Abstract
Plant responses to drought stress vary depending on the severity of stress and the stage of drought progression. To improve the understanding of such responses, the leaf physiology, abscisic acid (ABA) concentration, and expression of genes associated with ABA metabolism and signalling were investigated in Petunia × hybrida. Plants were exposed to different specific substrate water contents (θ = 0.10, 0.20, 0.30, or 0.40 m3·m–3) to induce varying levels of drought stress. Plant responses were investigated both during the drying period (θ decreased to the θ thresholds) and while those threshold θ were maintained. Stomatal conductance (gs) and net photosynthesis (A) decreased with decreasing midday leaf water potential (Ψleaf). Leaf ABA concentration increased with decreasing midday Ψleaf and was negatively correlated with gs (r = –0.92). Despite the increase in leaf ABA concentration under drought, no significant effects on the expression of ABA biosynthesis genes were observed. However, the ABA catabolism-related gene CYP707A2 was downregulated, primarily in plants under severe drought (θ = 0.10 m3∙m–3), suggesting a decrease in ABA catabolism under severe drought. Expression of phospholipase Dα (PLDα), involved in regulating stomatal responses to ABA, was enhanced under drought during the drying phase, but there was no relationship between PLDα expression and midday Ψleaf after the θ thresholds had been reached. The results show that drought response of plants depends on the severity of drought stress and the phase of drought progression.
Key words: abscisic acid, acclimation, automated irrigation, soil moisture sensor, stomatal conductance, substrate water content
Introduction
Drought is a common abiotic stress and considered to be the most limiting environmental factor for plant growth (Boyer, 1982). Over the last few decades, many studies have reported physiological, molecular, and biochemical plant responses to drought (Chaves et al., 2003). However, plant responses to water deficit may vary depending on the severity of the drought stress, the process of drought development, and the duration of drought stress (Bray, 1997; Chaves et al., 2003; Kim and van Iersel, 2011), and the application of the drought treatments varies considerably across studies (Jones, 2007). Inadequate descriptions of how the drought treatments are imposed complicate the interpretation of many previous studies (Pinheiro and Chaves, 2011). A commonly used approach is withholding irrigation until plants wilt, a method referred to as progressive drought (Kawaguchi et al., 2004; Harb et al., 2010). Other approaches for studying drought stress include the addition of an osmoticum such as mannitol (Kreps et al., 2002) and stress induction through desiccation (Seki et al., 2002). A comparison across these approaches indicated that only a small subset of the transcriptome was commonly regulated among these methods, indicating that plant responses to drought are critically dependent on the method of drought-stress imposition (Bray, 2004).
Several recent studies have underlined the importance of the method of drought-stress imposition. The ‘PHENOPSIS’ system was used to automate the maintenance of soil water content at predetermined levels across different Arabidopsis accessions, thereby enabling the identification of an Arabidopsis accession altered in its response to water deficit (Granier et al., 2006). Harb et al. (2010) studied responses of Arabidopsis to moderate drought stress (employed by maintaining the substrate at 30% of field capacity) and progressive drought, at the whole plant and the molecular levels, and identified different stages of plant responses to drought stress. These studies further highlight the importance of precise methods for controlling drought-stress imposition.
The severity of drought stress is also a critical factor that determines plant response. In loblolly pine (Pinus taeda L.), mild stress resulted in photosynthetic acclimation while severe stress inhibited it (Watkinson et al., 2003). Additionally, expression of drought-related genes was dependent on the level of drought stress (Watkinson et al., 2003). Under moderate stress, three stages of plant responses – pre-conditioning, acclimation, and post-acclimation homeostasis – were identified in Arabidopsis (Harb et al., 2010). These studies indicate that plant responses to drought stress that are generally evident under one level of stress (mild to moderate) may be absent under another level (severe stress). Monitoring and controlling the soil moisture status and frequent measurement of plant responses, both during the development of the drought and after a steady drought level has been imposed, may allow for the quantification of plant responses to different drought severities. Additionally, such analyses may allow a better understanding of the temporal changes in response to drought stress. Further, integration of changes in gene expression with whole plant responses may lead to a more comprehensive understanding of physiological responses to drought (Bray, 1997; Jones, 2007; Harb et al., 2010).
Abscisic acid (ABA) is the primary chemical signal for drought, increasing in concentration under drought stress and inducing stomatal closure to minimize water loss. ABA also alters the expression of a multitude of drought stress-related genes (Bray, 2004). This study focused on ABA-related mechanisms, since its anabolism and catabolism are well understood, key genes in its metabolic and regulatory pathways have been identified, it can be readily quantified, and its physiological effects at the leaf level are easily measured (Schachtman and Goodger, 2008; Schwartz and Zeevaart, 2010).
The endogenous ABA concentrations are controlled by ABA biosynthesis, catabolism, and conjugation mechanisms (Seiler et al., 2011). A key regulatory step in ABA biosynthesis is the cleavage of 9-cis-epoxycarotenoids by 9-cis-epoxycarotenoid dioxygenase (NCED) to form xanthoxin (Nambara and Marion-Poll, 2005). The NCED genes belong to a multigene family, and have been identified in many species (Thompson et al., 2000). In a subsequent step within the ABA biosynthesis pathway, abscisic aldehyde oxidase (AAO) converts the abscisic aldehyde derived from xanthoxin to ABA. In Arabidopsis, AAO3 was identified as the key gene encoding this enzyme (Seo et al., 2000; Nambara and Marion-Poll, 2005). ABA 8’-hydroxylases, members of the cytochrome P450 monoxygenase family (CYP707A), catalyse the ABA catabolic pathway by enabling the conversion of ABA to the inactive phaseic acid (Umezawa et al., 2006). ABA can also be inactivated and stored in the conjugated ABA glucosyl ester (ABA-GE) form, which may be subsequently reactivated by specific β-glucosidases (Lee et al., 2006).
ABA signalling in plants mediates stomatal opening and closing (Kim et al., 2010). Phospholipase Dα (PLDα) may play an important role in ABA signalling by mediating the effects of ABA on stomatal closing and opening. PLDα1 cleaves phospholipids to generate phosphatidic acid, a molecule with dual roles in promoting stomatal closure and inhibiting stomatal opening (Zhang et al., 2004; Mishra et al., 2006). Phosphatidic acid binds to a negative regulator of ABA responses, ABI1/PP2C (ABA insensitive/protein phosphatase 2C), thereby promoting ABA-induced stomatal closure (Zhang et al., 2004). Additionally, PLDα1 and phosphatidic acid also interact with the GTP-binding proteins (G-proteins), and may mediate ABA-dependent inhibition of stomatal opening (Mishra et al., 2006). ZPT2-3 encodes a Cys2/His2-type zinc finger protein, a class of transcription factors, and is upregulated in response to various abiotic stresses including drought in Petunia (Sugano et al., 2003). Overexpression of ZPT2-3 in Petunia increased drought tolerance and survival under drought (Sugano et al., 2003). Similar studies in Arabidopsis showed that ZPT homologues were upregulated under drought stress and aided in enhancing drought tolerance, indicating that it may be an important regulator of transcriptional responses to drought (Sakamoto et al., 2004; Shu-Jing et al., 2010).
