Abstract
Plant responses to abiotic stresses are coordinated by arrays of growth and developmental processes. Indole-3-acetic acid (IAA) and abscisic acid (ABA) play critical roles in developmental programmes and environmental responses, respectively, through complex signalling and metabolism networks. However, crosstalk between the two phytohormones in the stress responses remains largely unknown. Here, it is reported that a GH3 family gene, OsGH3-2, encoding an enzyme catalysing IAA conjugation to amino acids, is involved in the modulation of ABA level and stress tolerance. Expression of OsGH3-2 was induced by drought but was suppressed by cold. Overexpression of OsGH3-2 in rice caused significant morphological aberrations related to IAA deficiency, such as dwarfism, smaller leaves, and fewer crown roots and root hairs. The overexpressing line showed significantly reduced carotene, ABA, and free IAA levels, greater stomata aperture, and faster water loss, and was hypersensitive to drought stress. However, the overexpressing line showed increased cold tolerance, which was due to the combined effects of reduced free IAA content, alleviated oxidative damage, and decreased membrane penetrability. Furthermore, expression levels of some ABA synthesis- and stress-related genes were significantly changed in the overexpression line. It was conclude that OsGH3-2 modulates both endogenous free IAA and ABA homeostasis and differentially affects drought and cold tolerance in rice.
Key words: ABA, auxin, cold tolerance, drought resistance, GH3 family, Oryza sativa
Introduction
Abiotic stresses such as drought and cold are major limiting factors for crop growth and reproduction. To respond to these stresses, plants have evolved a variety of biochemical and physiological mechanisms that allow them to adapt to adverse conditions (Bressan et al., 2009). Many of the adaptation mechanisms are related to changes in endogenous abscisic acid (ABA) levels. For example, under drought conditions, the ABA level can rise about 40-fold, triggering closure of stomata and regulating an array of ABA-dependent stress-responsive genes (Verslues et al., 2006). Genetic modifications of genes for ABA homeostasis or ABA signalling often result in significant changes in drought tolerance (Hauser et al., 2011). Nevertheless, the role of ABA in other stresses, such as cold, remains unclear. Many genes are induced by cold stress but are not responsive to exogenous ABA treatment (Shinozaki et al., 2003), suggesting the existence of ABA-independent signalling pathways for cold stress. Among these, C-repeat/dehydration-responsive element binding factors and ICE1 were found to be essential for plant survival under cold stress (Yamaguchi-Shinozaki and Shinozaki, 2006).
Auxin or indole-3-acetic acid (IAA) is a well-known phytohormone required for plant morphogenesis, including tropistic growth, root patterning, vascular tissue differentiation, axillary bud formation, and flower organ development (Zhao, 2010). Increasing evidence suggests a potential link between stress and auxin responses. Transcript profiling has revealed that many auxin-responsive genes are also responsive to cold stress (Jain and Khurana, 2009). Basipetal auxin transport in the inflorescence is abolished under cold conditions and restored to the wild-type (WT) level soon after the plant is returned to room temperature (Nadella et al., 2006). A recent study demonstrated that the effect of cold stress on auxin is linked to the inhibition of intracellular trafficking of auxin efflux carriers (Shibasaki et al., 2009). Auxin may also play a crucial role in plant responses to drought stress. Transcript profiling has revealed that the expression levels of many auxin-related genes are changed under dehydration (Jain and Khurana, 2009). In Arabidopsis, the R2R3-type MYB transcription factor MYB96 regulates lateral root meristem activation under drought conditions, possibly through an ABA–auxin signalling crosstalk, and the MYB96-knockout mutant produced more lateral roots and was more susceptible to drought stress (Seo et al., 2009). Activation of the YUCCA7 (YUC7) gene, encoding a flavin monooxygenase belonging to the tryptophan-dependent auxin biosynthetic pathway, resulted in elevated endogenous auxin levels and enhanced drought resistance in Arabidopsis (Lee and Luan, 2012).
Maintenance of IAA homeostasis is achieved through conversion of free and active IAA to an inactive form via conjugation of IAA with amino acids (such as Asp, Ala, and Phe). This occurs by IAA-amido synthetases belonging to the GH3 family, which are conserved in monocots and dicots (Staswick et al., 2005). GH3 proteins have been classified into three groups. Members of group I can adenylate jasmonic acid (JA) in vitro and have JA-amino synthetase activity, whereas group II are able to adenylate IAA and catalyse IAA conjugation to amino acids through amide bonds (Staswick et al., 2002, 2005). Therefore, groups I and II are involved in the homeostasis of JA and IAA, respectively, through the conjugation of the hormones to amino acids. However, no adenylation activity on the substrates tested was found for group III members, and group III GH3 genes are absent in rice (Jain et al., 2006). In addition to the functions in growth and development, GH3 genes also participate in plant resistance to biotic and abiotic stresses. For example, OsGH3-8 and OsGH3-1, belonging to group II of the rice GH3 family, have been reported to have dual roles in development and fungal resistance through the regulation of auxin levels (Ding et al., 2008; Domingo et al., 2009). In rice, OsGH3-13, another group II member, is inducible by drought stress, and OsGH3-13-activated rice showed alterations in plant architecture and tissue patterning, enhanced drought tolerance, and reduced endogenous free IAA maxima and ABA synthesis (Zhang et al., 2009).
In a previous study, overexpression of the GH3 family gene OsGH3-2 resulted in basal disease resistance by suppressing pathogen-induced IAA accumulation (Fu et al., 2011). In this study, we examined further the morphological changes and stress responses resulting from OsGH3-2 overexpression. Our results demonstrated that OsGH3-2 overexpression resulted in drought hypersensitivity and reduced ABA levels. Strikingly, the OsGH3-2 overexpressing rice showed increased resistance to cold stress. These results provide evidence that a change in auxin homeostasis can influence ABA synthesis, and that the balance of auxin and ABA homeostasis plays a crucial role in diverse stress responses, as well as in developmental processes in rice.
Materials and methods
Plant materials
Transgenic rice for OsGH3-2 overexpression in a Mudanjiang 8 (Oryza sativa subsp. japonica) background produced in a previous study (Fu et al., 2011) and OsGH3-2 promoter fused to the β-glucuronidase gene (GUS) were used in this study.
Stress treatments
Positive OsGH3-2-overexpressing seedlings were selected by germinating transgenic seeds on Murashige and Skoog medium with 100mg l–1 hygromycin for 7 d, and the seedlings were then transferred into soil. To test drought tolerance at the seedling stage, overexpressing and control plants, each occupying half of each pot, were grown to the five-leaf stage and the water supply was then stopped. After severe drought stress (all leaves wilted, 4 d after stopping watering) and recovery with watering, survival performance was photographed and recorded. To induce cold stress at the seedling stage, plants at the five-leaf stage were transferred to a growth chamber at 4 °C with 14h light/10h dark for 5 d, and then moved back to normal growth conditions (30 °C, 14h light/10h dark) for recovery. Cold stress testing at the reproductive stage was performed in the same manner as at the seedling stage.
Physiological measurements
To measure water-loss rates, healthy and fully expanded leaves were sampled from rice plants and placed immediately in a dry plastic bag in an icebox. The leaves were weighted over a time course as indicated in Fig. 7D. Stomatal closure was monitored by scanning electron microscope (JSM-6390LV; JEOL, Japan). Cell-membrane penetrability was evaluated by the relative conductance (R1/R2) of the cell membrane under cold stress. The top fully expanded leaves were sampled and washed with dH2Oseveral times. The sampling was consistent in replicated experiments. The conductance of a leaf segment surging in 20ml ddH2O for 6h (R1) and after boiling for 15min (R2) was measured. All stress-tolerance testing experiments were repeated three times. The relative activity of superoxide dismutase (SOD) was measured as described previously (DeLong et al., 2002) with minor modifications (Du et al., 2010). A paired t-test was used to determine the difference between the overexpressing line (or mutant) and WT.
