Abstract
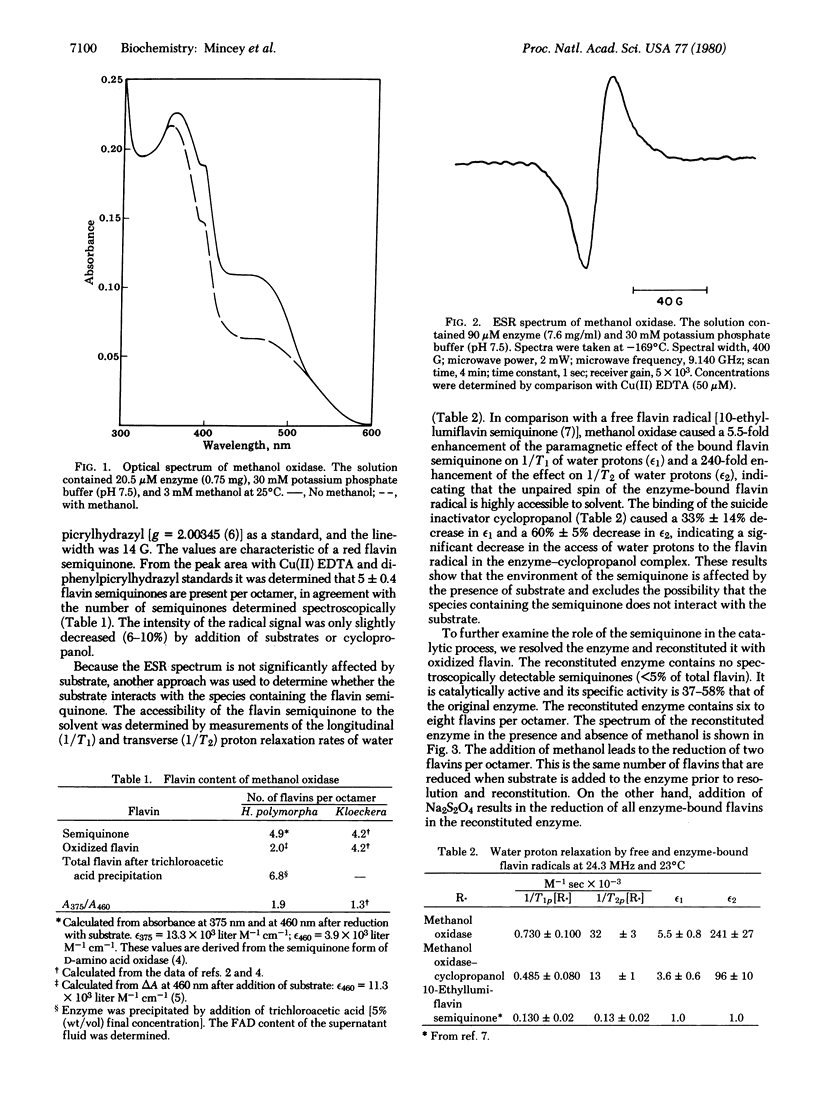
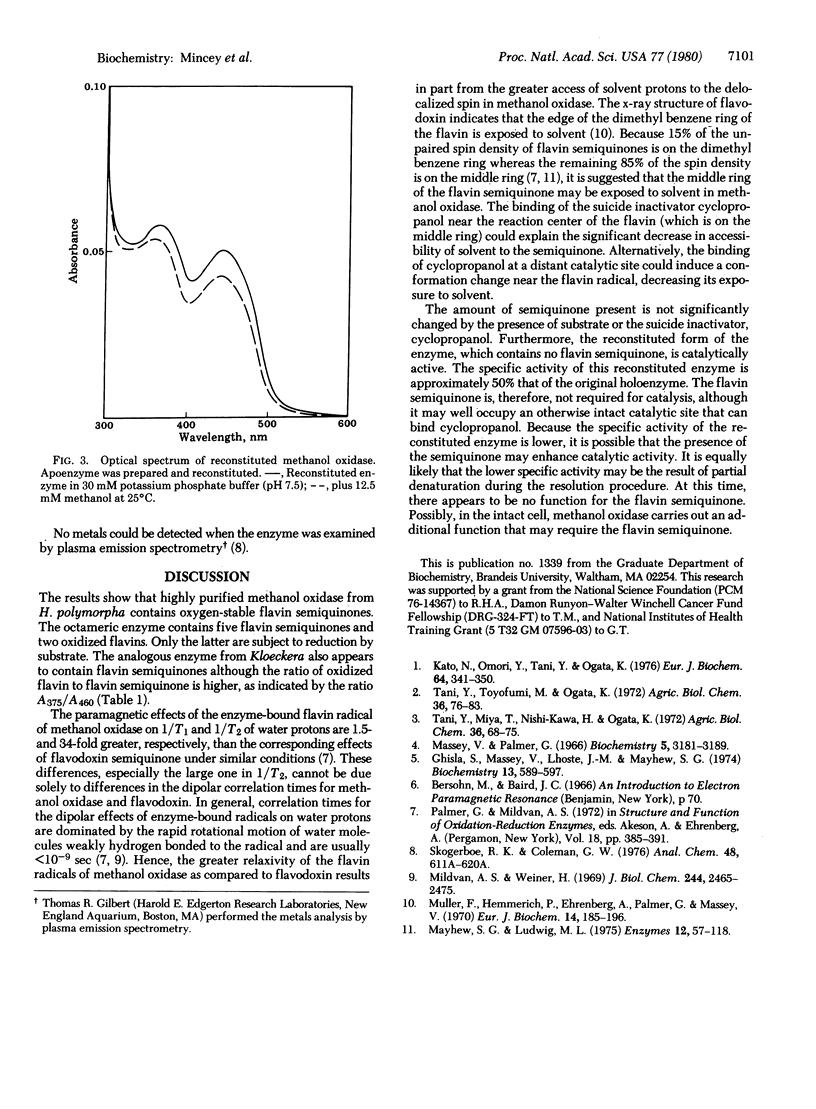
Methanol oxidase from Hansenula polymorpha contains five "red" flavin semiquinones and two oxidized flavins per octamer. Addition of substrate results in the reduction of the two oxidized flavins but does not affect the flavin semiquinones. Enhanced water proton relaxation rates indicate that the unpaired electron of the flavin semiquinones is accessible to the solvent and this accessibility is significantly decreased upon binding of the suicide inhibitor cyclopropanol. In the native enzyme, the semiquinones are not oxidizable by air. All flavins were resolved from the enzyme, and holoenzyme was reconstituted by addition of oxidized flavin. The reconstituted enzyme was catalytically active. The specific activity was 50% that of the original enzyme. It was concluded that the semiquinone is not required for the oxidation of methanol, although it may be present at an otherwise intact site.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ghisla S., Massey V., Lhoste J. M., Mayhew S. G. Fluorescence and optical characteristics of reduced flavines and flavoproteins. Biochemistry. 1974 Jan 29;13(3):589–597. doi: 10.1021/bi00700a029. [DOI] [PubMed] [Google Scholar]
- Kato N., Omori Y., Tani Y., Ogata K. Alcohol oxidases of Kloeckera sp. and Hansenula polymorpha. Catalytic properties and subunit structures. Eur J Biochem. 1976 May 1;64(2):341–350. doi: 10.1111/j.1432-1033.1976.tb10307.x. [DOI] [PubMed] [Google Scholar]
- Massey V., Palmer G. On the existence of spectrally distinct classes of flavoprotein semiquinones. A new method for the quantitative production of flavoprotein semiquinones. Biochemistry. 1966 Oct;5(10):3181–3189. doi: 10.1021/bi00874a016. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Weiner H. Interaction of a spin-labeled analogue of nicotinamide adenine dinucleotide with alcohol dehydrogenase. 3. Thermodynamic, kinetic, and structural properties of ternary complexes as determined by nuclear magnetic resonance. J Biol Chem. 1969 May 10;244(9):2465–2475. [PubMed] [Google Scholar]