To measure plant responses to well-controlled, specific drought conditions, this study controlled substrate water content (θ, v/v) using an automated irrigation system based on soil moisture sensor readings (Supplementary Fig. S1, available at JXB online). This system irrigates when θ drops below a specific threshold θ and allows for maintenance of different θ levels for prolonged periods (Nemali and van Iersel, 2006). This system has been previously used to quantify plants’ physiological responses to the rate of drought-stress imposition (Kim and van Iersel, 2011). The current study gradually exposed Petunias to different levels of drought by allowing the substrate to dry until specific θ thresholds had been reached, and maintained them at these thresholds. The objectives were to investigate the responses of Petunia to different severities and temporal stages of drought, from the level of gene expression to whole plant physiology, focusing on stomatal regulation through ABA biosynthesis, catabolism, and signalling. Plant responses were separated into two stages: (1) the period where the θ decreased to the θ threshold (drying period); and (2) the period after the threshold θ was attained, where the θ was stable.
Materials and methods
Plant materials and growth conditions
Eight Petunia × hybrida ‘Apple Blossom’ seedlings per experimental unit were transplanted into 16 8-l trays filled with soilless substrate (Fafard 2P, peat/perlite 60:40, Conrad Fafard, Agawam, MA, USA) mixed with a controlled-release fertilizer (Osmocote 14-14-14, N/P/K 14.0:6.2:11.6, The Scotts, Marysville, OH, USA) at a rate of 7.7 kg·m–3. Plants were grown for 3 weeks (from 23 March to 14 April 2010) in a greenhouse at the University of Georgia using a soil moisture sensor-based automated irrigation system (Nemali and van Iersel, 2006), which maintained θ at 0.40 m3·m–3. During the growing period, the average daily temperature and relative humidity in greenhouse were 21.0±1.0 °C and 53±10%, and the daily light integral averaged 26.9±12.2 mol·m–2·d–1 (mean ± SD).
Drought treatments
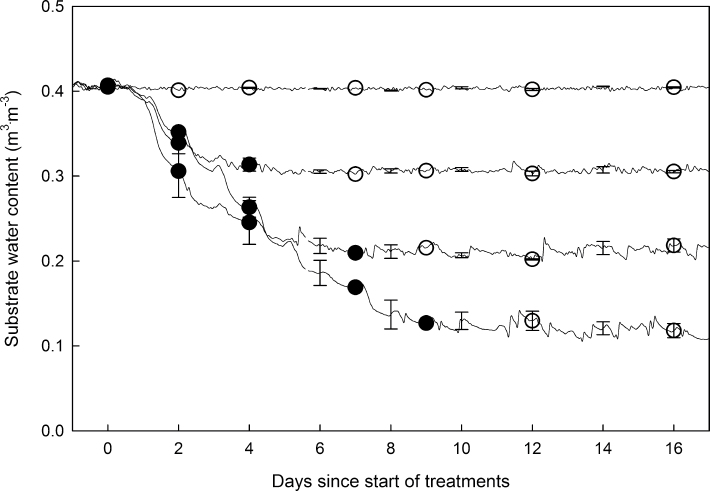
The automated irrigation system (Nemali and van Iersel, 2006) was modified for this research (Supplementary Fig. S1). Two capacitance soil moisture sensors (EC-5, Decagon Devices, Pullman, WA, USA) were placed in each tray and connected to a datalogger (CR10, Campbell Scientific, Logan, UT, USA) via a multiplexer (AM16/32, Campbell Scientific) to monitor and control θ. The soil moisture sensors were excited with 2.5V and substrate-specific calibration was used to convert the measured voltage to θ (θ = 1.7647×sensor output (V)–0.4745, r 2 = 0.95). When the average reading of the two sensors dropped below θ threshold for that tray, a datalogger opened a solenoid valve using a relay driver (SDM-CD16 AC/DC controller, Campbell Scientific) to irrigate the tray for 20 seconds (approximately 90ml per application), maintaining θ at the specific level. Each tray was watered with tap water using a custom grid, designed to apply the water as uniformly as possible, with two pressure compensated emitters (8 l h–1, Netafim USA, Fresno, CA, USA). The θ was measured every 10 minutes, and averages were logged hourly. The different θ thresholds, 0.40 (well-watered), 0.30 (mild drought), 0.20 (moderate drought), and 0.10 m3·m–3 (severe drought), were initiated at midnight, and irrigation was withheld until a tray θ reached its threshold θ. Once, the threshold θ was attained, the automated irrigation system maintained θ close to the threshold θ (± 0.02 m3·m–3, Fig. 1). Control plants were maintained at a θ of 0.40 m3·m–3 throughout the experiment, and the drought treatments reached their threshold θ of 0.30, 0.20, and 0.10 m3·m–3 after 2.5, 3.7, and 8.8 d, respectively. The collected data were separated into two different phases of drought stress: the drying period during which θ decreased to the threshold θ and the period after the threshold θ had been reached and θ was maintained at a stable level.
Fig. 1.
Substrate water contents (θ) changes of Petunia × hybrida with soil moisture sensor-based automatic irrigation over a 16-d period. A datalogger monitored θ using soil moisture sensors and maintained the θ in four treatments (θ = 0.40, 0.30, 0.20, and 0.10 m3·m–3). The drought treatments reached their threshold θ of 0.30, 0.20, and 0.10 m3·m–3 after 2.5, 3.7, and 8.8 d, respectively. Closed circles indicate data collected during the drying period and open circles indicate data collected after θ reached the thresholds. Error bars indicate the standard error (n = 4).
Leaf water potential and gas exchange measurement
Leaf physiological measurements and sampling were performed every 2 or 3 d from the start of the drying treatment. The uppermost, fully expanded leaves were used for data collection. Leaf discs for midday Ψleaf measurements were sampled at noon using leaf cutter thermocouple psychrometers (Model 76, J.R.D. Merrill Specialty Equipment, Logan, UT, USA), and midday Ψleaf was measured after equilibration of the psychrometers in a water bath at 25 °C for 4h. Stomatal conductance (gs), CO2 exchange rate (A), and quantum yield of PSII (ФPSII) were measured with a leaf photosynthesis system (CIRAS-2, PP Systems, Amesbury, MA, USA) equipped with a red/blue LED light source and a chlorophyll fluorescence module. Measurements were taken at a photosynthetic photon flux of 1000 µmol·m–2·s–1 and at a CO2 concentration of 388 µmol·mol–1 and were started approximately 5 hours after sunrise. ФPSII was calculated as (F’m – Ft)/F’m (Maxwell and Johnson, 2000). Leaf samples for RNA extraction and ABA assays were collected at noon by excising the leaf at the petiole using a razor blade and were immediately frozen in liquid N2 and stored at –80 °C.