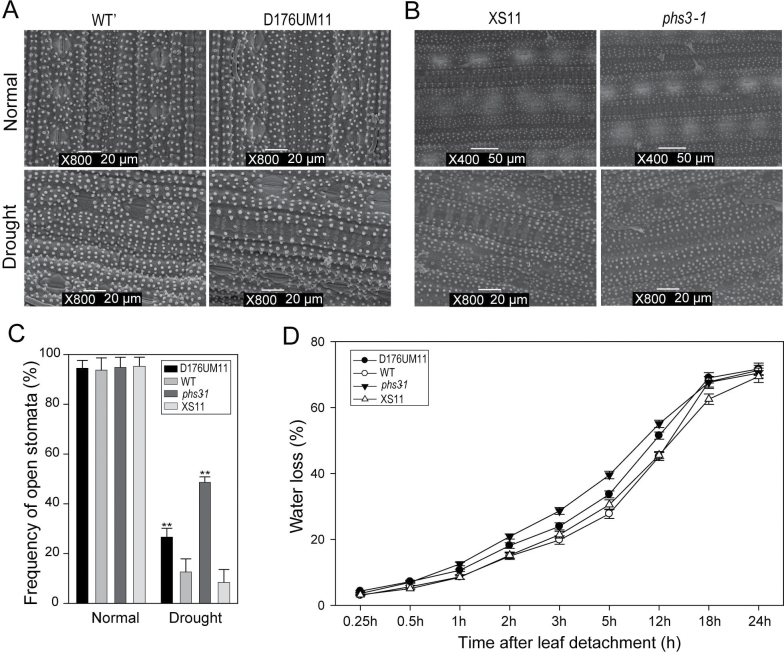
Fig. 7.
Stomatal aperture and water-loss assays. (A) Stomatal aperture of leaves of D176UM11 and WT’ plants observed with a scanning electron microscope. (B) Stomatal aperture of the ABA-deficient mutant phs3-1 and its WT (XS11). (C) Frequency of open stomata. Values are means ±SD (n=6). Statistical significance is indicated by ** (P <0.01, t-test). (D) Water-loss assay of leaves from D176UM11, phs3-1, and WT controls.
RNA extraction and real-time PCR
RNA was extracted using TRIzol reagent (Invitrogen, CA, USA). For real-time PCR, 5 µg of total RNA was digested by DNase I and reverse transcribed by Superscript III reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. The details of the procedure for real-time PCR have been described previously (Du et al., 2010). All amplification plots were analysed with an Rn threshold (reporter signal normalized to the fluorescence signal) of 0.2 to obtain threshold cycle (C T) values. The relative expression level was determined based on the 2∆∆CT method (Livak and Schmittgen, 2001) by using rice Actin1 as an internal control. The primers for real-time PCR are listed in Supplementary Table S1 at JXB online.
Histological assays
Stem node sections of plants were stained with haematoxylin to indicate lignin levels. The procedures of staining, dehydration, clearing, infiltration, and embedding were performed according to the method of Zhao et al. (2009). The microtome sections (8–10 µm) were mounted on glass slides for imaging. For the hydrochloric acid/phloroglucin stain, 0.3× phloroglucin was added to horizontal sections of leaves for 2min and then diluted with 0.3× hydrochloric acid. Photographs were taken using a Nikon 80i microscope. For diaminobenzidine (DAB) staining, leaves were vacuum infiltrated with 0.1mg m–1 of DAB in 50mM Tris/acetate buffer (pH 5.0). Samples were incubated for 24h at room temperature in the light. To remove chlorophyll, the stained samples were transferred to 95% ethanol and incubated at 100 °C for 15min, and this process was repeated several times.
Quantification of carotenoids, ABA, and auxin
To quantify carotenoid contents, samples were ground to a fine powder in liquid nitrogen. Carotenoid pigments were analysed by reverse-phase high-performance liquid chromatography (HPLC), with modifications from a previous method (Liu et al., 2006; Cao et al., 2012). Chromatography was carried out with an Agilent 1100 series (Agilent, USA). Carotenoids were eluted with methanol:methyl t-butyl ether:H2O (81:15:4, v/v; eluent A) and methanol:methyl t-butyl ether:H2O (10:90:4, v/v; eluent B) by a reverse-phase 5 µm C30 column (250×4.6mm; YMC, Japan). The linear elution gradients started with 100% eluent A and were followed by a gradual increase of eluent B, reaching 100% in 90min; the initial condition was then used for re-equilibration. The analysis was conducted under subdued lighting to avoid carotenoid degradation. The HPLC-grade β-carotene, violaxanthin, and lutein standards were obtained from Sigma (St Louis, MO, USA). Peaks were normalized relative to the internal standard, as described previously (Schaub et al., 2005). For ABA and auxin extraction, 100mg of four-leaf-stage seedlings were extracted twice with 900 µl of extracted buffer (methanol:H2O:acetonitrile, 90:9:1, v/v). Quantification was performed in an ABI 4000Q-TRAR LC-MS system (Applied Biosystems, USA) with stable-isotope-labelled ABA and auxin as standards (OlChemIm, Czech Specials), according to a method described previously (Liu et al., 2012).
Results
Morphological abnormality of OsGH3-2 overexpression in rice
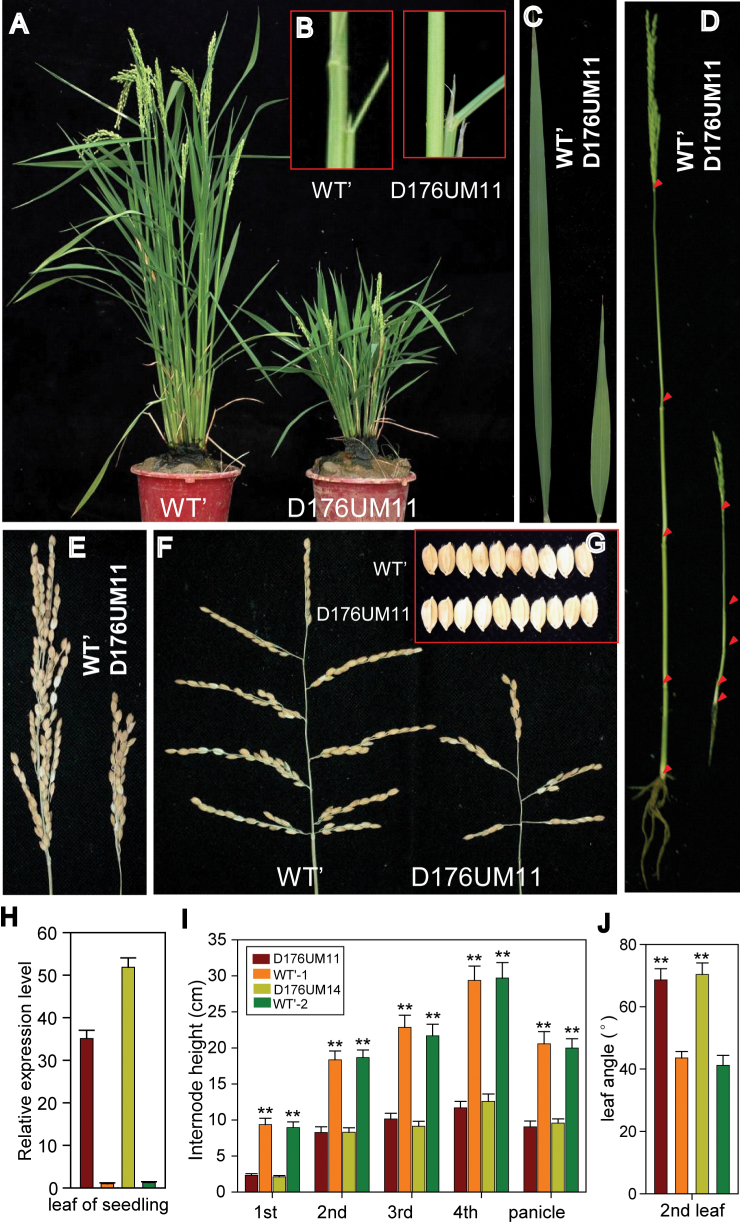
To examine whether auxin homeostasis had any effect on environmental stress tolerance in rice, plants overexpressing OsGH3-2 were selected because the auxin levels were significantly reduced in the OsGH3-2 overexpressing lines (Fu et al., 2011). We first examined the morphological changes resulting from impaired auxin homeostasis. Compared with the WT genotype (segregated from the heterozygous transgenic lines at the T0 generation and referred to as WT’ hereafter), the OsGH3-2 overexpressing rice lines (e.g. D176UM11) showed prominent morphological changes including dwarfism, increased leaf angle, shortened flag leaf length, and smaller panicles and internodes (Fig. 1A–G, I, J). In fact, at the mature stage, all the leaves of the overexpressing line were shorter than those of WT’ (Supplementary Fig. S1A at JXB online), but the relative water content in the leaves had showed no significant difference (Supplementary Fig. S1B at JXB online). Such extreme phenotypes imply that OsGH3-2 overexpression has detrimental effects on basic development in rice. The transgenic lines used in this study had been reproduced for a few generations, and we measured the transcript level of OsGH3-2 by real-time PCR and confirmed overexpression of this gene (Fig. 1H).
