Abstract
Flavonoids are one of the major plant pigments for flower colour. Not only coloured anthocyanins, but also co-pigment flavones or flavonols, accumulate in flowers. To study the regulation of early flavonoid biosynthesis, two R2R3-MYB transcription factors, GtMYBP3 and GtMYBP4, were identified from the petals of Japanese gentian (Gentiana triflora). Phylogenetic analysis showed that these two proteins belong to the subgroup 7 clade (flavonol-specific MYB), which includes Arabidopsis AtMYB12, grapevine VvMYBF1, and tomato SlMYB12. Gt MYBP3 and Gt MYBP4 transcripts were detected specifically in young petals and correlated with the profiles of flavone accumulation. Transient expression assays showed that GtMYBP3 and GtMYBP4 enhanced the promoter activities of early biosynthetic genes, including flavone synthase II (FNSII) and flavonoid 3′-hydroxylase (F3′H), but not the late biosynthetic gene, flavonoid 3′,5′-hydroxylase (F3′5′H). GtMYBP3 also enhanced the promoter activity of the chalcone synthase (CHS) gene. In transgenic Arabidopsis, overexpression of Gt MYBP3 and Gt MYBP4 activated the expression of endogenous flavonol biosynthesis genes and led to increased flavonol accumulation in seedlings. In transgenic tobacco petals, overexpression of Gt MYBP3 and Gt MYBP4 caused decreased anthocyanin levels, resulting in pale flower colours. Gt MYBP4-expressing transgenic tobacco flowers also showed increased flavonols. As far as is known, this is the first functional characterization of R2R3-MYB transcription factors regulating early flavonoid biosynthesis in petals.
Key words: Early flavonoid biosynthesis, flavone, flower colour, Japanese gentian, R2R3-MYB, transcription factor
Introduction
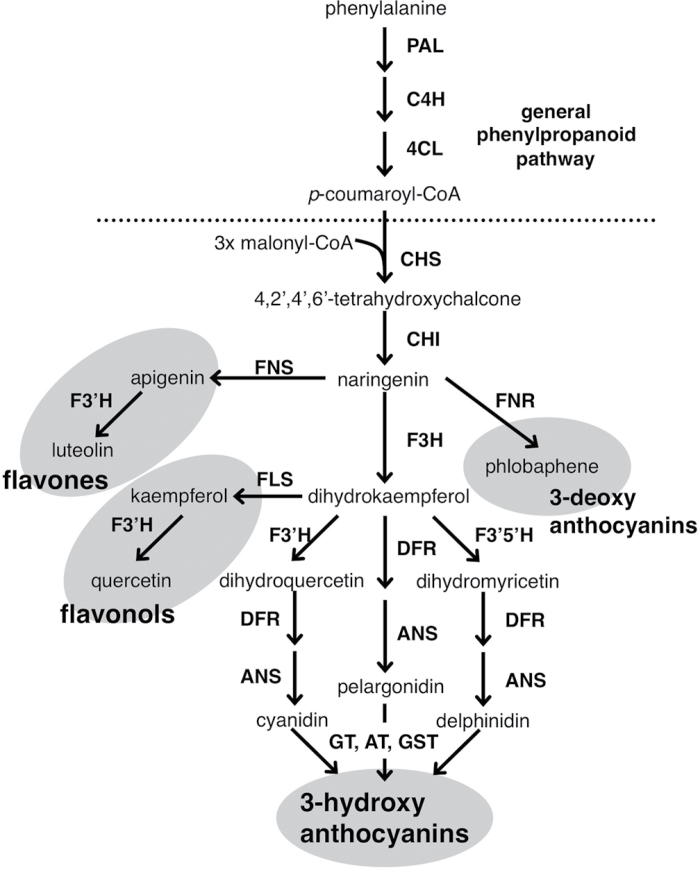
Natural flower pigments generally consist of complex secondary metabolites, such as flavonoids, carotenoids, and betalains, depending on the plant species. Flavonoids are the most characterized secondary metabolites in higher plants and have various biological functions, such as flower pigmentation, pollen fertility, plant–microbe interaction, and protection from UV radiation (reviewed in Mol et al., 1998). Determination of the flavonoid biosynthetic pathway is of great interest to plant scientists and many structural genes involved in flavonoid biosynthesis have been cloned and studied in maize kernels (Zea mays), petunia flowers (Petunia hybrida), snapdragon flowers (Antirrhinum majus), and in Arabidopsis seed coats (Arabidopsis thaliana) (Fig. 1; Holton and Cornish, 1995; Mol et al., 1998; Winkel, 2006).
Fig. 1.
Flavonoid biosynthetic pathway in higher plants. 4CL, 4-coumarate:CoA ligase; ANS, anthocyanidin synthase; AT, anthocyanin acyltransferase; C4H, cinnamate 4-hydroxylase; CHI, chalcone isomerase; CHS, chalcone synthase; DFR, dihydroflavonol 4-reductase; F3′5′H, flavonoid 3′,5′-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3H, flavanone 3-hydroxylase; FLS, flavonol synthase; FNR, flavanone reductase; FNS, flavone synthase; GST, glutathione S-transferase; GT, anthocyanin O-glucosyltransferase; PAL, phenylalanine ammonia lyase.
Japanese endemic gentian plants (Gentiana triflora) have brilliant blue flowers and are popular floricultural plants in Japan (Nishihara et al., 2008). The petals of Japanese gentian accumulate a polyacylated anthocyanin, termed gentiodelphin (delphinidin 3-O-glucoside-5,3′-O-caffeoylglucoside), and flavone derivatives (Goto et al., 1982; Yoshida et al., 2000; Nakatsuka et al., 2005). The structural genes involved in the gentiodelphin and flavone biosynthetic pathways have been isolated and characterized (Tanaka et al., 1996; Kobayashi et al., 1998; Nakatsuka et al., 2005, 2008a). Thus, the blue pigmentation and its development have been extensively studied in Japanese gentian (Yoshida et al., 2009).
In flavonoid biosynthesis, two clusters of co-regulated structural genes can generally be distinguished: early biosynthetic genes, which are involved in the synthesis of flavones, flavonols, and phlobaphenes; and late biosynthetic genes, which are involved in proanthocyanin and anthocyanin biosynthesis (Fig. 1; Mol et al., 1998; Quattrocchio et al., 2006; Gonzalez et al., 2008). Transcription factors for the anthocyanin and proanthocyanin biosynthetic pathways are mainly members of the R2R3-MYB, basic helix–loop–helix (bHLH), and WD40 repeats (WDR) protein families. Complexes of these transcription factors regulate the expression of structural genes in the late biosynthetic pathway (Broun, 2005; Koes et al., 2005). In maize, C1 (R2R3-MYB) requires interaction with R (bHLH) to activate anthocyanin biosynthesis (Lloyd et al., 1992). In petunia flowers, AN2 (R2R3-MYB) interacts with two distinct bHLH factors, JAF13 or AN1, both of which share a high sequence similarity with the maize R and snapdragon DELILA proteins (Spelt et al., 2000). PAP1/PAP2 are also C1 homologues in Arabidopsis and are involved in proanthocyanin accumulation in seed coats (Borevitz et al., 2000). In gentian, GtMYB3 interacts with GtbHLH1, and the complex of these two proteins activates the expression of flavonoid 3′,5′-hydroxylase (F3′5′H) and anthocyanin 5,3′-aromatic acyltransferase (5,3′AT′) genes, which belong to the late flavonoid biosynthetic pathway (Nakatsuka et al., 2008b). However, the GtMYB3/GtbHLH1 complex could not activate the expression of the chalcone synthase (CHS) gene, which belongs to the early biosynthetic pathway. Thus, the activation of many R2R3-MYB proteins regulating flavonoid biosynthetic pathways depends on an interaction with bHLH proteins. R2R3-MYB proteins that interact with bHLH proteins share a common motif in the N-terminal R3 repeat (Grotewold et al., 2000). However, the transcription factors regulating early flavonoid biosynthesis in gentian flowers remain unknown.