A/Ci curves, relative water content, leaf size, and shoot dry mass at harvest
At 16 d after initiation of the drought treatments, CO2 response curves (A/Ci curves) were collected on attached uppermost, fully expanded leaves using the CIRAS-2 system, by changing the CO2 concentration from 0 to 1200 µmol·mol–1 in 200 µmol·mol–1 increments. Measurements were started at 5 hours after sunrise and it took approximately 7 hours to collect all A/Ci curves. The CO2-saturated assimilation rate (Amax) was calculated using empirical A/Ci curve analysis (Photosyn Assistant, Dundee Scientific, Dundee, Scotland, UK). At noon, the uppermost, fully expanded leaf samples were collected and fresh weight of the leaves was measured immediately after excision. Fully turgid fresh weight of the leaves was obtained after floating the samples on deionized water at 4 °C for 6h, and dry weight was determined after drying the sample at 60 °C for 24h. Relative water content was calculated as (fresh weight – dry weight) / (turgid weight – dry weight) × 100%. Leaf size was measured on eight uppermost fully expanded leaves per tray using a leaf area meter (LI-3100, LI-COR, Lincoln, NE, USA) and shoot dry weight was obtained after drying samples in an oven at 70 °C for 4 d.
Identification of genes associated with ABA biosynthesis, catabolism, and signalling from Petunia
Petunia expressed sequence tags (ESTs) coding for ABA- and drought-related genes were identified from Petunia EST databases (http://biosrv.cab.unina.it/454petuniadb/) (Zenoni et al., 2011). All genes used in this study, except ZPT2-3 and the reference genes, were identified based on sequence similarity after tblastx analysis with related genes from tomato, potato (Solanaceae) and Arabidopsis. Primers were manually designed based on the EST sequences. Candidate sequences were further confirmed by sequencing after PCR amplification using the same primers. The Petunia homologues of NCED, NCED1 (contig PETIN084121) and NCED2 (contig PETIN041019), were identified based on sequence similarity with the tomato NCED, LeNCED1 (Taylor et al., 2000). The putative Petunia NCED1 displayed 93% identity with LeNCED2 while the putative NCED2 shared 86% identity with LeNCED1. Putative homologues of AAO31 (contig PETAX039740) and AAO32 (contig PETIN023342) were identified based on similarity with AAO3 from Arabidopsis (Seo et al., 2000). Also, the putative AAO31 and AAO32 had 80% and 90% identity with potato AAO (accession DQ206634.1), respectively. CYP707A1 (contig PETIN061494) and CYP707A2 (contig PETIN048226) were identified based on similarity to the tomato ABA catabolism gene, SlCYP707A1 (Nitsch et al., 2009), and also shared 91% identity with potato CYP707A1 (DQ206630.1) and 95% identity with potato CYP707A2 (DQ206631.1), respectively. PLDα (contig PETIN026822) was identified based on similarity to tomato LePLDα1 (AF154425.1)(Bargmann et al., 2009) with which it displayed 79% identity. The genes for cyclophilin-2 (CYP) and elongation factor 1α (EF1α) were used as reference genes in quantitative real-time PCR (qRT PCR). These genes were suggested as stably expressed reference genes based on previous research in Petunia (Mallona et al., 2010). Primers used for qRT PCR are presented in Supplementary Table S1.
RNA extraction, cDNA synthesis, and qRT PCR
RNA was extracted from ground, frozen leaf tissue using the guanidium isothiocyanate method (Chomczynski and Sacchi, 1987). Approximately 1g of ground sample was used for RNA extraction in 7ml of extraction buffer (38% acid phenol, 0.8M guanidine thiocyanate, 0.4M ammonium thiocyanate, 0.1M sodium acetate, and 5% glycerol). After centrifugation, the supernatant was extracted with chloroform/isoamyl alcohol (24:1, v/v). The aqueous supernatant was precipitated with isopropanol and a salt solution (0.8M sodium citrate and 1.2M NaCl). After centrifugation, the RNA was washed with 70% ethanol and dissolved in DEPC (diethylpyrocarbonate)-treated water. This mixture was extracted with 3M sodium acetate and chloroform/isoamyl alcohol, and the aqueous supernatant was precipitated in 70% ethanol overnight at –20 °C. After centrifugation, the RNA was washed with 70% ethanol, dissolved in DEPC-treated water and stored at –80 °C. RNA quality was analysed by gel electrophoresis and RNA quantification was performed using Nanodrop 8000 (Thermo Fisher scientific, Waltham, MA, USA).
RNA (1 µg) was treated with DNase (Promega, Madison, WI, USA) to remove genomic DNA contamination, according to the manufacturer’s instructions and used for reverse transcription. Reverse transcription was performed using oligo-dT (Promega) and ImPromII reverse transcriptase (Promega) in a 20-µl volume. Subsequently, the cDNA was diluted five times with autoclaved distilled water and stored at –20 °C until further analysis.
The qRT PCR analyses were performed on the Stratagene Mx3005P real-time PCR system (Agilent Technologies, Santa Clara, CA, USA) using 1 µl of diluted cDNA in a 14 µl reaction volume with 2×SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA). The reaction parameters were: 95 °C for 10min; 40 cycles of 95 °C for 30 s and 60 °C for 1min. The melting curve analysis indicated single, distinct peaks for each of the amplicons analysed indicating the amplification of a single product. Controls without the template were run to ensure the absence of non-specific amplification. Normalization of gene expression was performed using the geometric mean of expression of the reference genes, CYP and EF1α, according to Pfaffl (2001). The normalization factor determined from the geometric mean of the reference genes generally changed less than 2-fold among the samples, indicating that these genes were effective for normalization. All analyses were performed using four biological replicates.
Leaf ABA content determination
Leaf tissue was ground in liquid nitrogen and extracted in darkness in an ABA extraction buffer (80% methanol with butylated hydroxytoluene at 100 mg·l–1, and citric acid at 500 mg·l–1) for 16h with constant shaking at 4 °C. The supernatant was collected after centrifugation and diluted 10-fold with TBS buffer (50mM TRIS, 1mM MgCl2, 150mM NaCl, pH 7.8). Subsequently, the ABA concentration was quantified using ELISA with the Phytodetek ABA test kit (Agdia, Elkhart, IN, USA) following the manufacturer’s instructions.
Experimental design and statistical analysis of data
A randomized complete block design with four blocks was used in this experiment. Physiological data and relative gene expression data were analysed with the general linear models procedure using repeated measures at α = 0.05 (proc glm, SAS 9.2, SAS Systems, Cary, NC, USA). ABA concentration was analysed using log-transformed [ABA] data. To quantify relationships between midday Ψleaf and physiological and gene expression responses during the drying period, regression analysis was performed and normality of the residuals was tested using the Shapiro-Wilk test at α = 0.05 (SigmaPlot 11.1, Systat, Chicago, IL, USA). When the normality assumption was violated, data were log transformed or analysed using non-parametric Spearman’s rank order correlation (SigmaPlot 11.1). The regression analysis between gs, ABA concentration, midday Ψleaf, and the interaction between ABA concentration and midday Ψleaf was performed using the data from all sampling times after log transformation of ABA concentration and gs (SAS 9.2). Leaf size, leaf relative water content, and shoot dry weight were analysed using the analysis of variance procedure (proc anova, SAS 9.2). Mean separation for the harvest data was done using Fisher’s protected least-significant-difference procedure.
