expressed as a percentage of the baseline level (%increase). This statistic also showed a significant interaction effect (see Table 3 and Figure 2, Panel C). Indeed, inspection of the data indicates that both baseline GGEMG activity and the response to the NPP were greatest in the supine sleep condition. Thus, the greater responsiveness of GGEMG when subjects were asleep in the supine position was not likely to be due to differences in baseline activity levels.
Figure 2.
Genioglossus Group Data. This figure illustrates group data for genioglossus electromyogram (GGEMG) activity during basal breathing (2A) and following negative pressure pulses (NPP) expressed both as an absolute change (Δ, 2B) and as a percentage increase (%increase) (2C). w refers to wakefulness; s, stable non-rapid eye movement sleep; %maximum, percentage of maximum.
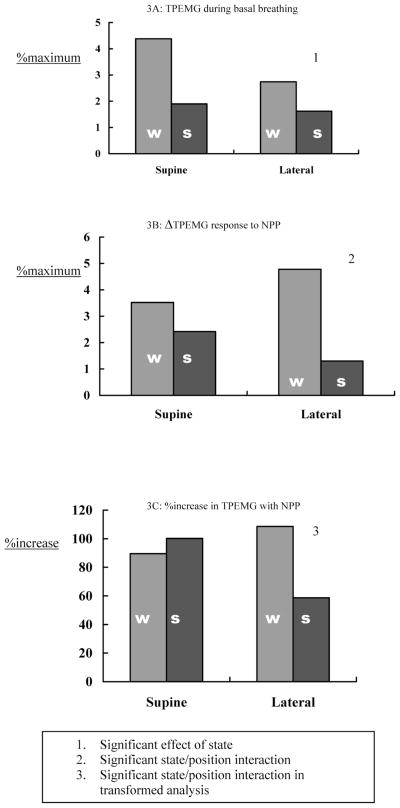
TPEMG did not show significant body position, or sleep-wake state, effects in response to the NPP, although there was a significant interaction effect, with TPEMG response to NPP falling to a greater extent during sleep in the lateral position (see Figure 3, Panel B).
Figure 3.
Tensor Palatini Group Data. This figure illustrates group data for tensor palatini electromyogram (TPEMG) activity during basal breathing (3A) and following negative pressure pulses expressed both as an absolute change (Δ, 3B) and as a percentage increase (%increase) (2C). w refers to wakefulness; s, stable non-rapid eye movement sleep; %maximum, percentage of maximum.
DISCUSSION
The major finding of this study was the greater responsiveness of GGEMG to negative pressure during sleep when subjects were in the supine position. Despite the augmentation of this protective reflex, pharyngeal collapsibility was significantly increased during supine sleep. Tensor palatini activity fell substantially from wakefulness to sleep but showed relatively minor positional effects from the standpoint of reflex activity. The observations of this study are important as they may explain some of the discordance in the existing literature regarding the responsiveness of pharyngeal dilator muscles during NREM sleep. In addition, the observed posture effects lead to interesting speculation regarding mechanism.
While some previous papers have suggested a marked attenuation of the reflexes important in controlling the pharyngeal dilator muscle during stable NREM sleep, others have implied the opposite. Following the original description of the NPR in humans, both Horner7 and Wheatley22 independently found minimal responsiveness of upper-airway muscles to NPPs during NREM sleep. Subsequently, Malhotra et al8 reported a markedly reduced responsiveness of these muscles to the negative pressures generated during inspiratory resistive loading compared to a robust response observed during wakefulness. Similarly, Pillar et al14 investigated the chemoreceptor sensitivity of upper-airway muscles, and despite an augmentation of ventilation, there was no important increase in pharyngeal dilator muscle activation with increased inspired CO2 during stable NREM sleep. All of these studies were done in the lateral posture. On the other hand, Basner et al30 observed increased GGEMG in 5 normal subjects during slow-wave sleep, compared with stages 1 and 2 NREM sleep in the supine posture, although the stimulus for this increase was unclear. Similarly, Worsnop et al23 observed increased GGEMG within 3 to 7 breaths of the alpha-theta transition in supine healthy men, although the mechanism mediating this recovery in activity was again not defined. In patients with OSA who were in the supine position, Berry and colleagues2 have reported locally mediated increases in muscle activity during apneic events prior to arousal. Finally, Stanchina et al31 have recently reported responsiveness of the genioglossus muscle to the combination of mechanoreceptive and chemoreceptive stimuli during stable NREM sleep in the lateral position. However, the muscle responded to neither stimulus alone.