Fig. 1.
Morphological phenotypes of OsGH3-2 overexpression in rice. (A) Adult plants of WT’ (WT genotype segregated from the OsGH3-2 overexpressing transgenic rice) and one positive overexpressing line, D176UM11, at the heading stage. (B) The angle of the second leaf at panicle development stage. (C, D) The flag leaf and main culm. (E, G) The panicle and seed. (H) Expression level of OsGH3-2 in overexpressing lines relative to WT’-1 or WT’-2 (normalized to 1). (I, J) Statistical analyses of the internode lengths and the angle of the second leaf at panicle development stage. Statistical significance is indicated by ** (P <0.01, t-test). Values are means ±standard deviation (SD) (n=3).
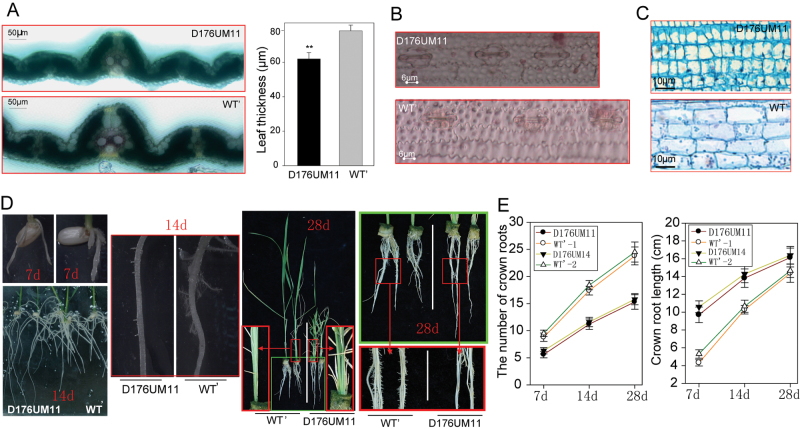
To elucidate the cellular basis of the morphological changes, cross-sections of leaves were measured and stained with hydrochloric acid/phloroglucin for indication of the lignin levels. The lignin levels in vascular tissues and leaf blade thickness (Fig. 2A) were significantly decreased in the overexpressing line. Detached epidermis from the leaf blade also showed a shortened cell size (Fig. 2B). Longitudinal sections of internodes showed that cell size was also significantly decreased in the overexpressing line (Fig. 2C). These results indicated that the reduced stature of the plants overexpressing OsGH3-2 was mainly due to the reduction in cell size, which is a typical characteristic of auxin deficiency in plants. Because auxin homeostasis plays an essential role in root development (Zhu et al., 2012), we examined the root system of the OsGH3-2-overexpressing rice plants. During the entire life cycle of rice grown under normal conditions, the overexpressing line had significantly fewer but longer crown roots with reduced root-hair density (Fig. 2D,2E). Nevertheless, there was sustained moderate drought-induced faster elongation of crown roots in the overexpressing line compared with WT’ (Supplementary Fig. S2 at JXB online).
Fig. 2.
Histological and dynamic analyses of WT’ and the OsGH3-2-overexpressing line. (A) Cross-section and hydrochloric acid/phloroglucin staining of the leaf blade at the seedling stage and statistical analysis of leaf thickness. Statistical significance is indicated by ** (P <0.01, t-test). (B) Detached epidermal cells of leaves from D176UM11 and WT’. (C) Longitudinal sections the first node of WT’ and D176UM11 plants. (D) Crown root and root hair of WT’ and D176UM11 at 7, 14, and 28 days after germination. (E) Statistical analysis of the number of crown roots and root length at 7, 14, and 28 days after germination.
Expression pattern of OsGH3-2
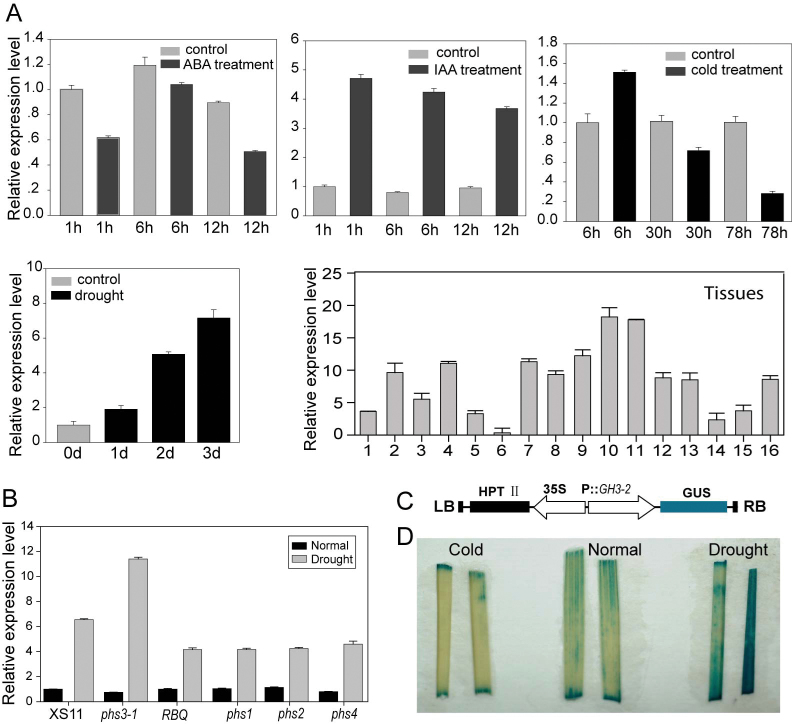
The tempo-spatial expression pattern of OsGH3-2 was investigated by searching the CREP rice gene-expression database containing microarray data for more than 30 tissues and organs covering the entire life cycle of rice (Wang et al., 2010), and the results were confirmed by quantitative PCR. OsGH3-2 was expressed in most of the tissues and organs and had relatively high levels in calli, leaves, and roots, and low levels in the panicles and stems (Fig. 3A and Supplementary Fig. S3A at JXB online). The OsGH3-2 transcript level was rapidly induced (four- to fivefold) by IAA, whereas it was slightly suppressed by ABA. OsGH3-2 expression was induced by drought, with the transcript level increasing to a peak of nearly eightfold on d 3 after the stress was applied. Contrary to our expectations, OsGH3-2 was notably suppressed by cold stress (Fig. 3A). To check whether the drought-induced expression of OsGH3-2 depended on ABA synthesis, we investigated the expression levels of OsGH3-2 in four ABA-deficient mutants, phs1–phs4 (Fang et al., 2008). Under normal growth conditions, the transcript levels of OsGH3-2 in the mutants were similar to those in the WT. Under drought stress, the induced levels of OsGH3-2 in the mutants were not significantly different compared with those in the WT (Fig. 3B). These results suggested that the drought-induced expression of OsGH3-2 may not depend on ABA synthesis. To examine further the expression profile of OsGH3-2, the OsGH3-2 promoter was fused to GUS (Fig. 3C) and transformed into rice Zhonghua 11. Strong GUS activity was detected in the calli, pulvinus, young leaf blades, primary roots, and leaf sheaths, whereas only slight activity was detected in the stems, spikelets, and immature seeds (Supplementary Fig. S3B–H). When the POsGH3-2::GUS transgenic plants were subjected to drought and cold stress conditions, strong induction and suppression of GUS was observed in the respective treatments (Fig. 3D), which is consistent with the quantitative PCR result.