Maize P1 (ZmP1) is an R2R3-MYB that is active without binding a bHLH protein. ZmP1 controls a subset of genes for 3-deoxyflavonoids and phlobaphene biosyntheses in kernel pericarp and cob tissue (Grotewold et al., 1994). A transient expression assay using a maize cell suspension demonstrated that P1 can activate the transcription of the flavanone reductase gene (A1, FNR) but cannot activate the flavonoid 3-O-glucosyltransferase gene (Bz1, 3GT). Genetic analysis also showed that the Zm P1 locus coincides with a major quantitative trait locus determining the levels of maysin, a C-glycosyl flavone (Byrne et al., 1996; Zhang et al., 2003; Cocciolone et al., 2005). In Arabidopsis, AtMYB12/PFG2, AtMYB11/PFG1, and AtMYB111/PFG3, which share amino acid sequence similarity with ZmP1, regulate individual flavonol accumulation in differential organs of developing seedlings (Mehrtens et al., 2005; Stracke et al., 2007). They activate the expression of CHS, chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonol synthase (FLS), and flavonoid 3′-hydroxylase (F3′H), but do not influence the expression of dihydroflavonol 4-reductase (DFR). In grapevine, the expression of Vv MYBF1 correlated with flavonol accumulation and the expression of Vv FLS1 (Czemmel et al., 2009). In addition, deficiency of SlMYB12, the tomato orthologue of AtMYB12, resulted in pink fruit lacking the ripening-dependent accumulation of the yellow flavonoid naringenin chalcone in the fruit peel (Ballester et al., 2010). These R2R3-MYBs were classified into subgroup 7 (Dubos et al., 2010) and activated specific gene sets of the early flavonoid biosynthetic pathway (Stracke et al., 2007). However, in spite of the demonstrated accumulation of early flavonoid biosynthetic metabolites, such as flavonol and flavone, no transcription factors regulating the early steps of flavonoid biosynthesis have been identified in the petals of floricultural plants.
In the present study, two P1 orthologues, Gt MYBP3 and Gt MYBP4, were isolated and characterized in Japanese gentian. The expression profiles of both genes correlated with flavone accumulation in petals and activated the expression of CHS, flavone synthase II (FNSII), and F3′H, belonging to early flavonoid biosynthetic pathway. Functional analyses of Gt MYBP3 and Gt MYBP4 were performed in transgenic Arabidopsis and tobacco plants. The results strongly suggested that both GtMYBP3 and GtMYBP4 regulate the early steps of the flavonoid biosynthetic pathway. This is the first report to investigate the function of P1 orthologues in floral organs.
Materials and methods
Plant materials
Japanese gentian (G. triflora cv. Maciry) plants were grown in the fields of the Iwate Agricultural Research Center (Iwate prefecture, Japan). The developmental stages of petal samples were defined as described by Nakatsuka et al. (2005).
Isolation of gentian R2R3-MYB transcription factors
Total RNA was isolated from petals at flower developmental stages 1–3. cDNA was synthesized using a Takara RNA PCR kit (AMV) version 3.0 (Takara-bio, Ohtsu, Japan). Degenerate primers were designed from the conserved DNA-binding domain of R2R3-MYBs controlling flavonoid biosynthesis from other plant species (Supplementary Table S1, available at JXB online). Other degenerate primers (pMybU and pMybL; Rabinowicz et al., 1999) were also used. Reaction conditions consisted of pre-heating at 94 °C for 90 s; 40 cycles of 95 °C for 20 s, 50 °C for 40 s, and 72 °C for 1min; and extension at 72 °C for 10min. Amplified fragments of approximately 180bp were subcloned into the pCR4 TA TOPO cloning vector (Invitrogen, Carlsbad, CA, USA) and sequenced using a Big-Dye terminal cycle sequencing kit version 1.1 and an ABI PRISM 3130 DNA sequencer (Applied Biosystems, Foster City, CA, USA). Nucleotide sequences were conceptually translated into amino acid sequences using GENETYX-MAC version 12 (GENETYX, Tokyo, Japan) and compared using the BLAST network service from the National Center for Biotechnology Information (NCBI). A phylogenetic tree was constructed using MEGA version 4 (Tamura et al., 2007).
To obtain full-length cDNAs of Gt MYBP3 and Gt MYBP4, 3′- and 5′-rapid amplification of cDNA ends (RACE) was performed using a GeneRacer kit (Invitrogen). The amplified fragments were subcloned and sequenced as described above.
Northern blot analysis was performed in gentian using Gt MYBP3 and Gt MYBP4 probes (Supplementary Table S1), as described by Nakatsuka et al. (2005).
Yeast two-hybrid analysis
The yeast two-hybrid assay was performed using the Matchmaker Yeast Two-Hybrid System 3 (Clontech, Mountain View, CA, USA) as described previously (Nakatsuka et al., 2008b). Briefly, the open reading frame sequences of GtMYBP3 and GtMYBP4 were cloned into the pGAD-T7 and pGBK-T7 vectors. GtMYB3 and GtbHLH1 constructs (Nakatsuka et al., 2008b) were also used.
Isolation of 5′-UTRs of GtFNSII and GtF3′H in gentian
The 5′-untranscribed regions of gentian Gt FNSII and Gt F3′H were identified by inverse PCR, using primers shown in Supplementary Table S1 and as described by Nakatsuka et al. (2008b). Amplified fragments of about 1.8kb for Gt FNSII and 1.6kb for Gt F3′H were subcloned and sequenced as described above. Reporter vectors were constructed to contain the firefly luciferase (LUC) gene under the control of the gentian Gt FNSII (accession number AB733018) or Gt F3′H (AB733017) promoters. GtCHSpro-LUC and GtF3′5′Hpro-LUC constructs (Nakatsuka et al., 2008b) were also used.
Transient expression assay using Arabidopsis suspension cells
To evaluate whether Gt MYBP3 and Gt MYBP4 are responsible for the regulation of early flavonoid biosynthesis, transient expression assays were performed using protoplasts from Arabidopsis T87 suspension cells (Axelos et al., 1992). The open reading frame sequences of Gt MYBP3 and Gt MYBP4 were inserted into the p35Spro expression vector under the control of the CaMV35S promoter and NOS terminator, resulting in p35Spro-GtMYBP3 and p35Spro-GtMYBP4, respectively. pBI221 (Clontech) was used as a negative control vector. p35Spro-RLUC, the Renilla luciferase (RLUC) gene under the control of the CaMV35S promoter, was used as a transformation control. Protoplast isolation and PEG-transfection experiments were performed as described by Hartmann et al. (1998). Firefly and Renilla luciferase activities were measured by the dual-Glo luciferase assay system (Promega, Madison, WI, USA) and Luminescencer JNR II (ATTO, Tokyo, Japan), according to the manufacturers′ instructions. At least five independent transfections were done for each plasmid combination to demonstrate reproducibility.
Vector construct and production of stable Arabidopsis and tobacco transformants
p35Spro-GtMYBP3 and p35Spro-GtMYBP4 was inserted into binary vectors, pSkan35SGUS (kanamycin resistance) and pSMABR35S-sGFP (Mishiba et al., 2010), to produce pSkan-35Spro-GtMYBP3 and pSMABR-35Spro-GtMYBP4, respectively. The constructs were then transformed into Agrobacterium tumefaciens EHA101. A. thaliana ecotype col-1 was transformed by floral dip method, as described by Clough and Bent (1998). Positive transformants were selected on germination medium supplemented with 50mg l–1 kanamycin or 6mg l–1 bialaphos, and then T2 seeds were obtained following self-pollination.
Tobacco plants (Nicotiana tabacum cv. SR-1), aseptically grown from seeds for about 1 month, were transformed via an A. tumefaciens-mediated leaf disc procedure (Horsch et al., 1985) and selected using 200mg l–1 kanamycin or 5mg l–1 bialaphos. After rooting and acclimatization, regenerated plants were grown in a greenhouse to set seeds by self-pollination. T1 transgenic plant lines were used for further analyses.
Flavonoid analysis in transgenic Arabidopsis and tobacco plants
The flavonol accumulation in T2 transgenic Arabidopsis seedlings was visualized by diphenylboric acid 2-aminoethyl ester (DPBA), as described by Stracke et al. (2007). Five-day-old seedlings grown on germination medium with 3mg l–1 norflurazon were stained by 0.25% (w/v) DPBA. Fluorescence images were visualized under UV light on a stereoscopic microscope (Olympus, Tokyo, Japan). The amount of anthocyanin and flavonol pigments in petals of transgenic tobacco plants were measured as described by Nakatsuka et al. (2007).
Quantitative real-time PCR in transgenic Arabidopsis and tobacco plants
Total RNA was isolated from the seedlings of transgenic Arabidopsis after germination for 7 days using an RNeasy Plant Mini kit (Qiagen, Venlo, The Netherlands). Total RNA of transgenic tobacco was also isolated from their petals at floral developmental stage 3 using a FastRNA Green kit (Q-Bio gene, Irvine, CA, USA).
Quantitative real-time PCR (qRT-PCR) was performed on a StepOne plus (Applied Biosystems) using SYBR GreenER qPCR Super Mix (Invitrogen). cDNA was synthesized from total RNA after removal of genomic DNA using PrimeScript RT reagent kit with gDNA eraser (Takara-bio), according to the manufacturer′s instructions. Reaction mixtures (10 µl) included the following components: 1×master mix, 0.2 µM of each primer (Supplementary Table S2) and 1 µl cDNA. Cycle conditions were 95 °C for 20 s and then 40 cycles of 95 °C for 1 s and 60 °C for 20 s. The expression levels of each gene were calibrated by the expression of actin (At ACT, Arabidopsis) or ubiquitin (Nt UBQ, tobacco) genes.
