Abstract
Variation in xylem structure and function has been extensively studied across different species with a wide taxonomic, geographical, and ecological coverage. In contrast, our understanding of how xylem of a single species can adjust to different growing condition remains limited. Here phenotypic and developmental plasticity in xylem traits of hybrid poplar (Populus trichocarpa×deltoides) was studied. Clonally propagated saplings were grown under experimental drought, nitrogen fertilization, and shade for >30 d. Xylem hydraulic and anatomical traits were subsequently examined in stem segments taken from two different vertical positions along the plant’s main axis. The experimental treatments affected growth and development and induced changes in xylem phenotype. Across all treatments, the amount of leaf area supported by stem segments (AL) scaled linearly with stem native hydraulic conductivity (K native), suggesting that the area of assimilating leaves is constrained by the xylem transport capacity. In turn, K native was mainly driven by the size of xylem cross-sectional area (AX). Moreover, the structural and functional properties of xylem varied significantly. Vulnerability to cavitation, measured as the xylem pressure inducing 50% loss of conductivity (P50), ranged from –1.71MPa to –0.15MPa in saplings subjected to drought and nitrogen fertilization, respectively. Across all treatments and stem segment positions, P50 was tightly correlated with wood density. In contrast, no relationship between P50 and xylem-specific conductivity (K S) was observed. The results of this study enhance our knowledge of plant hydraulic acclimation and provide insights into common trade-offs that exist in xylem structure and function.
Key words: Cavitation, hydraulic conductivity, phenotypic plasticity, vessels, wood density, xylem embolism.
Introduction
Light, nutrients, and water are primary resources required by plants for their growth and reproduction (Larcher, 2003). Over the course of evolution, plant species have acquired a suite of traits allowing them to utilize these resources and persist under environmental conditions characteristic for their habitat. This process is known as adaptation (Lambers et al., 1998). However, the availability of the resources can be rather variable during a plant’s lifetime. For instance, periods of sufficient soil moisture supply can be interrupted by drought (Hogg et al., 2008). Irradiance can rapidly increase when the surrounding vegetation is removed or decrease when a plant becomes shaded by faster-growing neighbours (Lieffers et al., 1999). The availability of inorganic nutrients such as nitrogen or phosphorus can become altered as a result of competition, flood pulse inundation, or increased runoff from fertilized fields (Rennenberg et al., 2010). Plants are, to a certain extent, able to adjust to such changes because plant functional traits exhibit phenotypic plasticity. This continuous adjustment of physiological and structural properties during a plant’s lifetime in order to optimize life processes under new environmental constraints is known as acclimation (Lambers et al., 1998).
Long-distance water transport in plants is a physiological process of paramount importance which is intimately linked with the acquisition and use of all three resources mentioned above. Both adaptation and acclimation can be seen in xylem structure and function. Xylem structure and function have been studied in a wide range of different species (e.g. Carlquist, 1988, 2001; Wheeler et al., 2007), and vast adaptive variation has been demonstrated (e.g. Maherali et al., 2004; Sperry et al., 2006; Chave et al., 2009; McCulloh et al., 2010). Among the most important hydraulic traits are the xylem-specific conductivity (K S) and the vulnerability to drought-induced cavitation. While the former is a measure of transport efficiency, the latter characterizes xylem safety. Both traits exhibit large interspecific variation. K S differs ~100-fold across diffuse porous angiosperms, reflecting differences in the size and number of vessels (Sperry et al., 2006; McCulloh et al., 2010). Vulnerability to cavitation measured as the pressure at 50% loss of hydraulic conductivity (P50) spans from values close to –0.2MPa to values below –14MPa (Maherali et al., 2004; Sperry, 2011). Moreover, these two traits seem to be in a trade-off relationship, such that xylem of a given species cannot be both highly transport efficient and highly resistant to cavitation (Maherali et al., 2004; Hacke et al., 2006). Wood density with values ranging from 0.1g cm–3 to 1.2g cm–3 is another functionally important trait (Chave et al., 2009). Wood density has been linked not only to wood mechanical strength (Niklas, 1992) but also to the resistance of xylem to cavitation (Hacke et al., 2001a ).
The structure and hydraulic function of xylem can also vary within a single species in response to growing conditions. Variation in ring widths, conduit diameters, or wood densities has often been used to reconstruct information about past environmental conditions and to infer the hydraulic function of xylem (for a review, see Fonti et al., 2010). While xylem anatomy can provide a good proxy of xylem function in some cases, solid knowledge of how specific growing conditions influence xylem anatomy and how patterns in xylem structure link with xylem function is an essential prerequisite for such approaches. Changes in K S and P50 in response to environmental conditions such as drought (Beikircher and Mayr, 2009; Fichot et al., 2010), irradiance (Cochard et al., 1999), salinity (Stiller, 2009), nutrient availability (Harvey and van den Driessche, 1997; Hacke et al., 2010), and soil type (Hacke et al., 2000; Mayr et al., 2010) have been shown. However, a broader range of species and environmental conditions should be tested and links to genetic underpinnings should be elucidated (Lamy et al., 2011; Wortemann et al., 2011) in order to gain a better understanding of the acclimation potential of xylem.
In this study, phenotypic plasticity of xylem traits was assessed using clonally propagated saplings of hybrid poplar (Populus trichocarpa×Populus deltoides, clone H11-11). Given its parentage, it was expected that highly vulnerable and highly conductive xylem (Tyree et al., 1994; Sperry, 2011) would be found in this hybrid poplar. These xylem characteristics are well suited for the exploitative ecological strategy of cottonwoods in their natural environment. The riverine floodplains that are inhabited by riparian cottonwoods are a highly dynamic environment which is continuously modified by repeated disturbances (Braatne et al., 1996). Therefore, it was expected that large phenotypic plasticity would be found in the hybrid poplar in response to experimental drought, nitrogen fertilization, and shading.
Variation in the anatomical and hydraulic parameters of xylem in response to treatments was assessed in stem segments sampled from two vertical positions along a plant’s main axis. Based on the above-mentioned literature, it was anticipated that increased vulnerability to cavitation in saplings subjected to fertilization and shade and increased resistance in response to drought would be found. Xylem-specific hydraulic conductivity was expected to increase in fertilized plants and decrease in drought-stressed and shaded plants. Furthermore, it was decided to test whether the hydraulic and anatomical traits were coupled in a similar way to that observed at the interspecific level. If so, it would be expected that correlations between vulnerability of xylem to cavitation and xylem transport efficiency, as well as between vulnerability and wood density, would be found.
Materials and methods
Plant material and experimental conditions
Saplings of hybrid poplar (Populus trichocarpa×Populus deltoides, clone H11-11) were grown under drought (DR), nitrogen fertilization (F), and shading (SH). Due to logistical concerns and a limited amount of space in the growth facility used, the experimental treatments were imposed one at a time in three independent, temporally separated experiments. For each experiment, saplings were produced from rooted cuttings and maintained in a growth chamber under the following standard growing conditions: 16/8h day/night cycle, 24/18 °C day/night temperature, ambient irradiance 350 µmol m–2 s–1. Plants were kept well watered, and fertilized with a complete water-soluble fertilizer (20-20-20 N-P-K, Plant Products, Brampton, ON, Canada) at 1g l–1 dilution on a weekly basis. After a 7–9 week long period of sapling establishment, plants were randomly assigned to either a treatment (DR, F, or SH) or a control (DRC, FC, or SHC) group. Plants subjected to the DR treatment received 50–200ml of water every other day. This limited irrigation resulted in repeated wilting but did not cause severe desiccation damage and extensive leaf die-off. In contrast, control plants (DRC) were kept well watered at all times, receiving 500–1000ml of water daily. Plants subjected to the F treatment were supplied with 400ml of 7.5mM NH4NO3 in 0.5× Hocking’s complete nutrient solution (Hocking, 1971) every other day, while control plants (FC) received 400ml of 0.75mM NH4NO3 in 0.5× Hocking’s complete nutrient solution. These two fertilization protocols were previously shown to provide high (F) and adequate (FC) levels of nitrogen (N) for the growth of this hybrid poplar genotype (Pitre et al., 2007a , b ; Hacke et al., 2010). In order to keep plants well watered, plants were irrigated with tap water on the days when the fertilizer solution was not applied. The general purpose N-P-K fertilizer was not applied in addition to F and FC treatments. The SH treatment was imposed by enclosing plants in shade boxes made of gardening fabric. The shade boxes reduced ambient irradiation by 80%, from 350 µmol m–2 s–1 in control plants (SHC) to 70 µmol m–2 s–1 in shaded plants (SH). Experimental treatments were imposed for ~5 weeks. The exact duration of the three individual experiments (i.e. the period between planting the rooted cuttings and plant harvesting) and the duration of the experimental treatments (i.e. the period between the onset of a treatment and plant harvesting) are indicated in Supplementary Table S1 available at JXB online.
