Abstract
Resolving the physiological mechanisms by which rhizobacteria enhance plant growth is difficult, since many such bacteria contain multiple plant growth-promoting properties. To understand further how the 1-aminocyclopropane-1-carboxylate (ACC) deaminase (ACCd)-containing rhizobacterium Variovorax paradoxus 5C-2 affects plant growth, the flows and partitioning of mineral nutrients and abscisic acid (ABA) and ABA metabolism were studied in pea (Pisum sativum) plants following rhizosphere bacterial inoculation. Although root architecture was not affected, inoculation increased root and shoot biomass, and stomatal conductance, by 20, 15, and 24%, respectively, and increased N, P, K, Ca, and Mg uptake by 16, 81, 50, 46, and 58%, respectively. P deposition in inoculated plant roots was 4.9 times higher than that in uninoculated controls. Rhizobacterial inoculation increased root to shoot xylem flows and shoot to root phloem flows of K by 1.8- and 2.1-fold, respectively. In control plants, major sinks for K deposition were the roots and upper shoot (43% and 49% of total uptake, respectively), while rhizobacterial inoculation increased K distribution to the lower shoot at the expense of other compartments (xylem, phloem, and upper shoot). Despite being unable to metabolize ABA in vitro, V. paradoxus 5C-2 decreased root ABA concentrations and accumulation by 40–60%. Although inoculation decreased xylem ABA flows, phloem ABA flows increased. Whether bacterial ACCd attenuates root to shoot ABA signalling requires further investigation, since ABA is critical to maintain growth of droughted plants, and ACCd-containing organisms have been advocated as a means of minimizing growth inhibition of plants in drying soil.
Key words: Abscisic acid, ACC deaminase, hormone flow modelling, nutrient uptake, pea, plant–microbe interaction, rhizobacteria, Variovorax paradoxus
Introduction
Plant growth-promoting rhizobacteria (PGPR) colonize the rhizosphere and can enhance plant growth via a set of biocontrol mechanisms (Lugtenberg and Kamilova, 2009) or by increasing plant nutrient uptake via multiple mechanisms including biological nitrogen fixation, siderophore production, and phosphate solubilization (Dey et al., 2004; Lugtenberg and Kamilova, 2009). Additionally, many rhizobacteria can alter plant hormone status by producing auxins and cytokinins (Costacurta and Vanderleyden, 1995; Dodd et al., 2010) or by decreasing plant ethylene levels via the bacterial enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase (ACCd) which hydrolyses the immediate ethylene precursor ACC into ammonia and α-ketobutyrate (Honma and Shimomura, 1978; Glick et al., 1998) for bacterial use.
Various ACCd-containing rhizobacteria were repeatedly shown to promote plant growth, particularly when plants were subjected to environmental stresses likely to stimulate stress-induced ethylene production (Glick et al., 2007; Belimov et al., 2009). Although bacterial auxin production by some ACCd-containing rhizobacteria (Glick et al., 1998, 2007) may stimulate root growth, the creation of bacterial mutants with severely diminished ACCd activity abolished their root growth-promoting effect (Glick et al., 1994; Belimov et al., 2007, 2009). ACCd-containing bacteria decreased root ACC concentrations and ethylene production (Penrose et al., 2001), whole plant ethylene production (Mayak et al., 2004), and the xylem ACC concentration of plants exposed to drying soil (Belimov et al., 2009). Since ethylene often acts as a growth inhibitor (Pierik et al., 2006), it seems likely that decreased ACC levels in planta lowered ethylene production, thereby increasing shoot growth and yield particularly under soil water deficit (Arshad et al., 2008; Belimov et al., 2009).
However, rhizobacterial impacts on in planta concentrations of one phytohormone may have feedback effects on the concentration of other hormones. Applying 0.5mM of the ethylene-releasing chemical ethephon (2-chloroethylphosphonic acid) to the roots of hydroponically or sand-grown plants increased endogenous ethylene production and stimulated abscisic acid (ABA) biosynthesis (Hansen and Grossmann, 2000; F. Jiang, unpublished results). Conceivably, decreased ethylene production of plants inoculated with ACCd-containing rhizobacteria (Mayak et al., 2004) may cause feedback reductions of plant ABA levels. In contrast, inoculation of pea (Pisum sativum) with Variovorax paradoxus 5C-2 apparently increased xylem ABA concentration of plants in drying soil, probably due to the greater soil drying of larger plants (Belimov et al., 2009). Another possibility, as yet untested for V. paradoxus 5C-2, is that it produces ABA, as do other rhizosphere bacteria (Cohen et al., 2008; Sgroy et al., 2009). However, rhizosphere inoculation of maize plants with V. paradoxus 5C-2 did not affect xylem ABA concentration over a wide range of soil water availability (Dodd et al., 2009 a). Limited evidence of systemic rhizobacterial effects on phytohormone relations (Dodd et al., 2010) suggests that detailed empirical hormone flow models (sensu Jeschke et al., 1995; Jiang et al., 2007) may be necessary to resolve subtle differences.
There has also been much recent interest in using PGPR inoculants to decrease the application of chemical fertilizers (Adesemoye et al., 2009), either by stimulating root growth (thereby increasing root foraging for nutrients) or by directly stimulating plant nutrient uptake. Some ACCd-containing rhizobacteria increased shoot and grain nutrient concentrations in specific plant–microbe interactions: pea and Pseudomonas brassicacearum Am3, P. marginalis Dp1, or Rhodococcus sp. Fp2 (Safronova et al., 2006); peanut (Arachis hypogea) and various Pseudomonas spp. isolates (Dey et al., 2004); and wheat (Triticum aestivum) and Azospirillum brasilense Sp245 (Creus et al., 2004). Following inoculation of pea with the ACCd-containing rhizobacterium V. paradoxus 5C-2, increased seed nitrogen concentration of plants grown in drying soil (Belimov et al., 2009) may have been due to enhanced nodulation, since ethylene typically inhibits nodulation (Guinel and Geil, 2002). The multiplicity of mechanisms by which a single bacterium can affect plant nutrient status suggests that more sophisticated methods of determining plant nutrient budgets are required.
Apart from these fundamental physiological impacts of PGPR on plant nutrient and hormone budgets, they have been much used to stimulate early plant growth and/or stand establishment, even in legume species which form nitrogen-fixing symbiosis with nodule bacteria of the Rhizobiaceae family (e.g. Dey et al., 2004; Ahmad et al., 2011). Since nodulation occurs comparatively late in legume ontogeny (commonly around flowering), there may be considerable agronomic benefits of applying PGPR such as V. paradoxus 5C-2 to improve early vegetative growth. Moreover, these ‘free-living’ PGPR may also stimulate legume nodulation (Dey et al., 2004; Belimov et al., 2009) by decreasing root ethylene production and/or other mechanisms.