The data of the present study bring some clarity to this literature as they demonstrate an important effect of body position on the responsiveness of upper-airway reflexes. Thus, some confusion in the existing literature can be resolved as a result of this new information. In addition, the responsiveness of the upper-airway muscles during supine sleep in normal subjects needs to the integrated into the working models on the pathogenesis of pharyngeal collapse.
One potential interpretation of the above studies is that upper-airway muscles become responsive to stimuli when the pharynx is vulnerable to collapse. For example, during supine NREM sleep, when the upper airway is most dependent on upper-airway muscles for the maintenance of pharyngeal patency, muscle responsiveness to protective reflexes is maximal. Similarly, when multiple challenges are presented to the upper airway (eg, CO2 plus negative pressure in normals or anatomic compromise plus negative pressure in patients with OSA), the muscles again become responsive. However, the mechanisms underlying these observations are unclear. There is teleologic appeal to the concept that upper-airway reflexes are relatively quiescent in normal subjects in the lateral posture, since the propensity for upper-airway collapse is minimal. Conversely, when the pharynx is vulnerable to collapse during supine NREM sleep, the protective reflexes are maximally active. Another way of interpreting these data would be that the genioglossus reflexes are maximal when tongue protrusion is critical to preventing pharyngeal collapse (supine posture). On the other hand, tongue protrusion is unlikely to be an effective method of maintaining pharyngeal patency in the lateral position. Recent research has shown that motor units tend to recruit maximally when a muscle is mechanically advantaged, as would be the case for the genioglossus in the supine posture.32 During wakefulness, when there are behavioral demands such as speech and swallowing that may require a more complex control system, such distinctions may become less relevant.
There are several mechanisms by which body or head position could be perceived. These include local receptors within the upper airway (eg, muscle spindles) or, potentially, the vestibular nervous system that is involved in postural perception.33 Because we observed no significant difference in the pharyngeal resistance or level of negative pressure within the upper airway as a function of position, we doubt that local upper-airway phenomena were important in mediating the positional effect. However, we have no direct proof of this vestibular hypothesis. Animal experiments indicate that stimulation of the vestibular nerve leads to a substantial increase in genioglossal activation.34 Along these lines, manipulations in head position led to important changes in genioglossus responsiveness in the cat.35 Thus, the vestibular system can interact with the upper-airway control mechanisms.35 However, in the present study, pharyngeal collapsibility remains higher in the supine posture despite this augmented reflex, suggesting that this posture-related adaptation is incomplete, although present. Despite the fact that the loss of the NPR during sleep is thought to be a major mechanism involved in OSA pathogenesis,1 the concept of reflex augmentation as a new therapeutic target remains untested.