Fig. 3.
Expression profile of OsGH3-2. Relative expression levels were compared with the first sample from the left (normalized to 1) in each graph. (A) Expression level of OsGH3-2 under treatments of ABA (200 µM), IAA (200 µM), cold and drought stress at the five-leaf-stage, and in various tissues or organs examined by quantitative real-time PCR: 1, culm; 2, node; 3, sheath; 4, three-leaf-stage shoot; 5, hull; 6, seed; 7, secondary branching of inflorescence; 8, anther; 9, calli induction stage; 10, calli screening stage; 11, calli differentiation stage; 12, young shoot; 13, young root; 14, flag leaf in the morning; 15, flag leaf in the evening; 16, pulvinus. (B) GH3-2 transcription levels in the ABA-deficient mutants and WT XS11 (for mutant phs3-1) and WT RBQ (Nipponbare, for mutants phs1, phs2, and phs4). (C) Diagram of the POsGH3-2::GUS construct and expression patterns of GUS driven by the OsGH3-2 promoter in transgenic rice plants. (D) GUS staining showing the leaf blade under cold and drought stress at the seedling stage.
Overexpression of OsGH3-2 decreases drought resistance
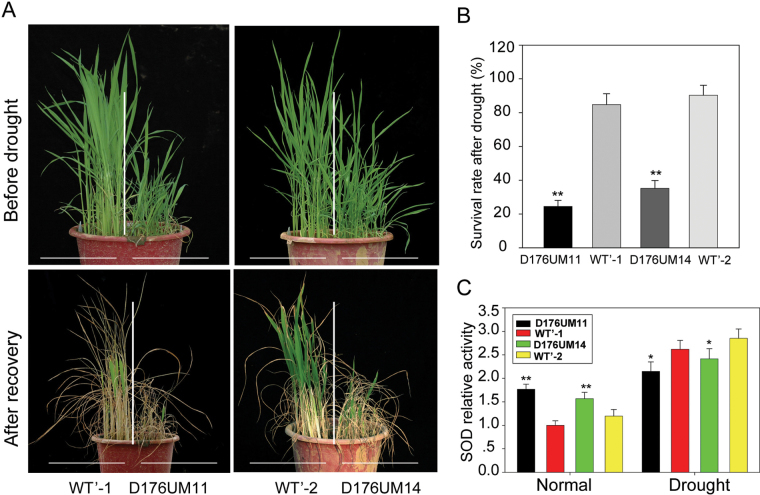
The induced expression of OsGH3-2 by drought stress prompted us to test the overexpressing transgenic rice for drought tolerance. Two OsGH3-2-overexpressing lines (D176UM11 and D176UM14 at the T4 generation) and WT’ were grown in a field with three biological repeats. Plants at the five-leaf stage were subjected to drought stress. During the stress, the transgenic plants showed drought-stressed symptoms (such as leaf wilting) earlier than the WT’ (Fig. 4A). After recovery for several days, the overexpressing lines had a significantly lower survival rate (~20–30%) than the WT’ (Fig. 4B). We measured SOD activity in the OsGH3-2-overexpressing and WT’ rice plants because SOD plays an important role in eliminating reactive oxygen species (ROS) induced by drought. Compared with WT’, the SOD activity in the overexpressing lines was slightly higher before the stress but was significantly lower after the stress (Fig. 4C). This result was similar to the SOD changes in a carotenoid-ABA-deficient mutant, dsm2, in our previous report (Du et al., 2010), implying that overexpression of OsGH3-2 may impair ROS scavenging under drought stress.
Fig. 4.
OsGH3-2-overexpressing rice shows increased drought sensitivity. (A) WT’, D176UM11, and D176UM14 plants before drought stress and after recovery. (B) Survival rate after recovery. (C) Determination of relative SOD activity in leaves. Statistical significance is indicated by * (P <0.05) and ** (P <0.01) (t-test). Values are means ±SD (n=3).
Improved cold and oxidative stress tolerance of OsGH3-2-overexpressing rice
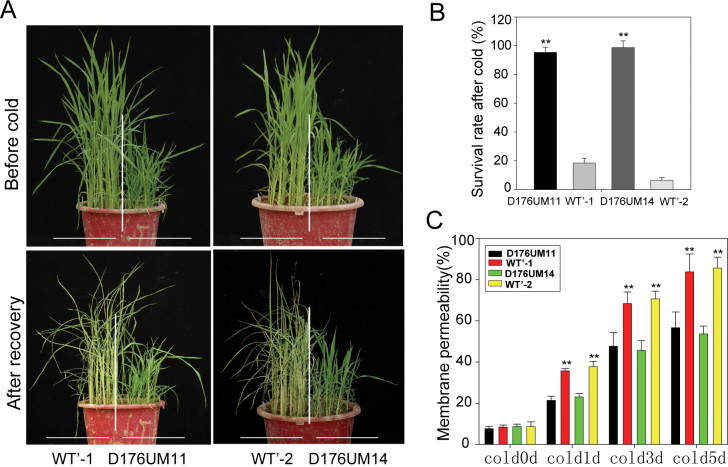
The strong suppression of OsGH3-2 by cold stress prompted us to test the OsGH3-2-overexpressing lines for cold tolerance. Two OsGH3-2-overexpressing lines were subjected to cold stress (see Materials and methods) with three biological repeats at the seedling stage. During the cold treatment, the WT’ plants wilted earlier than the overexpressing plants. During the recovery period (cold-stressed plants were transferred to normal growth conditions), the OsGH3-2-overexpressing plants performed significantly better than WT’ in terms of the leaves remaining green and survival rate (Fig. 5A). After 7 days of recovery, more than 80% of the transgenic plants remained vigorous, whereas almost all WT’ plants died (Fig. 5B). Cold stress often causes damage to cell membranes, so we further measured the cell-membrane penetrability, which has been well recognized as a parameter reflecting damage by cold stress. During the cold stress, the relative penetrability gradually increased but was significantly lower in the OsGH3-2-overexpressing rice than in WT’ (Fig. 5C).
Fig. 5.
OsGH3-2-overexpressing rice shows enhanced cold resistance. (A) WT’, D176UM11, and D176UM14 plants before cold stress and after recovery. (B) Survival rate after recovery. (C) Determination of membrane permeability. Statistical significance is indicated by ** (P <0.01, t-test). Values are means ±SD (n=3).