Results
Leaf physiological responses to specific θ
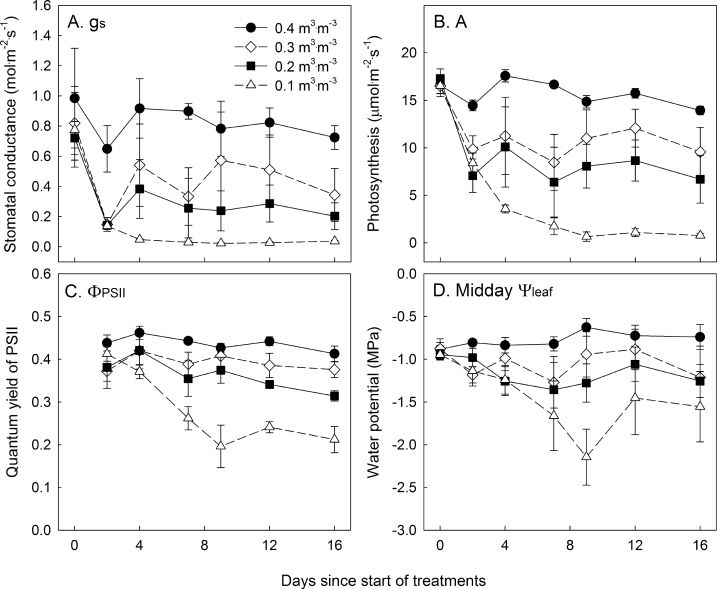
Withholding irrigation reduced gs to 20% (~150 mmol·m–2·s–1) of that of the control plants at 2 d after drought imposition (Fig. 2A). The gs of plants at θ of 0.30 and 0.20 m3·m–3 partially recovered after thresholds of 0.30 and 0.20 m3∙m–3 had been reached (50–70% and 30–40% of control gs for 0.30 and 0.20 m3·m–3, respectively). However, plants at 0.10 m3·m–3 maintained a low gs from day 4 until day 16 (< 50 mmol·m–2·s–1), less than 5% of the gs of control plants. Drought decreased A to 50–30% of the control at 2 d after drought initiation (Fig. 2B). After the θ thresholds had been reached, plants at θ of 0.30 and 0.20 m3·m–3 maintained A at ~67 and ~50% of control plants until the end of the experiment. However, A of plants at θ of 0.10 m3·m–3 subsequently decreased to 1 µmol·m–2·s–1, approximately 5% that of control plants. Only the 0.10 and 0.20 m3·m–3 treatments significantly decreased the quantum yield of photosystem II (ФPSII) (Fig. 2C). In contrast to the patterns of gs and A, the 0.30 m3·m–3 treatment did not show a significant decrease in ФPSII compared to control plants and maintained ФPSII at ~90% of that of control plants throughout the experiment. CO2-saturated assimilation rate (Amax) was lower as the threshold θ decreased (Supplementary Table S2), indicating that non-stomatal limitations contributed to the decreased A under drought.
Fig. 2.
Leaf physiological responses of Petunia × hybrida in response to substrate water content treatments (θ = 0.40, 0.30, 0.20, and 0.10 m3·m–3) over a 16 d period. Stomatal conductance (gs, panel A), photosynthesis (A, panel B), and quantum yield of PSII (ФPSII, panel C) were measured by a leaf photosynthesis measurement system and midday leaf water potential (Ψleaf, panel D) was measured using thermocouple psychrometers. All the measurements were conducted at noon. The drought treatments reached their threshold θ of 0.30, 0.20, and 0.10 m3·m–3 after 2.5, 3.7, and 8.8 d, respectively. Error bars indicate the standard error (n = 4).
Similar to gs, A, and ФPSII, midday Ψleaf decreased within 2 d of withholding irrigation (Fig. 2D). In general, midday Ψleaf was lower at lower θ levels, with the largest differences at 9 d after the start of the treatments. At harvest, midday Ψleaf of the plants at θ < 0.40 m3·m–3 were similar among the three drought treatments, but lower than that of the well-watered control. At harvest, plants in lower θ treatments had smaller leaves and lower shoot dry weight. Only a θ of 0.10 m3·m–3 reduced leaf relative water content (Supplementary Table S2).
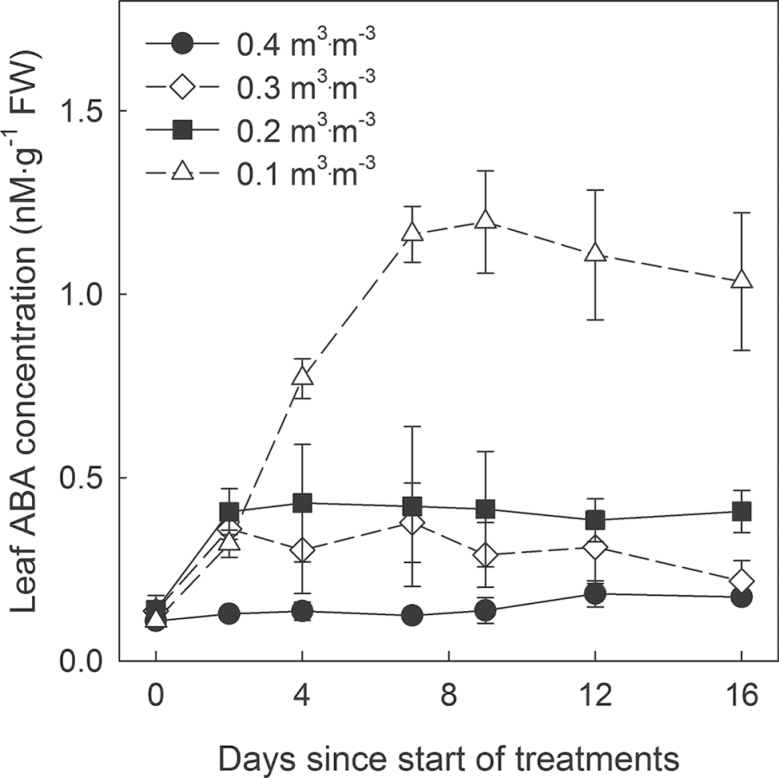
Effects of θ on ABA concentrations
Control plants maintained the leaf ABA concentration at ~0.15 nmol·(g fresh weight)–1, while all drought treatments resulted in a ~3-fold increase in leaf ABA concentration after 2 d of drought (Fig. 3). Plants at a θ of 0.30 m3·m–3 maintained a 2–3-fold higher ABA concentration for a week, but had ABA concentrations similar to that of the 0.40 m3·m–3 treatment from 9 to 16 d after the treatments. Plants in the 0.10 m3·m–3 treatment gradually increased their ABA concentration up to 1.2 nmol·(g fresh weight)–1 (8-fold that of control) as the substrate dried and subsequently maintained a high leaf ABA concentration until the end of the experiment.
Fig. 3.