Results
Cloning of P1 orthologues from gentian flowers
Degenerate PCR was used to isolate R2R3-MYB transcription factor genes from gentian flowers. More than 200 clones were classified into 24 groups based on a similarity search. Among them, the deduced amino acid sequences of two clones exhibited high similarities with ZmP1 and AtMYB12. Full-length cDNAs for these two clones were determined using 5′/3′ RACE techniques and designated GtMYBP3 and GtMYBP4.
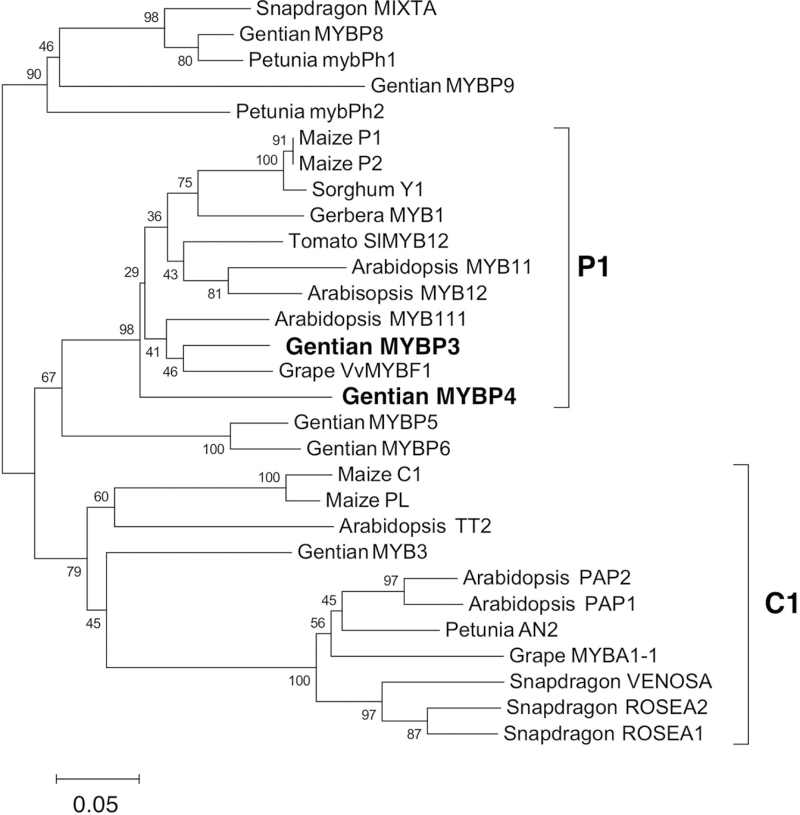
Phylogenetic analysis showed that GtMYBP3 and GtMYBP4 belonged to P1/subgroup 7 (Fig. 2). The GtMYBP3 cDNA (accession no. AB733016) was 1474bp in length and encoded a protein of 329 amino acid residues, whereas the Gt MYBP4 cDNA (accession no. AB289446) was 1303bp encoded a 376 amino acid protein. The deduced amino acid sequence of GtMYBP3 was 79.8% identical to that of GtMYBP4 in the R2R3-MYB DNA-binding domain, and 43.5% identical overall (Supplementary Fig. S1). GtMYBP3 was 84.6, 89.4, and 83.7% identical to Arabidopsis AtMYB12, grape VvMYBF1, and maize ZmP1, respectively, whereas GtMYBP4 was 78.8, 80.8, and 79.8% identical to those three genes, respectively. Although the sequence similarity between R2R3-MYB proteins is generally restricted to the N-terminus, the SG7 motif (GRTxRSxMK; Stracke et al., 2001) and the SG7-2 motif ([W/x][L/x]LS; Czemmel et al., 2009) are characteristic of R2R3-MYB proteins regulating flavonol biosynthesis. Both SG7 motifs were partially conserved in GtMYBP3 and GtMYBP4. GtMYBP3 and GtMYBP4 shared 56% identity (five out of nine amino acid residues) with the SG7-1 motif and 75% (3/4) and 50% (2/4) identity with the SG7-2 motif, respectively (Supplementary Fig. S1).
Fig. 2.
Phylogenetic analysis of deduced amino acid sequences of R2R3-MYB transcription factors in higher plants. The phylogenetic tree of the R2R3-MYB domain was generated using MEGA software (Tamura et al., 2007). Numerals next to branch nodes indicate bootstrap values from 1000 replications. The bar indicates an evolutionary distance of 0.05%. Accession numbers in the GenBank/EMBL/DDBJ database are as follows: gentian: MYBP3 (AB733016), MYBP4 (AB289446), MYBP5 (AB733616), MYBP6 (AB733617), MYBP8 (AB733618), MYBP9 (AB733619), and MYB3 (AB289445); Arabidopsis: MYB11 (NM_116126), MYB12 (DQ224277), MYB111 (NM_124310), TT2, (AJ299452), PAP1 (AF325123). and PAP2 (AF325124); gerbera: MYB1 (AJ554697); grape: MYBF1 (FJ948477), and MYBA1-1 (AB073010); maize: P1 (M73028), P2 (AF210616), C1 (MZEMYBAA), and PL (AF015268); petunia: mybPh1 (Z13996) and AN2 (AF146702);, snapdragon, MIXTA (X79108), VENOSA (DQ275531), ROSEA1 (DQ275529), and ROSEA2 (DQ275530); sorghum: Y1 (AY860968); and tomato: SlMYB12 (EU419748).
Expression profiles of GtMYBP3 and GtMYBP4 transcripts in gentian
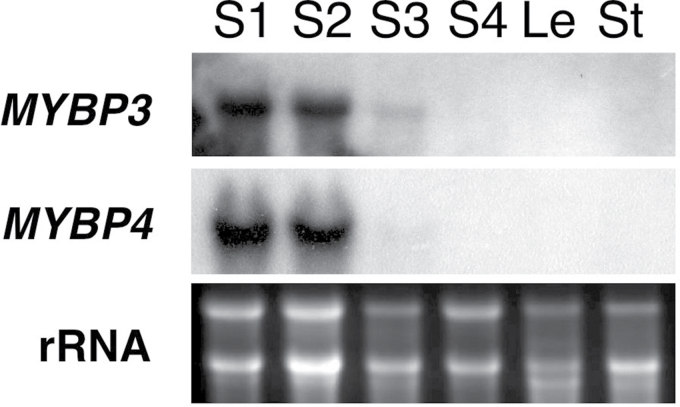
To reveal the temporal and spatial expression of Gt MYBP3 and Gt MYBP4 in gentian plants, northern blot analysis was performed using total RNA isolated from petals (at four developmental stages), leaves, and stems (Fig. 3). Gt MYBP3 and Gt MYBP4 transcripts were detected at the early flower development stages prior to pigmentation, but disappeared during later flower pigmented stages. Therefore, Gt MYBP3 and Gt MYBP4 would not be expected to regulate anthocyanin accumulation in gentian flowers. They could not be detected in vegetative tissues, such as leaves and stems. These expression profiles coincide with the accumulation profiles of flavones, which are abundantly present in young flower petals. The expression of Gt FNSII and GtF3′H genes, which are involved in flavone biosynthesis, were also detected at early flower developmental stages (Nakatsuka et al., 2005). Therefore, the temporal and spatial profiles of Gt MYBP3 and Gt MYBP4 transcripts correlated with those of flavone biosynthesis.
Fig. 3.
Accumulation profiles of GtMYBP3 and GtMYBP4 transcripts in gentian plants. Northern blot analysis was performed using total RNA isolated from petal samples at four different flower developmental stages (S1–S4) and from leaf (Le) and stem (St) samples. RNA was blotted onto nylon membrane and hybridized with DIG-labelled Gt MYBP3 or Gt MYBP4 probes. Ethidium bromide-stained rRNA bands are shown as loading controls.
Protein–protein interactions between GtMYBs and GtbHLH1
To investigate protein–protein interactions, this study employed a yeast two-hybrid system. A preliminary experiment showed that GAL4 DNA-binding domain-fused GtMYBs all led to false-positive results; therefore, the interaction among GtMYB proteins could not be revealed in this study. GtbHLH1, a transcription factor regulating the anthocyanin biosynthetic pathway, interacted with GtMYB3 (Supplementary Fig. S2). However, neither GtMYBP3 nor GtMYBP4 interacted with GtbHLH1 in this study (Supplementary Fig. S2). Therefore, it was assumed that the activities of GtMYBP3 and GtMYBP4 were independent of the bHLH co-activator, as well as ZmP1.