Previous work has demonstrated that V. paradoxus 5C-2 promoted pea vegetative growth and seed yield, especially of plants grown in drying soil, by attenuating a drought-induced increase in xylem sap ACC concentration in non-nodulated plants, and by preventing a drought-induced decrease in seed nitrogen content of nodulated plants by stimulating nodulation (Belimov et al., 2009). Since V. paradoxus 5C-2 apparently stimulated xylem ABA concentration in pea (Belimov et al., 2009) but had no effect in maize (Dodd et al., 2009 a), further work to understand these contrasting results, using hormonal flow models, seemed necessary. Since empirical flow models (Jeschke et al., 1995; Jiang et al., 2007) have never been used to evaluate PGPR impacts on plant nutrition and/or hormone homeostasis, the objectives of this study were to develop models to determine whether the ACCd-containing rhizobacterium V. paradoxus 5C-2 perturbed ABA metabolism and flows, and/or nutrient uptake, fluxes, and distribution in pea. A secondary objective was to determine whether this organism produced other phytohormones [e.g. ABA, gibberellin (GA), and indole-3-acetic acid (IAA)] in batch culture.
Materials and methods
Bacterial culture, phytohormone production, and ABA degradation
The PGPR strain V. paradoxus 5C-2 containing ACCd was obtained from the Russian Collection of Agricultural Microorganisms (Saint Petersburg) and maintained on Bacto-Pseudomonas F (BPF) agar medium as previously described (Belimov et al., 2005). Bacteria were grown on agar BPF medium for 3 d at 28 °C, and cells were suspended to a final concentration of 108 cells ml–1 in nutrient solution (µM): KNO3, 2800; Ca(NO3)2·4H2O, 1600; MgSO4·7H2O, 1000; NH4NO3, 2000; NaH2PO4, 60; and microelements NaFeEDTA, 40; H3BO3, 10; ZnSO4, 2; MnSO4·4H2O, 2; CuSO4·5H2O, 0.5; Co(NO3)2·6H2O, 0.2; H2MoO4, 0.08. Bacterial suspensions were added to the plants as described below.
V. paradoxus 5C-2 was cultivated in liquid BPF medium or in a modified minimal salt, minimal N (MSMN) medium (Belimov et al., 2005) containing (mg l–1): mannitol, 5000; glucose, 5000; yeast extract, 500; NH4NO3, 100; KNO3, 50; KH2PO4, 400; K2HPO4, 1600; MgSO4·7H2O, 200; CaCl2, 10; NaCl, 10; FeSO4, 5; biotin, 0.01; pyridoxal-HCl, 0.02; pH 6.4. In some cultures, the MSMN medium was supplemented with either 100mM NaCl to induce ABA biosynthesis (Cohen et al., 2008) or 500 µg ml–1 tryptophan as a precursor of auxin biosynthesis (Cohen et al., 2003). Batch cultures were incubated for 7 d at 25 °C, centrifuged at 13 000rpm for 5min, and sterilized using 0.2 µm filters (Corning, Germany). Supernatants were stored at –80 °C until used for phytohormone analysis.
For auxin determination, the supernatants and uninoculated media were acidified to pH 3.0 with 0.4 N hydrochloric acid and extracted with equal volumes of ethyl acetate. The organic phase containing auxins was evaporated to dryness under vacuum at 35 °C and suspended in 0.5ml of 20% acetonitrile (ACN). The obtained extracts were filtered through 0.22 µm nylon centrifuge tube filters Spin-X (Corning, USA) and fractionated by C18 reverse-phase ultra-performance liquid chromatography (UPLC) (Waters ACQUITY UPLC BEH Shield RP18 1.7 µm, 2.1×50mm column) with 5 µl sample injections. The UPLC system Waters ACQUITY H-Class (Waters, USA) with fluorescent detector (λex=280nm, λem=350nm) was used to detect auxins. Solvent conditions included a flow rate of 0.3ml min–1 with a 5min linear gradient from 1% ACN–0.1% acetic acid to 18% ACN–0.1% acetic acid, followed by a 3min isocratic elution and 3min column washing with 80% ACN–0.1% acetic acid. IAA, indole-3-carboxylic acid (ICA), and indole-3-lactic acid (ILA) supplied by Sigma-Aldrich were used as standards. The content of l-tryptophan was determined directly by 5 µl injections of initial media using the same conditions as for auxin analysis.
The supernatants and uninoculated media were analysed for ABA concentration via radioimmunoassay using the antibody MAC252 (Quarrie et al., 1988). For GA analysis, 1ml aliquots were diluted with 100ml of 80% methanol–water containing 2ng of each 2H-labelled internal standard (Professor Lewis Mander, Australian National University, Canberra, Australia) and the samples were then purified (Griffiths et al., 2006) and analysed by gas chromatography–mass spectrometry (GC-MS) (Rieu et al., 2008) as previously described.
To test whether V. paradoxus 5C-2 could utilize ABA as some other rhizobacteria can (A.A. Belimov and I.C. Dodd, unpublished observations), the MSMN medium (without mannitol, glucose, and yeast extract) was supplemented with 1mg ml–1 ABA as a sole carbon source. Bacteria were cultivated for 20 d at 25 °C with shaking at 200rpm. Bacterial growth was monitored daily via measurement of the optical density of batch cultures at 540nm against uninoculated medium used as a blank. At the end of the experiment, the ABA concentration in supernatants was determined as described above.
Plant culture and measurements
Pea (P. sativum L. cv. Alderman) seeds (Moles Seeds, UK) were selected for homogeneity of seed weight, surface-sterilized with 6% NaClO for 15min, rinsed carefully with sterile water, and germinated in vermiculite (LBS Horticulture, UK) at room temperature for 6 d. Afterwards, seedlings were washed with tap water to remove vermiculite from the roots and then transplanted into 1 litre pots (110mm diameter, 130mm height) containing washed sand (Leighton Buzzard 16/30, Sibelco, UK). Plants of similar size and developmental stage were separated into two groups. One group of plants was watered daily with the nutrient solution (see above) while the other was additionally supplied with a suspension of V. paradoxus 5C-2 (108 cells ml–1) every 3 d or 4 d, starting from the fifth day after transplanting. Plants were cultivated in a greenhouse with natural light and the temperature varying between 12 °C (night) and 25 °C (day).
Leaf stomatal resistance was measured 14 d after transplanting (10 d after inoculation of V. paradoxus 5C-2) with a transient-time porometer (Model AP4, Delta-T Devices, UK) between 10:00 h and 11:00 h. Following these measurements, the roots were carefully removed from the pots and adhering sand carefully washed away with tap water. The roots were spread on trays with water for scanning, and the length, diameter, and surface area of all roots were determined with WinRHIZO (Regent Instruments Inc., Canada).
Xylem sap was collected from the main vein of the pea leaves during the study period by placing the pots into a pressure chamber, sealing the shoot into the chamber using a silicone-based dental impression compound (a-gum vinyl polysiloxane impression materials, Dentsply DeTrey GmbH, Germany), and pressurizing the pots until xylem sap exuded from leaf incisions (Jeschke and Pate, 1991). Phloem exudates were obtained by placing excised pea shoots in vials containing 1.5 ml of 5 mM Na2EDTA, which were maintained in a humid atmosphere. By chelating Ca2+, EDTA prevents the formation of callose, which would otherwise seal the sieve tubes after wounding, and thus stimulates phloem exudation (Wolf et al., 1990). Sap samples were stored at –25 °C prior to analysis.