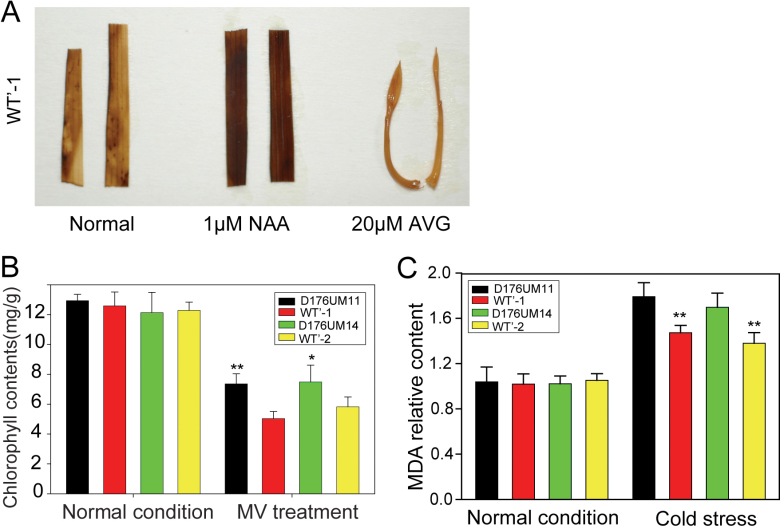
To examine whether the IAA deficiency in the OsGH3-2-overexpressing line was related to oxidative stress, seeds of the overexpressing line and WT’ were planted in Murashige and Skoog medium with 1 µM napthalene acetic acid (NAA), or 10 µM aminoethoxyvinylglycine (AVG), which is a strong inhibitor of auxin biosynthesis (Soeno et al., 2010). One-week-old seedlings were stained with DAB, and ROS production was induced by NAA, but was suppressed by AVG (Fig. 6A), suggesting that auxin may play an important role in ROS production, which is related to oxidative stress. Furthermore, we tested the response of the OsGH3-2-overexpressing rice to methylviologen (MV), a well-known oxidative stress inducer that inhibits electron transport during photosynthesis and generates hydrogen peroxide in chloroplasts under light (Cheng, 2006). Seven days after treatment with 30mM MV, the chlorophyll content in OsGH3-2-overexpressing rice was significantly higher than that in WT’ (Fig. 6B). We measured the level of monodehydroascorbate (MDA), an important intermediate in oxidative stress, and found that the OsGH3-2-overexpressing line had a significantly lower MDA level after cold stress (Fig. 6C). These results suggested that overexpression of OsGH3-2 may also result in increased oxidative stress tolerance.
Fig. 6.
Determination of ROS. (A) DAB staining of WT’ and D176UM11 leaves at the two-leaf stage after NAA and AVG treatments. (B) The chlorophyll content under normal conditions and after MV treatment. Statistical significance is indicated by * (P <0.05) and ** (P <0.01) (t-test). Values are means ±SD (n=3). (C) Relative MDA level of D176UM11 and D176UM14 plants after cold treatment. The MDA level under normal conditions was normalized to 1.
OsGH3-2 modulates auxin and ABA homeostasis
Because the transgenic rice had been propagated for a few generations, we rechecked the free IAA level in the OsGH3-2-overexpression lines at the seedling stage. The result showed that the free IAA level was significantly reduced in the OsGH3-2-overexpressing rice under normal conditions, which is consistent with a previous report (Fu et al., 2011). Under severe drought stress, there was a slight decrease in the free IAA level in the WT’, but overall the OsGH3-2-overexpressing lines contained significantly less free IAA than the WT’ plants (Table 1).
Table 1.
Carotenoids, ABA, and free IAA contents in the overexpressing lines and WT’ Leaves of D176UM11, D176UM14, and their controls were used for quantification by HPLC and HPLC mass spectrometry. Statistical significance is indicated by * (P <0.05) and ** (P <0.01) (t-test). Values are means ±SD (n=3). Unit are ng g–1 of fresh leaf tissue.
| Component | WT’-1 | D176UM11 | WT’-2 | D176UM14 | ||||
|---|---|---|---|---|---|---|---|---|
| Chlorophyll | 1741.82±91.28 | 1723.57±47.1 | 1687.572±82.94 | 1692.39±84.35 | ||||
| Trans-violaxanthin | 491.34±29.48 | 487.16±23.19 | 468.573±38.57 | 464.89±31.34 | ||||
| Cis-violaxanthin | 256.1±20.12 | 253.19±14.28 | 255.91±28.36 | 243.56±15.97 | ||||
| Lutein | 977.67±48.23 | 915.91±49.12 | 948.17±39.28 | 897.79±39.41 | ||||
| α-Carotene | 13.37±1.01 | 8.46±1.53** | 14.13±1.97 | 9.08±1.16** | ||||
| Trans-β-carotene | 354.76±20.27 | 275.38±17.29** | 362.13±22.3 | 287.23±11.29** | ||||
| Cis-β-carotene | 46.26±3.22 | 38.4±4.19* | 45.13±3.46 | 33.78±1.93** | ||||
| ABAn | 20.89±1.38 | 15.28±1.04** | 20.56±1.87 | 16.36±1.33** | ||||
| ABAd | 69.38±1.18 | 59.35±2.16** | 64.46±3.34 | 52.35±3.83** | ||||
| Free IAAn | 6.58±0.42 | 4.09±0.37** | 6.94±0.31 | 4.16±0.28** | ||||
| Free IAAd | 6.02±0.51 | 3.27±0.32** | 6.15±0.83 | 3.75±0.36** | ||||
| ABAp | 18.64±1.05 | 14.78±0.94** | 19.76±0.89 | 15.36±0.77** | ||||
| IAAp | 5.84±0.43 | 3.438±0.37** | 5.597±0.41 | 3.154±0.53** |
n Normal conditions at the five-leaf stage.
d Drought stress for 3 d at the five-leaf stage.
p Normal conditions at panicle development stage.
We further examined whether ABA and some of its precursors were influenced. ABA levels in the OsGH3-2-overexpressing lines at the seedling stage were markedly decreased to 76% of that in the WT’ (Table 1). After moderate drought stress, the ABA level was also significantly reduced in the OsGH3-2-overexpressing lines compared with WT’ (Table 1). At the panicle development stage, the free IAA and ABA levels were also significantly reduced in the OsGH3-2-overexpressing lines compared with the WT’ (Table 1). The levels of α-carotene and β-carotene were significantly reduced in the OsGH3-2-overexpressing lines under normal conditions (Table 1). However, the levels of violaxanthin and lutein, which are produced by β-carotene hydroxylase and α-carotene hydroxylase, respectively, were not changed. These results indicated that OsGH3-2 may contribute to carotenoid metabolism and ABA synthesis, especially at the level of α-carotene and β-carotene metabolism.
Decreased stomatal closure and increased water loss of OsGH3-2-overexpressing rice under dehydration stress
Reduced ABA levels in the OsGH3-2-overexpressing rice prompted us to check the stomatal density and aperture by scanning electron microscope. Significantly (P <0.01) more stomatal pores were open in the OsGH3-2-overexpressing rice under drought stress conditions but no difference in stomatal density was observed (Fig. 7A, C). This result was compared with the stomatal response of the ABA-deficient mutant psh3-1, which is partially impaired in ABA synthesis (Fang et al., 2008). We observed that significantly (P <0.01) more stomatal pores were open in the psh3-1 mutant than in the WT under drought stress conditions (Fig. 7B, C), and the proportion of open stomata in the psh3-1 mutant was slightly higher than that in the OsGH3-2-overexpressing line. In addition, we compared the water-loss rates of excised leaves from the OsGH3-2-overexpressing line and the psh3-1 mutant. Both the OsGH3-2-overexpressing line and the mutant lost water faster than the corresponding control (Fig. 7D), although the water-loss rate of the psh3-1 mutant leaf was slightly higher than that of the OsGH3-2-overexpressing line. These results suggested that the reduced ABA level in the OsGH3-2-overexpressing rice may be associated with the drought sensitivity phenotype.