Activation ability of GtMYBP3 and GtMYBP4 on promoters of flavonoid-biosynthetic genes by transient expression assay
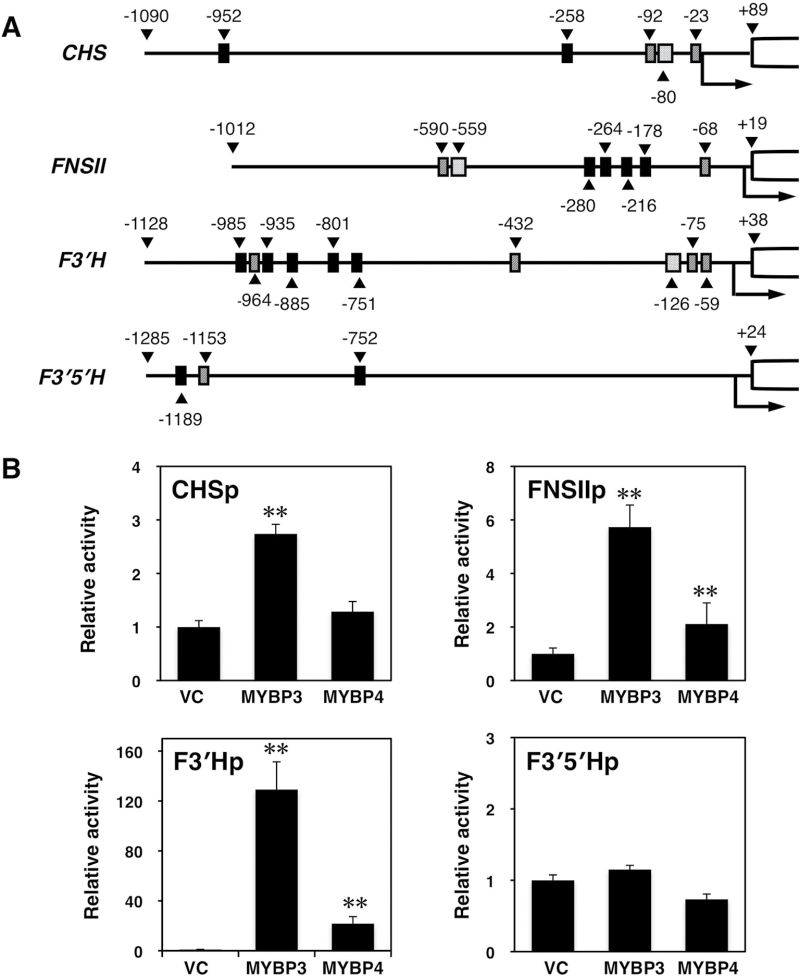
To gain an insight into the activation ability of GtMYBP3 and GtMYBP4 on flavonoid biosynthesis, a transient expression assay in protoplasts of cultured Arabidopsis T87 suspension cells was used. About 1kb of the 5′-upstream sequences of Gt CHS (Kobayashi et al., 1998), Gt F3′5′H (Nakatsuka et al., 2008b), Gt FNSII, and Gt F3′H were isolated from the gentian genome and connected to a reporter firefly luciferase (LUC) gene.
GtCHSpro, GtFNSIIpro, and GtF3′Hpro contained the binding sequences of vertebrate MYB protein (C/TAACT/GG, black box), a P-recognition element (CCT/AACC, diagonal box; Grotewold et al., 1994), and an ACGT-containing element (CACGT; Hartmann et al., 1998), as shown in Fig. 4A. By contrast, GtF3′5′Hpro contained the vertebrate MYB protein and P-recognition element, but not the ACGT-containing element (Nakatsuka et al., 2008b).
Fig. 4.
5′-flanking sequences of flavonoid biosynthetic genes from gentian and transient expression assay. (A) Structures of the promoters of Gt CHS, Gt FNSII, Gt F3′H, and GtF3′5′H. Filled, striped, and dotted boxes indicate MYB recognition elements, vertebrate MYB (Urao et al., 1993), P-recognition element (Grotewold et al. 1994), and an ACGT-containing element (ACE, Hartmann et al., 1998, 2005), respectively. The positions upstream of the transcription initiation site (arrow) are indicated as numerals above the filled triangles. The coding regions are indicated as open boxes. (B) Effect of GtMYBP3 and GtMYBP4 on promoter activities of four flavonoid biosynthetic genes. Transient expression assays were performed by transfecting the reporter and effector plasmid DNA into the protoplasts from Arabidopsis T87 cells. GtCHSpro-LUC, GtFNSIIpro-LUC, GtF3′Hpro-LUC, or GtF3′5′Hpro-LUC were used as reporters, and p35Spro-GtMYBP3, p35Spro-GtMYBP4, or pBI221 (negative control) were used as the effector. p35Spro-RLUC was also used as a transformation control. The promoter activation activities are indicated as relative values compared with that of the negative control. Asterisks indicate statistically significant differences between the means for negative control (pBI221) and tested genes, as judged by Student′s t-test (P < 0.01).
GtMYBP3 had about 2-, 5-, and 120-fold activation ability on Gt CHS, Gt FNSII and Gt F3′H promoters, respectively, compared with the vector control (Fig. 4B). Similarly, GtMYBP4 also enhanced Gt FNSII and Gt F3′H promoter activity, but its ability was weaker than GtMYBP3. Conversely, neither GtMYBP3 nor GtMYBP4 could induce Gt F3′5′H promoter activity. No synergistic effect of GtMYBP3 and GtMYBP4 was observed in the transient expression analysis (Supplementary Fig. S3).
GtMYBP3 and GtMYBP4 overexpression in transgenic Arabidopsis plants
To confirm whether the Gt MYBP3 and Gt MYBP4 genes can regulate early flavonoid biosynthesis in planta, this study produced transgenic Arabidopsis plants expressing Gt MYBP3 and Gt MYBP4 under the control of a constitutive CaMV35S promoter.
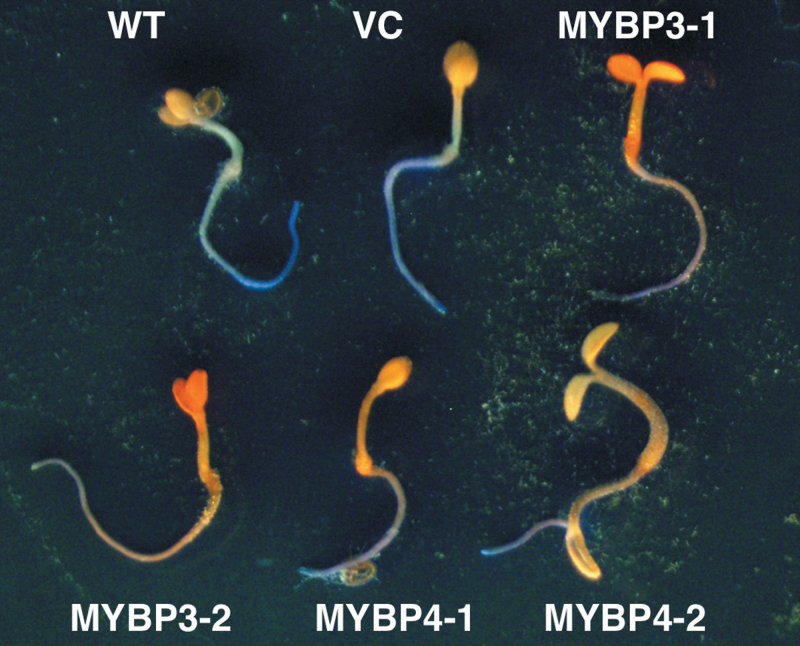
Both constructs were transformed into Arabidopsis plants using the floral dip method, and two independent T2 lines were produced, respectively. No morphological changes were observed in Gt MYBP3- and Gt MYBP4-expressing Arabidopsis plants compared with vector control plants (data not shown). Flavonoid accumulation of transgenic Arabidopsis seedlings was visualized with DPBA and imaged by epifluorescence microscopy (Fig. 5). In wild-type and vector control plants, cotyledons showed intense orange fluorescence implying abundant accumulation of flavonol derivatives. Their hypocotyls and roots showed yellow and blue fluorescence implying some flavonol accumulation. However, Gt MYBP3- and Gt MYBP4-expressing Arabidopsis showed intense orange fluorescence over the entire plant.
Fig. 5.
Visualized flavonoid accumulation in transgenic Arabidopsis seedlings. Flavonoid staining in wild type (WT), vector control (VC), and transgenic seedlings. Two independent transgenic lines are shown. Norflurazon-bleached seedlings were stained with diphenylboric acid 2-aminoethyl ester and photographed under UV light.