Sampling strategy
After measuring a plant’s final height and stem diamter at a height of 10cm above the root collar (Dstem), plants were cut at their base, placed in a dark plastic bag with a moist paper towel, and immediatelly brought to the lab. Bags with the plant material were stored at 4 °C in a refrigerator until they were used for hydraulic measurements (but no longer than for 4 d). For these measurements, stem segments 20–25cm in length were sampled from two different positions along the plant’s main stem. The first set of segments was sampled from a basal region of the stem at a fixed height of 5–30cm above the root collar (‘basal’ segments). The second set of segments was taken from a position closer to the apex of a plant (‘distal’ segments). The height at which the distal segments were collected differed among the three experiments, reflecting the different growth rates of plants exposed to different experimental treatments. Distal segments were located at ~60% of the final plant height. Hence, they were sampled at a height of 40–60cm above pots in DR, 60–85cm in DRC, 95–120cm in F, 85–110cm in FC, and 65–90cm in both SH and SHC plants. Distal segments underwent their entire growth and development under treatment conditions. Basal segments, in contrast, completed primary growth and initiated secondary growth under control conditions before experimental treatments began. Nevertheless, a significant portion of secondary xylem was formed after the onset of treatments.
Xylem pressure
Xylem pressure was measured using equilibrated mature leaves attached between the fifth and eighth node counted from the top of a plant. One leaf per plant was measured, from five plants per treatment group. Leaves were sealed in a plastic bag covered with aluminium foil the night before harvesting to inhibit transpiration and to ensure equilibration of water potentials. Immediately after bagged leaves were excised, xylem pressure was measured using a Scholander-type pressure chamber (Model 1000; PMS Instrument Company, Albany, OR, USA). Xylem pressure measurements were conducted for F and DR experiments only.
Growth measurements
Final height and stem basal diameter (Dstem) of each sapling were assessed with measuring tape and calipers, respectively. Leaf area (AL) was measured with an area meter (LI-3100, Li-Cor, Lincoln, NE, USA). A plant’s total AL and the AL supported by basal stem segments were determined for each plant. The supported AL was calculated as the sum of AL distal to the segment and half of the AL directly attached to the segment.
Hydraulic measurements
Segments used for hydraulic measurements were trimmed underwater to a final length of 14.2cm. To measure native hydraulic conductivity (K native), stem segments were fitted to a tubing apparatus, and the gravity-induced water flow rate through the segments was recorded with an electronic balance (CP225 D; Sartorius, Göttingen, Germany) interfaced with a computer. A pressure head of 4–5 kPa was used to induce the flow. Stem hydraulic conductivity was then calcuated as the flow rate for a given pressure gradient. Subsequently, segments were flushed for 15–20min at 50 kPa in order to remove native embolism. Conductivity measurements were repeated to determine hydraulic conductivity after flushing (K flush). Stem segments were then attached in a custom-built centrifuge rotor and spun to progressively more negative pressures. Hydraulic conductivity was measured after each pressure increment until it dropped below 90% of the K flush value. The percentage loss of hydraulic conductivity (PLC) relative to K flush was plotted against the corresponding xylem pressure to generate vulnerability curves. Data points were fitted with a Weibull function and the xylem pressure corresponding to 50% loss of conductivity (P50) was determined for each segment.
After hydraulic measurements were completed, stem segments were sectioned near the middle of their length. The exposed cross-sectional surface was captured with a digital camera attached to a stereomicroscope (MS5; Leica Microsystems, Wetzlar, Germany) at ×10–16 magnification. Xylem cross-sectional area (AX) excluding pith and bark was measured with image analysis software (ImagePro Plus version 6.1, Media Cybernetics, Silver Spring, MD, USA). Xylem-specific hydraulic conductivity (K S) was subsequently calculated as the maximal hydraulic conductivity (K max) of a stem segment divided by the corresponding AX. In the majority of stems, hydraulic conductivity increased after flushing. However, in few instances, K flush was slightly (i.e. <5%) lower then K native, possibly due to a wounding response or clogging of the pit membranes. Thus, the K max of a stem was determined as either K flush or K native, whichever value was higher.
Vessel diameter and wood density measurements
The same stem segments previously used for measuring hydraulic conductivity and cavitation resistance were used for measuring vessel diameter and wood density. Stem cross-sections (~40–60 µm thick) were prepared from the middle portion of stem segments using a sliding microtome (SM2400, Leica) or by hand with a fresh single-edge razor blade. Sections were stained with toluidine blue and observed with a light microscope (DM3000, Leica). Images were captured at ×100 magnification using a digital camera (DFC420C, Leica). Vessel diameters were measured in complete radial sectors delimited by xylem rays spanning from the pith to the cambium. Between three and 10 different sectors were selected for each stem, providing a total of 300–500 vessel diameter measurements per stem. The average vessel diameter (Dv) per stem was subsequently calculated. Five to six stems were analysed for each treatment and position.
For measuring wood density (dw), samples 2cm in length were excised from stem segments and split longitudinally into two subsamples. The bark was peeled off and the pith was carefully removed. Wood specimens were then submersed in a beaker filled with water placed on an electronic balance (CP224 S, Sartorius). The displaced water weight was recorded and converted into fresh wood volume. Specimens were then oven-dried at 70 °C for 2 d. dw was calculated as the ratio beteween a sample’s dry weight and its fresh volume. The dw of each stem segment was finally determined by averaging the values of the two subsamples. Five to eight stems were analysed for each treatment and position.
Statistical analyses
Since the three experimental treatments were imposed as three independent and temporally separated experiments, the effects of treatments were statistically evaluated by comparing treated plants with their controls within a single experiment. In order to evaluate the effect of treatments on plant growth parameters, independent two-sample t-tests were performed. Analysis of variance (ANOVA) was carried out in order to dissect the effect of treatment and stem segment position on xylem hydraulic and structural parameters (AX, Dv, dw, K S, and P50). The following linear model was used to fit the measured data:
| var~treat+pos+(treat×pos) |
where var represents the tested variable, treat and pos are the fixed effect factors ‘treatment’ and ‘position’, and treat×pos is the interaction term. Planned comparisons between means of treated versus control plants within the same stem segment position (i.e. either basal or distal) and between means of basal and distal stem segments within either treated or control plant groups were carried out using the least significance difference procedure (Sokal and Rohlf, 1995). To elucidate potential relationships between selected growth, hydraulic, and anatomical parameters, we tested for significant linear correlations between the group means across all three experiments. The results of all statistical analyses were deemed significant at P ≤ 0.05. Probability (P) values for the planned comparisons were adjusted to 0.0125 using the Bonferroni correction procedure for multiple comparisons (Sokal and Rohlf, 1995). The statistical software package R 2.10.1 (R Development CoreTeam 2009, Auckland, New Zealand) was used to perform the analyses. Throughout this manuscript, group means are cited with their standard deviations.