Changes in leaf abscisic acid (ABA) concentration of Petunia × hybrida under drought with different substrate water content (θ = 0.40, 0.30, 0.20, and 0.10 m3∙m–3) over a 16 d period. Error bars indicate the standard error (n = 4).
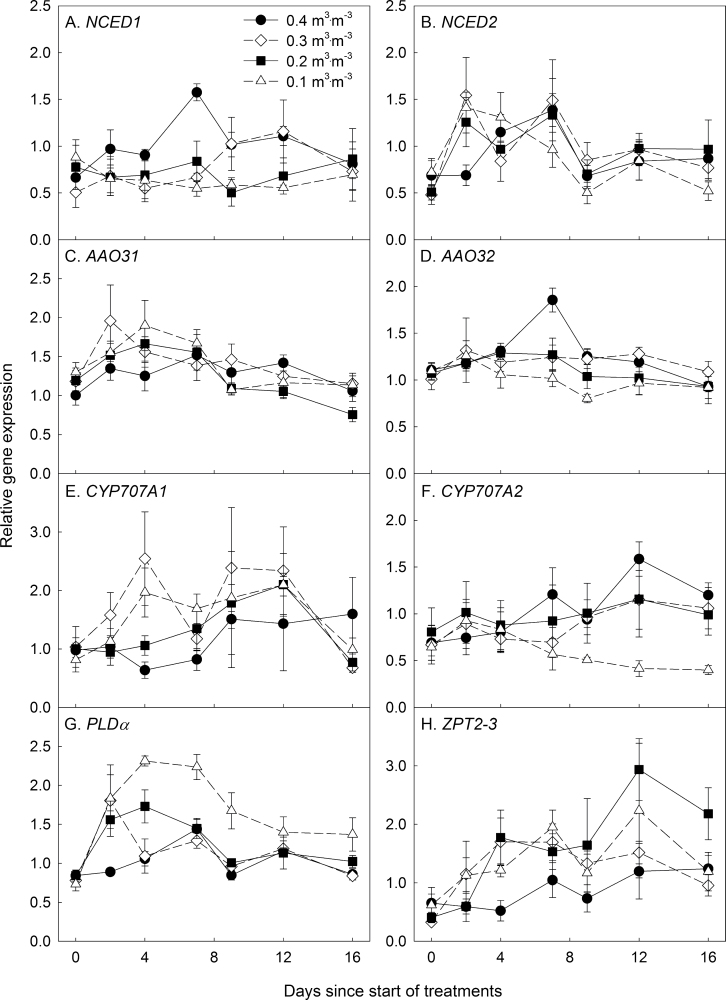
Expression of genes associated with ABA biosynthesis, catabolism, and signalling
Drought did not affect the expression of the ABA biosynthesis-related NCED and AAO3 genes in Petunia leaves (Figs. 4A–D). However, expression of the ABA catabolism-related gene CYP707A2 in the 0.10 m3·m–3 treatment was ~3-fold lower than in the control and 2-fold lower than in other drought treatments as θ reached the threshold and when it was stable at 0.10 m3·m–3 (Fig. 4F), suggesting a decrease in ABA catabolic activity.
Fig. 4.
Relative expression of abscisic acid metabolism-, signalling-, and drought tolerance-related genes (A, NCED1; B, NCED2; C, AAO31; D, AAO32; E, CYP707A1; F, CYP707A2; G, PLDα; H, ZPT2-3) in response to various substrate water contents (θ = 0.40, 0.30, 0.20, and 0.10 m3·m–3) during a 16 d period in Petunia × hybrid leaves. The drought treatments reached their threshold θ of 0.30, 0.20, and 0.10 m3·m–3 after 2.5, 3.7, and 8.8 d, respectively. Error bars indicate standard error (n = 4). Expression data were normalized using EF1α and CYP.
Relative expression of PLDα in Petunia leaves increased rapidly during the drying period (Fig. 4G). All drought treatments displayed a ~2-fold increase in the expression of PLDα after 2 d. The relative expression of PLDα in plants at a θ of 0.30 m3·m–3 decreased at 4 d after treatment, when the θ reached the threshold level, and a similar pattern was observed with the 0.20 m3·m–3 treatment. Plants in the 0.10 m3·m–3 treatment displayed ~2.5-fold higher relative expression of PLDα than control plants and maintained higher expression rates than other treatments until θ reached the threshold. Withholding irrigation increased the expression of ZPT2-3 within 4 d in all the drought treatments, but only plants at a θ of 0.20 m3·m–3 maintained higher expression levels than control plants throughout the study (Fig. 4H).
Relationships between ABA metabolism- and signalling-related genes and leaf ABA concentration
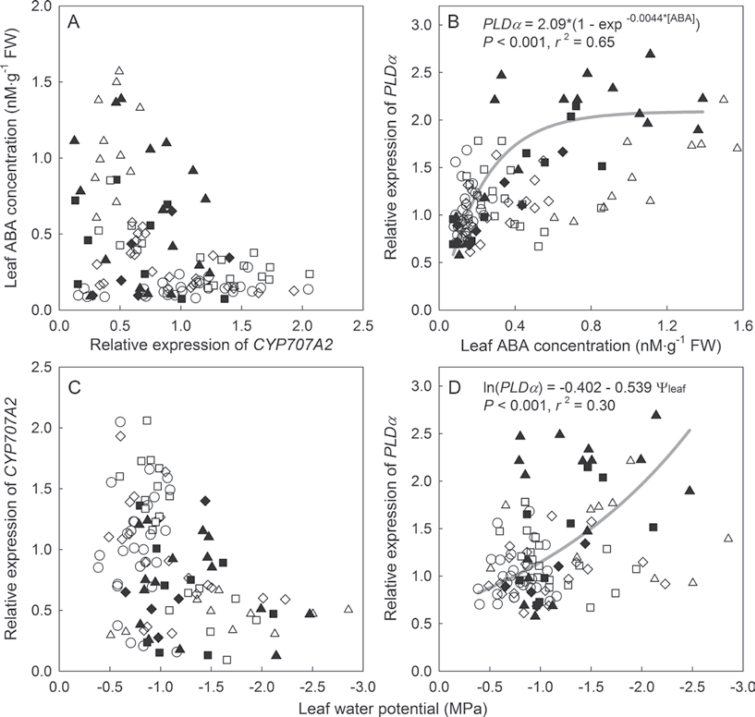
Although leaf ABA concentration was increased in all three drought treatments, the relative expression of ABA biosynthesis genes (NCED1, NCED2, AAO31, and AAO32) did not display an apparent relationship with either θ or leaf ABA concentration. However, when midday Ψleaf was low, the expression of CYP707A2 was low, and this was associated with high concentrations of ABA in the leaves (Fig. 5A and 5C).
Fig. 5.
Relationships between relative expression of CYP707A2 and PLDα in Petunia leaves and leaf abscisic acid (ABA) concentration (A and B) and midday leaf water potential (Ψleaf; C and D). Substrate water content treatments: 0.40 (circles), 0.30 (diamonds); 0.20 (squares), and 0.10 m3·m–3 (triangles). Closed symbols indicate data collected during the drying period and open symbols indicate data collected after substrate water content thresholds had been reached. Regression curves indicate significant relationships between gene expression and leaf ABA concentration/midday leaf water potential during the drying period.