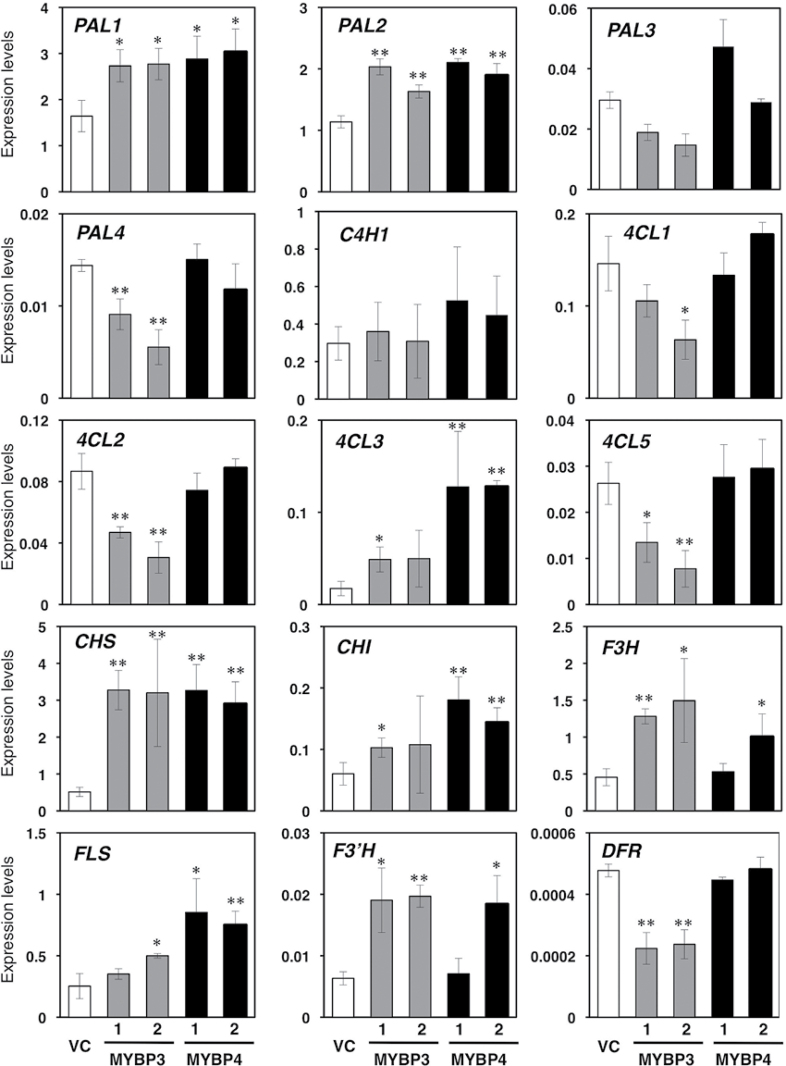
In both Gt MYBP3- and Gt MYBP4-expressing transgenic seedlings, the expression levels of each transgene in clone no. 1 was approximately 2-fold higher than in clone no. 2 (Supplementary Fig. S4A). Among genes involved in phenylpropanoid pathway, the expression levels of At PAL1 and At PAL2 were significantly enhanced in transgenic plants. At PAL1 and At PAL2 transcripts were upregulated by 1.4–1.8-fold in both Gt MYBP3- and GtMYBP4-expressing plants compared with the vector control (Fig. 6). Induction of Arabidopsis 4-coumarate:CoA ligase (At 4CL3) expression, which contributes to flavonoid biosynthesis, was observed during overexpression of Gt MYBP3 (2.8-fold) and Gt MYBP4 (7.3-fold). In GtMYBP3-expressing transgenic seedlings, the expression levels of several genes, including At PAL4, At 4CL2, and At 4CL5, were reduced in both lines.
Fig. 6.
Expression analysis of endogenous flavonoid biosynthetic genes in transgenic Arabidopsis. The effects of Gt MYBP3 and Gt MYBP4 overexpression on endogenous phenylpropanoid and flavonoid biosynthetic genes were investigated using qRT-PCR analyses in vector control (VC) and 5-day-old transgenic seedlings. Two independent transgenic lines shown in Fig. 5 were analysed. Asterisks indicate statistically significant differences between the means for vector control and transgenic lines, as judged by Student’s t-test (*, P < 0.05; **, P < 0.01).
With regard to flavonoid biosynthetic genes, the expression of At CHS, At CHI, At F3H and At F3′H were upregulated by 6.3-, 2.7-, 3.2-, and 3.0-fold, respectively, in Gt MYBP3-expressing Arabidopsis plants compared with the vector control. Conversely, the overexpression of GtMYBP4 induced the expression of At CHS, At CHI, and At FLS transcripts by 6.3-, 4.8-, and 2.9-fold. The At F3H and At F3′H genes were also induced in Gt MYBP4-expressing Arabidopsis line no. 2. The expression of At DFR, encoding an enzyme of a later step of the anthocyanin biosynthetic pathway, was suppressed in Gt MYBP3-expressing plants, whereas no difference was detected in Gt MYBP4-expressing plants. Expression of endogenous flavonol-specific R2R3-MYB genes, including At MYB12, At MYB11, and At MYB111, did not markedly change in Gt MYBP3 and Gt MYBP4-expressing seedlings (Supplementary Fig. S5). Thus, the overexpression of Gt MYBP3 and Gt MYBP4 in Arabidopsis enhanced or decreased the expression of several genes encoding enzymes of the phenylpropanoid and flavonoid biosynthetic pathways. However, there was a slight difference in the affected gene sets and intensities between Gt MYBP3 and Gt MYBP4.
GtMYBP3 and GtMYBP4 overexpression in transgenic tobacco plants
As shown above, transgenic Arabidopsis showed clear changes in flavonoid biosynthesis in vegetative tissues. However, flavonoid compositions, including anthocyanin pigments, were difficult to analyse in the floral tissues. Thus, to investigate the effect of Gt MYBP3 and Gt MYBP4 overexpression on floral flavonoid biosynthesis, this study produced transgenic tobacco plants. The petals of tobacco accumulate flavonol and anthocyanin derivatives (Nakatsuka et al., 2007). Tobacco leaf sections were transformed by A. tumefaciens harbouring the same binary vector construct used for Arabidopsis transformation. Over 20 independent transgenic lines were grown in a greenhouse, and the T1 seeds were collected after self-pollination. Two representative lines for each construct were chosen and subjected to further analysis.
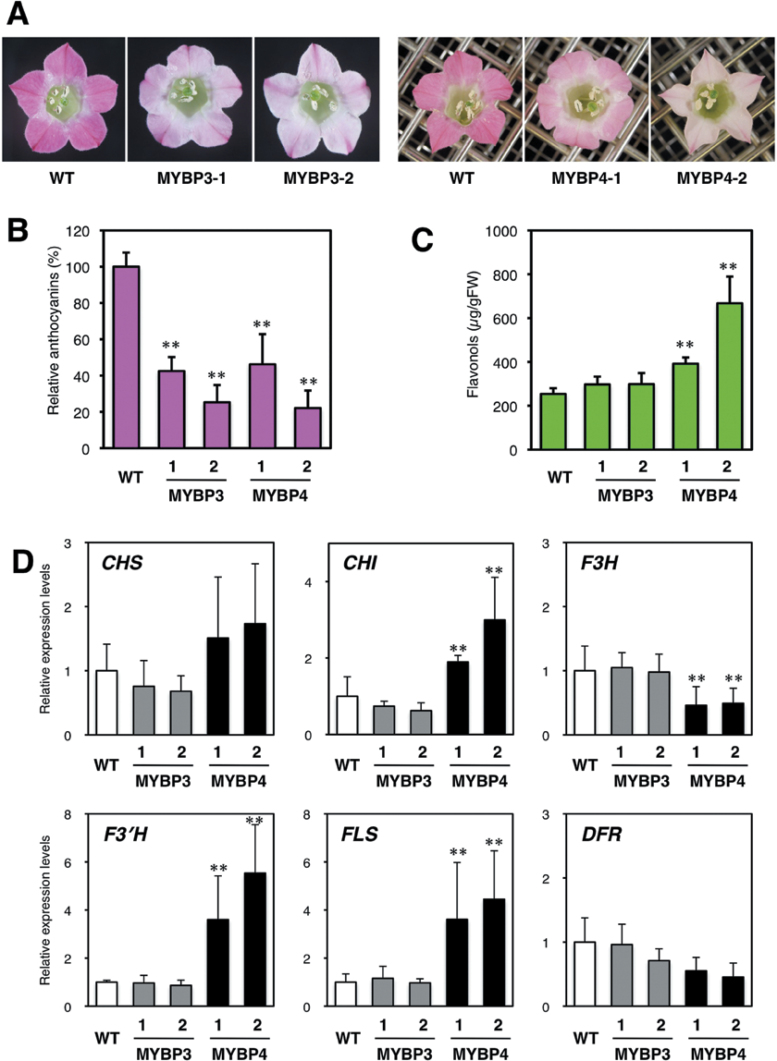
The pigmentation of the petals of Gt MYBP3- and Gt MYBP4-expressing tobacco plants was decreased compared with the wild type (Fig. 7A). Anthocyanin levels in Gt MYBP3-expressing transgenic tobacco petals were 22–46% lower compared with the wild type (Fig. 7B). However, the amounts of flavonol were the same between wild-type and Gt MYBP3-expressing plants (Fig. 7C). Anthocyanin levels in Gt MYBP4-expressing transgenic tobacco petals were 25–28% lower compared with wild type (Fig. 7B). In addition, the amounts of flavonols were increased by 1.5–2.6-fold in the petals of Gt MYBP4-expressing tobacco plants (Fig. 7C) and were inversely correlated with the accumulation levels of anthocyanins. No difference in flavonol and anthocyanin components was observed between any transgenic and wild-type petals by HPLC analysis (data not shown).
