Abstract
Orthophosphate (Pi) is an essential but limiting macronutrient for plant growth. Extensive soil P reserves exist in the form of organic P (Po), which is unavailable for root uptake until hydrolysed by secretory acid phosphatases (APases). The predominant purple APase (PAP) isozymes secreted by roots of Pi-deficient (–Pi) Arabidopsis thaliana were recently identified as AtPAP12 (At2g27190) and AtPAP26 (At5g34850). The present study demonstrated that exogenous Po compounds such as glycerol-3-phosphate or herring sperm DNA: (i) effectively substituted for Pi in supporting the P nutrition of Arabidopsis seedlings, and (ii) caused upregulation and secretion of AtPAP12 and AtPAP26 into the growth medium. When cultivated under –Pi conditions or supplied with Po as its sole source of P nutrition, an atpap26/atpap12 T-DNA double insertion mutant exhibited impaired growth coupled with >60 and >30% decreases in root secretory APase activity and rosette total Pi concentration, respectively. Development of the atpap12/atpap26 mutant was unaffected during growth on Pi-replete medium but was completely arrested when 7-day-old Pi-sufficient seedlings were transplanted into a –Pi, Po-containing soil mix. Both PAPs were also strongly upregulated on root surfaces and in shoot cell-wall extracts of –Pi seedlings. It is hypothesized that secreted AtPAP12 and AtPAP26 facilitate the acclimation of Arabidopsis to nutritional Pi deficiency by: (i) functioning in the rhizosphere to scavenge Pi from the soil’s accessible Po pool, while (ii) recycling Pi from endogenous phosphomonoesters that have been leaked into cell walls from the cytoplasm. Thus, AtPAP12 and AtPAP26 are promising targets for improving crop P-use efficiency.
Key words: Arabidopsis thaliana, cell walls, extracellular phosphate scavenging, functional genomics, phosphate nutrition, purple acid phosphatase, secreted hydrolases
Introduction
Acid phosphatases (APases, EC 3.1.3.2) catalyse the hydrolysis of orthophosphate (Pi) from a broad range of phosphomonoesters and anhydrides with an acidic pH optimum. They function in the production, transport, and recycling of Pi, a critical macronutrient for cellular metabolism and bioenergetics. The induction of extra- and intracellular APases is a ubiquitous plant response to nutritional Pi deprivation, a common abiotic stress that frequently limits plant growth in natural ecosystems (Plaxton and Tran, 2011). Extracellular APases belong to a group of Pi-starvation-inducible (PSI) phosphohydrolases secreted by roots of Pi-deficient (–Pi) plants to hydrolyse Pi from external phosphomonoesters and phosphodiesters derived from decomposing biomaterial, referred to as organic P (Po). For example, the combined activities of secreted nucleases, phosphodiesterases, and APases allows –Pi plants to efficiently scavenge extracellular nucleic acids as their sole source of P nutrition (Abel et al., 2000; Chen et al., 2000). Po generally accounts for around 50% of the soil’s total P content (Richardson et al., 2009). Owing to microbial activity, extended periods of P-fertilizer application increase the proportion of applied P that accumulates in agricultural soils as labile Po (George et al., 2007). Given the abundance of Po in most soils and its steady accumulation under various Pi fertilizer regimes, soil Po makes an important contribution to plant P nutrition and overall efficiency of crop Pi uptake from applied fertilizers (Richardson et al., 2009, 2011).
Soils have demonstrable APase activity, and substantial increases in APase activity have been documented in the rhizosphere of –Pi plants, with several studies showing this to be associated with soil Po depletion (Tarafdar and Claassen, 1988; Miller et al., 2001; Nuruzzaman et al., 2006; Richardson et al., 2009). However, which soil Po pools are accessible to roots remains unclear, and most plants have a limited capacity to obtain Pi from phytate (myo-inositol hexaphosphate), an abundant Po component of certain soils (Richardson, 2009; Richardson et al., 2009, 2011). Hydrolysis of extracellular Po substrates to release Pi is essential, because Pi anions (primarily H2PO4 − or HPO4 2−) are translocated across the root plasmalemma by low- or PSI high-affinity Pi transporters (Plaxton and Tran, 2011). There is no evidence to support direct import of Po substrates into plant cells, although Po uptake followed by hydrolysis within the apoplast may occur (Richardson et al., 2009, 2011). PSI APases are also secreted into cell walls where they may contribute to Pi recapture from phosphomonoesters leaked by the –Pi cells (Bieleski and Johnson, 1972; Lefebvre et al., 1990; Barrett-Lennard et al., 1993; Zhang and McManus, 2000; Wasaki et al., 2008; Tran et al., 2010b ). Similarly, PSI vacuolar APases appear to be involved in Pi scavenging and remobilization from expendable intracellular phosphomonoesters and anhydrides (Veljanovski et al., 2006; Hurley et al., 2010; Tran et al., 2010b ). This is accompanied by a marked reduction in levels of cytoplasmic Po metabolites during long-term Pi deprivation (Plaxton and Tran, 2011).
Purple acid phosphatases (PAPs), the most important class of plant PSI APases, are characterized by their distinctive purple or pink colour in solution (due to a bimetallic active centre; Tran et al., 2010b ). Genome annotation identified 29 PAP genes in the model plant Arabidopsis thaliana, several of which are transcriptionally induced during Pi deprivation (del Pozo et al., 1999; Haran et al., 2000; Li et al., 2002; Tran et al., 2010a , b; Wang et al., 2011). These and subsequent studies have demonstrated the complexity and variation of AtPAP1–29 expression and regulation. The principal PAP isozymes that contribute to extracellular Pi scavenging by –Pi Arabidopsis were evaluated recently using a combination of biochemical and genomic approaches. The results established that AtPAP12 and AtPAP26 are the major root- and suspension cell culture secretory APases upregulated by –Pi Arabidopsis (Tran et al., 2010a ). AtPAP26 is also the predominant vacuolar APase that functions to recycle intracellular Pi during Pi stress, as well as to remobilize Pi from the Po pool of senescing leaves (Veljanovski et al., 2006; Hurley et al., 2010; Robinson et al., 2012). The overlapping but non-identical substrate selectivities and pH-activity profiles, and the high specific APase activities of secreted AtPAP12 and AtPAP26 (Tran et al., 2010a ) support the hypothesis that their combined activities help –Pi Arabidopsis to scavenge efficiently Pi from a wide range of extracellular phosphomonoesters over a broad pH range. Analysis of atpap12 and atpap26 T-DNA insertional mutants has indicated that AtPAP12 and AtPAP26 account for the majority of APase activity secreted by the roots of –Pi Arabidopsis (Tran et al., 2010a ). Furthermore, AtPAP10 was shown recently to be associated predominantly with the root surface and to be induced by Pi limitation to help Arabidopsis acclimatize to Pi deprivation (Wang et al., 2011). In the present study, analysis of a double atpap12/atpap26 loss-of-function mutant established conclusively that AtPAP12 and AtPAP26 are secreted by –Pi Arabidopsis to scavenge Pi from exogenous Po. The results also revealed that AtPAP12 and AtPAP26 are important contributors to the PSI APase activity of the root surface as well as the cell walls of –Pi Arabidopsis shoots.
Materials and methods
Plant material and growth conditions
For mutant isolation and routine plant growth, Arabidopsis (Col-0 ecotype) seeds were sown in a standard soil mixture (Sunshine Aggregate Plus Mix 1; SunGro) and stratified at 4 °C for 3 d. Plants were cultivated in growth chambers at 23 °C (16/8h photoperiod at 100 µmol m– s–1 photosynthetically active radiation) and fertilized twice weekly by subirrigation with 0.25× Hoagland’s medium (pH 6.0). To assess the influence of Pi deprivation on soil-grown plants, seedlings were established for 7 d in a 24-well microtitre plate (one seedling per well) containing 0.5ml per well of 0.5× Murashige and Skoog (MS) medium supplemented with 1% (w/v) sucrose and 0.2mM Pi, and then transplanted into a 75–85% sphagnum peat moss/perlite soil mix lacking all nutrients (Sunshine Mix 2; SunGro). Plants were cultivated in growth chambers as described above for an additional 14 d and fertilized twice weekly with 0.25× Hoagland’s medium containing either 0 or 2mM KH2PO4. Whenever Pi was eliminated, it was replaced by 2mM KH2SO4 and 0.5mM MES.