At the beginning and end of the study (10 d and 16 d after transplanting), plants were separated into lower leaves and internodes (nodes 1–4 numbering from the base of the plant), upper leaves and internodes, and roots. All plant parts were weighed, and placed in liquid nitrogen prior to freeze-drying. Dry tissues were weighed and then finely ground. Ions in different organs and xylem sap were analysed using an ICP spectrometer (JY Plus, Division d’Instruments S.A., France). Total N was analysed by use of a CHN analyser (Elementar, Germany). Cotyledons (which were very small and shrunken at the time of the first harvest) were discarded.
Bacterial root colonization
At the end of the experiment (22 d after germination), roots were removed from the pot and adhering sand particles gently removed. Three random samples of inoculated fresh pea root tissue were weighed and homogenized in sterile tap water with a sterile mortar and pestle. Homogenates were serially diluted in 10-fold steps and 50 µl aliquots were plated in two replicates on BPF agar supplemented with 30 µg ml–1 kanamycin and 20 µg ml–1 rifampicin, to which V. paradoxus 5C-2 naturally shows resistance, and 40 µg ml–1 nystatin to prevent fungal growth. The characteristic colonies of V. paradoxus 5C-2 were counted after incubation at 28 °C for 4 d. The average number of colony-forming units (CFU) of inoculated pea roots was 1.9×106 g–1 fresh weight (FW), while no colonies were recovered from uninoculated plants.
ABA analysis of plant samples
Freeze-dried tissue samples were homogenized and extracted in 80% aqueous methanol solution. Extracts were passed through Sep Pak C18-cartridges. Methanol was removed under reduced pressure and the aqueous residue was taken up in phosphate buffer mixed with 0.1% Tween-20 and 0.1% gelatin, and then subjected to an immunological ABA assay (enzyme-linked immunosorbent assay; as described in Yang et al., 2001). For xylem sap and phloem exudates, no further purification was necessary. The percentage recovery of ABA was >90% and all sample extract dilution curves paralleled the standard curves, indicating the absence of non-specific inhibitors in the extracts.
Modelling plant internal flows
Based on the assumption that (i) calcium is transported in the xylem only and (ii) mass flow occurs in the xylem, net xylem potassium flow (moles per plant) from root to shoot (Jk,x) was calculated from the ratio of potassium to calcium (K/Ca)x in xylem sap and the increment of calcium in the shoot, ΔCa (Armstrong and Kirkby, 1979):
| Jk,x=(K/Ca)x×ΔCa | (1) |
Net potassium flow in the phloem (Jk,p) was calculated from the difference between the potassium increment ΔK in each organ and the net xylem import to that organ, Jk,x:
| Jk,p=ΔK–Jk,x | (2) |
The content of each element in the organs in moles per plant and increments in moles per plant over the study period were then calculated from the concentrations and the dry weights; see Table 2.
Table 2.
Biomass (mg dry weight plant–1), total nitrogen, phosphorus, potassium, calcium, and magnesium contents (µmol plant–1) and ABA contents (pmol plant–1) and their increments in pea plants (control and inoculated by V. paradoxus 5C-2) at the beginning and end of the study period 10 d and 16 d after transplanting, ~6 and 12 d after inoculation.
| Internodes 1–4 and leaves | Internodes 5–7 and leaves | Roots | Control | V. paradoxus 5C-2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | V. paradoxus 5C-2 | Control | V. paradoxus 5C-2 | Control | V. paradoxus 5C-2 | ||||
| Biomass | Harvest 1 | 109±3.23 | 123±5.27* | 25.3±3.40 | 36.1±1.18* | 84.5±3.03 | 87.2±4.31 | ||
| Harvest 2 | 121±3.90 | 139±4.43* | 143±7.65 | 165±5.95* | 143±5.47 | 172±5.53* | |||
| Increment | 12±1.82 | 16±1.41 | 118±3.78 | 129±3.79 | 58.5±3.06 | 85±3.25** | |||
| Total N | Harvest 1 | 517±15 | 520±23 | 147±20 | 189±6 | 425±15 | 421±21 | ||
| Harvest 2 | 508±16 | 542±17 | 702±37 | 747±27 | 636±24 | 719±23* | |||
| Increment | –9±3.51 | 22±9.47 | 556±20.1 | 558±17.5 | 211±10.9 | 298±11.1** | |||
| Uptake of N by roots | 758±87.9 | 878±30.3 | |||||||
| P | Harvest 1 | 15.3±0.45 | 15.5±0.65 | 8.23±1.11 | 10.1±0.33 | 12.3±0.44 | 13.6±0.67 | ||
| Harvest 2 | 10.3±0.33 | 15.1±0.45* | 24.1±1.27 | 26.2±0.95 | 13.6±0.52 | 20.0±0.64* | |||
| Increment | –5±0.17 | –0.4±0.4** | 15.9±0.16 | 16.1±0.52 | 1.3±0.22 | 6.4±0.32** | |||
| Uptake of P by roots | 12.2±2.85 | 22.1±0.91* | |||||||
| K | Harvest 1 | 65.1±1.94 | 72.4±2.98 | 16.8±2.22 | 22.8±0.74* | 71.0±2.55 | 103±5.08* | ||
| Harvest 2 | 85.8±2.82 | 165±5.00* | 143±7.84 | 166±5.90* | 182±6.97 | 254±8.14* | |||
| Increment | 21±0.88 | 93±3.18** | 126±5.45 | 143±3.94* | 111±4.42 | 151±4.49** | |||
| Uptake of K by roots | 258±18.0 | 387±9.67** | |||||||
| Ca | Harvest 1 | 22.9±0.68 | 23.1±1.01 | 2.47±0.33 | 3.38±0.11* | 8.96±0.32 | 9.59±0.47 | ||
| Harvest 2 | 31.4±1.00 | 45.1±1.41* | 31.9±1.70 | 35.1±1.26 | 16.7±0.64 | 22.7±0.73* | |||
| Increment | 8.5±0.39 | 22±0.64** | 29.5±1.34 | 31.7±0.95 | 7.7±0.33 | 13.1±0.39** | |||
| Uptake of Ca by roots | 45.7±3.37 | 66.8±1.74 | |||||||
| Mg | Harvest 1 | 12.4±0.37 | 12.1±0.51 | 2.23±0.30 | 2.81±0.09 | 3.66±0.13 | 3.59±0.18 | ||
| Harvest 2 | 13.3±0.43 | 19.5±0.59* | 16.2±0.87 | 17.4±0.62 | 8.36±0.32 | 12.5±0.40* | |||
| Increment | 0.9±0.12 | 7.4±0.27** | 13.9±0.54 | 14.5±0.42 | 4.7±0.19 | 8.9±0.26** | |||
| Uptake of Mg by roots | 19.5±1.69 | 30.8±0.82** | |||||||
| ABA | Harvest 1 | 39.4±1.20 | 60.4±2.78* | 12.0±1.65 | 19.4±0.66* | 29.6±1.06 | 28.4±1.40 | ||
| Harvest 2 | 33.3±1.05 | 31.8±1.04 | 53.2±2.84 | 56.1±2.02 | 59.1±2.26 | 41.7±1.34* | |||
| Increment | –6±0.42 | –29±1.61** | 41±1.56 | 37±1.54* | 29±1.27 | 13±0.49** | |||
Data are shown as means ±SE; n=11–12.