Fig. 7.
Phenotype and flavonoid analysis in transgenic tobacco flowers. (A) Typical flower phenotypes of wild-type (WT) and Gt MYBP3- and Gt MYBP4-expressing transgenic tobacco plants. Two independent transgenic lines per construct are shown. (B) Anthocyanin concentrations of WT and Gt MYBP3- and Gt MYBP4-expressing transgenic petals. (C) Flavonol concentrations of WT and Gt MYBP3- and Gt MYBP4-expressing transgenic petals. (D) Expression analyses of endogenous flavonoid biosynthetic genes in transgenic flowers. The effects of Gt MYBP3 and Gt MYBP4 overexpression on endogenous flavonoid biosynthetic genes were investigated using qRT-PCR analyses in WT and transgenic petals. Asterisks represent statistically significant differences between the means for wild-type and transgenic lines, as judged by Student’s t-test (*, P < 0.05; **, P < 0.01).
The expression of the transgenes were confirmed (Supplementary Fig. S4B). Expression of Nt CHS, Nt CHI, Nt F3′H, and Nt FLS was enhanced 2.1-, 3.5, 6.0-, and 5.1-fold in the petals of Gt MYBP4-expressing transgenic plants (Fig. 7D). The expression of the Nt F3H gene decreased in GtMYBP4-expressing plants. However, no change was detected in the Nt DFR gene, which is involved in late flavonoid biosynthesis. On the other hand, no significant change in expression of flavonoid biosynthetic genes was observed in Gt MYBP3-expressing tobacco plants (Fig. 7D).
Discussion
A previous study revealed that two transcription factors, GtMYB3 and GtbHLH1, regulate gentiodelphin biosynthesis in the petals of Japanese gentian (Nakatsuka et al., 2008b). The complex of GtMYB3 and GtbHLH1 proteins activated the expression of genes encoding enzymes involved in the anthocyanin biosynthetic pathway after the Gt F3H gene, but could not induce the transcripts of early flavonoid biosynthetic genes. Recently, some transcription factors controlling early flavonoid biosynthesis, especially flavonol accumulation, have been reported in several plant species, including Arabidopsis seedlings (Mehrtens et al., 2005; Stracke et al., 2007), grapes (Czemmel et al., 2009), and tomatoes (Ballester et al., 2010). However, no transcription factor controlling early flavonoid biosynthesis in the petals has been identified.
In this study, two R2R3-MYB transcription factors from the petals of Japanese gentian were identified. GtMYBP3 and GtMYBP4 were classified into P1/subgroup 7 (Fig. 2), which were reported to regulate early flavonoid biosynthesis (Dubos et al., 2010). Arabidopsis R2R3-MYB subgroup 7 contains AtMYB12/PFG2, AtMYB11/PFG1, and AtMYB111/PFG1, and these are known to control flavonol biosynthesis individually in the different organs (Mehrtens et al., 2005; Stracke et al., 2007; Dubos et al., 2010). The deduced amino acid sequence of GtMYBP3 is highly similar to GtMYBP4 in the R2R3-MYB DNA-binding domain (79.8%), suggesting that they might be functionally redundant genes (Fig. 2 and Supplementary Fig. S1). SG7 and SG7-2 motifs are conserved in the C-termini of R3R3-MYBs belonging to subgroup 7 in Arabidopsis and grapevine (Stracke et al., 2001; Czemmel et al., 2009; Dubos et al., 2010). However, the SG7 or SG7-2 motifs were not conserved in tomato SlMYB12, which has been identified as a flavonol regulator in tomato fruits (Ballester et al., 2010). The SG7 and SG7-2 motifs of GtMYBP3 and GtMYBP4 also had amino acid substitutions at some positions (Supplementary Fig. S1). Therefore, conservation of SG7 motifs in GtMYBP3 and GtMYBP4 might not be so important for regulation of early flavonoid biosynthesis in gentian petals.
Gt MYBP3 and Gt MYBP4 had similar temporal and spatial expression profiles, expressing strongly during early developmental stages (stages 1 and 2) of flower petals in gentians (Fig. 3). These expression profiles corresponded well with the accumulation profiles of FNSII and F3′H transcripts and flavone derivatives (Nakatsuka et al., 2005). In grapevine, Vv MYBF1 transcripts were detected during flowering and in skins of ripening berries and were correlated with the accumulation of flavonol and expression of Vv FLS1 (Czemmel et al., 2009).
Transient expression in Arabidopsis suspension cells showed that both GtMYBP3 and GtMYB4 could enhance the promoter activities of gentian Gt FNSII and Gt F3′H, which encode enzymes of the early flavonoid biosynthesis pathway (Fig. 4B). GtMYBP3 also enhanced the promoter activity of gentian Gt CHS. However, the promoter activity of Gt F3′5′H, encoding an enzyme of the late flavonoid biosynthesis pathway, could not be activated by either GtMYBP3 or GtMYBP4. Nakatsuka et al. (2008b) demonstrated that GtMYB3 and GtbHLH1, which are anthocyanin biosynthetic regulators in gentian flowers, induced promoter activity of Gt F3′5′H, but not of Gt CHS. These results suggested that GtMYBP3 and GtMYBP4 control the expression of early flavonoid biosynthetic genes, unlike GtMYB3 and GtbHLH1. For the promoters of all early flavonoid biosynthetic genes, the activation intensities of GtMYBP3 were higher than those of GtMYBP4 (Fig. 4B). The Gt FNSII promoter was also activated by GtMYBP3 or GtMYBP4 in a transient assay using gentian mesophyll protoplasts, whereas Gt CHS was activated by GtMYBP3 only (Supplementary Fig. S6), confirming the results of transient expression assays in Arabidopsis suspension cells (Fig. 5B). The reason for the different activation ability between GtMYBP3 and GtMYBP4 is not yet clearly understood, but it might depend on the different DNA-binding activities on each promoter and/or on interactions with other transcription factor(s) to regulate flavonoid biosynthesis. Two cis-elements, a P-recognition element and an ACGT-containing element, were present in close proximity to the transcription initiation site of the promoters of Gt CHS, Gt FNSII, and Gt F3′H, but not GtF3′5′H (Fig. 4A). The P-recognition element has been identified as a cis-binding site for the maize P1 protein, controlling 3-deoxyanthocyanin phlobaphene and C-glycosyl flavone maysin biosynthesis (Grotewold et al., 1994). Therefore, GtMYBP3 and GtMYBP4 might bind to the P-recognition element and activate the transcription of downstream genes. This is reasonable, because yeast two-hybrid analysis also showed that the GtMYBP3 or GtMYBP4 proteins did not interact with GtbHLH1 (Supplementary Fig. S2). Moreover, ACGT-containing element potentially binds to bZIP transcription factors, which work together with R2R3-MYB in light-inducible pigmentation (Hartmann et al., 2005). Further analysis is necessary to elucidate the functions of GtMYBP3 and GtMYBP4 in relation to other transcription factors, including bZIP.
Overexpression of Gt MYBP3 and Gt MYBP4 led to flavonol accumulation in transgenic Arabidopsis seedlings (Fig. 5). Overexpression of At MYB12 also resulted in increased flavonol amounts in Arabidopsis plants (Mehrtens et al., 2005). qRT-PCR analyses of endogenous phenylpropanoid and flavonol biosynthetic genes showed the activated endogenous gene sets differed somewhat between Gt MYBP3- and Gt MYBP4-expressing Arabidopsis (Fig. 6). The expression levels of three endogenous flavonol-specific transcription factor genes, At MYB12, At MYB11, and At MYB111, were not markedly affected in the transformants and there was no relationship between the expression levels of foreign genes and endogenous genes (Supplementary Figs. S4A and S5); therefore, an indirect effect via activation of endogenous transcription factors was excluded. GtMYBP3 activated the transcripts of At PAL1, At PAL2, At 4CL3, At CHS, At CHI, At F3H, and At F3′H, whereas GtMYBP4 enhanced At PAL1, At PAL2, At 4CL3, At CHS, At CHI, and At FLS transcripts in transgenic Arabidopsis (Fig. 6). Arabidopsis genome comprises four PAL genes (At PAL1 to At PAL4), among them At PAL1 and At PAL2 have a redundant role in flavonoid biosynthesis (Huang et al., 2010). 4CL also comprises a multigene family, At 4CL1, At 4CL2, At 4CL3, and At 4CL5, in Arabidopsis (Ehlting et al., 1999; Costa et al., 2005). It is notable that At 4CL3 is likely to participate in the biosynthetic pathway leading to flavonoids, whereas At 4CL1 and At 4CL2 are probably involved in lignin formation and in the production of additional phenolic compounds other than flavonoids (Ehlting et al., 1999). Therefore, the observed activation of At PAL1, At PAL2, and At 4CL3 transcripts in transgenic Arabidopsis plants would be reasonable in light of the functions of GtMYBP3 and GtMYBP4. GtMYBP4 induced stronger expression of almost all phenylpropanoid and flavonol biosynthetic genes, except for At F3H and At F3′H, than GtMYBP3 (Fig. 6). At F3′H was not activated by AtMYB12 or ZmP1 (Mehrtens et al., 2005). The differences in the controlled gene sets between GtMYBP3 and GtMYBP4 might reflect the differences in their C-terminal regions. ZmP1 activates the promoters of At CHS, At F3H, and At FLS, although At FLS induction was only 18% of that observed for AtMYB12 (Mehrtens et al., 2005). Thus, the intensity of transcriptional activation for individual flavonol biosynthetic genes seems to be different among P1 orthologues. The suppression of several biosynthetic genes in the Gt MYBP3-expressing transgenic Arabidopsis seedlings also suggested that other phenylpropanoid metabolism, such as lignins, organic acids, and proanthocyanidins, were regulated; therefore, detailed analyses are necessary in the future.