For liquid cultures, 5mg of seeds were surface sterilized, stratified, and placed in 250ml Magenta boxes containing 50ml of 0.5× MS medium (pH 5.7) with 1% (w/v) sucrose and 0.2mM KH2PO4, and placed on an orbital shaker (80 r.p.m.) at 24 °C under continuous illumination (100 µmol m– s–1). After 7 d, the medium was replaced with fresh medium containing filter-sterilized 0 or 1.5mM KH2PO4, or 1.5mM dl-glycerol-3-phosphate (G3P; Sigma Chemical Co.), or 0.6mg ml–1 of DNA. The DNA (crude oligonucleotides from herring sperm; Sigma Chemical Co.) was purified by repeated extractions with phenol/chloroform followed by gel permeation chromatography on a Sephadex G-25 column as described previously (Chen et al., 2000). It was assumed that 0.6mg ml–1 DNA equated to ~2mM total P (Chen et al., 2000). All Po stocks contained negligible free Pi. The 14-d-old seedlings were blotted dry, snap frozen in liquid N2, and stored at –80 °C, whereas growth medium was filtered through 0.45 µm membranes and concentrated over 250-fold using Amicon Ultra-15 centrifugal filter units (30kDa cut-off). For growth on agar-solidified nutrient medium, stratified seeds were placed on horizontal or vertically oriented 1% (w/v) agar (Micropropagation Type I Agar; Caisson Laboratories) plates containing 0.5× MS medium and 1% (w/v) sucrose supplemented with 50 µM or 1.5mM KH2PO4, 0.6mg ml–1 of DNA, 1.5mM G3P, or 1.5mM glucose-6-phosphate (Glc-6-P) and cultivated at 24 °C under continuous illumination (100 µmol m–2 s–1) for 14–21 d.
Extraction of shoot cell-wall proteins
Shoots (2.5g) of 14 d-old seedlings cultivated in Pi-sufficient (+Pi) or –Pi liquid medium as described above were powdered under liquid N2 and homogenized (1:15; w/v) using a mortar and pestle in ice-cold buffer [25mM TES/KOH (pH 7.4) containing 10mM MgCl2, 1mM EDTA, 1mM dithiothreitol, 1% (v/v) Triton X-100, and 1% (w/v) polyvinylpolypyrrolidone]. The mixture was clarified by centrifugation at 20 000g at 4 °C for 20min and the supernatant collected as the soluble cytoplasmic extract. The pellet underwent three more washes by resuspending with homogenizing buffer and recentrifugation as above. The insoluble fraction was extracted with 5ml of 0.2M CaCl2 in 5mM acetate/NaOH (pH 4.6) and centrifuged at 23 700g for 15min. The supernatant was collected as the cell-wall extract (Barrett-Lennard et al., 1993). The pellet was re-extracted with the same buffer and recentrifuged as above. The supernatant was combined with the first cell-wall extract to yield a final volume of ~10ml. Cytoplasmic and cell-wall extracts were filtered through Miracloth and dialysed overnight against 500ml of 40mM Tricine/KOH (pH 7.4) containing 10mM MgCl2, 1mM EDTA, 1mM dithiothreitol, and 1% (v/v) Triton X-100. Both samples were concentrated ~40-fold as described above to a protein concentration of at least 2mg ml–1.
APase activity determination
APase activity was routinely measured by coupling the hydrolysis of phosphoenolpyruvate (PEP) to pyruvate to the lactate dehydrogenase reaction at 24 °C and continuously monitoring NADH oxidation at 340nm using a Molecular Devices Spectromax Plus Microplate spectrophotometer. Optimized assay conditions were: 50mM sodium acetate (pH 5.6), 5mM PEP, 10mM MgCl2, 0.2mM NADH, and 3U of rabbit muscle lactate dehydrogenase in a final volume of 0.2ml. Assays were corrected for any background NADH oxidation by omitting PEP from the reaction mixture. APase assays were also carried out in an assay mix containing 50mM sodium acetate (pH 5.6), 5mM para-nitrophenol phosphate (pNPP), and 10mM MgCl2 by monitoring the formation of para-nitrophenol at 405nm (ɛ=18.2mM–1 cm–1). All APase assays were linear with respect to time and concentration of enzyme assayed. One unit of activity was defined as the amount of enzyme resulting in the hydrolysis of 1 µmol of substrate min–1 at 24 °C.
Protein electrophoresis and immunoblotting
SDS-PAGE, immunoblotting onto polyvinylidene difluoride membranes and chromogenic detection of antigenic polypeptides using an alkaline phosphatase-tagged secondary antibody were conducted as described previously (Hurley et al., 2010; Tran et al., 2010a ). All immunoblot results were replicated a minimum of three times, with representative results shown in the various figures.
Determination of protein, total and free Pi, and anthocyanin concentrations
Protein concentrations were determined using a modified Bradford assay (Bozzo et al., 2002) with bovine γ-globulin as the standard. Total Pi, free Pi, and anthocyanin determinations were carried out as described previously (Hurley et al., 2010).
RNA isolation and semi-quantitative RT-PCR
Total RNA was extracted and purified as described previously (Gregory et al., 2009). RNA samples were assessed for purity via their A 260/A 280 ratio and integrity by resolving 1 µg of total RNA on a 1.2% (w/v) denaturing agarose gel. RNA (5 µg) was reverse transcribed with Superscript III (Invitrogen), and non-competitive RT-PCR was performed with appropriate primers as previously described (Gregory et al., 2009; Hurley et al., 2010; Tran et al., 2010a ); all PCR products were sequenced for verification. Conditions were optimized for all RT-PCRs to ensure linearity of response for comparison between samples.
β-Glucuronidase (GUS) analysis
The AtPAP12 and AtPAP26 promoters (2010 and 3853bp sequences upstream of the start codon of the AtPAP12 or AtPAP26 genes, respectively) were amplified from genomic DNA using the following primer pairs: AtPAP12:GUS 12ProFull-InfF: 5’-TGATTACGCCAAGC TTTTTCTTCTCCGGTGAAACC-3’ and 12ProFull-InfR: 5’-CCGGG GATCCTCTAGACTTCAAGATTAGTTTCTCTGAATCC-3’; and AtPA P26:GUS 26ProFull-InfF: 5’-TGATTACGCCAAGCTTATTTGTAATG TCATCACCTCGG-3’ and 26ProFull-InfR: 5’-CCGGGGATCCTCTAG ACACGTCACCAAATCTCGA-3’. The amplified promoter region of AtPAP12 or AtPAP26 was mixed at a 3:1 molar ratio with pBI101-N1 linearized by HindIII and XbaI, incubated with In-Fusion reaction mix, and transformed according to the manufacturer’s protocol (Clontech) to yield AtPAP12:GUS or AtPAP26:GUS. Each construct was transferred into Agrobacterium tumefaciens strain LBA4404 and transformed into Arabidopsis plants via the floral dip method (Clough and Bent, 1998). Transformed plants were selected on 0.8% (w/v) agar plates containing 0.5× MS medium, 1% (w/v) sucrose, and 30 µg ml–1 of kanamycin, and transferred to soil for self-pollination and propagation. For analysis of mature plants, seeds were planted in soil and grown for 28 d while being fertilized twice weekly with 0.25× Hoagland’s medium containing 2mM Pi.
Histochemical staining of GUS activity was performed as described previously (Jefferson et al., 1987). Tissues were incubated at 37 °C overnight in GUS staining buffer [100mM NaPi (pH 7.0), containing 1mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronide, 0.5mM EDTA, 0.1% (v/v) Triton X-100, 2mM K3Fe(CN)6, and 2mM K4Fe (CN)6]. Cyanide was omitted for AtPAP12:GUS staining. The stained tissues were cleared with 70% ethanol prior to imaging using a dissecting microscope.
Isolation and backcross of the atpap12/atpap26 double-knockout mutant
Homozygous atpap26 and atpap12 T-DNA insertion mutants (Salk_152821 and SAIL_1187_A05, respectively) were obtained as reported previously (Hurley et al., 2010; Tran et al., 2010a ). Mutant plants had been isolated by PCR-screening using T-DNA left-border and gene-specific primers (Supplementary Fig. S1 at JXB online). All PCR products were sequenced for verification (Centre for Applied Genomics, The Hospital for Sick Children, Toronto, ON, Canada). To generate atpap26/atpap12 double mutants, the atpap26 mutant (pollen donor) was crossed into the atpap12 mutant (pollen receptors). Seeds obtained from these crosses were germinated and grown to obtain F1 seeds. The presence of T-DNA insertions in both AtPAP12 and AtPAP26 in the respective F1 plants was verified by PCR-screening. F1 plants were self-pollinated and individual F2 plants were screened on BASTA-containing MS medium. From the BASTA-resistant (for the atpap12 allele) plants, genomic DNA was extracted and PCR-screened for homozygous double mutants. Of 20 individual F2 plants screened by PCR, three plants were homozygous for both atpap26 and atpap12. To generate backcross lines to restore either AtPAP12 or AtPAP26 expression, the atpap26/atpap12 mutant was crossed with atpap12 and atpap26 mutants. F1 plants were self-pollinated and leaf extracts of F2 plants screened using anti-AtPAP12 immunoblot analysis for restoration of AtPAP12 or AtPAP26 expression.