Results
Plant growth
The growth characteristics of hybrid poplar saplings differed between treated and control plants as well as between experiments (Supplementary Table S2 at JXB online). The average final height ranged from 77.1±2.1cm to 171.3±6.1cm in DR and F plants, respectively. Similarly, the Dstem was smallest in DR (6.3±0.2mm) and largest in F (9.5±0.4mm). Furthermore, total AL exhibited differences ranging from 0.27±0.02 m2 to 0.99±0.07 m2. As for the effect of treatments, DR plants exhibited reduced growth in height and girth and had smaller AL compared with controls. The opposite trend in growth was observed in F relative to FC plants. The height growth increment was higher in SH, while their radial growth was reduced in comparison with controls.
Leaf area, xylem area, and stem hydraulic conductivity
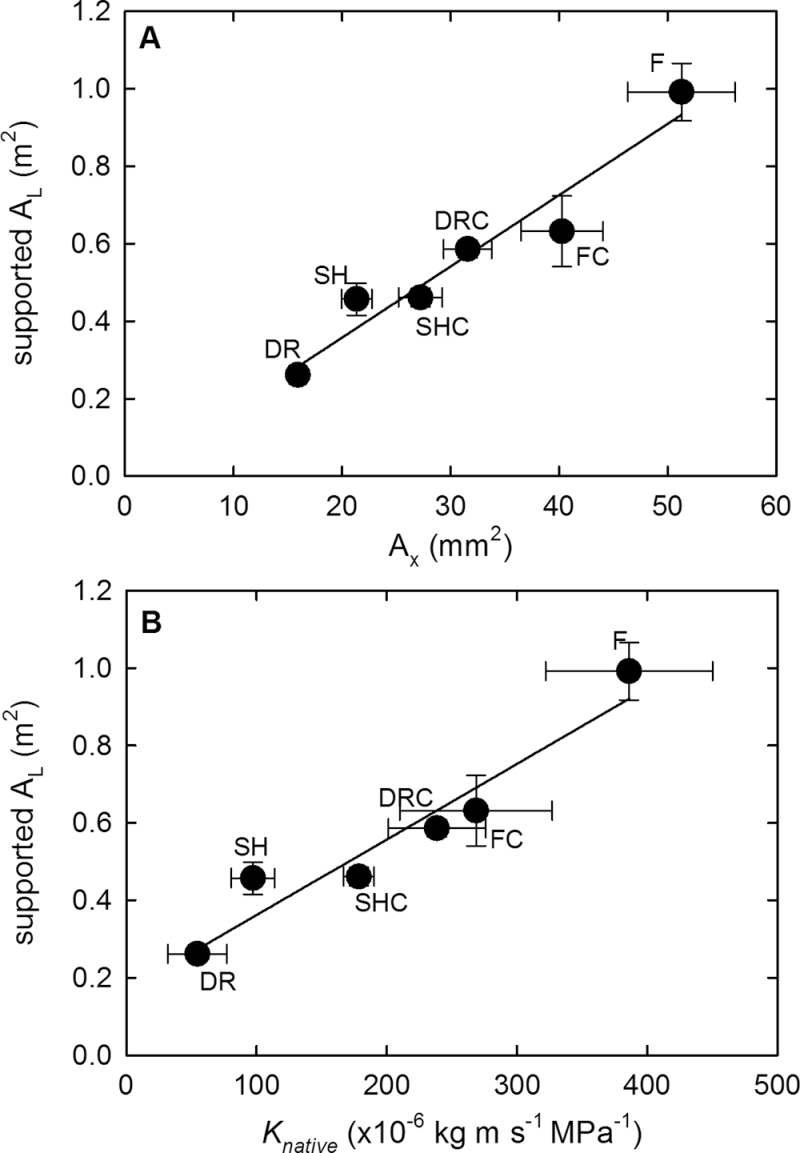
The supported AL scaled linearly with AX across all plant groups (R 2=0.933, P=0.002) (Fig. 1A). AX was tightly correlated with a stem’s K max (R 2=0.976, P < 0.001). The level of native embolism was low in most basal stem segments; therefore, their K max corresponded well with their K native. As a result, the supported AL was positively correlated with both K native (R 2=0.921, P=0.002) (Fig. 1B) and K max (R 2=0.921, P=0.002).
Fig. 1.
Relationship between supported leaf area (AL) and a stem’s capacity to transport water measured as (A) xylem cross-sectional area (AX) and (B) native hydraulic conductivity (K native) in hybrid poplar saplings grown under drought (DR), nitrogen fertilization (F), shade (SH), and control conditions (DRC, FC, and SHC). Means ±SD are shown.
Variation in stem morphology and xylem anatomy
The structural and hydraulic parameters of xylem varied between treated and control plants and between the two stem segment positions (Table 1). In all xylem parameters tested, the ANOVA results showed a significant effect of at least one of the factors (treat, pos) or of their interaction (treat×pos) (Supplementary Table S3 at JXB online). The results of planned comparisons testing for a significant difference between group means are presented in Table 2.
Table 1.
Structural and hydraulic parameters of xylem of basal (upper case letters) and distal (lower case letters) stem segments in hybrid poplar saplings grown under drought (DR, dr), nitrogen fertilization (F, f), shade (SH, sh), and control conditions (DRC, drc; FC, fc; SHC, shc).
| AX (mm2) | DV (µm) | dw (g cm–3) | KS (kg m–1s–1 MPa–1) | P50 (MPa) | |
|---|---|---|---|---|---|
| DR | 15.9 (0.7) | 38.0 (1.8) | 0.332 (0.021) | 5.6 (0.6) | –1.18 (0.16) |
| DRC | 31.6 (2.1) | 43.4 (0.8) | 0.349 (0.008) | 7.9 (0.7) | –1.38 (0.04) |
| dr | 6.3 (0.5) | 30.1 (1.2) | 0.334 (0.027) | 3.1 (0.6) | –1.71 (0.02) |
| drc | 12.8 (2.3) | 45.9 (0.9) | 0.288 (0.009) | 3.8 (0.5) | –0.96 (0.20) |
| Fa | 51.3 (5.0) | 42.6 (1.6) | 0.311 (0.014) | 8.3 (1.0) | –1.14 (0.16) |
| FCa | 40.3 (3.8) | 38.9 (0.4) | 0.372 (0.021) | 7.2 (0.6) | –1.42 (0.07) |
| f | 28.1 (3.5) | 48.6 (1.2) | 0.244 (0.016) | 6.5 (1.9) | –0.15 (0.08) |
| fc | 18.0 (2.9) | 45.3 (1.0) | 0.295 (0.008) | 7.9 (0.7) | –0.79 (0.19) |
| SHb | 21.3 (1.4) | 35.2 (1.1) | 0.318 (0.005) | 4.8 (0.3) | –1.14 (0.11) |
| SHCb | 27.2 (2.0) | 36.7 (0.8) | 0.404 (0.010) | 6.6 (0.1) | –1.53 (0.06) |
| sh | 11.4 (2.0) | 41.4 (1.2) | 0.276 (0.010) | 5.1 (0.7) | –0.63 (0.06) |
| shc | 11.6 (1.8) | 40.6 (1.5) | 0.314 (0.013) | 2.7 (0.3) | –1.03 (0.24) |
Values are means with standard deviations given in parentheses (n=5–8)
AX, xylem cross-sectional area; DV, mean vessel diameter; dw, wood density; K S, xylem-specific hydraulic conductivity; P50, the pressure at 50% loss of hydraulic conductivity.
a Data from Plavcová et al. (2012)
b Data from Plavcová et al. (2011).
Table 2.