As ABA concentrations increased during the drying period, the expression of PLDα increased as well, suggesting that drought imposition not only increased leaf ABA concentrations, but also ABA-dependent signalling mechanisms associated with stomatal closing/opening (Fig. 5B). PLDα expression during the drying period was negatively correlated with midday Ψleaf (P < 0.001). However, expression of PLDα decreased in all drought treatments after the θ reached the threshold levels and was no longer correlated with midday Ψleaf (Fig. 5D).
Discussion
Physiological responses depend on the severity of drought stress
Stomatal conductance, A, ФPSII, and midday Ψleaf decreased during the drying period, but gs and A of plants exposed to mild and moderate drought partially recovered after the θ reached the threshold levels (Fig. 2). This partial recovery of gs and A suggests that the drought stress became less severe after the θ thresholds of 0.20 and 0.30 m3∙m–3 had been reached. This may be related to the physical properties of soilless substrates. Moisture release curves for soilless substrates are hysteretic (Wallach, 2008). The frequent addition of small amounts of irrigation water after θ thresholds had been reached may have resulted in a higher substrate Ψ than that at the end of the drying period, despite similar θ, thus partially alleviating the drought stress. Therefore, θ may have limitations as a quantitative indicator of the drought severity.
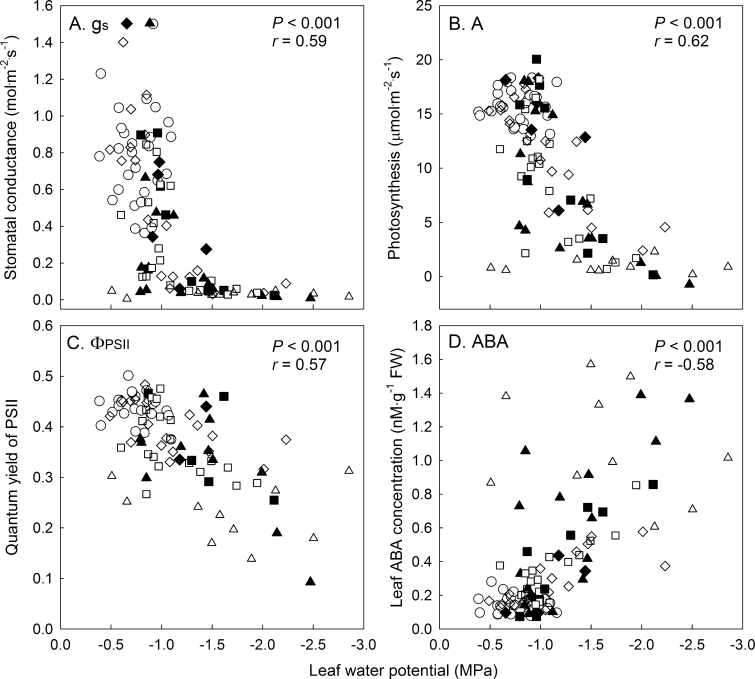
Leaf water potential is commonly used for determining the severity of drought stress (Jones, 2007), but there is no agreement about the best time of day to collect these data. Jones (2007) indicates that predawn measurement are less sensitive to environmental fluctuations and provide a better integrated short-term measure of plant water status than midday Ψleaf. However, Williams and Araujo (2002) found that predawn, midday Ψleaf, and midday Ψstem were all highly correlated with each other and that they were equally viable methods for assessing plant water status and physiological responses of grape (Vitis vinifera L.). Shackel et al. (1997) also concluded that midday Ψstem was a reliable method to quantify drought stress of fruit trees. The current results show clear relationships (P < 0.001) between midday Ψleaf and leaf responses to drought (Fig. 6), confirming that midday Ψleaf is a useful indicator of drought-stress severity. Stomatal conductance, A, and ФPSII decreased with decreasing midday Ψleaf (P < 0.001), while leaf ABA concentration increased with lower midday Ψleaf (P < 0.001). These results suggest that midday Ψleaf is a better indicator of drought-stress severity than θ, but it is important to realize that correlations between midday Ψleaf and other physiological responses do not indicate a causal relationship. There is no evidence that regulatory systems in plants are controlled by Ψleaf, and cell volume or turgor may be the signal perceived by plants (Jones, 2007). Quantifying the severity of the stress in drought studies is important and these results show that maintenance of θ at specific thresholds results in differences in midday Ψleaf and can be used to achieve different severities of drought. The differences in midday Ψleaf were achieved by controlled changes and maintenance of θ at specific thresholds, indicating that θ can be used to achieve different severities of drought. Further, these results indicate that quantifying the severity of stress in drought studies is important.
Fig. 6.
Leaf physiological responses of Petunia as a function of midday leaf water potential (Ψleaf). Stomatal conductance (gs, panel A), photosynthesis (A, panel B), and quantum yield of PSII (ФPSII, panel C) were measured by a leaf photosynthesis measurement system and midday leaf water potential (Ψleaf, panel D) was measured using thermocouple psychrometers. Substrate water content treatments: 0.40 (circles), 0.30 (diamonds); 0.20 (squares), and 0.10 m3·m–3 (triangles). Closed symbols indicate data collected during the drying period and open symbols indicate data collected after substrate water content thresholds had been reached. P- and r-values indicate the results from Spearman’s rank order correlation performed on the combined data.
Severity of drought stress, leaf ABA, and gs
A clear and major increase in ABA concentration was observed in all the drought treatments. As with the physiological responses, the extent of increase in ABA concentrations depended on the severity of drought, and more specifically on midday Ψleaf (Fig. 6), with severe drought resulting in the highest increase in ABA concentrations.
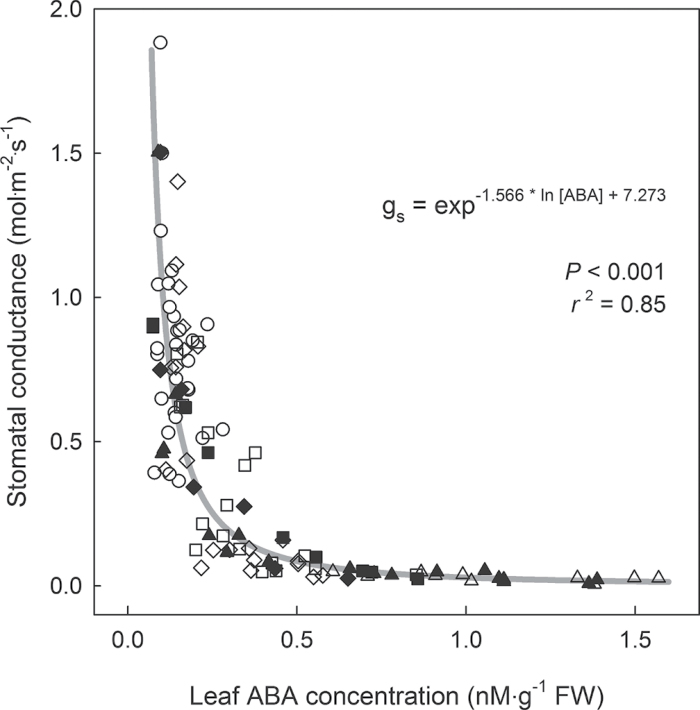
Previous research suggested that gs is better correlated with xylem ABA concentration than with leaf ABA concentration (Tardieu and Davies, 1993; Heilmeier et al., 2007; Jiang and Hartung, 2008). However, the current results show a strong correlation between leaf ABA concentration and gs in Petunia, regardless of θ or time, suggesting that gs is controlled by leaf ABA concentration (Fig. 7). Tardieu and Davies (1993) also reported that the effect of xylem ABA on gs depends on midday Ψleaf. Using multiple regression analysis, the current study found that gs was correlated with both leaf ABA concentration and midday Ψleaf, but their interaction was not significant. The partial r 2 of ABA and midday Ψleaf in the regression were 0.84 and 0.02, respectively, indicating that most variation in gs could be explained by changes in leaf ABA concentration, with only a minor contribution of midday Ψleaf.