Expression level changes of ABA and auxin-related genes in OsGH3-2-overexpressing rice
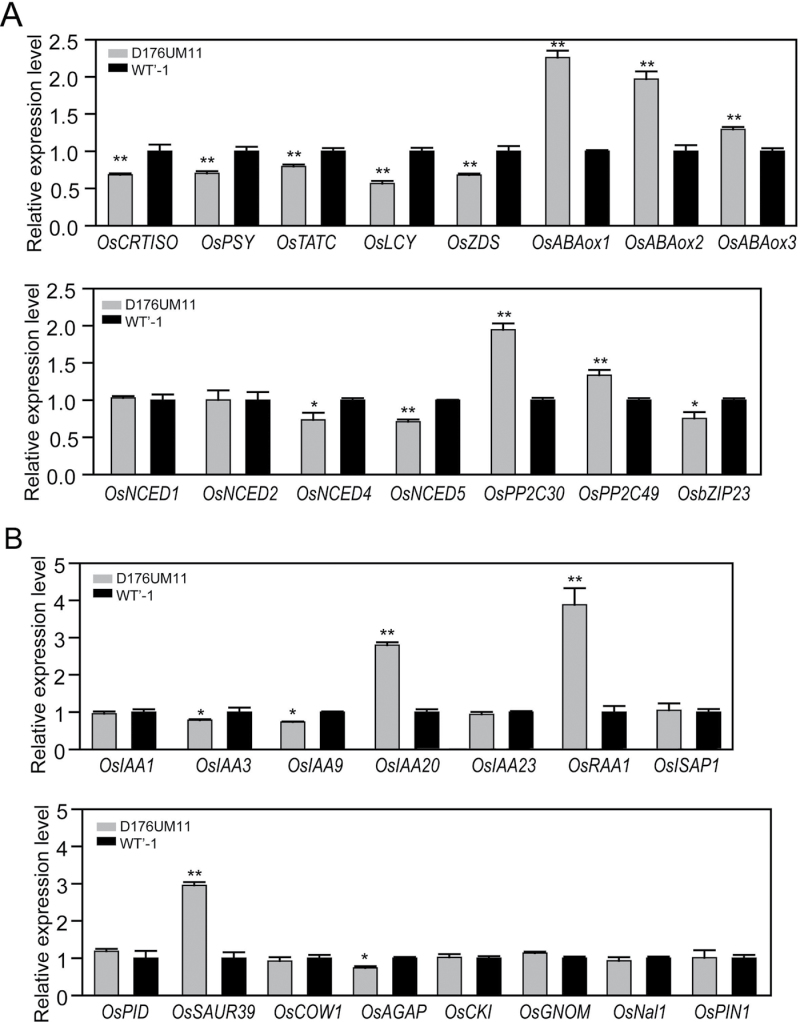
We tried to explain the decreased ABA levels in OsGH3-2-overexpressing rice by checking the expression levels of well-characterized genes for ABA synthesis and catabolism in rice. Many ABA synthesis genes, such as OsCRTISO, OsPSY, OsTATC, OsNCEDs, OsLCY, OsZDS, NCED4, and NCED5, were suppressed in the OsGH3-2-overexpressing rice, and ABA 8’-hydroxylase genes involved in ABA catabolism were significantly upregulated (Fig. 8A). However, the expression levels of most genes related to ABA signalling were unchanged, except for OsPP2C30 and OsPP2C49 (slightly increased) and OsbZIP23 (slightly decreased) in the OsGH3-2-overexpressing rice plants (Fig. 8A).
Fig. 8.
Expression levels of hormone-related genes. (A) Quantitative PCR analysis of genes related to ABA synthesis or signalling. (B) Quantitative PCR analysis of genes related to auxin signalling. Statistical significance is indicated by * (P <0.05) and ** (P <0.01) (t-test). Values are means ±SD (n=3).
We also examined the expression of well-characterized auxin signalling genes in the OsGH3-2-overexpressing rice. The mRNA levels of OsIAA20, OsRAA1, and OsSAUR39 were upregulated in OsGH3-2-overexpressing rice, whereas the expression levels of the other genes were unaffected (Fig. 8B). These results suggested that the disruption of OsGH3-2 affected the expression of many ABA synthesis and auxin-related genes, which may have contributed to the different phenotypes under drought and cold stresses.
Discussion
Overexpression of OsGH3 family genes, including OsGH3-1, OsGH3-2, OsGH3-8, and OsGH3-13, in transgenic rice plants results in a decrease in free IAA content and similar morphological phenotypes including dwarfism, larger leaf angle, shortened flag leaves, and smaller panicles (Ding et al., 2008; Domingo et al., 2009; Zhang et al., 2009; Fu et al., 2011), and these characteristic phenotypes have been observed frequently in other auxin-deficient mutants. For example, double, triple, and quadruple mutants of Arabidopsis YUCCA genes display severe defects in plant architecture (Cheng, 2006). A double-mutant of CYP79B2 and CYP79B3 genes in Arabidopsis showed a slight reduction in IAA levels and weak growth related to auxin deficiency (Zhao et al., 2002). In addition, OsGH3-1, OsGH3-2, and OsGH3-8 overexpression in rice was shown to result in enhanced defence responses and resistance to pathogens (Ding et al., 2008; Domingo et al., 2009; Zhang et al., 2009; Fu et al., 2011).
We tried to examine the function of OsGH3-2 by suppressing its expression. The expression of OsGH3-2 was significantly suppressed by RNA interference (Supplementary Fig. S4 at JXB online), but no significant change in the IAA and ABA levels was detected. The OsGH3-2-suprressed plants showed no difference compared with the WT under normal, drought, and cold stress conditions (Supplementary Figs S4 and S5 at JXB online). We suspect that the function of OsGH3-2 might be compensated by other members of the OsGH3 family, as there are eight OsGH3 members including OsGH3-2 in group II of the GH3 family.
The functions of OsGH3 members may differ. For example, OsGH3-13-overexpressing rice exhibited downregulation of IAA, with an increased number of cell layers, smaller seeds, and fewer lateral roots during its life cycle (Zhang et al., 2009). In contrast, OsGH3-2-overexpressing rice, with decreased endogenous IAA (Table 1), displayed thinner leaves and longer and fewer crown roots, but the seed size was not changed according to our data (Fig. 1G and Fig. 2A–D). Sequence analysis showed that some conserved amino acids of the GH3 family were incomplete in OsGH3-13 (Supplementary Fig. S6 and Table S2 at JXB online). Such sequence specificity may partially explain the phenotypic difference between the OsGH3-2- and OsGH3-13-overexpressing lines. Compared with OsGH3-13, OsGH3-2 showed a distinctive expression profile in tissues and organs during vegetative and reproductive development (Supplementary Fig. S3A). In addition, the ABA level was significantly increased in OsGH3-13-overexpressing rice compared with that in the control. OsGH3-13 was strongly induced by drought but only slightly induced by ABA and cold stress (Supplementary Fig. S7 at JXB online), and the OsGH3-13 overexpression rice showed increased drought tolerance. Compared with the expression pattern of OsGH3-13, OsGH3-2 was induced by drought but significantly suppressed by cold stress (Fig. 3A). Furthermore, drought-induced expression of OsGH3-2 was independent of ABA synthesis (Fig. 3B). These differences suggest that OsGH3-2 and OsGH3-13 each play specific roles in stress responses.
Previous studies revealed that crosstalk between ABA and auxin responses occurs mainly during seed germination and early seedling development, during which the ABA-dependent repression of growth is potentiated by auxin (Nakabayashi et al., 2005; Liu et al., 2007). A previous study indicated that both ABA and auxin may be involved in the promotion of drought-induced rhizogenesis (Vartanian et al., 1994). RCN1 was characterized previously as a molecular component affecting auxin transport and gravitropism in Arabidopsis, and further studies have revealed that RCN1 also functions as a general positive transducer of early ABA signalling (Garbers et al., 1996; Kwak et al., 2002). These results indicate that ABA signalling crosstalks with auxin signalling at many levels. Furthermore, auxin stimulates lateral root formation and growth, but auxin-induced lateral root formation is completely suppressed by ABA, as seen in the abi3 mutant, indicating that ABI3 may mediate the crosstalk between ABA and auxin signalling (Suzuki et al., 2001). Another study showed that ABI3 is necessary for correct auxin signalling during lateral root formation, and the authors speculated that ABI3 interacts with ARF or AUX proteins (Brady et al., 2003). In this study, OsGH3-2-overexpressing rice had fewer but longer crown roots and fewer root hairs than WT’ under normal conditions (Fig. 2D and Supplementary Fig. S2). Under mild drought stress, however, the crown root length of OsGH3-2-overexpressing rice was significantly shorter than that of WT’ (Supplementary Fig. S2B). ABA has been shown to have an important role in suppressing lateral root development (de Smet et al., 2006). Therefore, we speculate that the root-related phenotypes of the OsGH3-2-overexpressing rice are due mainly to the decreased ABA and/or auxin level(s), and that the defect in root growth under drought stress may also affect water uptake in the OsGH3-2-overexpressing rice (Supplementary Fig. S2B). Recently, ABI5-like1 was reported to mediate the crosstalk of ABA and auxin responses by directly regulating a series of downstream genes containing an ABA-responsive element, providing new insights into the ABA and auxin signalling interactions (Yang et al., 2011). We also investigated the responses of OsGH3-2-overexpressing rice to exogenous auxin and ABA. Although OsGH3-2 was induced by both auxin and ABA, no significant difference was observed after treatment with 0.1 µM NAA, 1 µM NAA, or 3 µM ABA compared with the WT’ (Supplementary Fig. S8 at JXB online). This result suggests that OsGH3-2 overexpression mainly influences ABA synthesis, although some signalling-related genes showed slight changes in the OsGH3-2-overexpressing line (Fig. 8).