Root-surface APase activity staining
This was conducted using enzyme-labelled fluorescent (ELF)-97 phosphate (Invitrogen) with hydroponically cultivated 14-d-old seedlings. Individual seedlings were rinsed with 75mM sodium acetate (pH 5.6) and incubated at 23 °C for 1h with 1ml of this buffer containing 25 µM ELF-97 phosphate. As a negative control, replicate seedlings were incubated in acetate buffer alone. Roots were washed three times with acetate buffer containing 25mM EDTA for 15min. ELF-97, the fluorescent product of APase activity, was imaged using a Zeiss 710 confocal laser scanning microscope equipped with a Zeiss 63× plan apochromat oil-immersion objective and 340 and 450nm for excitation and emission, respectively. Image processing was carried out using Adobe Photoshop CS (Adobe Systems Inc.). Root-surface APase activity staining was also conducted using β-naphthyl phosphate and 5-bromo-4-chloro-3-indolyl phosphate (BCIP), as previously described (Gilbert et al., 1999; Wang et al., 2011). All root-surface APase activity staining images are representative results obtained from experiments that were replicated at least three times.
Statistics
All values are presented as means ±standard error (SE). Data were analysed using a one-tailed Student’s t-test, and deemed significant for P <0.05.
Results and discussion
Influence of different P supplements on growth, total Pi concentration, secretory APase activity, and secreted AtPAP12 and AtPAP26 polypeptides of wild-type Arabidopsis seedlings
The ability of exogenous Pi, G3P, or purified herring sperm DNA to support growth and P nutrition of wild-type (Col-0) Arabidopsis seedlings was compared. G3P and nucleic acids are common soil Po components (Tarafdar and Claassen, 1988; Ticconi and Abel, 2004; Richardson et al., 2009), whereas G3P is an effective in vitro substrate for native AtPAP12 and AtPAP26 purified from the secretome of –Pi Arabidopsis suspension cells (Tran et al., 2010a ). Seedling dry weight biomass and total Pi concentration of rosette leaves of 14-d-old Col-0 seedlings cultivated over the previous 7 d in liquid medium containing 1.5mM Pi and 1.5mM G3P (–Pi/+G3P) or 0.6mg ml–1 DNA (–Pi/+DNA) (equivalent to ~2mM total Pi) were generally comparable, whereas biomass and total shoot Pi concentration of –Pi seedlings were both reduced by ~50% (Fig. 1A, B). These results agree with previous studies showing that plants cultivated in sterile culture were able to use exogenous Po substrates, such as G3P, glucose-1-phosphate, ATP, or nucleic acids as equivalent sources to Pi for growth (Ticconi and Abel, 2004; Richardson et al., 2009; Liang et al., 2010; Richardson et al., 2011). Our results also corroborate previous studies demonstrating that Arabidopsis seedlings and tomato cell cultures efficiently scavenge Pi from exogenous nucleic acids as their sole source of P nutrition owing to secretion of PSI nucleases, phosphodiesterases, and APases (Abel et al., 2000; Chen et al., 2000; Ticconi and Abel, 2004).
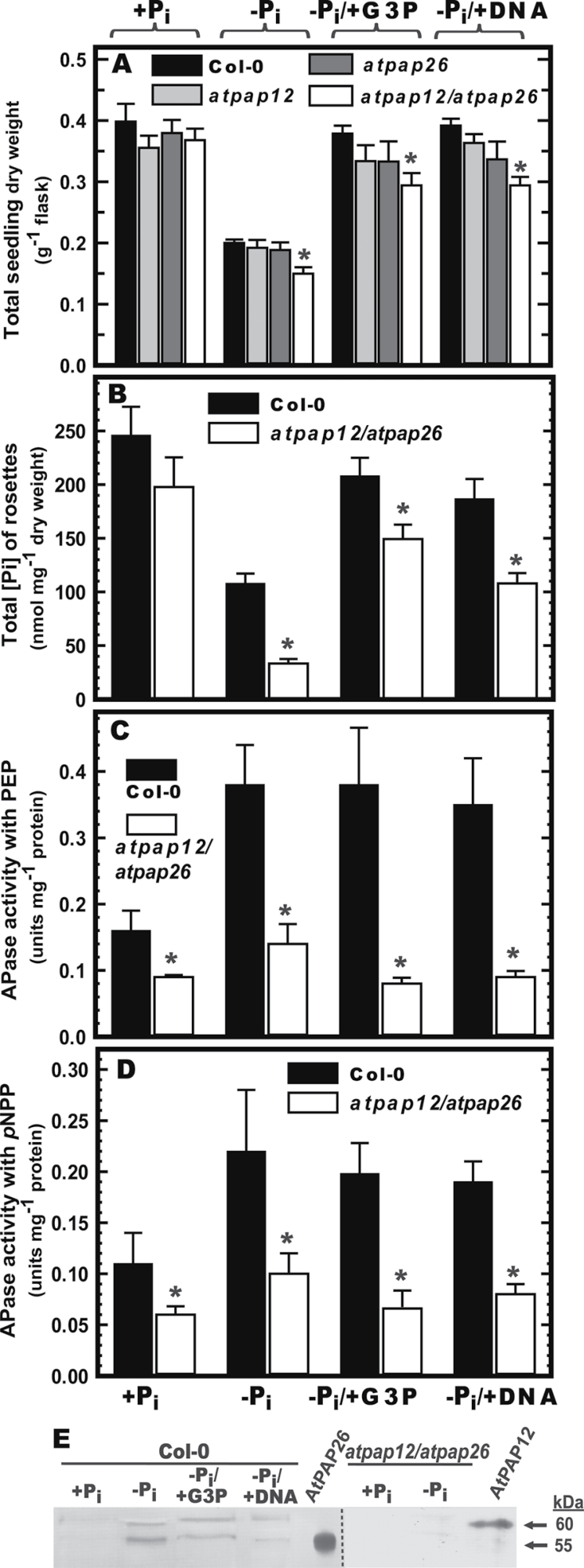
Fig. 1.
Influence of different P supplements on biomass accumulation, rosette Pi concentration, secretory APase activity, and secreted AtPAP12 and AtPAP26 polypeptides of Col-0 and mutant Arabidopsis seedlings. Seeds (5mg) of Col-0, atpap12 and atpap26 single mutants, and atpap12/atpap26 double mutants were placed in 50ml of 0.5 MS medium containing 0.2mM Pi and cultivated on an orbital shaker at 24 C under continuous illumination (100 mol m2 s1). After 7 d, the seedlings were transferred into fresh medium containing 0 or 1.5mM Pi (Pi and +Pi, respectively), 1.5mM G3P (Pi/+G3P), or 0.6mg ml1 DNA (Pi/+DNA) and cultured for an additional 7 d. (A) Seedling dry weight per flask. (B) Total Pi concentration of rosette leaves. (C, D) Secreted APase activity of concentrated seedling culture filtrates of Col-0 and atpap12/atpap26 plants. Spectrophotometric APase activity assays were conducted using 5mM PEP (C) or 5mM pNPP (D) as described in Materials and methods. All values represent means SE of duplicate determinations for three biological replicates; asterisks indicate values that are significantly different from those of Col-0 (P <0.01). (E) Concentrated secreted proteins (15 g per lane) of Col-0 and atpap12/atpap26 mutant seedlings, and secretory AtPAP12 and AtPAP26 (25ng each) purified from the culture medium of Pi Arabidopsis suspension cells (Tran et al., 2010a ) were resolved by SDS-PAGE and electroblotted onto a polyvinylidene difluoride membrane. Blots were probed with anti-AtPAP12 immune serum (Tran et al., 2010a ) and immunoreactive polypeptides were detected as described in Materials and methods.
We next assessed whether the capacity of Col-0 seedlings to scavenge Pi from G3P or DNA was correlated with secretory APase activity or immunoreactive AtPAP12 or AtPAP26 polypeptides. APase activities were determined using both 5mM PEP and 5mM pNPP as substrates. Irrespective of which substrate was used, the growth medium of Col-0 seedlings cultivated under –Pi, –Pi/+G3P, or –Pi/+DNA conditions exhibited a significant increase in secreted APase activity relative to +Pi seedlings (Fig. 1C, D). Immunoblotting using anti-AtPAP12 immune serum (which cross-reacts with both AtPAP12 and AtPAP26; Tran et al., 2010a ) indicated that 60kDa AtPAP12 and 55kDa AtPAP26 immunoreactive polypeptides were upregulated in the growth medium of the –Pi, –Pi/+G3P, and –Pi/+DNA Col-0 seedlings (Fig. 1E). These results suggested that AtPAP12 and AtPAP26 were secreted into the medium in order to hydrolyse Pi from the exogenous Po sources.