Fig. 7.
Leaf ABA concentration effects on stomatal conductance. Data from all treatments and the entire study period are combined. Substrate water content treatments: 0.40 (circles), 0.30 (diamonds); 0.20 (squares), and 0.10 m3·m–3 (triangles). Closed symbols indicate data collected during the drying period and open symbols indicate data collected after substrate water content thresholds had been reached.
Expression of ABA-related genes responds to the severity of drought stress
Despite the increase in leaf ABA concentration under drought, there were no significant changes in the expression of the ABA biosynthesis-related genes NCED and AAO3 in Petunia leaves. Previous studies indicated that NCED (Thompson et al., 2000; Harb et al., 2010) and AAO3 (Seo et al., 2000) are key regulatory genes involved in ABA biosynthesis (Nambara and Marion-Poll, 2005). This suggests that de novo ABA synthesis may not be a significant contributor to the increase in ABA concentrations in Petunia leaves during drought. It also is possible that the putative NCED genes identified in the current study, NCED1 and NCED2, do not regulate ABA biosynthesis in response to drought, but may have primary roles in maintaining normal ABA concentrations. It is possible that other, as yet unidentified, members of the NCED family in Petunia may specifically regulate ABA biosynthesis under drought. In Arabidopsis, at least three NCED genes have been identified, among which primarily AtNCED3 was responsive to drought stress (Iuchi et al., 2001; Harb et al., 2010).
Interestingly, the relative expression of the ABA catabolism-related gene CYP707A2 was related to the ABA concentration. Petunia leaves with low midday Ψleaf had lower expression of CYP707A2 and higher ABA concentration than those at higher midday Ψleaf (Fig. 5A and 5C), suggesting that severe drought decreases ABA catabolism. The results of the expression of the putative CYP707A2 agree with previous findings, implicating it in regulating ABA homeostasis (Umezawa et al., 2006). These data suggest that low ABA catabolic activity may be important in maintaining high ABA concentrations in Petunia leaves during drought.
PLDα was upregulated in Petunia leaves as θ decreased with the highest expression noted under severe drought. Further, the increase in PLDα expression was evident primarily during the drying period and was correlated with gs (Fig. 5B), suggesting that decreasing θ enhanced the expression of PLDα thereby promoting stomatal closing. Harb et al. (2010) also reported an increase of PLDα1 as an early response in Arabidopsis leaves under moderate drought stress (employed by maintaining the substrate at 30% of field capacity). Phosphatidic acid generated through PLDα1 activity binds to ABI1/PP2C, a key component of ABA signalling involved in stomatal regulation in Arabidopsis (Mishra et al., 2006). Hence, increase in PLDα1 expression and subsequent PLDα1 activity may therefore mediate ABA-dependent stomatal closure during the drying phase of drought imposition. PLDα expression decreased in all drought treatments after the θ thresholds were reached, which may be related to a partial alleviation of the drought stress.
ZPT2-3 was upregulated under cold, drought, and heavy metal stress (Sugano et al., 2003). Consistent with this report, in the current study, ZPT2-3 expression increased in the drought treatments during the drying period. Overexpression of ZPT2-3 and its homologues enhanced drought tolerance in Petunia and Arabidopsis, respectively (Sugano et al., 2003; Sakamoto et al., 2004). Hence, increase in ZPT2-3 expression during the drying period may result in the transcriptional regulation of downstream genes associated with mechanisms that mediate drought tolerance. Higher levels of ZPT2-3 expression at later stages in the moderate drought treatment further support a role for this gene in regulating drought tolerance mechanisms.
In conclusion, by precisely controlling θ and conducting temporal analyses of physiological and molecular responses, this study demonstrates differences in plant responses to varying severity of drought stress. All Petunia plants at θ < 0.40 m3·m–3 displayed an increase in leaf ABA concentrations, but no significant changes in the relative expression of ABA biosynthesis-related genes were observed. There was a significant decrease in the expression of CYP707A2, a putative ABA catabolic gene, in plants under severe drought stress. This suggests that maintaining high ABA concentration in leaves of plants under severe drought is at least partially associated with decreased ABA catabolism. The results also show a close correlation between leaf ABA concentration and gs, regardless of stage of drought or θ, suggesting that gs was regulated by leaf ABA concentration. Higher expression of PLDα during the drying period observed in all the drought treatments may promote ABA-dependent stomatal closure. Leaf physiological responses (gs, A, and ФPSII) and ABA concentration were correlated with midday Ψleaf. The results indicate that the drought response of plants varies depending on the severity of drought stress and that this severity can be manipulated by controlling θ. Accurate descriptions of the severity and method of imposing drought stress deserve more attention, since they can greatly affect plant responses.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Schematic diagram of the soil moisture sensor-based irrigation system.
Supplementary Table S1. List of genes and sequence of the primers used for qRT PCR.
Supplementary Table S2. Morphological and physiological changes of Petunia × hybrida ‘Apple Blossom’ in response to various substrate water contents at 16 d after the start of the drying treatment.
Supplementary Material
Acknowledgements
This work was supported by Valent BioSciences and USDA-NIFA-SCRI award no. 2009-51181-05768. The authors acknowledge the advice of Dr. David Clark from the University of Florida, and Drs. Igor Kostenyuk and Jackie Burns from the CREC, University of Florida for helping in training of ABA determination. They also thank Sue Dove and Lisa Johnson for their technical support.