Given the findings of the present study, the fall in GGEMG activity that has previously been reported in both patients with OSA and normal individuals during the wake-sleep (alpha-theta) transition becomes somewhat difficult to explain. We and others36 have previously speculated that the large fall in GGEMG activity observed at the alpha-theta transition is a result of the loss of this protective reflex. Several possible explanations for these apparently discordant findings exist. First, several authors37,38 have reported impairment in upper-airway sensation in patients with OSA compared to controls. This would imply that the behavior of these reflexes may be different in patients with OSA as compared to healthy subjects. Second, some have argued that the neurochemical milieu in the brainstem of the patient with OSA may have important differences from that of healthy subjects (eg, with respect to serotonin).39 Thus upper-airway reflexes mediated via the brainstem could behave differently in these 2 different populations (patients versus controls). Third, because our observations are limited to stable NREM sleep, we can only speculate about the wake-sleep transition. Because many of the neurochemical events that occur during sleep have a gradual time course, there is ample reason to believe that the wake-sleep transition and stable NREM sleep may be very different neurobiologic states. Fourth and finally, there are many factors contributing to muscle activity during wakefulness, including the wakefulness drive to breathe, respiratory premotor neurons, the NPR, and chemoreceptive inputs among others.28,40 In theory, the fall in GGEMG that occurs in patients with OSA and normal subjects may reflect the loss of 1 or all of these inputs rather than the NPR alone. Indeed, the pattern reported by Worsnop et al,23 in which GGEMG activity fell at the alpha-theta transition and then rose several breaths later, suggests an initial loss of wakefulness drive, with subsequent recruitment through the NPR. Further work is clearly needed to define the relevance of our findings to the patient with OSA.
This study has a number of limitations. First, as our ultimate goal is to understand the disease, OSA syndrome, one could argue that the investigation of healthy control subjects has little relevance. However, by definition patients with OSA do not achieve stable NREM sleep, limiting the feasibility of the present study in populations with this disease. In addition, we believe that an improved understanding of normal pharyngeal motor control will ultimately advance our understanding of the pathogenesis of OSA. Second, the measurement of EMG as a percentage of maximum activity is potentially problematic due to variability of effort during maximal maneuvers and inconsistent electrode placement. To overcome this, we have analyzed the NPR data both as a percentage of maximum activity as well as normalized to baseline during wakefulness. In addition, because our primary hypotheses involved within-subject comparisons, these results were unaffected by the use of percentage of maximum values. Third, one could argue that suction pressure applied nasally is not a physiologic stimulus and that the upper-airway muscles could behave differently if the pressure were generated diaphragmatically. Although we acknowledge this limitation, we believe, based on prior work, that nasal or laryngeal mechanoreceptors will likely respond to negative pressure similarly, regardless of the source of the pressure. In addition, diaphragmatically generated negative pressure has a potentially confounding effect because increased output from the central respiratory pattern generator affects both the phrenic nerve and the hypoglossal nerve. However, we do plan to conduct future experiments using an iron-lung model of passive ventilation to extend our current findings. Thus, despite these limitations, we believe that the findings of our study are robust and importantly contribute to our existing knowledge in this area.
CONCLUSIONS
The responsiveness of the pharyngeal dilator muscles to negative pressure stimulation is augmented in the supine as compared to the lateral decubitus posture and in the supine posture is largely maintained during stable NREM sleep. Although a number of interpretations are possible, we believe that this reflex may alter responsiveness depending on pharyngeal mechanics and the potential effectiveness of tongue protrusion. Further work is necessary to determine whether the vestibular nervous system has any role in mediating these findings.
Acknowledgments
This work was supported by NIH 1 P50 HL60292 (Specialized Center of Research on the Neurobiology of Sleep and Sleep Apnea), RO1 HL48531, T32 HL07633, and Sleep Medicine and Education Research Foundation of the American Academy of Sleep Medicine. This work was also supported by the NIH Grant NCRR GCRC MO1 RR02635 to the Brigham and Women’s Hospital General Clinical Research Center. Dr. Malhotra has received grants from the Medical Research Council of Canada, NIA (AG024837-01), and the American Heart Association. The authors thank Dr. Shiva Gautam for his statistical input in this manuscript.
Footnotes
Disclosure Statement
This is not an industry-sponsored study. Dr. Malhotra receives research support from Respironics. Dr. Fogel serves on the visiting speaker’s bureau for Wyeth Pharmaceuticals. Dr. White receives research support and consulting fees from Respironics, Itamar Medical, Aspire Medical, and the Alfred E. Mann Foundation. Drs. Trinder, Stanchina, Patel, Schory, and Kleverlaan have indicated no financial conflicts of interest.