Influence of inorganic versus organic phosphate supply on AtPAP12 and AtPAP26 gene expression
Semi-quantitative RT-PCR was used to assess the relationship between exogenous P source and the relative shoot versus root expression of several PSI genes. The results of Fig. 2A confirmed previous studies documenting the constitutive expression of AtPAP26, whereas AtPAP12, AtPAP17, RNS1, and AtPPCK1 transcripts are significantly induced in shoots and roots of –Pi Arabidopsis (del Pozo et al., 1999; Haran et al., 2000; Veljanovski et al., 2006; Gregory et al., 2009; Hurley et al., 2010; Tran et al., 2010a ). AtPAP12 was also induced in both shoots and roots when the seedlings were grown on –Pi/+G3P or –Pi/+DNA, whereas transcripts for AtPAP17 or AtPPCK1 were either undetectable or expressed at a lower level relative to plants grown on –Pi medium (Fig. 2A). AtPAP17 was the first PSI PAP to be characterized in Arabidopsis (del Pozo et al., 1999), although its cellular location and biological function(s) remain elusive. AtPPCK encodes a protein kinase that specifically phosphorylates and thereby activates the cytosolic enzyme PEP carboxylase (PEPC) in –Pi Arabidopsis (Gregory et al., 2009). RNS1 encodes a nuclease that is upregulated and secreted by roots of –Pi Arabidopsis, or during cultivation on exogenous RNA or DNA as the sole source of nutritional P (Chen et al., 2000). RNS1 transcripts were induced in both shoots and roots when the seedlings were grown on –Pi and –Pi/+DNA but not –Pi/+G3P. These findings suggest a selective upregulation of genes based on the type of Po supplied to the seedlings. A challenging yet intriguing aspect for future studies will be to delineate the respective signal transduction pathways that appear to result in differential expression of secretory hydrolases such as AtPAP12, AtPAP26, and RNS1 during Arabidopsis growth on exogenous Po sources such as G3P and DNA.
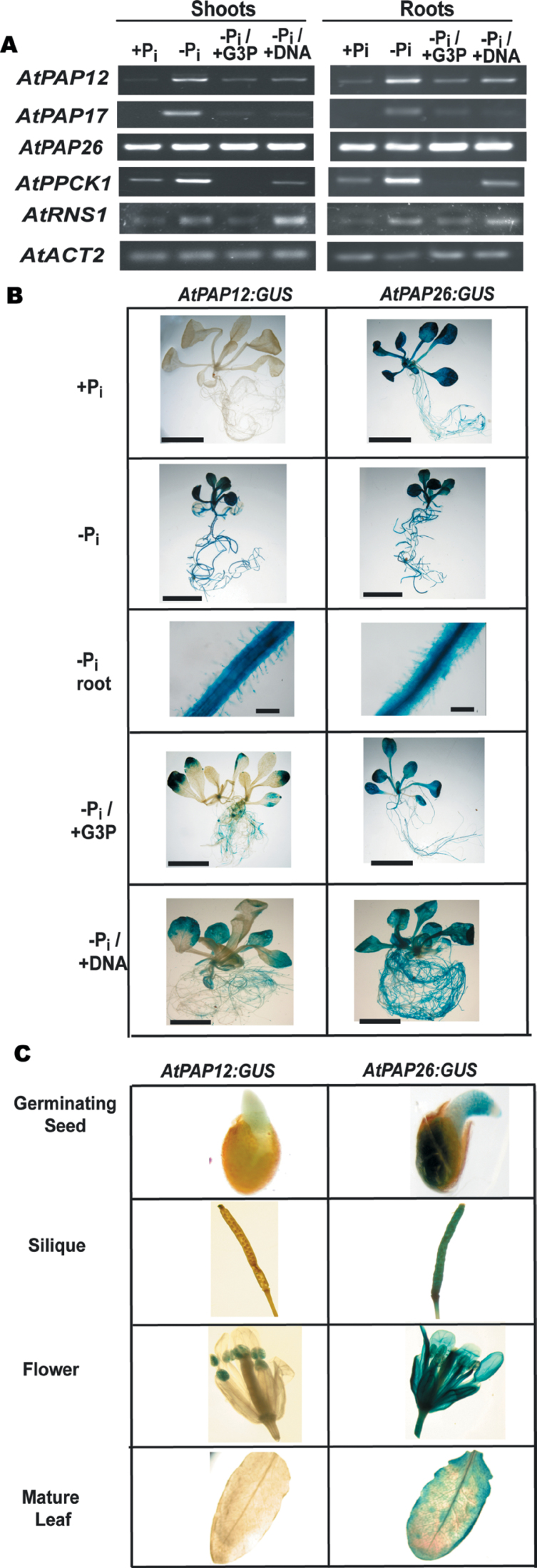
Fig. 2.
Analysis of AtPAP12 and AtPAP26 gene expression. (A) Levels of mRNA were analysed by semi-quantitative RT-PCR using gene-specific primers for AtPAP12, AtPAP17, AtPAP26, RNS1, and AtPPCK1. AtACT2 was used as a reference to ensure equal template loading. Seedlings were cultivated as described in the legend for Fig. 1. All PCR products were taken at cycle numbers determined to be non-saturating. Control RT–PCRs lacking reverse transcriptase did not produce any bands. (B) AtPAP12:GUS and AtPAP26:GUS transgenic lines were cultivated in 24-well microtitre plates in liquid MS medium containing 0.2mM Pi for 7 d, before being transferred into medium containing 0 or 1.5mM KH2PO4 (–Pi and +Pi, respectively), 1.5mM G3P (–Pi/+G3P), or 0.6mg ml–1 DNA (–Pi/+DNA) for another 7 d. Bars, 1cm, except for ‘–Pi root’ (bar, 100 µm). (C) AtPAP12:GUS and AtPAP26:GUS expression was also examined in several aerial tissues of 4-week-old +Pi plants that had been cultivated in soil under a regular light/dark diurnal cycle. ‘Germinating seed’ is a representative image of seeds that had been placed on moist filter paper and allowed to germinate for 1 d before GUS staining.
To determine the tissue specificity of AtPAP12 and AtPAP26 expression, promoter:GUS reporter gene fusions were generated. The expression of GUS activity was examined in 12 AtPAP12:GUS and five AtPAP26:GUS independent transgenic lines, which all exhibited similar tissue-specific expression patterns. The GUS expression patterns of representative lines are reported here. In agreement with the results of Fig. 2A: (i) the AtPAP26:GUS plants showed widespread GUS activity in all tissues, irrespective of the plant’s age or P status, whereas (ii) GUS activity was generally undetectable in +Pi AtPAP12:GUS tissues (other than in anthers) but was prevalent in shoots and roots of seedlings cultivated on –Pi or –Pi/G3P medium (Fig. 2B, C). AtPAP12 induction in shoots and roots of –Pi Arabidopsis seedlings has been well documented (Haran et al., 2000; Tran et al., 2010a ). To the best of our knowledge, however, the present study is first to observe the induction of a PSI PAP isozyme such as AtPAP12 during plant growth on medium in which the only accessible form of P nutrition is exogenous Po.
Identification and validation of an atpap12/atpap26 double mutant
To assess further the role that secreted AtPAP12 and AtPAP26 play in scavenging extracellular Po, a double atpap12/atpap26 knockout mutant was isolated by crossing homozygous atpap12 and atpap26 T-DNA insertion lines (Salk_152821 and SAIL_1187_A05, respectively) (Hurley et al., 2010; Tran et al., 2010a ). Confirmation of loss of AtPAP12 and/or AtPAP26 gene expression in the atpap12, atpap26, and atpap12/atpap26 mutants was confirmed by PCR of genomic DNA using AtPAP12- and AtPAP26-specific primers (Supplementary Fig. S1). Immunoblotting indicated that AtPAP12 or AtPAP26 polypeptides were absent in the concentrated secretome of +Pi or –Pi atpap12/atpap26 seedlings (Fig. 1E). This correlated with a >60% reduction in secreted APase activity during Pi deprivation (Fig. 1C, D). These results agreed with our earlier study of atpap12 and atpap26 single mutants, which concluded that AtPAP12 and AtPAP26 account for most of the APase activity secreted by roots of –Pi Arabidopsis seedlings (Tran et al., 2010a ).