Asterisks indicate significant (*P < 0.05) and highly significant (**P < 0.01) differences between inoculated and control plants.
Estimation of ABA flows was based on the assumption that mass flow occurs in the xylem and phloem, hence solutes are translocated according to their relative concentrations. Potassium flows were the basis for the calculation of ABA flows.
According to this assumption, net xylem flows of ABA (JABA,x) from root to shoot are given by the flows of potassium (JK,x) and the ratio of ABA to K in xylem sap [ABA/K]x:
| JABA,x=JK,x×[ABA/K]x | (3) |
The phloem flow of ABA (JABA,p) was estimated on the basis of the obtained K flows, as the product of the phloem K flow (JK,p) and the ratio of ABA to K in phloem exudates [ABA/K]p:
| JABA,p=JK,p×[ABA/K]p | (4) |
The differences between the estimated net flows of ABA moving in or out of an organ and its increment (ΔABA) in that organ yielded the net metabolic changes of ABA (JABA,met) either by degradation or by synthesis of ABA:
| JABA,met=ΔABA–JABA,x–JABA,p | (5) |
(with an influx into, or an efflux from, an organ being a positive or negative flow, respectively). Net degradation must have occurred if the resulting metabolic changes were negative, whereas, if they were positive, net synthesis was indicated.
The flow modelling approach presented herein has been used previously (Jeschke et al., 1995; Jiang et al., 2007) and depends on increments of nutrient and ABA contents between first and second harvest, the standard errors of which are presented (Table 2).
Results
V. paradoxus 5C-2 produced auxins, but not ABA and GAs, in selected media
Variovorax paradoxus 5C-2 produced IAA and ILA most actively after adding l-tryptophan to MSMN medium, whereas the maximum ICA concentration was detected in supernatants of bacteria grown in BPF medium, which also contained a relatively high concentration of l-tryptophan (Table 1). In contrast, neither biologically active GAs nor their precursors could be detected (limits were 30, 70, and 80 pg ml–1 for GA1, GA3, and GA4, respectively) by GC-MS.
Table 1.
Production of phytohormones by V. paradoxus 5C-2 in batch culture.
| Medium | L-Tryptophan in growth media (µg ml–1) | Phytohormone production (ng ml–1) | ||||
|---|---|---|---|---|---|---|
| IAA | ICA | ILA | ABA | GA1, 3, 4 | ||
| MSMN | 0.025±0.002 | 0.9±0.04 | 0.23±0.01 | 2.6±0.1 | ND | ND |
| MSMN + l-tryptophan | 500±10 | 75±6 | ND | 156±10 | ND | ND |
| BPF | 67±5 | 19±2 | 121±11 | 2.6±0.1 | ND | ND |
Data are shown as means ±SE; n=2.
ND, not detected.
Using a radioimmunoassay, ABA was not detected (the limit was 0.2ng ABA ml–1) in all culture media, suggesting that V. paradoxus 5C-2 was not able to produce this hormone in these media, unlike another organism (Achromobacter xylosoxidans) that was tested. No bacterial growth was observed on MSMN medium supplemented with 1mg ABA ml–1 as a sole carbon source, and the ABA concentration in the medium at the end of the experiment did not change (data not shown). Thus V. paradoxus 5C-2 was not able to metabolize ABA under the conditions tested.
V. paradoxus 5C-2 stimulated growth, stomatal conductance, and nutrient uptake
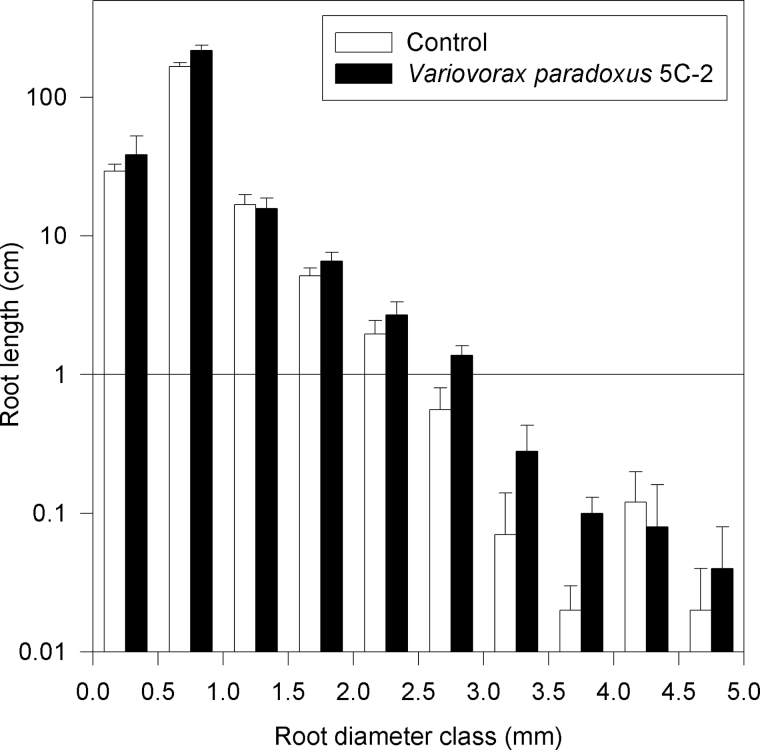
Variovorax paradoxus 5C-2 significantly (P < 0.05) increased pea root and shoot biomass (Table 2) by 20% and 15%, respectively. However, bacterial inoculation did not significantly affect total root length and surface area (data not shown) or root length distribution according to diameter (Fig. 1). In Leaf 3 (counting from the base of the plant), rhizobacterial inoculation significantly (P < 0.05) decreased stomatal resistance (from 3.66±0.25 s cm–1 in control plants to 2.67±0.29 s cm–1 in inoculated plants, n=4), while no significant effect was detected in Leaf 4 (data not shown).
Fig. 1.
Relationship between root length and diameter for peas grown in sand that were uninoculated (hollow bars) or inoculated with V. paradoxus 5C-2 (filled bars). Data are means ±SE; n=4.