References
- 1.Malhotra A, White D. Seminar: Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Berry RB, McNellis MI, Kouchi K, Light RW. Upper airway anesthesia reduces phasic genioglossus activity during sleep apnea. Am J Respir Crit Care Med. 1997;156:127–32. doi: 10.1164/ajrccm.156.1.9608037. [DOI] [PubMed] [Google Scholar]
- 3.Badr MS. Pathogenesis of obstructive sleep apnea. Prog Cardiovasc Dis. 1999;41:323–30. doi: 10.1053/pcad.1999.0410323. [DOI] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 5.Lavie P, Silverberg D, Oksenberg A, Hoffstein V. Obstructive sleep apnea and hypertension: from correlative to causative relationship. J Clin Hypertens. 2001;3:296–301. doi: 10.1111/j.1524-6175.2001.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sajkov D, Wang T, Saunders NA, Bune AJ, McEvoy RD. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:152–8. doi: 10.1164/ajrccm.165.2.2010092. [DOI] [PubMed] [Google Scholar]
- 7.Horner R, Innes J, Morrell M, Shea S, Guz A. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol. 1994;476:141–51. [PMC free article] [PubMed] [Google Scholar]
- 8.Malhotra A, Pillar G, Fogel R, Beauregard J, White D. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162:1058–62. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- 9.Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus and muscle respiratory activity. J Appl Physiol. 1982;52:438. doi: 10.1152/jappl.1982.52.2.438. [DOI] [PubMed] [Google Scholar]
- 10.Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol. 1991;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi I, et al. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol. 1996;80:1595–604. doi: 10.1152/jappl.1996.80.5.1595. [DOI] [PubMed] [Google Scholar]
- 12.Tangel DT, Mezzanotte WS, White DP. The influence of sleep on tensor palatini EMG and upper airway resistance in normal subjects. J Appl Physiol. 1991;70:2574–81. doi: 10.1152/jappl.1991.70.6.2574. [DOI] [PubMed] [Google Scholar]
- 13.Onal E, Lopata M, O’ Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol. 1981;50:1052–5. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- 14.Pillar G, Malhotra A, Fogel R, Beuregard J, Slamowitz D, Shea S, White D. Upper airway muscle responsiveness to rising PCO2 during NREM sleep. J Appl Physiol. 2000;89:1275–82. doi: 10.1152/jappl.2000.89.4.1275. [DOI] [PubMed] [Google Scholar]
- 15.Orem J. The nature of the wakefulness stimulus for breathing. Prog Clin Biol Res. 1990;345:23–31. [PubMed] [Google Scholar]
- 16.Malhotra A, Fogel R, Edwards J, Shea S, White D. Local Mechanisms drive genioglossus muscle activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1746–9. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- 17.Horner RL. Impact of brainstem sleep mechanisms on pharyngeal motor control. Respir Physiol. 2000;119:113–21. doi: 10.1016/s0034-5687(99)00106-1. [DOI] [PubMed] [Google Scholar]
- 18.Hudgel D, Mulholland M, Hendricks C. Neuromuscular and mechanical responses to inspiratory resistive loading during sleep. J Appl Physiol. 1987;63:603–8. doi: 10.1152/jappl.1987.63.2.603. [DOI] [PubMed] [Google Scholar]
- 19.Kuna S, Smickley J. Response of genioglossus muscle activity to nasal airway occlusion in normal sleeping adults. J Appl Physiol. 1988;64:347–53. doi: 10.1152/jappl.1988.64.1.347. [DOI] [PubMed] [Google Scholar]
- 20.Wiegand L, Zwillich CW, White DP. Collapsibility of the human upper airway during normal sleep. J Appl Physiol. 1989;66:1800–8. doi: 10.1152/jappl.1989.66.4.1800. [DOI] [PubMed] [Google Scholar]