Probability (P) values of least significance difference (LSD) tests comparing means of treated versus control plants within the same stem segment position (i.e. either basal or distal) and between means of basal and distal stem segments within either treated or control plant groups.
| Comparison | AX (mm2) | DV (µm) | dw (g cm–3) | KS (kg m–1s–1 MPa–1) | P50 (MPa) |
|---|---|---|---|---|---|
| DR–DRC | <0.001 | <0.001 | NS | <0.001 | NS |
| dr–drc | <0.001 | <0.001 | <0.001 | NS | <0.001 |
| DR–dr | <0.001 | <0.001 | NS | <0.001 | <0.001 |
| DRC–drc | <0.001 | 0.022 | <0.001 | <0.001 | <0.001 |
| F–FC | <0.001 | <0.001 | <0.001 | NS | <0.001 |
| f–fc | <0.001 | <0.001 | <0.001 | NS | <0.001 |
| F–f | <0.001 | <0.001 | <0.001 | 0.0045 | <0.001 |
| FC–fc | <0.001 | <0.001 | <0.001 | NS | <0.001 |
| SH–SHC | <0.001 | NS | <0.001 | <0.001 | <0.001 |
| sh–shc | NS | NS | <0.001 | <0.001 | <0.001 |
| SH–sh | <0.001 | <0.001 | <0.001 | NS | <0.001 |
| SHC–shc | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
DR, dr, drought-stressed plants; F, f, plants fertilized with high levels of nitrogen; SH, sh, shaded plants; DRC, drc, FC, fc, SHC, shc, control plants.
Upper case letters indicate basal segments, while lower case letters refer to distal segments.
AX, xylem cross-sectional area; DV, mean vessel diameter; dw, wood density; K S, xylem-specific hydraulic conductivity; P50, the pressure at 50% loss of hydraulic conductivity; NS, non-significant (P > 0.0125).
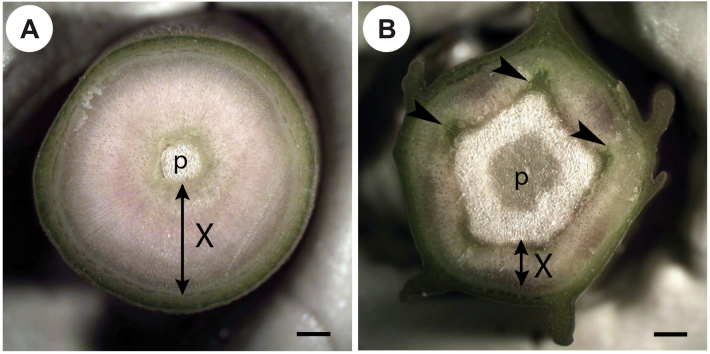
Variation in AX in response to treatments reflected changes in plant radial growth. In basal segments, AX decreased in DR and SH plants and increased in F plants in comparison with their controls (Table 1). Similar patterns in xylem formation were observed in distal segments although there was no significant difference in AX between SH and SHC plants (Table 2). The AX of distal segments was 45–60% smaller compared with basal segments even though the external stem diameters of distal and basal segments were often similar. The difference in AX was mainly caused by a larger pith area in distal segments compared with their basal counterparts (Fig. 2). Furthermore, the vascular cylinder in distal segments had an irregular (rather than more or less cylindrical) shape, and the patterns associated with primary growth were still apparent in stem cross-sections. For instance, clusters of primary xylem could be readily distinguished adjacent to the vertices of the pentagonal pith (arrowheads in Fig. 2B). Despite these signs of juvenility, the transition from primary to secondary growth was clearly completed and a substantial amount of secondary xylem was produced along the entire length of distal segments.
Fig. 2.
Representative cross-sections of (A) basal and (B) distal stem segments from one of the control plants (FC). In the basal segment (A), secondary xylem (X) represents the majority of stem cross-sectional area, while the area of pith (p) is relatively small. In contrast, a large pentagonal pith is surrounded by a relatively narrow layer of secondary xylem in the distal segment (B). Signs of juvenility are apparent in the distal segment. Clusters of primary xylem and secondary xylem formed early after the transition to secondary growth are apparent in the distal segment cross-section (arrowheads). Scale bars=1 mm.
Differences in xylem development and differentiation were paralleled by variation in xylem anatomy. In basal segments, mean vessel diameters (Dv) ranged between 35.2±1.1 µm and 43.4±0.8 µm in SHC and DRC plants, respectively (Table 1). In basal segments, Dv decreased in DR while it increased in F plants when compared with their controls. Dv was not significantly different between SH and SHC plants (Table 2). In distal segments, Dv showed even more variation in response to treatments. The smallest (30.1±1.2 µm) and the largest Dv (48.6±1.2 µm) were measured in DR and F plants, respectively (Table 1). The relative changes in Dv in response to treatments were consistent with the trends observed in basal segments. With the exception of DR plants, distal segments exhibited wider vessels than their basal counterparts.
Large differences were also observed in wood density (dw), with values ranging from 0.244±0.016g cm–3 in the distal segments of F plants to 0.404±0.010g cm–3 in the basal segments of SHC plants (Table 1). Basal segments typically showed higher dw than the corresponding distal segments. In basal segments, dw was significantly lower in F and SH plants in comparison with their respective controls, while it did not differ between DR and DRC plants (Table 1). In distal segments, dw was lower in F and SH segments relative to their controls, following the same pattern as in basal segments. In contrast, the distal segments in DR exhibited denser wood than DRC plants (Table 1).
Variation in the hydraulic parameters of xylem
Changes in xylem structure were paralleled by differences in xylem hydraulic parameters (Tables 1, 2). Xylem-specific hydraulic conductivity (K S) varied ~3-fold, from 2.7±0.3kg m–1 s–1 MPa–1 to 8.3±1.0kg m–1 s–1 MPa–1 in DR and F plants, respectively (Table 1). In basal segments, K S was lower in DR and SH plants in comparison with their controls. K S tended to be higher in F than in FC plants; however, the difference was not statistically significant (Table 2). In distal segments, K S was higher in SH and did not differ in DR and F plants with respect to their respective controls.
Cavitation resistance varied profoundly in response to experimental treatments and segment location (Tables 1, 2). The P50 of basal segments differed <0.5MPa across all three treatments, ranging from –1.14MPa in both SH and F plants to –1.53MPa in SHC plants. While the vulnerability of basal stem segments did not change significantly in response to the DR treatment, stems of SH and F plants were more vulnerable than their controls. In distal segments, P50 values exhibited a large variation of 1.5MPa between the most vulnerable (F plants, –0.15±0.08MPa) and the most resistant plants (DR plants, –1.71±0.20MPa). With the exception of the DR treatment, distal segments were more vulnerable than their basal counterparts. In fact, distal segments of DR plants had the most resistant xylem across all the plant groups and segment positions tested in this study (Table 1).
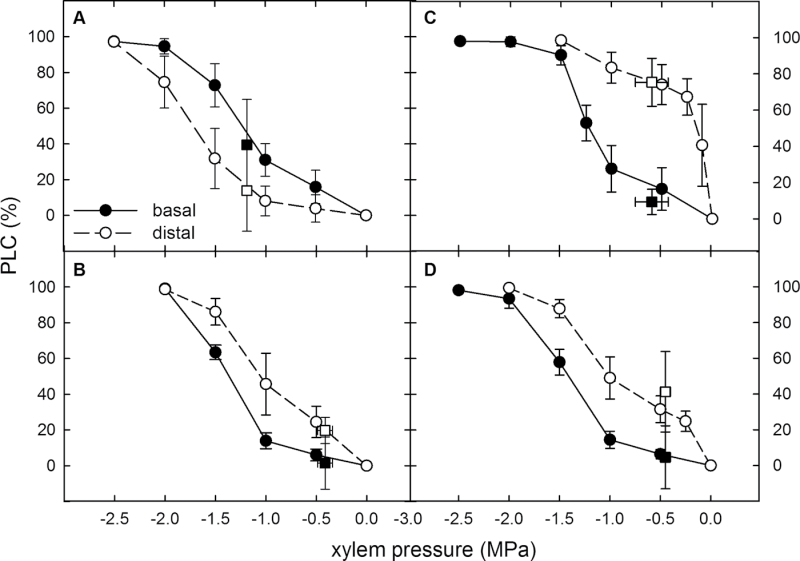
The differences in P50 between basal and distal segments were associated with a marked change in the shape of their vulnerability curves (Fig. 3). While all basal segments showed typical sigmoidal-shaped vulnerability curves, the shape of the curves of distal segments varied from sigmoidal through linear to r-shaped, depending on the treatment. The distal segments of F plants were extremely vulnerable. These segments exhibited 75% PLC at a modest xylem pressure of –0.25MPa, resulting in an r-shaped vulnerability curve (Fig. 3C). In distal segments of DR plants, in contrast, embolism did not exceed 20% at xylem pressures less negative than –1.25MPa (Fig. 3A). Vulnerability curves generated by the centrifuge method were in good agreement with the native PLC values plotted against the native xylem pressures measured in the F and DR experiments (square symbols in Fig. 3). Xylem pressure was not measured for the SH experiment.