AtPAP12 and AtPAP26 are major cell-wall acid phosphatases upregulated by Pi-deprived Arabidopsis
Pi-starvation-inducible root surface and/or cell-wall APase activities have been reported for numerous plant species including Arabidopsis (Lefebvre et al., 1990; Duff et al., 1991; Barrett-Lennard et al., 1993; Gilbert et al., 1999; Wasaki et al., 2000, 2008; Zhang and McManus, 2000; Kaida et al., 2008; Richardson et al., 2009; Tran et al., 2010b ; Wang et al., 2011). For example, AtPAP10 is a PSI-secreted PAP that is predominantly associated with the surface of root epidermal cells (but undetectable in culture medium), and that functions in the acclimation of Arabidopsis to Pi limitation (Wang et al., 2011). Cell-wall-associated PSI APases have been hypothesized to facilitate maintenance of the plant’s P status either by scavenging Pi from Po compounds present in the rhizosphere or by recycling Pi from endogenous phosphomonoesters that have been leaked from the cytoplasm across the plasma membrane (Lefebvre et al., 1990; Barrett-Lennard et al., 1993; Zhang and McManus, 2000; Tran et al., 2010b ; Wang et al., 2011). Classic studies by Bieliski’s group with the small aquatic plant Spirodela oligorrhiza demonstrated that significant levels of phosphomonoesters can be leaked during –Pi growth, and that failure to recapture this lost P can seriously compromise the overall P economy of the plant (Bieleski and Johnson, 1972).
Histochemical localization using ELF-97 phosphate as a substrate was applied to root samples of hydroponically cultivated seedlings. ELF-97 phosphate produces a fluorescent precipitate at the site of enzymatic hydrolysis, thus localizing active APases when viewed by fluorescence microscopy (Wasaki et al., 2008). Strong PSI APase activity was observed on the root surface and particularly at the root meristematic (tip) region of –Pi Col-0 seedlings. This activity was noticeably diminished in the atpap12 and atpap26 single mutants, and almost negligible in the atpap12/atpap26 double mutant (Fig. 3A). The atpap12/atpap26 plants also showed decreased root-surface APase staining when incubated with β-naphthyl phosphate (Supplementary Fig. S2 at JXB online), an excellent in vitro substrate for purified AtPAP12 and AtPAP26 (Tran et al., 2010a ). However, when incubated with BCIP instead of ELF-97 phosphate or β-naphthyl phosphate there was no obvious decrease in root-surface APase activity staining between Col-0 and the atpap12/atpap26 mutant (Supplementary Fig. S2). This result can be explained by the very low in vitro activity of purified native AtPAP12 and AtPAP26 with BCIP (H. Del Vecchio and W. Plaxton, unpublished data), coupled with AtPAP10’s known contribution to the BCIP-dependent APase activity of –Pi Arabidopsis root surfaces (Wang et al., 2011). Our ELF-97 phosphate and β-naphthyl phosphate results indicated that AtPAP12 and AtPAP26 account for a substantial proportion of root-surface-localized PSI APase activity. The results of Fig. 3A, coupled with the transcriptional activation of AtPAP12 in –Pi Arabidopsis shoots (Fig. 2), prompted us to investigate the influence of Pi deprivation on extractable APase activity and immunoreactive AtPAP12 and AtPAP26 polypeptides of shoot cell-wall extracts of hydroponically cultivated Col-0 and atpap12/atpap26 plants.
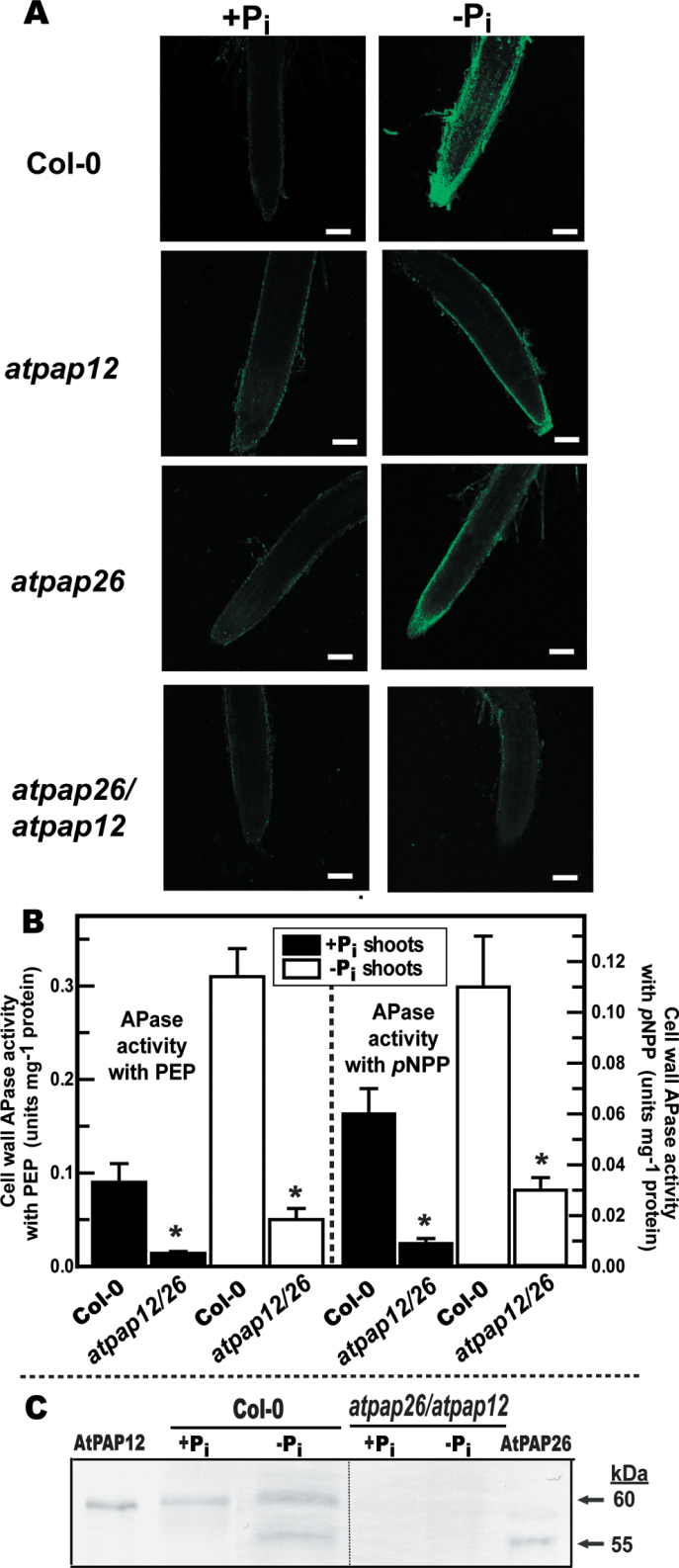
Fig. 3.
AtPAP12 and AtPAP26 make an important contribution to Pi-starvation-inducible APase activity of Arabidopsis root surfaces and shoot cell walls. (A) Histochemical staining of root-surface APase activity of Col-0, atpap12, atpap26 and atpap26/atpap12 seedlings using ELF-97 phosphate as a substrate. Green fluorescent precipitates of the APase product ELF-97 were observed using a confocal-laser scanning microscope. Bars, 100 µm. Seedlings were cultivated as described in the legend for Fig. 2B. (B) Concentrated cell-wall proteins extracted from shoots of Col-0 and atpap26/atpap12 seedlings were assayed for APase activity using 5mM PEP or 5mM pNPP as substrate. Values represent means ±SE of duplicate determinations on three biological replicates; asterisks indicate values that are significantly different from those of Col-0 (P <0.01). Seedlings were cultivated as described in the legend for Fig. 1. (C) Concentrated shoot cell-wall proteins (15 µg per lane) and purified native AtPAP26 and AtPAP12 (25ng per lane) (Tran et al., 2010a ) were subjected to immunoblot analysis with anti-AtPAP12 immune serum.