References
- Bargmann BOR, Laxalt AM, Riet Bt, van Schooten B, Merquiol E, Testerink C, Haring MA, Bartels D, Munnik T. 2009. Multiple PLDs required for high salinity and water deficit tolerance in plants Plant and Cell Physiology 50 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS. 1982. Plant productivity and environment Science 218 443–448 [DOI] [PubMed] [Google Scholar]
- Bray EA. 1997. Plant responses to water deficit Trends in Plant Science 2 48–54 [Google Scholar]
- Bray EA. 2004. Genes commonly regulated by water-deficit stress in Arabidopsis thaliana Journal of Experimental Botany 55 2331–2341 [DOI] [PubMed] [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. 2003. Understanding plant responses to drought – from genes to the whole plant Functional Plant Biology 30 239–264 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction Analytical Biochemistry 162 156–159 [DOI] [PubMed] [Google Scholar]
- Granier C, Aguirrezabal L, Chenu K, et al. 2006. PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit New Phytologist 169 623–635 [DOI] [PubMed] [Google Scholar]
- Harb A, Krishnan A, Ambavaram MMR, Pereira A. 2010. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth Plant Physiology 154 1254–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmeier H, Schulze E-D, Fan J, Hartung W. 2007. General relations of stomatal responses to xylem sap abscisic acid under stress in the rooting zone – a global perspective Flora 202 624–636 [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. 2001. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis The Plant Journal 27 325–333 [DOI] [PubMed] [Google Scholar]
- Jiang F, Hartung W. 2008. Long-distance signalling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal Journal of Experimental Botany 59 37–43 [DOI] [PubMed] [Google Scholar]
- Jones HG. 2007. Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance Journal of Experimental Botany 58 119–130 [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Girke T, Bray EA, Bailey-Serres J. 2004. Differential mRNA translation contributes to gene regulation under non-stress and dehydration stress conditions in Arabidopsis thaliana The Plant Journal 38 823–839 [DOI] [PubMed] [Google Scholar]
- Kim J, van Iersel MW. 2011. Slowly developing drought stress increases photosynthetic acclimation of Catharanthus roseus Physiologia Plantarum 143 166–177 [DOI] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu HH, Nishimura N, Schroeder JI. 2010. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling Annual Review of Plant Biology 61 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreps JA, Wu YJ, Chang HS, Zhu T, Wang X, Harper JF. 2002. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress Plant Physiology 130 2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ. 2006. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid Cell 126 1109–1120 [DOI] [PubMed] [Google Scholar]
- Mallona I, Lischewski S, Weiss J, Hause B, Egea-Cortines M. 2010. Validation of reference genes for quantitative real-time PCR during leaf and flower development in Petunia hybrida BMC Plant Biology 104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. 2000. Chlorophyll fluorescence – a practical guide Journal of Experimental Botany 51 659–668 [DOI] [PubMed] [Google Scholar]
- Mishra G, Zhang WH, Deng F, Zhao J, Wang XM. 2006. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis Science 312 264–266 [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. 2005. Abscisic acid biosynthesis and catabolism Annual Review of Plant Biology 56 165–185 [DOI] [PubMed] [Google Scholar]
- Nemali KS, van Iersel MW. 2006. An automated system for controlling drought stress and irrigation in potted plants Scientia Horticulturae 110 292–297 [Google Scholar]
- Nitsch L, Oplaat C, Feron R, Ma Q, Wolters-Arts M, Hedden P, Mariani C, Vriezen W. 2009. Abscisic acid levels in tomato ovaries are regulated by LeNCED1 and SlCYP707A1 Planta 229 1335–1346 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C, Chaves MM. 2011. Photosynthesis and drought: can we make metabolic connections from available data? Journal of Experimental Botany 62 869–882 [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-Shinozaki K. 2004. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions Plant Physiology 136 2734–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Goodger JQD. 2008. Chemical root to shoot signaling under drought Trends in Plant Science 13 281–287 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Zeevaart JAD. 2010. Abscisic acid biosynthesis and metabolism. In: Davies PJ, ed, Plant hormones: biosynthesis, signal transduction, action! New York: Springer; pp 137–155 [Google Scholar]
- Seiler C, Harshavardhan VT, Rajesh K, Reddy PS, Strickert M, Rolletschek H, Scholz U, Wobus U, Sreenivasulu N. 2011. ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions Journal of Experimental Botany 62 2615–2632 [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Ishida J, et al. 2002. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray The Plant Journal 31 279–292 [DOI] [PubMed] [Google Scholar]
- Seo M, Peeters AJM, Koiwai H, Oritani T, Marion-Poll A, Zeevaart JAD, Koornneef M, Kamiya Y, Koshiba T. 2000. The Arabidopsis aldehyde oxidase 3 (AA03) gene product catalyzes the final step in abscisic acid biosynthesis in leaves Proceedings of the National Academy of Sciences, USA 97 12908–12913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackel KA, Ahmadi H, Biasi W, et al. 1997. Plant water status as an index of irrigation need in deciduous fruit trees HortTechnology 7 23–29 [Google Scholar]
- Shu-Jing S, Shu-Qiao G, Xia Y, Yong-Mei B, Hai-Juan T, Hui S, Ji H, Hong-Sheng Z. 2010. Functional analysis of a novel Cys2/His2-type zinc finger protein involved in salt tolerance in rice Journal of Experimental Botany 61 2807–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano S, Kaminaka H, Rybka Z, Catala R, Salinas J, Matsui K, Ohme-Takagi M, Takatsuji H. 2003. Stress-responsive zinc finger gene ZPT2-3 plays a role in drought tolerance in petunia The Plant Journal 36 830–841 [DOI] [PubMed] [Google Scholar]
- Tardieu F, Davies WJ. 1993. Integration of hydraulic and chemical signalling in the control of stomatal conductance and water status of droughted plants Plant, Cell and Environment 16 341–349 [Google Scholar]
- Taylor IB, Burbidge A, Thompson AJ. 2000. Control of abscisic acid synthesis Journal of Experimental Botany 51 1563–1574 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Jackson AC, Parker RA, Morpeth DR, Burbidge A, Taylor IB. 2000. Abscisic acid biosynthesis in tomato: regulation of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid Plant Molecular Biology 42 833–845 [DOI] [PubMed] [Google Scholar]
- Umezawa T, Okamoto M, Kushiro T, Nambara E, Oono Y, Seki M, Kobayashi M, Koshiba T, Kamiya Y, Shinozaki K. 2006. CYP707A3, a major ABA 8’-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana The Plant Journal 46 171–182 [DOI] [PubMed] [Google Scholar]
- Wallach R. 2008. Physical characteristics of soilless media. In: Raviv M, Lieth JH, eds, Soilless culture: theory and practice Amsterdam: Elsevier; pp 41–116 [Google Scholar]
- Watkinson JI, Sioson AA, Vasquez-Robinet C, et al. 2003. Photosynthetic acclimation is reflected in specific patterns of gene expression in drought-stressed loblolly pine Plant Physiology 133 1702–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Araujo FJ. 2002. Corelations among predawn leaf, midday leaf, and midday stem water potential and their correlations with other measures of soil and plant water status in Vitis vinifera Journal of the American Society for Horticultural Science 127 448–454 [Google Scholar]
- Zenoni S, D’Agostino N, Tornielli GB, et al. 2011. Revealing impaired pathways in the an11 mutant by high-throughput characterization of Petunia axillaris and Petunia inflata transcriptomes The Plant Journal 68 11–27 [DOI] [PubMed] [Google Scholar]
- Zhang WH, Qin CB, Zhao J, Wang XM. 2004. Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling Proceedings of the National Academy of Sciences, USA 101 9508–9513 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.