Fig. 3.
Vulnerability curves (circles) and native values of percentage loss of conductivity (PLC) plotted against the native xylem pressure (squares) for basal (filled symbols) and distal (open symbols) stem segments in saplings grown under (A) drought (DR) and (B) well-watered conditions (DRC), and under (C) high N (F) and (D) adequate N (FC) fertilization. Note the profoundly different shape of the vulnerability curves in distal segments (open circles), ranging from (A) sigmoidal through (B and D) linear to (C) r-shaped. Means ±SD are shown.
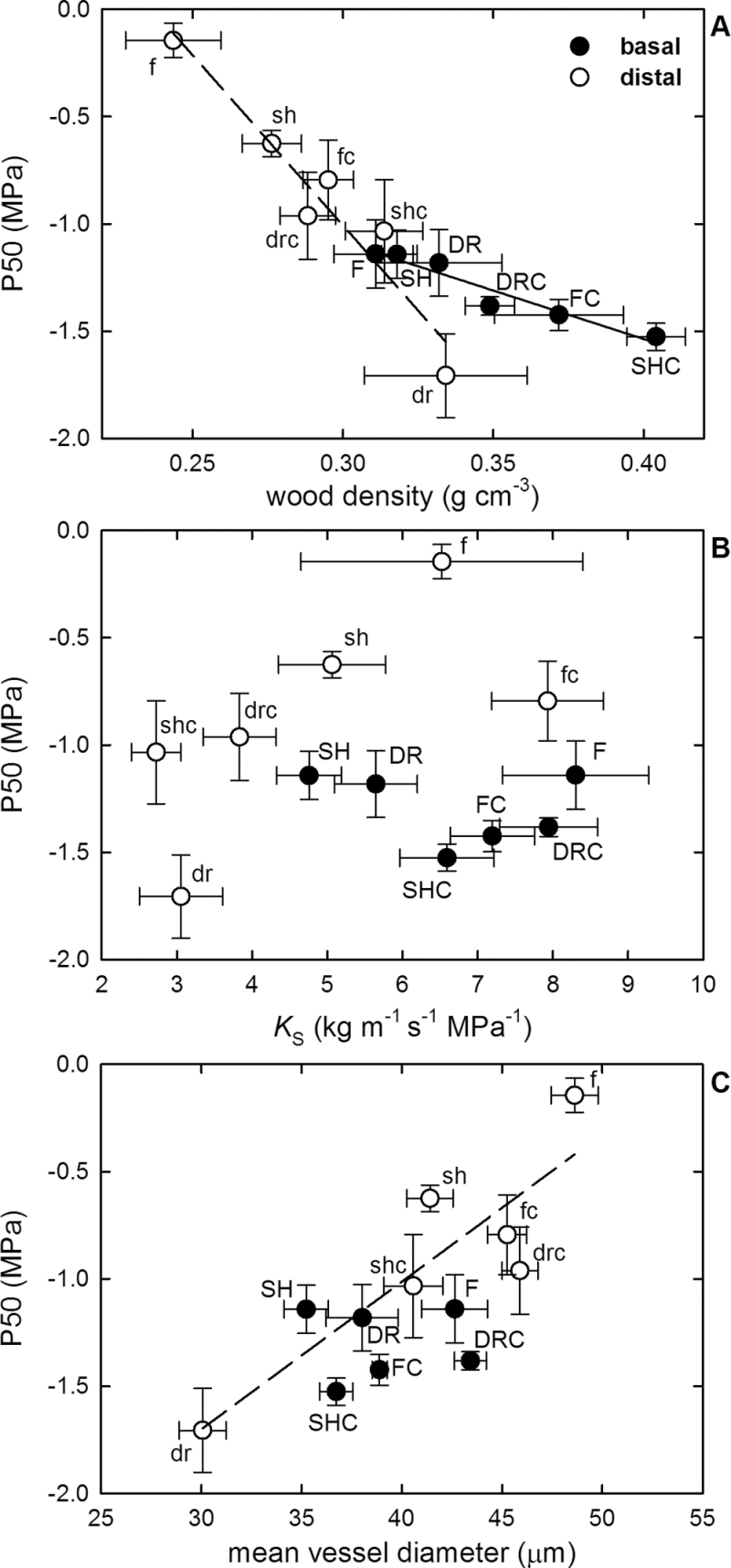
P50s of both basal and distal segments scaled tightly with dw (basal R 2= 0.928, P=0.002; distal R 2= 0.925, P=0.002) (Fig. 4A). In contrast, P50 was not correlated with K S (Fig. 4B). P50 was significantly correlated with Dv in distal segments (distal R 2= 0.766, P=0.023); however, this correlation was mainly driven by the two extremely different distal segments in DR and F plants (Fig. 4C).
Fig. 4.
Relationship between P50 and (A) wood density, (B) xylem-specific hydraulic conductivity (K S), and (C) mean vessel diameter for basal (filled circles, upper case letters) and distal (open circles, lower case letters) stem segments in hybrid poplar saplings grown under drought (DR, dr), nitrogen fertilization (F, f), shade (SH, sh), and control conditions (DRC, drc; FC, fc; SHC, shc). Means ±SD are shown. Solid and dashed lines represent significant linear correlations for basal and distal segments, respectively.
Discussion
Scaling of AX and AL and variation in K S
Plant growth and development were greatly affected when saplings of hybrid poplar were grown under experimental drought (DR), shade (SH), or nitrogen fertilization (F) for >30 d (Supplementary Table S2 at JXB online). In addition, some variability in growth and hydraulic parameters existed between the three sets of control plants (DRC, SHC, and FC) that grew under similar but not identical conditions. Across all plant groups, the AX was tightly correlated with the supported AL (Fig. 1A). Since AX represents the principal anatomical basis of a stem’s capacity to transport water, the allometric relationship between AX and AL translates into tight scaling between AL and a stem’s hydraulic conductivity (K native) (Fig. 1B). These relationships, reflecting a well-established allometric relationship between stem basal diameter and leaf area (e.g. Harrington and Fownes, 1993; McCulloh et al., 2012), arguably help to maintain an adequate supply of water from roots to transpiring leaves (Shinozaki et al., 1964).
A tight correlation between AL and AX would in principle not be required if a plant could radically change the hydraulic parameters of its xylem. For instance, if the xylem could become much more transport efficient, a smaller AX per unit AL would be sufficient to provide an adequate water supply. Despite an almost 2-fold variation in K S values across the basal segments in the current data set, these differences in transport efficiency did not significantly alter the overall linear relationship between AL and AX across the experimental group averages.
The changes in K S observed in response to individual experimental treatments (Tables 1, 2) were largely consistent with the initial hypotheses. In basal segments, K S values were lower in resource-limited DR and SH plants. In contrast, the highest value of K S was found in basal segments of F plants which received luxurious levels of nitrogen and were kept well irrigated all the time. These findings are in agreement with changes in K S observed in other studies in response to low water availability (Beikircher and Mayr, 2009), shade (Raimondo et al., 2009), and high nutrient supply (Hacke et al., 2010).