The complement of ionically bound (0.2M CaCl2-extractable) cell-wall proteins in shoots of +Pi and –Pi Col-0 seedlings was compared. The effectiveness of our extraction procedure was evaluated by testing for cytoplasmic contamination of the cell-wall fraction, using PEPC as a cytoplasmic marker enzyme. Immunoblots probed with anti- (castor bean PEPC) IgG demonstrated a lack of cytoplasmic contamination in the concentrated cell-wall fraction, as reflected by the absence of 107kDa immunoreactive PEPC polypeptides in the cell wall but not in corresponding cytoplasmic fractions (Supplementary Fig. S3A at JXB online). Comparison of the cytoplasmic and cell-wall fractions on protein-stained SDS gels indicated clear differences in their respective proteomes (Supplementary Fig. S3B). The –Pi Col-0 seedlings exhibited a large increase in shoot cell-wall APase activity compared with +Pi seedlings; this was correlated with the upregulation of immunoreactive 60kDa AtPAP12 and 55kDa AtPAP26 polypeptides (Fig. 3B, C). By contrast, immunoreactive AtPAP12 and AtPAP26 polypeptides were absent on immunoblots of cell-wall extracts prepared from the +Pi or –Pi atpap12/atpap26 mutant (Fig. 3C). This was paralleled by a >70% reduction in extractable cell-wall APase activity of –Pi atpap12/atpap26 shoots relative to Col-0, irrespective of whether PEP or pNPP was used as the APase substrate (Fig. 3B). These results demonstrated that AtPAP12 and AtPAP26 account for most of the APase activity secreted into the cell walls of –Pi Arabidopsis shoots. Pi recycling by PSI cell-wall-targeted AtPAP12 and AtPAP26 could be critical in maintaining cytoplasmic Pi and thus photosynthetic metabolism in the leaves of –Pi plants.
It is likely that the residual extracellular APase activity of –Pi atpap12/atpap26 seedlings (Figs 1C, D, and 3B) is at least partially due to a low-molecular-mass APase that has been shown previously to be upregulated and secreted by roots of –Pi Arabidopsis (Hurley et al., 2010). This APase may correspond to AtPAP17, a PSI ~35kDa PAP isozyme that is also induced (with AtPAP26) during leaf senescence (del Pozo et al., 1999; Robinson et al., 2012). However, the remaining extracellular APase activity of atpap12/atpap26 seedlings was unable to fully compensate for the loss of AtPAP12 and AtPAP26 function, as overall seedling growth and Pi acquisition efficiency was clearly compromised during cultivation on –Pi or –Pi/+Po medium (Figs. 1A and 4).
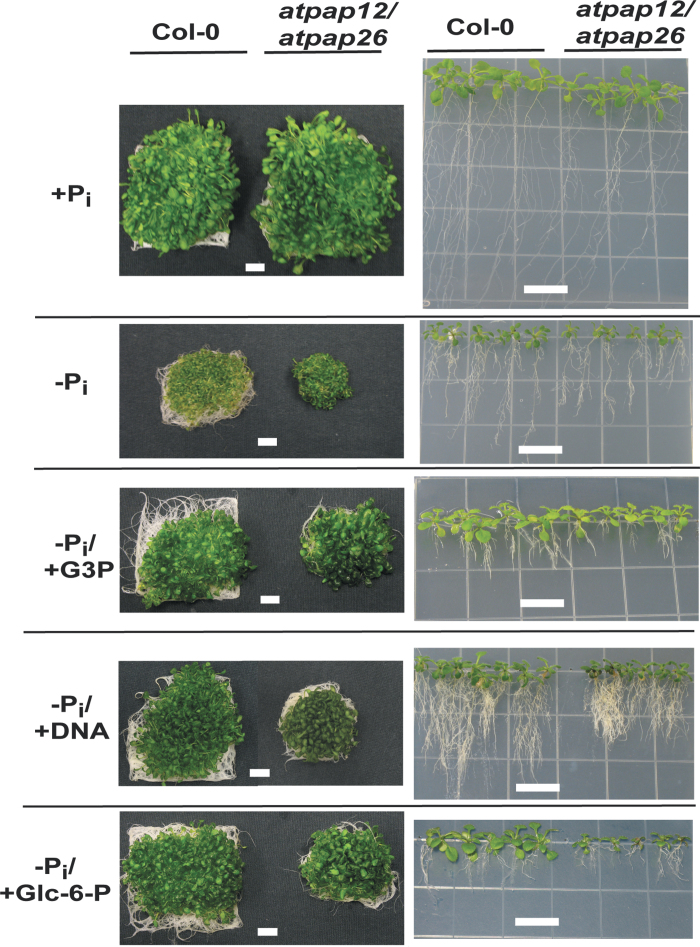
Fig. 4.
Impact of different P sources on appearance and root morphology of Col-0 and atpap26/atpap12 mutant seedlings. Left panels: seedlings were cultivated for 14 d in liquid medium as described in the legend for Fig. 1. Right panels: plants were cultivated for 21 d on vertically oriented agar plates containing 0.5× MS medium, 1% (w/v) sucrose, and 50 µM or 1.5mM Pi (–Pi and +Pi, respectively), 1.5mM G3P (–Pi/+G3P), 0.6mg ml–1 DNA (–Pi/+DNA), or 1.5mM Glc-6-P (–Pi/Glc-6-P). Images are representative of at least five replicates. Bars, 1cm.
Secreted AtPAP12 and AtPAP26 scavenge phosphate from extracellular organic phosphates
The growth of Col-0 versus atpap12, atpap26, and atpap12/atpap26 mutant plants was examined by cultivating 7 d +Pi seedlings for an additional 7 d on +Pi, –Pi, –Pi/+G3P, or –Pi/+DNA liquid medium. No differences were noted in the growth or appearance of +Pi plants (Figs 1A and 4). However, under –Pi, –Pi/+G3P, or –Pi/+DNA conditions, biomass yield of atpap12/atpap26 plants was significantly reduced (by up to ~25%) relative to the Col-0, or atpap12 and atpap26 single mutant plants (Figs 1A and 4). This suggests that the absence of AtPAP12 was largely compensated for by AtPAP26 and vice versa during cultivation of the single mutants in –Pi, –Pi/+G3P, or –Pi/+DNA liquid medium. However, when expression of both PAP isozymes was eliminated in the atpap12/atpap26 mutant, their absence could not be fully compensated by other PSI PAP isozymes such as AtPAP10 or AtPAP17 (del Pozo et al., 1999; Wang et al., 2011). Diminished growth of the –Pi, –Pi/+G3P, and –Pi/+DNA atpap12/atpap26 seedlings was probably due to the marked reductions in their total Pi concentration, particularly during –Pi growth (Fig. 1B). The reduced biomass accumulation of –Pi atpap12/atpap26 seedlings relative to Col-0 appeared to be specific to Pi deprivation, as no phenotypic differences were apparent when +Pi seedlings were subjected to nitrogen or potassium deficiency, or oxidative stress imposed by paraquat treatment (Supplementary Fig. S4 at JXB online).
The impaired development of atpap12/atpap26 seedlings during growth on –Pi, –Pi/+G3P, or –Pi/+DNA medium was also evident during their cultivation on vertically oriented agar plates (Fig. 4, right panels). Similar results were obtained when the plants were cultured in –Pi liquid medium or vertical agar plates supplemented with 1.5mM Glc-6-P, which like G3P is also efficiently hydrolysed by the native AtPAP12 or AtPAP26 purified from the secretome of –Pi Arabidopsis (Tran et al., 2010a ). It was notable that Col-0 or atpap12/atpap26 plants cultivated on –Pi agar plates supplemented with G3P, DNA, or Glc-6-P showed typical root architectural adaptations to Pi limitation (e.g. decreased primary root growth and increased lateral branching; Williamson et al., 2001), even though total biomass accumulation and shoot Pi concentration of Col-0 plants paralleled that of the respective +Pi seedlings (Figs 1A, B and 4). A rationale for this observation is that intracellular Pi status appears to be irrelevant to the reprogramming of root architecture in –Pi Arabidopsis, whereas low extracellular Pi in the area surrounding the root tip appears to trigger this response (Svistoonoff et al., 2007). Presumably, the root-cap Pi sensor complex that mediates adaptive modifications in root structure to Pi limitation does not perceive exogenous Po sources such as G3P, DNA, or Glc-6-P as a potential source of P nutrition, despite the fact that these compounds supported growth and Pi assimilation typical of Pi-fertilized plants.