- 21.Aronson ROE, Carley DW, Lopata M. Upper airway and respiratory muscle responses to continuous negative airway pressure. J Appl Physiol. 1989;66:1373–82. doi: 10.1152/jappl.1989.66.3.1373. [DOI] [PubMed] [Google Scholar]
- 22.Wheatley J, Mezzanotte W, Tangel D, White D. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- 23.Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–20. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra A, Pillar G, Fogel RB, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–7. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- 25.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and easurement techniques in adults. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington: Government Printing Office; 1968. NIH Pub #204. [Google Scholar]
- 27.Malhotra A, Pillar G, Fogel R, Edwards J, Beauregard J, White DP. Upper airway collapsibility: measurement and sleep effects. Chest. 2001;120:156–61. doi: 10.1378/chest.120.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillar G, Fogel RB, Malhotra A, et al. Genioglossal inspiratory activation: central respiratory vs mechanoreceptor influences. Respir Physiol. 2001;127:23–38. doi: 10.1016/s0034-5687(01)00230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little R, Rubin D. Statistical Analysis with Missing Data Points. Chichester: Wiley; 1987. [Google Scholar]
- 30.Basner RC, Ringler J, Schwartzstein RM, Weinberger SE, Weiss JW. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir Physiol. 1991;83:189–200. doi: 10.1016/0034-5687(91)90028-h. [DOI] [PubMed] [Google Scholar]
- 31.Stanchina M, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical loading during NREM sleep. Am J Respir Crit Care Med. 2002;165:945–9. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 32.De Troyer A, Gorman RB, Gandevia SC. Distribution of inspiratory drive to the external intercostal muscles in humans. J Physiol. 2003;546:943–54. doi: 10.1113/jphysiol.2002.028696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harper RM. Autonomic control during sleep and risk for sudden death in infancy. Arch Ital Biol. 2001;139:185–94. [PubMed] [Google Scholar]
- 34.Anker A, Ali A, Arendt H, et al. Use of electrical vestibular stimulation to alter genioglossal muscle activity in awake cats. J Vestib Res. 2003;13:1–8. [PubMed] [Google Scholar]
- 35.Bonora M, Bartlett D, Knuth S. Changes in upper airway muscle activity related to head position in awake cats. Respir Physiol. 1985;60:181–92. doi: 10.1016/0034-5687(85)90102-1. [DOI] [PubMed] [Google Scholar]
- 36.Mezzanotte WS, Tangel DJ, White DP. Waking and sleeping upper airway muscle activity in apnea patients versus normal control. Am J Respir Crit Care Med. 1996;153:1880–7. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- 37.Kimoff R, Sforza E, Champagne V, Ofiara L, Gendron D. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 2001;160:250–5. doi: 10.1164/ajrccm.164.2.2010012. [DOI] [PubMed] [Google Scholar]
- 38.Friberg D, Gazelius B, Hokfelt T, Nordlander B. Abnormal afferent nerve endings in the soft palatal mucosa of sleep apneics and habitual snorers. Regul Pept. 1997;71:29–36. doi: 10.1016/s0167-0115(97)01016-1. [DOI] [PubMed] [Google Scholar]
- 39.Hudgel DW, Gordon EA. Serotonin-induced cortisol release in CPAP-treated obstructive sleep apnea patients. Chest. 1997;111:632–8. doi: 10.1378/chest.111.3.632. [DOI] [PubMed] [Google Scholar]
- 40.Fogel R, Trinder J, Malhotra A, et al. Within-breath control of genioglossal muscle activation in humans: effect of sleep-wake state. J Physiol. 2003;550:899–910. doi: 10.1113/jphysiol.2003.038810. [DOI] [PMC free article] [PubMed] [Google Scholar]