Variation in cavitation resistance
In agreement with our initial hypothesis, xylem vulnerability to drought-induced cavitation changed in response to experimental treatments (Tables 1, 2). More vulnerable xylem in comparison with controls was found in both basal and distal segments of SH and F plants. These findings are in line with results obtained in other species in response to similar environmental cues (Cochard et al., 1999; Harvey and van den Driessche, 1999; Barigah et al., 2006). P50 was not statistically different between DR and DRC plants in basal segments; however, cavitation resistance increased in distal segments of DR saplings. As growth was significantly reduced in DR plants, the amount of xylem produced under treatment conditions was small in basal segments, and hence did not affect the overall vulnerability of the bulk xylem tissue. However, the increased resistance in distal segments of DR plants indicates that their xylem adjusted to sustain lower xylem pressures and prevent excessive cavitation. An increase in cavitation resistance in response to low water availability has been previously documented in several species, including poplar, exposed to various levels of drought severity (Beikircher and Mayr, 2009; Awad et al., 2010; Fichot et al., 2010), while only a limited change has been found in three willow clones (Wikberg and Ögren, 2007).
Across the three experiments conducted in the current study, the average difference in P50 between treated and control plants was 0.3MPa and 0.6MPa for basal and distal segments, respectively. Even a relatively subtle change in P50 may have important implications for plant hydraulic performance because the loss of conductivity due to embolism increases very steeply around the P50 value. The smaller variation in P50 observed in basal in comparison with distal segments is not surprising given the experimental design. The basal segments completed their primary growth and started their secondary growth under the same conditions prior to the commencement of treatment. In distal segments, in contrast, the entire growth and development took place under treatment conditions.
With the exception of DR plants, distal segments were more vulnerable than their basal counterparts. The difference between basal and distal segments across the treatment groups was on average 0.6MPa, with the largest difference of almost 1MPa found in F plants. Age-related differences in xylem vulnerability have been previously studied using branches of field-grown trees. While some studies found that younger branches and roots were more vulnerable to cavitation (Sperry and Ikeda, 1997; Choat et al., 2005), other studies do not support this trend (Sperry and Saliendra, 1994; Hacke and Sauter, 1996). Based on the vulnerability segmentation hypothesis (Zimmermann, 1983; Tyree and Ewers, 1991), it has been proposed that more distal organs such as leaf petioles and terminal branches should be more vulnerable than trunk xylem in mature trees. Distal plant parts are arguably expendable; their sacrifice can help to maintain favourable plant water balance by reducing the total transpiring surface (Rood et al., 2000). This strategy might be particularly vital in poplar which is well known for its ability to regenerate by root suckering and re-sprouting from auxiliary buds (Galvez and Tyree, 2009; Lu et al., 2010). Nevertheless, it is questionable whether such reasoning is relevant to the relatively young saplings measured in this current study.
The increased vulnerability of the distal segments could potentially be linked to their juvenility. In distal segments, primary xylem represented a substantial proportion of the bulk xylem (Fig. 2). Primary xylem has been shown to be more vulnerable than secondary xylem in 1-year-old branches of sugar maple (Choat et al., 2005). However, little evidence was found for increased vulnerability of primary xylem in hybrid poplar in the current study. When native and artificially induced embolism was visualized by perfusing distal stem segments with safranin dye, more embolized vessels were detected in secondary xylem while the clusters of primary xylem vessels were largely functional (Supplementary Fig. S1 at JXB online). Thus, the increased proportion of primary xylem in distal segments cannot explain their increased vulnerability.
The distal segments of F plants appeared particularly vulnerable, as indicated by their r-shaped vulnerability curve (Fig. 3C). While some regarded r-shaped vulnerability curves as measurement artefacts (Choat et al., 2010; Cochard et al., 2010), others provided evidence that these curves can be valid and that they are associated with the presence of extremely vulnerable vessels that embolize at near-atmospheric pressures (Christman et al., 2012; Jacobsen and Pratt, 2012; Sperry et al., 2012). r-shaped curves have been typically measured in large-vesselled plants such as oaks and grapevine (Taneda and Sperry, 2008; Jacobsen and Pratt, 2012); however, similar curves have been observed in poplar under distinct conditions of cavitation fatigue (Hacke et al., 2001b ) and xylem senescence (Sperry et al., 1991). In the current study, native PLC measured in distal segments of F plants plotted against the values of native xylem pressure corresponded well with the vulnerability curves (Fig. 3C), providing support that these curves are valid in the present study. According to the air-seeding hypothesis, the population of particularly vulnerable vessels is characterized by extremely leaky pits. Thus, the pit membranes in distal segments of F plants might have been inherently more porous or more susceptible to pore enlargement during pit membrane deflection. Alternatively, it is possible that the low mechanical reinforcement of vessels, as evidenced by low wood density, resulted in irreversible damage to some of the pit membranes, rendering them extremely permeable to air.
Relationship between wood density and cavitation resistance
The correlation between wood density (dw) and P50 was remarkably strong across all basal and distal segments measured in this study (Fig. 4A). This finding agrees with previous studies that identified a trade-off between vulnerability and xylem construction cost at the interspecific level (Hacke et al., 2001a ; Jacobsen et al., 2005; Pratt et al., 2007). Results from intraspecific comparisons are less conclusive regarding this trade-off. No relationship between dw and P50 was found in a recent study comparing eight different genotypes of Populus deltoides×Populus nigra grown under two levels of irrigation (Fichot et al., 2010). However, a significant correlation between P50 and another parameter related to xylem mechanical strength, the double vessel wall thickness, was found in the same study. Similarly, Awad et al. (2010) found that increased vulnerability scaled with decreasing cell wall thickness and vessel thickness to span ratio in plants of a single Populus tremula×Populus alba clone grown under three contrasting water regimes, while no significant correlation was found between P50 and dw. In the current study, saplings were maintained in a controlled environment with their stems secured to supporting stakes. In plants supported by stakes, wood density may strongly reflect demands arising from cohesion-driven water transport, while the role of xylem in providing structural support to the plant body is likely to be less important than in plants growing in a natural environment. This might have contributed to the tight correlation observed between P50 and dw in this study.
Safety versus efficiency trade-off
The mean values of K S and P50 across all basal segments were 6.7kg m–1 s–1 MPa–1 and –1.3MPa, respectively, which ranks hybrid poplar among diffuse-porous species that are relatively transport efficient but vulnerable to cavitation. Thus, these data support the notion that xylem cannot be superior in both cavitation resistance and transport efficiency (Maherali et al., 2004; Hacke et al., 2006; Lens et al., 2011). However, within the present data set, there was no significant correlation between P50 and K S in either basal or distal stem segments (Fig. 4B). A correlation between Dv and P50, which can also be regarded as an indicator of a safety versus efficiency trade-off, was significant only in distal segments, and this trend was mainly driven by two extreme data points (Fig. 4C). Thus, these findings indicate that increased resistance to cavitation is not necessarily associated with reduced transport efficiency. This is good news for tree breeders because it suggests that there is some limited room for simultaneous improvement of both hydraulic efficiency and cavitation resistance. In the current data set, basal segments were closer to this optimum than the distal segments (Fig. 4B). Interestingly, basal segments under treatments in which either water or light resources were severely limited (i.e. DR and SH plants) appeared less hydraulically optimized compared with the basal segments of F and all three control plant groups.