For all measured elements, nutrient contents of all parts of inoculated pea plants were generally slightly higher at the first harvest (6 d after inoculation), and were more pronounced after a further 6 d. Thus inoculation substantially increased root nutrient increments over 6 d by 41% for N, 4.9-fold for P, 36% for K, 70% for Ca, and 89% for Mg (Table 2). In comparison, inoculation effects on nutrient increments in the lower part of the shoot were much stronger: 4.4-fold for K, 2.6-fold for Ca, and 8.2-fold for Mg. Following inoculation, the nitrogen content of the lower part of the pea shoot increased between the two harvests, but it decreased in control plants. Less P was mobilized from the lower part of the inoculated pea plants in comparison with the control plants. The effects of rhizobacterial inoculation on nutrient increments in the upper part of the shoot were much weaker than in other organs (Table 2).
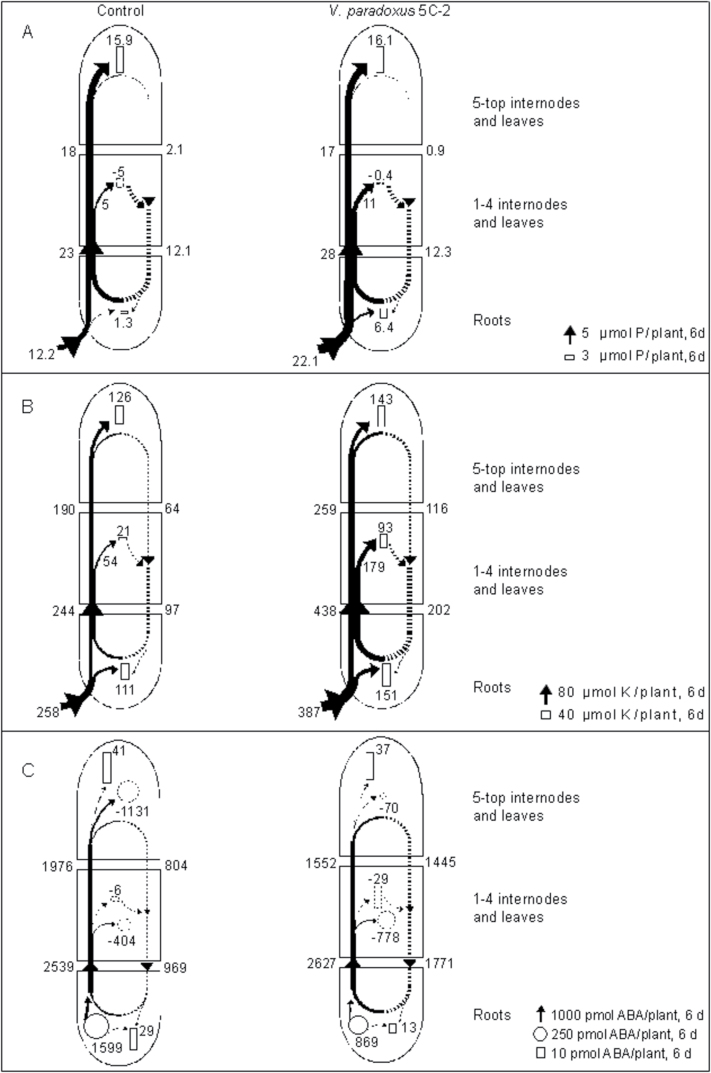
Rhizobacterial effects on nutrient budgets were investigated by constructing flow models of phosphorus (Fig. 2A) and potassium (Fig. 2B) from the data of Tables 2 and 3. In control plants, the upper part of the shoot was the major P sink. A substantial amount of P was mobilized from the lower shoot. Consequently, P re-translocation in the phloem (52% of xylem flow) exceeded P deposition in the root and hence led to a recirculation of P towards the upper shoot (Fig. 2A).
Fig. 2.
Empirical models of the uptake, transport, and utilization of total phosphorus (A) and potassium (B), or the metabolism, transport, and deposition of ABA (C) in whole pea plants over a 6 d study period. Arrow widths [net flows in xylem sap (black) or phloem (dotted)] and rectangle heights (deposition in each organ) are drawn in proportion to flow rates and the magnitude of deposition, respectively. Circled areas in (C) are drawn in proportion to the rates of metabolism.
Table 3.
The concentrations and ratios of Ca, K, P, and ABA in xylem sap in different parts of the shoot, and the ratio of ABA to potassium (K) in phloem exudates obtained with the EDTA technique in pea plants. Further details are as described in Table 2.
| Control | V. paradoxus 5C-2 | |
|---|---|---|
| Xylem sap concentrations in internodes 1–4 | ||
| Abscisic acid (ABA) (nM) | 15.5±1.76 | 12.6±1.79 |
| Calcium (Ca) (mM) | 0.25±0.03 | 0.23±0.02 |
| Potassium (K) (mM) | 1.35±0.33 | 2.05±0.33 |
| Phosphorus (P) (mM) | 0.14±0.02 | 0.14±0.05 |
| Xylem sap concentrations in internodes 5–7 | ||
| Abscisic acid (ABA) (nM) | 15.3±1.67 | 9.20±2.31 |
| Calcium (Ca) (mM) | 0.26±0.05 | 0.36±0.03 |
| Potassium (K) (mM) | 1.84±0.26 | 2.49±0.24 |
| Phosphorus (P) (mM) | 0.15±0.02 | 0.14±0.01 |
| Xylem sap ratios (entire shoot) | ||
| K/Ca | 6.45±0.72 | 8.17±0.74 |
| P/Ca | 0.60±0.07 | 0.52±0.12 |
| ABA/K | 10.4±1.39 | 6.0±1.37* |
| ABA/K in phloem exudates | ||
| Internodes 1–4 | 10.0±3.2 | 8.8±2.7 |
| Internodes 5–7 | 12.6±4.0 | 12.5±2.6 |
Data are shown as means ±SE; n=4–8. An asterisk indicates a significant difference at the P < 0.05 level.
Rhizobacterial inoculation increased total P uptake by 81%, and most of this was used for upper shoot growth. Total P deposition in the roots increased 4.9-fold, while P re-translocation in phloem was relatively lower (44% of xylem flow).
In control plants, major sinks for K were the roots and upper shoot, where 43% and 49% of root K uptake were deposited over 6 d. Only 8% of the K was deposited in the lower shoot. Xylem K transport into the lower and upper shoot exceeded K deposition and resulted in phloem K re-translocation (40% of the xylem flow).
Rhizobacterial inoculation increased K uptake substantially (by 1.5 times), and this increased xylem K flow to the shoot (by 1.8-fold). Since xylem K flow was clearly higher than K accumulation, K flow in the phloem approximately doubled and led to a higher K re-translocation (46% of xylem flow). K was distributed homogeneously, with 39, 24, and 37% in the roots, lower parts, and upper parts of the shoot, respectively.