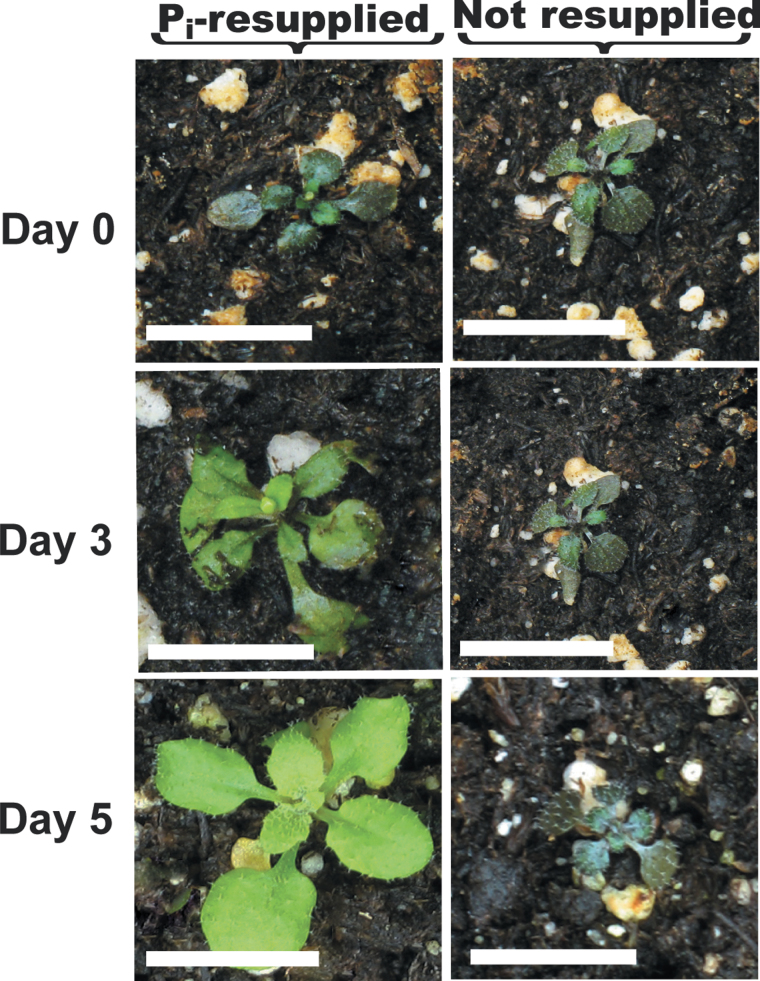
We also examined the phenotype of soil-grown plants. Seedlings were cultivated in +Pi liquid medium for 7 d, before being transferred into a nutrient-depleted soil mixture and cultivated in growth cabinets under a regular light/dark regime for an additional 14 d. All Pi present in the peat/vermiculite soil mix used for these experiments was in the form of Po; it contained 12.8±0.5 µmol total Pi g–1 of dry weight but undetectable free Pi. No obvious phenotypic differences were noted when any of the soil-grown plants were provided with a regular Pi fertilizer treatment (Fig. 5A). However, the growth of the atpap12 and atpap26 single mutants was obviously compromised during their cultivation on the –Pi soil, as reflected by the ~50% reduction in their rosette dry weights relative to Col-0 plants (Fig. 5B). Impaired growth of atpap26 seedlings on a –Pi soil mixture has been noted previously (Hurley et al., 2010). It is remarkable, however, that development of atpap12/atpap26 plants was completely arrested when +Pi seedlings were transplanted into the –Pi soil mix (Figs. 5 and 6). In addition, shoots of soil-grown –Pi atpap12/atpap26 plants rapidly turned purple, reflecting their anthocyanin accumulation, a typical symptom of severe Pi stress (Plaxton and Tran, 2011); the leaf anthocyanin concentration of the soil-grown –Pi Col-0 and atpap12/atpap26 plants was 70±8 and 900±12 nmol mg–1 of fresh weight, respectively (means ±SE of duplicate determinations on three biological replicates). Shoots of soil-grown –Pi atpap12/atpap26 plants also contained significantly less free Pi; the free Pi concentration of leaves of the –Pi Col-0 and atpap12/atpap26 plants was 1.7±0.2 and 0.38±0.09 µmol g–1 of fresh weight, respectively (means ±SE of duplicate determinations on three biological replicates). The arrested development of soil-cultivated 21-d-old –Pi atpap12/atpap26 plants was quickly reversed when they were fertilized with medium containing 2mM Pi and cultivated for an additional 5 d; this was paralleled by rapid leaf colour conversion from purple to green (Fig. 6).
Fig. 5.
Effect of Pi deprivation on appearance and shoot biomass accumulation of soil-grown Col-0 and mutant Arabidopsis seedlings. (A) Seedlings were cultivated for 7 d in liquid medium containing 0.2mM Pi, then transplanted into a Pi-deficient soil mix and grown for an additional 14 d. Fertilization occurred twice weekly with 0.25× Hoagland’s medium containing 0 or 2mM Pi (–Pi and +Pi, respectively). Solid bars, 1cm; dashed bar, 0.5cm. (B) Rosette dry weights of soil grown seedlings. All values represent means ±SE of ten different seedlings; asterisks indicate values that were significantly different from those of Col-0 (P <0.01).
Fig. 6.
Influence of Pi resupply on appearance of soil-grown –Pi atpap12/atpap26 plants. Seedlings were grown for 7 d in liquid medium containing 0.2mM Pi and then transplanted into the –Pi soil mix and grown for an additional 14 d. Seedlings were then fertilized with 0.25× Hoagland’s medium containing 0 or 2mM Pi and cultivated for an additional 5 d. Bars, 1cm.
Backcrossing atpap12/atpap26 plants with each of the atpap12 and atpap26 single mutants restored AtPAP12 or AtPAP26 expression (Supplementary Fig. S5 at JXB online), as well as the –Pi soil growth phenotype characteristic of the respective single mutants (Fig. 5). This supports the ability of AtPAP12 to partially compensate for the absence AtPAP26 and vice versa. It is hypothesized that decreased scavenging of soil-localized Po reduced the amount of Pi assimilated by the atpap12 or atpap26 mutants, and that this was particularly exacerbated in the atpap12/atpap26 double mutant. It is important to note that direct hydrolysis of rhizosphere Po and subsequent assimilation of released Pi by APase-secreting roots has been demonstrated in soil-grown plants (Richardson et al., 2009, 2011). However, while both monoester and diester (e.g. nucleic acid) Po pools were depleted,: (i) the precise chemical nature of the specific Po substrates remains unclear, and (ii) the relative contributions of APases secreted by roots of –Pi plants relative to those secreted by soil-dwelling bacteria remain to be established. Nevertheless, mineralization of soil Po by plant and microbial APases does occur in the rhizosphere and appears to make an important contribution to the Pi nutrition of –Pi plants (Richardson et al., 2009, 2011). As both AtPAP12 and AtPAP26 were also markedly upregulated in the cell walls of –Pi Col-0 Arabidopsis shoots (Fig. 3B and 3C), diminished Pi recapture from leaked phosphomonoesters is also suggested to contribute to the prominent phenotype of atpap12/atpap26 mutant plants cultivated on–Pi soil.
Concluding remarks
The de novo synthesis and secretion of APases by roots or suspension cell cultures has long been recognized as a widespread response of –Pi plants (Tran et al., 2010b ; Plaxton and Tran, 2011). Conversely, the molecular identities, biochemical properties, and genetic control of PSI-secreted APases are not fully understood. However, such an understanding is likely to contribute towards exploiting biotechnological strategies for improving crop P acquisition from the abundant Po sources prevalent in agricultural soils (Richardson, 2009). The results of the current study corroborate our earlier report indicating that AtPAP12 and AtPAP26 are the predominant secretory APases of –Pi Arabidopsis seedlings (Tran et al., 2010a ). Their upregulation and secretion during growth on –Pi/+Po medium clearly helps Arabidopsis to exploit exogenous Po compounds such as G3P, Glc-6-P, and DNA as alternative sources of P nutrition (Figs 1 and 4). AtPAP12 and AtPAP26 were also upregulated in shoot cell walls and on the root surface of –Pi plants (Fig. 3), indicating that they have an additional function to recycle Pi from leaked phosphomonoesters. Cell-wall-localized or root secretory PSI AtPAP12 orthologues have been described in a variety of plant species including white lupin, tobacco, barrel medic, and tomato (Wasaki et al., 2000, 2008; Miller et al., 2001; Bozzo et al., 2002, 2006; Xiao et al., 2006; Kaida et al., 2008). Wasaki et al. (2009) recently overexpressed a secreted AtPAP12 orthologue (LaSAP2) in tobacco; the transgenic plants exhibited enhanced Pi uptake and growth during cultivation on –Pi soils. To the best of our knowledge, however, the involvement of AtPAP26 orthologues in scavenging Pi from extracellular Po has not yet been reported in any other species.