Conclusions
The hybrid poplar genotype used in this study inherited a genetic blueprint from the riparian cottonwoods P. trichocarpa and P. deltoides, which defines the general characteristics of its anatomy, morphology, and physiology. This study has specifically focused on the anatomy and hydraulic function of xylem. The data show that the xylem of hybrid poplar is efficient and highly vulnerable to drought-induced cavitation, as expected given its parentage. While these general properties of xylem cannot be radically changed, the data demonstrate that the xylem structure and function are, to a certain extent, variable. Differences in xylem cross-sectional area, mean vessel diameter, wood density, xylem-specific hydraulic conductivity, and vulnerability to cavitation were detected not only in response to experimental treatments (drought, nitrogen fertilization, and shade) but also in stem segments sampled from different vertical positions along a plant’s main axis (basal and distal segments). Such developmental and phenotypic plasticity in xylem traits can potentially be used to cope with different and changing environmental conditions. However, it is difficult to predict what xylem phenotypes would be produced under field conditions.
The results also provide insights into xylem structure–function trade-offs and can help to elucidate mechanistic underpinnings of some of these patterns. The close correlation between wood density and P50 observed in this study is intriguing and suggests that there might have been a true functional link between these two traits in this study. It is likely that stronger cell walls helped to stabilize the pit fields, thereby protecting pit membranes from mechanically induced damage. More research is necessary to show if this situation is unique to this highly vulnerable hybrid poplar clone grown with stems structurally supported by stakes or if it can be extrapolated to a broader array of species and growing conditions. In contrast, no correlation between P50 and K S was found across the data set, indicating that the safety versus efficiency trade-off is not, within certain bounds, inevitable.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Cross-section of a distal stem segment perfused with safranin dye to visualize functional and embolized xylem conduits.
Table S1. Overview of the experimental design: the duration of experiments, and the duration of treatments, and a brief description of the treatment conditions for treated and control plants.
Table S2. Growth-related parameters of hybrid poplar saplings grown under drought (DR), nitrogen fertilization (F), shade (SH), and control conditions (DRC, FC, SHC).
Table S2. Analysis of variance results: variation in xylem structural and hydraulic parameters in response to treatments and between stem segment positions.
Acknowledgements
UH acknowledges funding by an Alberta Ingenuity New Faculty Award, the Canada Research Chair Program, and the Canada Foundation for Innovation.
References
- Awad H, Barigah T, Badel E, Cochard H, Herbette S. 2010. Poplar vulnerability to xylem cavitation acclimates to drier soil conditions Physiologia Plantarum 139 280–288 [DOI] [PubMed] [Google Scholar]
- Barigah TS, Ibrahim T, Bogard A, Faivre-Vuillin B, Lagneau LA, Montpied P, Dreyer E. 2006. Irradiance-induced plasticity in the hydraulic properties of saplings of different temperate broad-leaved forest tree species Tree Physiology 26 1505–1516 [DOI] [PubMed] [Google Scholar]
- Beikircher B, Mayr S. 2009. Intraspecific differences in drought tolerance and acclimation in hydraulics of Ligustrum vulgare and Viburnum lantana Tree Physiology 29 765–775 [DOI] [PubMed] [Google Scholar]
- Braatne J, Rood S, Heilman P. 1996. Life history, ecology, and conservation of riparian cottonwoods in North America. In: Stettler R, Bradshaw HJ, Heilman P, Hinckley T, eds. Biology of Populus and its implications for management and conservation Ottawa, ON, Canada: NRC Research Press; 57–85 [Google Scholar]
- Carlquist S. Comparative wood anatomy. Berlin: Springer-Verlag; 1988. [Google Scholar]
- Carlquist S. Comparative wood anatomy: systematic, ecological, and evolutionary aspects of dicotyledon wood. Berlin: Springer Verlag; 2001. [Google Scholar]
- Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. 2009. Towards a worldwide wood economics spectrum Ecology Letters 12 351–366 [DOI] [PubMed] [Google Scholar]
- Choat B, Drayton WM, Brodersen C, Matthews MA, Shackel KA, Wada H, McElrone AJ. 2010. Measurement of vulnerability to water stress-induced cavitation in grapevine: a comparison of four techniques applied to a long-vesseled species Plant, Cell and Environment 33 1502–1512 [DOI] [PubMed] [Google Scholar]
- Choat B, Lahr EC, Melcher PJ, Zwieniecki MA, Holbrook NM. 2005. The spatial pattern of air seeding thresholds in mature sugar maple trees Plant, Cell and Environment 28 1082–1089 [Google Scholar]
- Christman MA, Sperry JS, Smith DD. 2012. Rare pits, large vessels and extreme vulnerability to cavitation in a ring-porous tree species New Phytologist 193 713–720 [DOI] [PubMed] [Google Scholar]
- Cochard H, Herbette S, Barigah T, Badel E, Ennajeh M, Vilagrosa A. 2010. Does sample length influence the shape of xylem embolism vulnerability curves? A test with the Cavitron spinning technique Plant, Cell and Environment 33 1543–1552 [DOI] [PubMed] [Google Scholar]
- Cochard H, Lemoine D, Dreyer E. 1999. The effects of acclimation to sunlight on the xylem vulnerability to embolism in Fagus sylvatica L Plant, Cell and Environment 22 101–108 [Google Scholar]
- Fichot R, Barigah TS, Chamaillard S, Le Thiec D, Laurans F, Cochard H, Brignolas F. 2010. Common trade-offs between xylem resistance to cavitation and other physiological traits do not hold among unrelated Populus deltoides×Populus nigra hybrids Plant, Cell and Environment 33 1553–1568 [DOI] [PubMed] [Google Scholar]
- Fonti P, von Arx G, Garcia-Gonzalez I, Eilmann B, Sass-Klaassen U, Gaertner H, Eckstein D. 2010. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings New Phytologist 185 42–53 [DOI] [PubMed] [Google Scholar]
- Galvez DA, Tyree MT. 2009. Impact of simulated herbivory on water relations of aspen (Populus tremuloides) seedlings: the role of new tissue in the hydraulic conductivity recovery cycle Oecologia 161 665–671 [DOI] [PubMed] [Google Scholar]
- Hacke UG, Plavcová L, Almeida-Rodriguez A, King-Jones S, Zhou W, Cooke JEK. 2010. Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar Tree Physiology 30 1016–1025 [DOI] [PubMed] [Google Scholar]
- Hacke U, Sauter JJ. 1996. Drought-induced xylem dysfunction in petioles, branches, and roots of Populus balsamifera L and Alnus glutinosa (L.) Gaertn Plant Physiology 111 413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Ewers BE, Ellsworth DS, Schäfer KVR, Oren R. 2000. Influence of soil porosity on water use in Pinus taeda Oecologia 124 495–505 [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. 2001a. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure Oecologia 126 457–461 [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Wheeler JK, Castro L. 2006. Scaling of angiosperm xylem structure with safety and efficiency Tree Physiology 26 689–701 [DOI] [PubMed] [Google Scholar]
- Hacke UG, Stiller V, Sperry JS, Pittermann J, McCulloh KA. 2001b. Cavitation fatigue. Embolism and refilling cycles can weaken the cavitation resistance of xylem Plant Physiology 125 779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington RA, Fownes JH. 1993. Allometry and growth of planted versus coppice stands of four fast-growing tropical tree species Forest Ecology and Management 56 315–327 [Google Scholar]
- Harvey HP, van den Driessche R. 1997. Nutrition, xylem cavitation and drought resistance in hybrid poplar Tree Physiology 17 647–654 [DOI] [PubMed] [Google Scholar]
- Harvey HP, van den Driessche R. 1999. Nitrogen and potassium effects on xylem cavitation and water-use efficiency in poplars Tree Physiology 19 943–950 [DOI] [PubMed] [Google Scholar]