V. paradoxus 5C-2 decreased root ABA concentration despite increased phloem ABA flow
ABA flows within the plants (Fig. 2C) were calculated using the data from Tables 2 and 3. Rhizobacterial inoculation tended to decrease xylem ABA concentration (Table 3) and ABA concentrations in all shoot parts, while root ABA concentrations decreased significantly by 41% (Table 2). After inoculation, xylem ABA flows from the lower to the upper part of the shoots decreased by 21%. Estimated ABA degradation in the upper shoot decreased by 94%. However, ABA deposition in the upper shoot was similar to that of control plants, consistent with increased phloem ABA flow (80% higher than the control) from the upper to the lower part of the shoots. The lower part of the shoots released much more ABA into the phloem, which also contributed to a higher ABA flow (83% higher than in the control) from shoot to roots. The ratio of ABA shoot to root phloem flows to ABA root to shoot xylem flows was also increased 76%. Root ABA biosynthesis and accumulation were decreased by 46% and 55%, respectively.
Discussion
Adding the ACCd-containing rhizobacterium V. paradoxus 5C-2 to the substrate of well-watered, well-fertilized pea plants increased root and shoot growth by 20% and 15%, respectively (Table 2) as previously described (Belimov et al., 2009), independently of any effect on root nodulation. Since bacterial mutants having low ACCd activity (including a transposome mutant of V. paradoxus 5C-2) did not stimulate plant growth (Glick et al., 1994; Belimov et al., 2007, 2009), the growth promotion observed here was most probably due to decreased plant production of the growth-inhibitory phytohormone ethylene.
However, many rhizobacteria can also produce multiple plant hormones when cultured in vitro (e.g. Sgroy et al., 2009), thus these were assayed in V. paradoxus 5C-2 culture filtrate (Table 1). Although V. paradoxus 5C-2 apparently did not produce GA in vitro, in contrast to some other rhizobacteria such as Bacillus pumilis and B. licheniformis (Gutierrez-Manero et al., 2001), previous measurements (with a colorimetric method based on Salkovsky’s reagent) indicated that V. paradoxus 5C-2 produced putative auxins or other indoles (Belimov et al., 2005). While this technique can be non-specific for auxins (Ehman, 1977), UPLC confirmed the production of several auxins by this strain (Table 1), indicating that V. paradoxus 5C-2 might synthesize auxins from l-tryptophan, a common root exudate (Kamilova et al., 2006). Irrespective of the mechanism(s) by which bacteria synthesize auxin(s), their effects in planta will be concentration dependent, with 10nM IAA inhibiting pea root growth (Eliasson et al., 1989) yet stimulating bean (Vicia faba) root growth (El-Antably and Larsen, 1974) in hydroponics. Since bacterial mutants with decreased auxin production failed to stimulate root growth (Patten and Glick, 2002), further work with V. paradoxus 5C-2 should down-regulate its auxin production and assay effects on root growth.
Enhanced root growth following V. paradoxus 5C-2 inoculation probably improved nutrient uptake (Fig. 2A, 2B). These nutritional effects seem partially specific to V. paradoxus 5C-2, as other ACCd-containing rhizobacteria (P. brassicacearum Am3, P. marginalis Dp1, or Rhodococcus sp. Fp2) had positive effects on pea foliar N, Ca, S, and Fe concentrations, but not on P and K concentrations (Safronova et al., 2006). Rhizobacterial stimulation of nutrient uptake (81, 50, 46, and 58% for total P, K, Ca, and Mg, respectively) was proportionally greater for many nutrients than the enhancement of root growth (20%). In contrast, the similar enhancement of nitrogen uptake (16%) and root growth (20%) suggests a cause–effect relationship. Increases in nutrient uptake larger than the increased root growth suggest that alternative mechanisms (e.g. ion transporters or channels) may be stimulated by rhizobacterial inoculation.
While some ACCd-containing rhizobacteria (but not V. paradoxus 5C-2; A.A. Belimov, unpublished data) can solubilize phosphate (Dey et al., 2004), this is unlikely to benefit plants in the present experiments, to which P was supplied as the soluble H2PO4 – ion. Although altered root morphology of inoculated plants may enhance phosphorus uptake, ACCd-containing rhizobacteria did not affect lateral root development or root architecture in Arabidopsis thaliana (Contesto et al., 2008) and Cucumis sativus (Gamalero et al., 2008), and P. sativum here (Fig. 1). Root hair abundance and length are also positively correlated with increased uptake of relatively immobile elements such as P (Bates and Lynch, 2001; Gahoonia and Nielsen, 2003), yet in vitro application of bacterial mutants with decreased ACCd activity resulted in plants with longer root hairs (Contesto et al., 2008; A.A. Belimov and I.C. Dodd unpublished results) than those inoculated with wild-type ACCd-containing rhizobacteria. Nevertheless, V. paradoxus 5C-2 stimulated root hair elongation of tomato in vitro (Belimov, 2012), which may enhance P uptake of soil-grown plants.
Since V. paradoxus 5C-2 decreased xylem ACC concentrations in pea (Belimov et al., 2009), shoot ethylene production should diminish, which may alter the concentrations of other phytohormones in planta. Although plant ABA status moderates ethylene production (Sharp et al., 2000; Dodd et al., 2009 b), the converse effect is equivocal. However, V. paradoxus 5C-2 inoculation decreased ABA biosynthesis and deposition in pea roots (Fig. 2C), more so than in comparable experiments with maize (Dodd et al., 2009 a). Unlike maize, where the root hypodermis (=exodermis) strongly inhibits exudation of solutes and plant hormones to the rhizosphere (Hose et al., 2001), legumes never form exodermal Casparian bands (Enstone and Peterson, 1992), and it has been argued that rhizobacterial inoculation will have greater effects on plant hormonal relations in legumes than cereals (Belimov et al., 2009; Dodd et al., 2009 a). However, further experiments with a range of leguminous and non-leguminous species are required to determine the generality of this hypothesis. The mechanism(s) by which V. paradoxus 5C-2 decreased root ABA concentration is not known, as it was not capable of metabolizing ABA in vitro when grown on ABA as a sole carbon source.
Decreased ABA levels following inoculation with ACCd-containing rhizobacteria may regulate plant growth. Although ABA application inhibited root growth of hydroponically grown peas (Tietz, 1973), the ABA-deficient wilty pea mutant showed decreased root growth and allocation of biomass to the roots compared with wild-type plants (I.C. Dodd, unpublished data), suggesting instead that normal ABA levels are required to maintain root growth. Similarly, rhizobacterial alterations in shoot ABA homeostasis (Fig. 2C) may influence shoot growth. However, the ABA-deficient wilty pea mutant (with shoot ABA concentrations 50% lower than those of wild-type peas) had a similar relative foliage expansion rate to wild-type peas when grown in sand at two different external N concentrations (Dodd, 2003). Following rhizobacterial inoculation, the spatial distribution of changes in ABA status suggests that ABA did not mediate leaf growth since there were only limited impacts in the upper shoot where leaves were expanding (Fig. 2C). Nevertheless, greater ABA degradation in the lower nodes and leaves (93% higher than control plants) probably accounted for the increased stomatal conductance of more mature leaves.