Mutants defective in synthesizing carotenoid precursors for endogenous ABA synthesis often show drought sensitivity (Nambara and Marion-Poll, 2005). Compared with WT’, the OsGH3-2-overexpressing rice exhibited reduced ABA content (Table 1) and greater stomatal aperture, which resulted in faster water loss under drought stress (Fig. 7A, D). This phenotype is very similar to that of the ABA-deficient mutant phs3-1, implying that OsGH3-2-mediated ABA deficiency may be related to the increased drought sensitivity.
Cell-membrane systems are the primary sites of freezing injury in plants (Steponkus and Lynch, 1989). During cold stress conditions, OsGH3-2-overexpressing rice showed significantly lower cell-membrane penetrability compared with WT’ (Fig. 5C). Recent reports showed that cold stress affects auxin transport in Arabidopsis and causes downregulation of active auxin in wheat (Shibasaki et al., 2009; Kosova et al., 2012). MDA is an important intermediate in ROS scavenging, and a high level of MDA is toxic to plant cells. After cold stress, the OsGH3-2-overexpressing line had significantly lower MDA content (Fig. 6C). Together, these results suggest that overexpression of OsGH3-2 can improve cold tolerance in rice.
Unilateral application of auxin to vertical roots induced a transient increase in intracellular ROS in root convex endodermis related to root gravitropism (Joo et al., 2005). A mutant of gene AtGRXS17 that was defective in auxin sensitivity and polar auxin transport had a high level of ROS and severe cellular membrane damage under high temperature stress (Cheng et al., 2011). In this study, NAA treatment induced ROS in rice, while AVG treatment suppressed ROS production (Fig. 6A). The OsGH3-2-overexpressing rice had a significantly higher chlorophyll content after MV treatment (Fig. 6B) and a lower MDA level after cold treatment, suggesting a possible involvement of ROS in the stress tolerance.
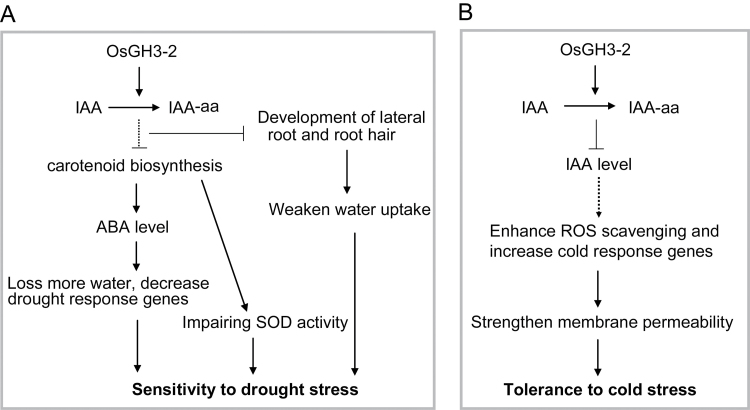
Drought and cold cause different changes at transcriptional and physiological levels in plants (Yamaguchi-Shinozaki and Shinozaki, 2006). Here, we showed that overexpression of the OsGH3-2 gene had opposite effects on drought and cold tolerance. Based on our data, a simple model was proposed to explain the increased drought sensitivity and cold tolerance of the OsGH3-2-overexpressing rice (Fig. 9). In contrast to the intensive molecular and genetic research of ABA deficiency related to drought, the function of auxin under cold stress and the balance of auxin and ABA metabolism under cold stress have received limited attention.
Fig. 9.
Working model for the function of OsGH3-2 in response to drought and cold stress. (A) Overexpression of OsGH3-2 causes a decrease in IAA. The decrease in IAA results in a decrease in the ABA level, which further results in decreased expression of some drought-response genes and faster water loss under drought stress conditions. The decrease in IAA also results in reduced numbers of lateral roots and root hairs, which may weaken water uptake. Decreases in IAA may also impair SOD activity. Together, these contribute to the drought sensitivity of OsGH3-2-overexpressing rice. (B) Overexpression of OsGH3-2 impairs the accumulation of free IAA, leading to enhanced ROS scavenging ability and increased expression of some genes related to cold response and membrane permeability, which ultimately contributes to cold tolerance.
Taken together, our findings indicate that OsGH3-2 plays important roles not only in modulating auxin homeostasis but also in regulating the endogenous ABA level. We propose that the balance of auxin and ABA homeostasis is important for rice to respond to drought and cold stress via different mechanisms. Further investigation of ABA and auxin crosstalk at the levels of biosynthesis and signalling, as well as their roles in plant growth and responses to abiotic stresses, will help to elucidate the integrative effect of auxin and ABA homeostasis on plant growth and response to stresses.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. (A) Leaf blade length of mature OsGH3-2-overexpressing rice. (B) Relative water content (RWC) in OsGH3-2-overexpression rice and WT’.
Supplementary Fig. S2. Root phenotypes under different growth conditions.
Supplementary Fig. S3. Expression profile of OsGH3-2 and other members of the GH3 family.
Supplementary Fig. S4. Relative expression level (A) and phenotype (B) of OsGH3-2-RNAi transgenic rice under drought stress.
Supplementary Fig. S5. Phenotype of OsGH3-2-RNAi transgenic rice under cold stress.
Supplementary Fig. S6. Sequence alignment of OsGH3-2 and OsGH3-13.
Supplementary Fig. S7. Expression profiles of 13 OsGH3 family genes under drought or cold stress.
Supplementary Fig. S8. Phenotypes of D176UM11, D176UM14, and WT’ plants grown in medium with NAA or ABA treatment.
Supplementary Table S1. Primer sequences used in this study.
Supplementary Table S2. Accession numbers and protein sequences for OsGH3-2 and OsGH3-13.
Acknowledgments
We thank Ning Yang for vector construction, and Fei Huang and Lei Sun for measuring MDA. This work was supported by grants from the National Program for Basic Research of China (2012CB114305), the National Program on High Technology Development (2012AA10A303), the National Natural Science Foundation of China (30921091 and J1103510 to N.W.), and the Ministry of Agriculture of China for Transgenic Research (2011ZX08009-003-002, 2011ZX08001-003).