- Hocking D. 1971. Preparation and use of a nutrient solution for cultruring seedlings of lodgepole pine and white spruce, with selected bibliography Northern Forest Research Centre Information Report Nor-Xl. Edmonton, Canada: Canadian Forest Service, Department of the Environment; [Google Scholar]
- Hogg EH, Brandt JP, Michaellian M. 2008. Impacts of a regional drought on the productivity, dieback, and biomass of western Canadian aspen forests Canadian Journal of Forest Research 38 1373–1384 [Google Scholar]
- Jacobsen AL, Ewers FW, Pratt RB, Paddock WA, Davis SD. 2005. Do xylem fibers affect vessel cavitation resistance? Plant Physiology 139 546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen AL, Pratt RB. 2012. No evidence for an open vessel effect in centrifuge-based vulnerability curves of a long-vesselled liana (Vitis vinifera) New Phytologist 194 982–990 [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapin FS, Pons TL. Plant physiological ecology. New York: Springer-Verlag; 1998. [Google Scholar]
- Lamy JB, Bouffier L, Brulett R, Plomion C, Cochard H, Dlezon S. Uniform selection as a primary force reducing population genetic differentiation of cavitation resistance across a species range. PLoS One. 2011;6:e23476. doi: 10.1371/journal.pone.0023476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher W. Physiological plant ecology. Berlin:; Springer-Verlag: 2003. [Google Scholar]
- Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S. 2011. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer New Phytologist 190 709–723 [DOI] [PubMed] [Google Scholar]
- Lieffers VJ, Messier C, Stadt KJ, Gendron F, Comeau PG. 1999. Predicting and managing light in the understory of boreal forests Canadian Journal of Forest Research 29 796–811 [Google Scholar]
- Lu Y, Equiza MA, Deng X, Tyree MT. 2010. Recovery of Populus tremuloides seedlings following severe drought causing total leaf mortality and extreme stem embolism. Physiologia Plantarum 140 246–257 [DOI] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. 2004. Adaptive variation in the vulnerability of woody plants to xylem cavitation Ecology 85 2184–2199 [Google Scholar]
- Mayr S, Beikircher B, Obkircher M-A, Schmid P. 2010. Hydraulic plasticity and limitations of alpine Rhododendron species Oecologia 164 321–330 [DOI] [PubMed] [Google Scholar]
- McCulloh K, Sperry JS, Lachenbruch B, Meinzer FC, Reich PB, Voelker S. 2010. Moving water well: comparing hydraulic efficiency in twigs and trunks of coniferous, ring-porous, and diffuse-porous saplings from temperate and tropical forests New Phytologist 186 439–450 [DOI] [PubMed] [Google Scholar]
- McCulloh KA, Johnson DM, Meinzer FC, Voelker SL, Lachenbruch B, Domec J-C. 2012. Hydraulic architecture of two species differing in wood density: opposing strategies in co-occurring tropical pioneer trees Plant, Cell and Environment 35 116–125 [DOI] [PubMed] [Google Scholar]
- Niklas KJ. Plant biomechanics. Chicago: University of Chicago Press; 1992. [Google Scholar]
- Pitre FE, Cooke JEK, Mackay JJ. 2007a. Short-term effects of nitrogen availability on wood formation and fibre properties in hybrid poplar Trees-Structure and Function 21 249–259 [Google Scholar]
- Pitre FE, Pollet B, Lafarguette F, Cooke JEK, MacKay JJ, Lapierre C. 2007b. Effects of increased nitrogen supply on the lignification of poplar wood Journal of Agricultural and Food Chemistry 55 10306–10314 [DOI] [PubMed] [Google Scholar]
- Plavcová L, Hacke UG, Sperry JS. 2011. Linking irradiance-induced changes in pit membrane ultrastructure with xylem vulnerability to cavitation Plant, Cell and Environment 34 501–513 [DOI] [PubMed] [Google Scholar]
- Plavcová L, Hacke UG, Almeida-Rodriguez AM, Li Eryang Douglas JC. 2012. Gene expression patterns underlying changes in xylem structure and function in response to increased nitrogen availability in hybrid poplar Plant, Cell and Environment(in press) [DOI] [PubMed] [Google Scholar]
- Pratt RB, Jacobsen AL, Ewers FW, Davis SD. 2007. Relationships among xylem transport, biomechanics and storage in stems and roots of nine Rhamnaceae species of the California chaparral New Phytologist 174 787–798 [DOI] [PubMed] [Google Scholar]
- Raimondo F, Trifilo P, Lo Gullo MA, Buffa R, Nardini A, Salleo S. 2009. Effects of reduced irradiance on hydraulic architecture and water relations of two olive clones with different growth potentials Environmental and Experimental Botany 66 249–256 [Google Scholar]
- Rennenberg H, Wildhagen H, Ehlting B. 2010. Nitrogen nutrition of poplar trees Plant Biology 12 275–291 [DOI] [PubMed] [Google Scholar]
- Rood SB, Patino S, Coombs K, Tyree MT. 2000. Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods Trees 14 248–257 [Google Scholar]
- Shinozaki K, Yoda K, Hozumi K, Kira T. 1964. A quantitative analysis of plant form—the Pipe Model Theory: I. Basic analysis Japanese Journal of Ecology 14 97–105 [Google Scholar]
- Sokal RR, Rohlf F. Biometry: the principles and practice of statistics in biological research. New York: W.H. Freeman & Company; 1995. [Google Scholar]
- Sperry JS. 2011. Hydraulics of vascular water transport. In: Wojtaszek P, ed. Mechanical integration of plant cells and plants Berlin: Springer-Verlag; 303–327 [Google Scholar]
- Sperry JS, Christman MA, Torres-Ruiz JM, Taneda H, Smith DD. 2012. Vulnerability curves by centrifugation: is there an open vessel artefact, and are ‘r’ shaped curves necessarily invalid? Plant, Cell and Environment 35 601–610 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Pittermann J. 2006. Size and function in conifer tracheids and angiosperm vessels American Journal of Botany 93 1490–1500 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Ikeda T. 1997. Xylem cavitation in roots and stems of Douglas-fir and white fir Tree Physiology 17 275–280 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Perry AH, Sullivan JEM. 1991. Pit membrane degradation and air-embolism formation in ageing xylem vessels of Populus tremuloides Michx Journal of Experimental Botany 42 1399–1406 [Google Scholar]
- Sperry JS, Saliendra NZ. 1994. Intra- and inter-plant variation in xylem cavitation in Betula occidentalis Plant, Cell and Environment 17 1233–1241 [Google Scholar]
- Stiller V. 2009. Soil salinity and drought alter wood density and vulnerability to xylem cavitation of baldcypress (Taxodium distichum (L.) Rich.) seedlings Environmental and Experimental Botany 67 164–171 [Google Scholar]
- Taneda H, Sperry JS. 2008. A case-study of water transport in co-occurring ring- versus diffuse-porous trees: contrasts in water-status, conducting capacity, cavitation and vessel refilling Tree Physiology 28 1641–1651 [DOI] [PubMed] [Google Scholar]
- Tyree MT, Ewers FW. 1991. Tansley Review no. 34. The hydraulic architecture of trees and other woody plants New Phytologist 119 345–360 [Google Scholar]
- Tyree MT, Kolb KJ, Rood SB, Patino S. 1994. Vulnerability to drought-induced cavitation of riparian cottonwoods in Alberta: a possible factor in the decline of the ecosystem? Tree Physiology 14 455–466 [DOI] [PubMed] [Google Scholar]
- Wheeler EA, Baas P, Rodgers S. 2007. Variations in dicot wood anatomy: a global analysis based on the insidewood database IAWA Journal 28 229–258 [Google Scholar]
- Wikberg J, Ögren E. 2007. Variation in drought resistance, drought acclimation and water conservation in four willow cultivars used for biomass production Tree Physiology 27 1339–1346 [DOI] [PubMed] [Google Scholar]
- Wortemann R, Herbette S, Barigah TS, Fumanal B, Alia R, Ducousso A, Gomory D, Roeckel-Drevet P, Coachard H. 2011. Genotypic variablility and phenotypic plasticity of cavitation resistance in Fagus sylvatica L. across Europe Tree Physiology 31 1175–1182 [DOI] [PubMed] [Google Scholar]
- Zimmermann MH. Xylem structure and the ascent of sap. Berlin: Springer-Verlag; 1983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.