To further investigate the functions of GtMYBP3 and GtMYBP4 in flowers, they were expressed in tobacco plants, which are well known for flavonoid biosynthesis in their petals (Nishihara et al., 2005; Nakatsuka et al., 2007, 2008a). The flowers of Gt MYBP3- and Gt MYBP4-expressing transgenic tobacco plants showed decreased colour intensity (Fig. 7A). The phenotype of Gt MYBP4-expressing tobacco flowers arose from increased flavonol and reduced anthocyanin accumulation (Fig. 7B, C). qRT-PCR analysis also confirmed that Gt MYBP4 increased the expression levels of four endogenous tobacco genes, CHI, F3′H, and FLS, by 3–6-fold, but did not activate the expression of flavonoid biosynthesis genes, F3H and DFR (Fig. 7D). These results suggested the increased flavonol accumulation resulted from the activation of genes encoding enzymes catalysing the early steps in flavonoid biosynthesis, but not from the suppression of anthocyanin biosynthetic genes in transgenic tobacco plants. The inverse correlation between anthocyanin and flavonol levels in the flowers probably reflects competition between these two branches for flavonoid metabolites (Davies et al., 2003). The overexpression of At MYB12, a regulator of flavonol biosynthesis, induced the enhanced expression of Nt PAL, Nt CHS, Nt CHI, and Nt FLS and increased flavonol accumulation in tobacco petals (Luo et al., 2008). Therefore, heterologous expression of Gt MYBP4 could control the early flavonoid biosynthesis in tobacco plants. Conversely, Gt MYBP3-expressing tobacco flowers showed reduced anthocyanin accumulation, but showed no increase in flavonol accumulation (Fig. 7B, C). No significantly different expression levels of flavonol and anthocyanin biosynthetic genes were detected between wild-type and Gt MYBP3-expressing plants (Fig. 7D). Arabidopsis At MYB4 and strawberry Fa MYB1, classified into subgroup 4, suppress the expression of cinnamate 4-hydroxylase and anthocyanidin synthase, respectively (Jin et al., 2000; Aharoni et al., 2001). Therefore, GtMYBP3 might affect the expression of genes that were not investigated in this study, such as the phenylpropanoid biosynthetic and flavonoid modification genes, in tobacco petals. Proanthocyanidins (condensed tannins) compounds also derived from the flavonoid biosynthetic pathway, and two key enzymes, anthocyanidin reductase (ANR) and leucoanthocyanidin reductase (LAR), are involved in their biosynthesis (Tanner et al., 2003; Xie et al., 2003). Recently, Han et al. (2012) reported that ectopic expression of apple ANR in tobacco led to downregulation of both CHI and DFR in the flowers, leading to loss of anthocyanin. Similarly, in some cases, metabolic engineering of the flavonoid biosynthetic pathway has been known to induce feedback suppression of endogenous related genes. Therefore, the reduced anthocyanin accumulation in Gt MYBP3-expressing tobacco flowers might result from such unexpected regulation of genes encoding phenylpropanoid and flavonoid biosynthetic enzymes, although further metabolic and gene expression analyses are necessary to explain this observation. As shown above, the endogenous gene sets activated by GtMYBP3 and GtMYBP4 were different between transgenic Arabidopsis seedlings and tobacco flowers (Figs. 6 and 7). However, the targeted gene sets of GtMYBP3 and GtMYBP4 mostly overlapped in the early flavonoid biosynthetic genes; therefore, the functions of these two genes were thought to be complementary in gentian flowers. Moreover, the differences in the targeted gene sets among plant hosts (Arabidopsis and tobacco) suggested that full activation of GtMYBP3 and GtMYBP4 transcription factors probably required cofactors, such as bZIP (Hartmann et al., 2005).
Petunia flowers have been extensively studied as models for flavonoid biosynthesis and are proposed as a model showing that early- and late-biosynthetic genes are controlled by two different regulators (Quattrocchio et al., 1993). However, although it has been revealed that AN1/AN2 regulate the expression of anthocyanin biosynthetic genes, no transcription factor involved in the flavonol biosynthetic pathway has been identified. Uniquely, GhMYB1 was isolated from the petals of Gerbera hybrida, but its function has not been characterized (Elomaa et al., 2003). Therefore, Gt MYBP3 and Gt MYBP4 are the first characterized transcription factors that are involved in the early flavonoid biosynthesis in petal organs. Their identification will advance the understanding of flavonoid biosynthesis in floricultural crops.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Alignment of P1 orthologue proteins in higher plants.
Supplementary Fig. S2. Yeast two-hybrid analysis to examine the protein–protein interaction between GtMYBs and GtbHLH1.
Supplementary Fig. S3. Effect of co-transfection of GtMYBP3 and GtMYBP4 on promoter activities of Gt CHS, Gt FNSII, and GtF3′H.
Supplementary Fig. S4. Confirmation of the expression of transgenes in transgenic Arabidopsis and tobacco.
Supplementary Fig. S5. Expression analyses of endogenous flavonol-specific transcription factor genes in transgenic Arabidopsis.
Supplementary Fig. S6. Transient expression assay in gentian mesophyll protoplast.
Supplementary Table S1. Degenerate PCR, probe, and inverse PCR primers used in this study.
Supplementary Table S2. Primers used for quantitative RT-PCR analysis.
Acknowledgements
The authors thank Messrs. K. Fujiwara and T. Nakasato, Iwate Agricultural Research Center, for providing the Japanese gentian materials. They also thank Dr. M. Ohme-Takagi (National Institute of Advanced Industrial Technology and Science, Japan) for providing p35Spro-RLUC. The authors are grateful to Mses. A. Kubota and C. Yoshida, Iwate Biotechnology Research Center, for their technical support. This work was financially supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to T.N. (no. 18789005), M.N. (no. 24380024), and by the Iwate prefectural government.