Glossary
Abbreviations:
- ABA
abscisic acid
- AVG
aminoethoxyvinylglycine
- DAB
diaminobenzidine
- GUS
glucuronidase
- HPLC
high-performance liquid chromatography
- IAA
indole-3-acetic acid
- JA
jasmonic acid
- MDA
monodehydroascorbate
- MV
methylviologen
- NAA
napthalene acetic acid
- ROS
reactive oxygen species
- SD
standard deviation
- SOD
superoxide dismutase
- WT
wild type
References
- Brady SM, Sarkar SF, Bonetta D, McCourt P. 2003. The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis The Plant Journal 34 67–75 [DOI] [PubMed] [Google Scholar]
- Bressan R, Bohnert H, Zhu JK. 2009. Abiotic stress tolerance: from gene discovery in model organisms to crop improvement Molecular Plant 2 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Zhang J, Xu J, Ye J, Yun Z, Xu Q, Xu J, Deng X. 2012. Comprehending crystalline β-carotene accumulation by comparing engineered cell models and the natural carotenoid-rich system of citrus Journal of Experimental Botany 63 4403–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Liu JZ, Liu X, et al. 2011. Arabidopsis monothiol glutaredoxin AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response Journal of Biological Chemistry 286 20398–20406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q. 2006. Structural diversity and functional novelty of new carotenoid biosynthesis genes Journal of Industrial Microbiology and Biotechnology 33 552–559 16609853 [Google Scholar]
- de Smet I, Zhang H, Inze D, Beeckman T. 2006. A novel role for abscisic acid emerges from underground Trends in Plant Science 11 434–439 [DOI] [PubMed] [Google Scholar]
- DeLong JM, Prange RK, Hodges DM, Forney CF, Bishop MC, Quilliam M. 2002. Using a modified ferrous oxidation-xylenol orange (FOX) assay for detection of lipid hydroperoxides in plant tissue Journal of Agricultural and Food Chemistry 50 248–254 [DOI] [PubMed] [Google Scholar]
- Ding X, Cao Y, Huang L, Zhao J, Xu C, Li X, Wang S. 2008. Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice The Plant Cell 20 228–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo C, Andres F, Tharreau D, Iglesias DJ, Talon M. 2009. Constitutive expression of OsGH3.1 reduces auxin content and enhances defense response and resistance to a fungal pathogen in rice Molecular Plant–Microbe Interactions 22 201–210 [DOI] [PubMed] [Google Scholar]
- Du H, Wang N, Cui F, Li X, Xiao J, Xiong L. 2010. Characterization of the β-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice Plant Physiology 154 1304–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Chai C, Qian Q, et al. 2008. Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice The Plant Journal 54 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Liu H, Li Y, Yu H, Li X, Xiao J, Wang S. 2011. Manipulating broad-spectrum disease resistance by suppressing pathogen-induced auxin accumulation in rice Plant Physiology 155 589–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruere J, Bernasconi P, Soll D. 1996. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis EMBO Journal 15 2115–2124 [PMC free article] [PubMed] [Google Scholar]
- Hauser F, Waadt R, Schroeder JI. 2011. Evolution of abscisic acid synthesis and signaling mechanisms Current Biology 21 R346–R355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Khurana JP. 2009. Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice FEBS Journal 276 3148–3162 [DOI] [PubMed] [Google Scholar]
- Jain M, Kaur N, Tyagi AK, Khurana JP. 2006. The auxin-responsive GH3 gene family in rice (Oryza sativa) Functional and Integrative Genomics 6 36–46 [DOI] [PubMed] [Google Scholar]
- Joo JH, Yoo HJ, Hwang I, Lee JS, Nam KH, Bae YS. 2005. Auxin-induced reactive oxygen species production requires the activation of phosphatidylinositol 3-kinase FEBS Letters 579 1243–1248 [DOI] [PubMed] [Google Scholar]
- Kosova K, Prasil IT, Vitamvas P, et al. 2012. Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra Journal of Plant Physiology 169 567–576 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Moon JH, Murata Y, Kuchitsu K, Leonhardt N, DeLong A, Schroeder JI. 2002. Disruption of a guard cell-expressed protein phosphatase 2A regulatory subunit, RCN1, confers abscisic acid insensitivity in Arabidopsis The Plant Cell 14 2849–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Luan S. 2012. ABA signal transduction at the crossroad of biotic and abiotic stress responses The Plant Cell Environment 35 53–60 [DOI] [PubMed] [Google Scholar]
- Liu H, Li X, Xiao J, Wang S. A convenient method for simultaneous quantification of multiple phytohormones and metabolites: application in study of rice–bacterium interaction. Plant Methods. 2012;8:2. doi: 10.1186/1746-4811-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PP, Montgomery TA, Fahlgren N, Kasschau KD, Nonogaki H, Carrington JC. 2007. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages The Plant Journal 52 133–146 [DOI] [PubMed] [Google Scholar]
- Liu Q, Xu J, Liu Y, Zhao X, Deng X, Guo L, Gu J. 2006. A novel bud mutation that confers abnormal patterns of lycopene accumulation in sweet orange fruit (Citrus sinensis L. Osbeck) Journal of Experimental Botany 58 4161–4171 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−⊠⊠CT method Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Nadella V, Shipp MJ, Muday GK, Wyatt SE. 2006. Evidence for altered polar and lateral auxin transport in the gravity persistent signal (gps) mutants of Arabidopsis Plant, Cell and Environment 29 682–690 [DOI] [PubMed] [Google Scholar]
- Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E. 2005. Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. The Plant Journal 41 697–709 [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. 2005. Abscisic acid biosynthesis and catabolism Annual Review of Plant Biology 56 165–185 [DOI] [PubMed] [Google Scholar]
- Schaub P, Al-Babili S, Drake R, Beyer P. 2005. Why is golden rice golden (yellow) instead of red? Plant Physiology 138 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo PJ, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, Lee YH, Park WJ, Park CM. 2009. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis Plant Physiology 151 275–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Uemura M, Tsurumi S, Rahman A. 2009. Auxin response in Arabidopsis under cold stress: underlying molecular mechanisms The Plant Cell 21 3823–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K, Seki M. 2003. Regulatory network of gene expression in the drought and cold stress responses Current Opinion in Plant Biology 6 410–417 [DOI] [PubMed] [Google Scholar]
- Soeno K, Goda H, Ishii T, Ogura T, Tachikawa T, Sasaki E, Yoshida S, Fujioka S, Asami T, Shimada Y. 2010. Auxin biosynthesis inhibitors, identified by a genomics-based approach, provide insights into auxin biosynthesis Plant and Cell Physiology 51 524–536 [DOI] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. 2005. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid The Plant Cell 17 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I, Rowe ML. 2002. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation The Plant Cell 14 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus PL, Lynch DV. 1989. Freeze/thaw-induced destabilization of the plasma membrane and the effects of cold acclimation Journal of Bioenergetics and Biomembranes 21 21–41 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kao CY, Cocciolone S, McCarty DR. 2001. Maize VP1 complements Arabidopsis abi3 and confers a novel ABA/auxin interaction in roots The Plant Journal 28 409–418 [DOI] [PubMed] [Google Scholar]
- Vartanian N, Marcotte L, Giraudat J. 1994. Drought rhizogenesis in Arabidopsis thaliana (differential responses of hormonal mutants) Plant Physiology 104 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK. 2006. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status The Plant Journal 45 523–539 [DOI] [PubMed] [Google Scholar]
- Wang L, Xie W, Chen Y, et al. 2010. A dynamic gene expression atlas covering the entire life cycle of rice The Plant Journal 61 752–766 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. 2006. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses Annual Review of Plant Biology 57 781–803 [DOI] [PubMed] [Google Scholar]
- Yang X, Yang YN, Xue LJ, Zou MJ, Liu JY, Chen F, Xue HW. 2011. Rice ABI5-Like1 regulates abscisic acid and auxin responses by affecting the expression of ABRE-containing genes Plant Physiology 156 1397–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SW, Li CH, Cao J, Zhang YC, Zhang SQ, Xia YF, Sun DY, Sun Y. 2009. Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3.13 activation Plant Physiology 151 1889–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hu Y, Dai M, Huang L, Zhou DX. 2009. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice The Plant Cell 21 736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL. 2002. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3 Genes and Development 16 3100–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. 2010. Auxin biosynthesis and its role in plant development Annual Review of Plant Biology 61 49–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZX, Liu Y, Liu SJ, Mao CZ, Wu YR, Wu P. 2012. A gain-of-function mutation in OsIAA11 affects lateral root development in rice Molecular Plant 5 154–161 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.