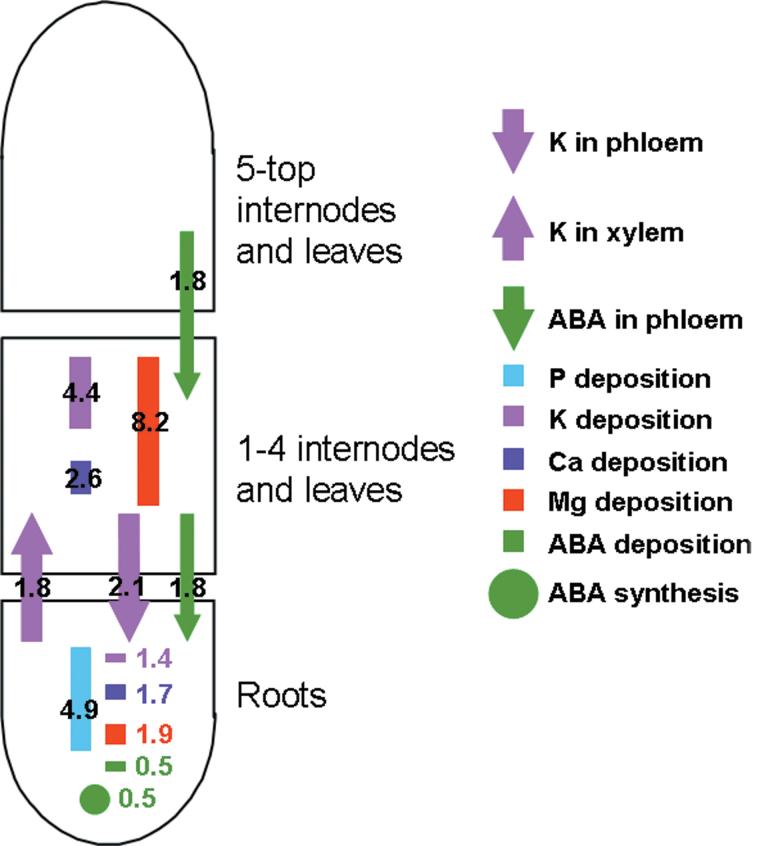
While interpreting the effects of ACCd-containing rhizobacteria has logically focused on attenuating the growth-inhibitory effects of ethylene, other mechanisms such as improved nutrient uptake (Fig. 2A, 2B) and CO2 fixation (because of increased stomatal conductance) may also be important. Empirical modelling of nutrient flows revealed specific changes in plant nutrient relations induced by rhizobacterial inoculation (Fig. 3) that were not apparent from conventional measurements of tissue concentrations. However, rhizobacterial inoculation decreased root ABA concentrations (Table 2), which may mitigate plant adaptation to water deficits, suggesting that the strategy of applying ACCd-containing PGPR to overcome the effects of soil drying (Dodd, 2009) requires re-evaluation.
Fig. 3.
Fold changes in xylem sap or phloem flows (arrows), deposition (rectangles), and metabolism (circles) of ABA and plant macronutrients induced by inoculation with V. paradoxus 5C-2.
Acknowledgements
Financial support was provided to FJ by the NSFC (31121003), the State Key Basic Research and Development Plan (2007CB106802), and the Scientific Research Foundation of Beijing Normal University (2009SAT-3). FJ’s visit to Lancaster was part supported by an RCUK China Bridge project (to WJD); ICD and AAB thank DEFRA (WU0121), the Royal Society, and MSE RF (GK 16.512.11.2162) for financial support. Rothamsted Research receives strategic funding from the Biotechnology and Biological Sciences Research Council (BBSRC).
References
- Adesemoye AO, Torbert HA, Kloepper JW. 2009. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers Microbial Ecology 58 921–929 [DOI] [PubMed] [Google Scholar]
- Ahmad M, Zahir ZA, Asghar HN, Ashgar M. 2011. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase Canadian Journal of Microbiology 57 578–589 [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Kirkby EA. 1979. Estimation of potassium recirculation in tomato plants by comparison of the rates of potassium and calcium accumulation in the tops with their fluxes in the xylem stream Plant Physiology 63 1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad M, Shaharoona B, Mahmood T. 2008. Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth, yield and ripening of pea (Pisum sativum L.) Pedosphere 18 611–620 [Google Scholar]
- Bates TR, Lynch JP. 2001. Root hairs confer a competitive advantage under low phosphorus availability Plant and Soil 236 243–250 [Google Scholar]
- Belimov AA. Interactions between associative bacteria and plants: the role of biotic and abiotic factors. Moscow: Palmarium Academic Publishers; 2012. [Google Scholar]
- Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI, Davies WJ. 2009. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signaling New Phytologist 181 413–423 [DOI] [PubMed] [Google Scholar]
- Belimov AA, Dodd IC, Safronova VI, Hontzeas N, Davies WJ. 2007. Pseudomonas brassicacearum strain Am3 containing 1-aminocyclopropane-1-carboxylate deaminase can show both pathogenic and growth-promoting properties in its interaction with tomato Journal of Experimental Botany 58 1485–1495 [DOI] [PubMed] [Google Scholar]
- Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR. 2005. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biology and Biochemistry 37 241–250 [Google Scholar]
- Cohen AC, Bottini R, Piccoli PN. 2008. Azospirillum brasilense Sp245 produces ABA in chemically-defined culture medium and increases ABA content in Arabidopsis plants Plant Growth Regulation 54 97–103 [Google Scholar]
- Cohen JD, Slovin JP, Hendrickson AM. 2003. Two genetically discrete pathways convert tryptophan to auxin: more redundancy in auxin biosynthesis Trends in Plant Science 8 197–199 [DOI] [PubMed] [Google Scholar]
- Contesto C, Desbrosses G, Lefoulon C, Béna G, Borel F, Galland M, Gamet L, Varoquaux F, Touraine B. 2008. Effects of rhizobacterial ACC deaminase activity on Arabidopsis indicate that ethylene mediates local root responses to plant growth-promoting rhizobacteria Plant Science 175 178–189 [Google Scholar]
- Costacurta A, Vanderleyden J. 1995. Synthesis of phytohormones by plant-associated bacteria Critical Reviews in Microbiology 21 1–18 [DOI] [PubMed] [Google Scholar]
- Creus CM, Sueldo RJ, Barassi CA. 2004. Water relations and yield in Azospirillum inoculated wheat exposed to drought in the field Canadian Journal of Botany 82 273–281 [Google Scholar]
- Dey R, Pal KK, Bhatt DM, Chauhan SM. 2004. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria Microbiological Research 159 371–394 [DOI] [PubMed] [Google Scholar]
- Dodd IC. 2003. Leaf area development of ABA deficient and wild-type peas at two levels of nitrogen supply Functional Plant Biology 30 777–783 [DOI] [PubMed] [Google Scholar]
- Dodd IC. 2009. Rhizosphere manipulations to maximise ‘crop per drop’ during deficit irrigation Journal of Experimental Botany 60 2454–2459 [DOI] [PubMed] [Google Scholar]
- Dodd IC, Jiang F, Teijeiro RG, Belimov AA, Hartung W. 2009. a The rhizosphere bacterium Variovorax paradoxus 5C-2 containing ACC deaminase does not increase systemic ABA signalling in maize (Zea mays L.) Plant Signaling and Behavior 4 519–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC, Theobald JC, Richer SK, Davies WJ. 2009. b Partial phenotypic reversion of ABA-deficient flacca tomato (Solanum lycopersicum) scions by a wild-type rootstock: normalising shoot ethylene relations promotes leaf area but does not diminish whole plant transpiration rate Journal of Experimental Botany 60 4029–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC, Zinovkina NY, Safronova VI, Belimov AA. 2010. Rhizobacterial mediation of plant hormone status Annals of Applied Biology 157 361–379 [Google Scholar]
- Ehman A. 1977. The van Urk–Salkovski reagent—a sensitive and specific chromatogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives Journal of Chromatography 132 267–276 [DOI] [PubMed] [Google Scholar]
- El-Antably HMM, Larsen P. 1974. Distribution of gibberellins and abscisic acid in geotropically stimulated Vicia faba roots Physiologia Plantarum 32 322–329 [Google Scholar]