References
- Aharoni A, De Vos CH, Wein M, Sun Z, Greco R, Kroon A, Mol JN, O’Connell AP. 2001. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco The Plant Journal 28 319–332 [DOI] [PubMed] [Google Scholar]
- Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B. 1992. A protocol for transient gene expression in Arabidopsis thaliana protoplast isolated from cell suspension cultures Plant Physiology and Biochemistry 30 123–128 [Google Scholar]
- Ballester AR, Molthoff J, de Vos R, et al. 2010. Biochemical and molecular analysis of pink tomatoes: deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color Plant Physiology 152 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. 2000. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis The Plant Cell 12 2383–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broun P. 2005. Transcriptional control of flavonoid biosynthesis: a complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis Current Opinion in Plant Biology 8 272–279 [DOI] [PubMed] [Google Scholar]
- Byrne PF, McMullen MD, Snook ME, Musket TA, Theuri JM, Widstrom NW, Wiseman BR, Coe EH. 1996. Quantitative trait loci and metabolic pathways: genetic control of the concentration of maysin, a corn earworm resistance factor, in maize silks Proceedings of the National Academy of Sciences, USA 93 8820–8825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana The Plant Journal 16 735–743 [DOI] [PubMed] [Google Scholar]
- Cocciolone SM, Nettleton D, Snook ME, Peterson T. 2005. Transformation of maize with the p1 transcription factor directs production of silk maysin, a corn earworm resistance factor, in concordance with a hierarchy of floral organ pigmentation Plant Biotechnology Journal 3 225–235 [DOI] [PubMed] [Google Scholar]
- Costa MA, Bedgar DL, Moinuddin SG, et al. 2005. Characterization in vitro and in vivo of the putative multigene 4-coumarate:CoA ligase network in Arabidopsis: syringyl lignin and sinapate/sinapyl alcohol derivative formation Phytochemistry 66 2072–2091 [DOI] [PubMed] [Google Scholar]
- Czemmel S, Stracke R, Weisshaar B, Cordon N, Harris NN, Walker AR, Robinson SP, Bogs J. 2009. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries Plant Physiology 151 1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KM, Schwinn KE, Deroles SC, Manson DG, Lewis DH, Bloor SJ, Bradley JM. 2003. Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase Euphytica 131 259–268 [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis Trends in Plant Science 15 573–581 [DOI] [PubMed] [Google Scholar]
- Ehlting J, Buttner D, Wang Q, Douglas CJ, Somssich IE, Kombrink E. 1999. Three 4-coumarate:coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms The Plant Journal 19 9–20 [DOI] [PubMed] [Google Scholar]
- Elomaa P, Uimari A, Mehto M, Albert VA, Laitinen RA, Teeri TH. 2003. Activation of anthocyanin biosynthesis in Gerbera hybrida (Asteraceae) suggests conserved protein–protein and protein–promoter interactions between the anciently diverged monocots and eudicots Plant Physiology 133 1831–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. 2008. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings The Plant Journal 53 814–827 [DOI] [PubMed] [Google Scholar]
- Goto T, Kondo T, Tamura H, Imagawa H, Iino A, Takeda K. 1982. Structure of gentiodelphin, an acylated anthocyanin isolated from Gentiana makinori that is stable in dilute aqueous solution Tetrahedron Letters 23 3695–3698 [Google Scholar]
- Grotewold E, Drummond BJ, Bowen B, Peterson T. 1994. The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset Cell 76 543–553 [DOI] [PubMed] [Google Scholar]
- Grotewold E, Sainz MB, Tagliani L, Hernandez JM, Bowen B, Chandler VL. 2000. Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R Proceedings of the National Academy of Sciences, USA 97 13579–13584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Vimolmangkang S, Soria-Guerra RE, Korban SS. 2012. Introduction of apple ANR genes into tobacco inhibits expression of both CHI and DFR genes in flowers, leading to loss of anthocyanin Journal of Experimental Botany 63 2437–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann U, Sagasser M, Mehrtens F, Stracke R, Weisshaar B. 2005. Differential combinatorial interactions of cis-acting elements recognized by R2R3-MYB, BZIP, and BHLH factors control light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes Plant Molecular Biology 57 155–171 [DOI] [PubMed] [Google Scholar]
- Hartmann U, Valentine WJ, Christie JM, Hays J, Jenkins GI, Weisshaar B. 1998. Identification of UV/blue light-response elements in the Arabidopsis thaliana chalcone synthase promoter using a homologous protoplast transient expression system Plant Molecular Biology 36 741–754 [DOI] [PubMed] [Google Scholar]
- Holton TA, Cornish EC. 1995. Genetics and biochemistry of anthocyanin biosynthesis The Plant Cell 7 1071–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eicholtz D, Rogers SG, Fraley RT. 1985. A simple method for transferring genes into plants Science 227 1229–1231 [DOI] [PubMed] [Google Scholar]
- Huang J, Gu M, Lai Z, Fan B, Shi K, Zhou YH, Yu JQ, Chen Z. 2010. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress Plant Physiology 153 1526–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C. 2000. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis The EMBO Journal 19 6150–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Oikawa Y, Koiwa H, Yamamura S. 1998. Flower-specific expression directed by the promoter of a chalcone synthase gene from Gentiana triflora in Petunia hybrida Plant Science 131 173–180 [Google Scholar]
- Koes R, Verweij W, Quattrocchio F. 2005. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways Trends in Plant Science 10 236–242 [DOI] [PubMed] [Google Scholar]
- Lloyd AM, Walbot V, Davis RW. 1992. Arabidopsis and Nicotiana anthocyanin production activated by maize regulators R and C1 Science 258 1773–1775 [DOI] [PubMed] [Google Scholar]
- Luo J, Butelli E, Hill L, Parr A, Niggeweg R, Bailey P, Weisshaar B, Martin C. 2008. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: expression in fruit results in very high levels of both types of polyphenol The Plant Journal 56 316–326 [DOI] [PubMed] [Google Scholar]
- Mehrtens F, Kranz H, Bednarek P, Weisshaar B. 2005. The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis Plant Physiology 138 1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishiba K, Yamasaki S, Nakatsuka T, Abe Y, Daimon H, Oda M, Nishihara M. 2010. Strict de novo methylation of the 35S enhancer sequence in gentian PLoS One 5–e9670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol J, Grotewold E, Koes R. 1998. How genes paint flowers and seeds Trends in Plant Science 3 212–217 [Google Scholar]
- Nakatsuka T, Abe Y, Kakizaki Y, Yamamura S, Nishihara M. 2007. Production of red-flowered plants by genetic engineering of multiple flavonoid biosynthetic genes Plant Cell Reports 26 1951–1959 [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Haruta KS, Pitaksutheepong C, Abe Y, Kakizaki Y, Yamamoto K, Shimada N, Yamamura S, Nishihara M. 2008b. Identification and characterization of R2R3-MYB and bHLH transcription factors regulating anthocyanin biosynthesis in gentian flowers Plant and Cell Physiology 49 1818–1829 [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Nishihara M, Mishiba K, Yamamura S. 2005. Temporal expression of flavonoid biosynthesis-related genes regulates flower pigmentation in gentian plants Plant Science 168 1309–1318 [Google Scholar]
- Nakatsuka T, Sato K, Takahashi H, Yamamura S, Nishihara M. 2008a. Cloning and characterization of the UDP-glucose:anthocyanin 5-O-glucosyltransferase gene from blue-flowered gentian Journal of Experimental Botany 59 1241–1252 [DOI] [PubMed] [Google Scholar]
- Nishihara M, Nakatsuka T, Mizutani-Fukuchi M, Tanaka Y, Yamamura S. 2008. Gentians: from gene cloning to molecular breeding. In: Jaime A., da Silva T., editors, Floricultural and ornamental biotechnology V. UK: Global Science Books; 57–67 [Google Scholar]
- Nishihara M, Nakatsuka T, Yamamura S. 2005. Flavonoid components and flower color change in transgenic tobacco plants by suppression of chalcone isomerase gene FEBS Letters 579 6074–6078 [DOI] [PubMed] [Google Scholar]
- Quattrocchio F, Baudry A, Lepiniec L, Grotewold E. 2006. The regulation of flavonoid biosynthesis. In: Grotewold E., editor, The science of flavonoids New York: Springer; 97–122 [Google Scholar]
- Quattrocchio F, Wing JF, Leppen H, Mol J, Koes RE. 1993. Regulatory genes controlling anthocyanin pigmentation are functionally conserved among plant species and have distinct sets of target genes The Plant Cell 5 1497–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowicz PD, Braun EL, Wolfe AD, Bowen B, Grotewold E. 1999. Maize R2R3 Myb genes: sequence analysis reveals amplification in the higher plants Genetics 153 427–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelt C, Quattrocchio F, Mol JN, Koes R. 2000. Anthocyanin1 of petunia encodes a basic helix-loop-helix protein that directly activates transcription of structural anthocyanin genes The Plant Cell 12 1619–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B. 2007. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling The Plant Journal 50 660–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B. 2001. The R2R3-MYB gene family in Arabidopsis thaliana Current Opinion in Plant Biology 4 447–456 [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24 1596–1599 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Yonekura K, Fukuchi-Mizutani M, Fukui Y, Fujiwara H, Ashikari T, Kusumi T. 1996. Molecular and biochemical characterization of three anthocyanin synthetic enzymes from Gentiana triflora Plant and Cell Physiology 37 711–716 [DOI] [PubMed] [Google Scholar]
- Tanner GJ, Francki KT, Abrahams S, Watson JM, Larkin PJ, Ashton AR. 2003. Proanthocyanidin biosynthesis in plants. Purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA Journal of Biological Chemistry 278 31647–31656 [DOI] [PubMed] [Google Scholar]
- Urao T, Yamaguchi-Shinozaki K, Urao S, Shinozaki K. 1993. An Arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence The Plant Cell 5 1529–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel BSJ. 2006. The biosynthesis of flavonoid. In: Grotewold E., editor, The science of flavonoids New York: Springer; 71–96 [Google Scholar]
- Xie D-Y, Sharma SB, Paiva NL, Ferreira D, Dixon RA. 2003. Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis Science 299 396–399 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Mori M, Kondo T. 2009. Blue flower color development by anthocyanins: from chemical structure to cell physiology Natural Product Reports 26 884–915 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Toyama Y, Kameda K, Kondo T. 2000. Contribution of each caffeoyl residue of the pigment molecule of gentiodelphin to blue color development Phytochemistry 54 85–92 [DOI] [PubMed] [Google Scholar]
- Zhang P, Wang Y, Zhang J, Maddock S, Snook M, Peterson T. 2003. A maize QTL for silk maysin levels contains duplicated Myb-homologous genes which jointly regulate flavone biosynthesis Plant Molecular Biology 52 1–15 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.