- Eliasson L, Bertel G, Bolander E. 1989. Inhibitory action of auxin on root elongation not mediated by ethylene Plant Physiology 91 310–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA. 1992. The apoplastic permeability of root apices Canadian Journal of Botany 70 1502–1512 [Google Scholar]
- Gahoonia TS, Nielsen NE. 2003. Phosphorus (P) uptake and growth of a root hairless barley mutant (bald root barley, brb) and wild type in low- and high-P soils Plant, Cell and Environment 26 1759–1766 [Google Scholar]
- Gamalero E, Berta G, Massa N, Glick BR, Lingua G. 2008. Synergistic interactions between the ACC deaminase-producing bacterium Pseudomonas putida UW4 and the AM fungus Gigaspora rosea positively affect cucumber plant growth FEMS Microbiology Ecology 64 459–467 [DOI] [PubMed] [Google Scholar]
- Glick BR, Cheng Z, Czarny J, Duan J. 2007. Promotion of plant growth by ACC deaminase-producing soil bacteria European Journal of Plant Pathology 119 329–339 [Google Scholar]
- Glick BR, Jacobson CB, Schwarze MMK, Pasternak JJ. 1994. 1-Aminocyclopropane-1-carboxylic acid deaminase mutants of the plant-growth promoting rhizobacterium Pseudomonas putida GR12-2 do not stimulate canola root elongation Canadian Journal of Microbiology 40 911–915 [Google Scholar]
- Glick BR, Penrose DM, Li JP. 1998. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria Journal of Theoretical Biology 190 63–68 [DOI] [PubMed] [Google Scholar]
- Griffiths J, Murase K, Rieu I, et al. 2006. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis The Plant Cell 18 3399–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinel FC, Geil RD. 2002. A model for the development of the rhizobial and arbuscular mycorrhizal symbioses in legumes and its use to understand the roles of ethylene in the establishment of these two symbioses Canadian Journal of Botany 80 695–720 [Google Scholar]
- Gutierrez-Manero FJ, Ramos-Solano B, Robanza A, Mehouachi J, Tadeo FR, Talon M. 2001. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins Physiologia Plantarum 111 206–211 [Google Scholar]
- Hansen H, Grossmann K. 2000. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition Plant Physiology 124 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M, Shimomura T. 1978. Metabolism of 1-aminocyclopropane-1-carboxylic acid Agricultural Biology and Chemistry 42 1825–1831 [Google Scholar]
- Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W. 2001. The exodermis—a variable apoplastic barrier Journal of Experimental Botany 52 2245–2254 [DOI] [PubMed] [Google Scholar]
- Jeschke WD, Klagges S, Hilpert A, Bhatti AS, Sarwar G. 1995. Partitioning and flows of ions and nutrients in salt-treated plants of Leptochloa fusca L. Kunth. I. Cations and chloride New Phytologist 130 23–35 [Google Scholar]
- Jeschke WD, Pate JS. 1991. Modelling of the uptake, flow and utilization of C, N and H2O within whole plants of Ricinus communis L. based on empirical data Journal of Plant Physiology 137 488–498 [Google Scholar]
- Jiang F, Heilmeier H, Hartung W. 2007. Abscisic acid relations of plants grown on tungsten enriched substrates Plant and Soil 301 37–49 [Google Scholar]
- Kamilova F, Kravchenko L, Shaposhnikov A, Makarova N, Azarova T, Lugtenberg B. 2006. Organic acids, sugars and l-tryptophan in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria Molecular Plant-Microbe Interactions 19 250–256 [DOI] [PubMed] [Google Scholar]
- Lugtenberg B, Kamilova F. 2009. Plant-growth-promoting rhizobacteria Annual Reviews of Microbiology 63 541–546 [DOI] [PubMed] [Google Scholar]
- Mayak S, Tirosh T, Glick BR. 2004. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers Plant Science 166 525–530 [Google Scholar]
- Pate JS, Layzell DB, McNeil DL. 1979. Modeling the transport and utilization of carbon and nitrogen in a nodulated legume Plant Physiology 63 730–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten C, Glick BR. 2002. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system Applied and Environmental Microbiology 68 3795–3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose DM, Moffatt BA, Glick BR. 2001. Determination of 1-aminocyclopropane-1-carboxylic acid (ACC) to assess the effects of ACCdeaminase-containing bacteria on roots of canola seedlings Canadian Journal of Microbiology 47 77–80 [DOI] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ. 2006. The Janus face of ethylene: growth inhibition and stimulation Trends in Plant Science 11 176–183 [DOI] [PubMed] [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, Wang TL, Cook SK, Henson IE, Loveys BR. 1988. A monoclonal antibody to (S)-abscisic acid: its characterisation and use in a radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves Planta 173 330–339 [DOI] [PubMed] [Google Scholar]
- Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, et al. 2008. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle The Plant Journal 53 488–504 [DOI] [PubMed] [Google Scholar]
- Safronova VI, Stepanok VV, Engqvist GL, Alekseyev YV, Belimov AA. 2006. Root associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil Biology and Fertility of Soils 42 267–272 [Google Scholar]
- Sgroy V, Cassan F, Masciarelli O, Del Papa MF, Lagares A, Luna V. 2009. Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera Applied Microbiology and Biotechnology 85 371–381 [DOI] [PubMed] [Google Scholar]
- Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F. 2000. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene Journal of Experimental Botany 51 1575–1584 [DOI] [PubMed] [Google Scholar]
- Tietz A. 1973. Abscisinaure und Keimlingwachten Zeitschrift für Pflanzenphysiologie 68 382–384 [Google Scholar]
- Wolf O, Munns R, Tonnet ML, Jeschke WD. 1990. Concentrations and transport of solutes in xylem and phloem along the leaf axis of NaCl-treated Hordeum vulgare Journal of Experimental Botany 41 1133–1141 [Google Scholar]
- Yang YM, Xu CN, Wang BM, Jia JZ. 2001. Effects of plant growth regulators on secondary wall thickening of cotton fibres Plant Growth Regulation 35 233–237 [Google Scholar]