During the cultivation of atpap12 and atpap26 single mutants on sterile –Pi/+G3P or –Pi/+DNA liquid medium, it was apparent that AtPAP12 could compensate for the absence of AtPAP26 and vice versa (Fig. 1A). However, this was not evident when either of the single mutants was cultivated on a more physiologically relevant –Pi, Po-containing soil mix, as both groups showed poorer growth relative to Col-0 control plants (Fig. 5). It was particularly noteworthy that development of the atpap12/atpap26 double mutant was totally blocked when seedlings were transplanted into the –Pi soil. This highlights the critical role that AtPAP12 and AtPAP26 have in facilitating acclimation of Arabidopsis to nutritional Pi deprivation. AtPAP10, AtPAP12, and AtPAP26 are closely related high-molecular-mass PSI PAPs that comprise subgroup Ia-2 of the Arabidopsis PAP family (Supplementary Fig. S6 at JXB online) (Li et al., 2002; Tran et al., 2010b ). Evolution of this PAP subgroup appears to have endowed Arabidopsis with an effective hydrolytic machinery for scavenging Pi from exogenous Po compounds prevalent in the –Pi soils typical of most ecosystems (Tran et al., 2010a ; Wang et al., 2011). As the susceptibility of soil Po to enzymatic hydrolysis is a probable constraint for crop Pi acquisition (Richardson, 2009), it will be of interest to determine whether AtPAP12 and/or AtPAP26 overexpression could facilitate the production of P-use-efficient crops needed to reduce the use of Pi fertilizers in agriculture.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Confirmation of T-DNA insert location and loss of AtPAP12 and/or AtPAP26 gene expression in atpap12, atpap26, and atpap26/atpap12 mutants.
Supplementary Fig. S2. Histochemical staining of root-surface APase activity in Col-0 and atpap12/atpap26 seedlings using β-naphthyl phosphate or BCIP.
Supplementary Fig. S3. Immunoblot and SDS-PAGE analysis of cytoplasmic and cell-wall extracts isolated from shoots of +Pi versus –Pi Col-0 Arabidopsis seedlings.
Supplementary Fig. S4. Influence of nutrient deprivation or oxidative stress on growth of atpap26/atpap12 and Col-0 seedlings.
Supplementary Fig. S5. Immunoblot analysis of AtPAP12 and AtPAP26 polypeptides in clarified rosette extracts of 21-d-old Arabidopsis plants cultivated in –Pi soil.
Supplementary Fig. S6. A classification scheme for Arabidopsis PAPs based on clustering analysis of amino acid sequences.
Acknowledgements
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Queen’s Research Chairs program (to W.C.P.). We are also grateful to Professor Wayne Snedden (Queen’s University) and his research team for helpful discussions and advice regarding atpap12/atpap26 mutant selection and analyses.
Glossary
Abbreviations:
- APase
acid phosphatase
- BCIP
5-bromo-4-chloro-3-indolyl phosphate
- ELF
enzyme-labelled fluorescent
- G3P
glycerol-3-phosphate
- Glc-6-P
glucose-6-phosphate
- GUS
β-glucuronidase
- MS
Murashige and Skoog
- PAP
purple acid phosphatase
- PEP
phosphoenolpyruvate
- PEPC
PEP carboxylase
- +Pi
Pi sufficient
- –Pi
Pi deficient
- pNPP
para-nitrophenol phosphate
- Po
organic P
- PSI
Pi starvation inducible
References
- Abel S, Nurnberger T, Ahnert V, Krauss GJ, Glund K. 2000. Induction of an extracellular cyclic nucleotide phosphodiesterase as an accessory ribonucleolytic activity during phosphate starvation of cultured tomato cells Plant Physiology 122 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Lennard EG, Dracup M, Greenway H. 1993. Role of extracellular phosphatases in the phosphorus-nutrition of cover Journal of Experimental Botany 44 1595–1600 [Google Scholar]
- Bieleski RL, Johnson PN. 1972. External location of phosphatase-activity in phosphorus-deficient Spirodela oligorrhiza Australian Journal of Biological Sciences 25 707–720 [Google Scholar]
- Bozzo GG, Dunn EL, Plaxton WC. 2006. Differential synthesis of phosphate-starvation inducible purple acid phosphatase isozymes in tomato (Lycopersicon esculentum) suspension cells and seedlings Plant Cell and Environment 29 303–313 [DOI] [PubMed] [Google Scholar]
- Bozzo GG, Raghothama KG, Plaxton WC. 2002. Purification and characterization of two secreted purple acid phosphatase isozymes from phosphate-starved tomato (Lycopersicon esculentum) cell cultures European Journal of Biochemistry 269 6278–6286 [DOI] [PubMed] [Google Scholar]
- Chen DL, Delatorre CA, Bakker A, Abel S. 2000. Conditional identification of phosphate-starvation-response mutants in Arabidopsis thaliana Planta 211 13–22 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana The Plant Journal 16 735–743 [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Allona I, Rubio V, Leyva A, de la Pena A, Aragoncillo C, Paz-Ares J. 1999. A type 5 acid phosphatase gene from Arabidopsis thaliana is induced by phosphate starvation and by some other types of phosphate mobilising/oxidative stress conditions The Plant Journal 19 579–589 [DOI] [PubMed] [Google Scholar]
- Duff SMG, Lefebvre DD, Plaxton WC. 1991. Purification, characterization, and subcellular-localization of an acid-phosphatase from black mustard cell-suspension cultures: comparison with phosphoenolpyruvate phosphatase Archives of Biochemistry and Biophysics 286 226–232 [DOI] [PubMed] [Google Scholar]
- George TS, Simpson RJ, Hadobas PA, Marshall DJ, Richardson AE. 2007. Accumulation and phosphatase-lability of organic phosphorus in fertilised pasture soils Australian Journal of Agricultural Research 58 47–55 [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL. 1999. Acid phosphatase activity in phosphorus-deficient white lupin roots Plant, Cell & Environment 22 801–810 [Google Scholar]
- Gregory AL, Hurley BA, Tran HT, Valentine AJ, She YM, Knowles VL, Plaxton WC. 2009. In vivo regulatory phosphorylation of the phosphoenolpyruvate carboxylase AtPPC1 in phosphate-starved Arabidopsis thaliana Biochemical Journal 420 57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran S, Logendra S, Seskar M, Bratanova M, Raskin I. 2000. Characterization of Arabidopsis acid phosphatase promoter and regulation of acid phosphatase expression Plant Physiology 124 615–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley BA, Tran HT, Marty NJ, Park J, Snedden WA, Mullen RT, Plaxton WC. 2010. The dual-targeted purple acid phosphatase AtPAP26 is essential for efficient acclimation of Arabidopsis thaliana to nutritional phosphate deprivation Plant Physiology 153 1112–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. Gus fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher-plants EMBO Journal 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida R, Hayashi T, Kaneko TS. 2008. Purple acid phosphatase in the walls of tobacco cells Phytochemistry 69 2546–2551 [DOI] [PubMed] [Google Scholar]
- Lefebvre DD, Duff SMG, Fife CA, Julien-Inalsingh C, Plaxton WC. 1990. Response to phosphate deprivation in Brassica nigra suspension cells: enhancement of intracellular, cell-surface, and secreted phosphatase-activities compared to increases in Pi-absorption rate Plant Physiology 93 504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Zhu HF, Liu KF, Liu X, Leggewie G, Udvardi M, Wang DW. 2002. Purple acid phosphatases of Arabidopsis thaliana: comparative analysis and differential regulation by phosphate deprivation Journal of Biological Chemistry 277 27772–27781 [DOI] [PubMed] [Google Scholar]
- Liang C, Tian J, Lam H, Lim BL, Yan X, Liao H. 2010. Biochemical and molecular characterization of PvPAP3, a novel purple acid phosphatase isolated from common bean enhancing extracellular ATP utilization Plant Physiology 152 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SS, Liu J, Allan DL, Menzhuber CJ, Fedorova M, Vance CP. 2001. Molecular control of acid phosphatase secretion into the rhizosphere of proteoid roots from phosphorus-stressed white lupin Plant Physiology 127 594–606 [PMC free article] [PubMed] [Google Scholar]
- Nuruzzaman M, Lambers H, Bolland MDA. 2006. Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes Plant and Soil 281 109–120 [Google Scholar]
- Plaxton WC, Tran HT. 2011. Metabolic adaptations of phosphate-starved plants Plant Physiology 156 1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AE. 2009. Regulating the phosphorus nutrition of plants: molecular biology meeting agronomic needs Plant and Soil 322 17–24 [Google Scholar]
- Richardson AE, Hocking PJ, Simpson RJ, George TS. 2009. Plant mechanisms to optimise access to soil phosphorus Crop and Pasture Science 60 124–143 [Google Scholar]
- Richardson AE, Lynch JP, Ryan PR, et al. 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture Plant and Soil 349 121–156 [Google Scholar]
- Robinson WD, Carson I, Ying S, Ellis K, Plaxton WC. 2012. Eliminating the purple acid phosphatase AtPAP26 in Arabidopsis thaliana delays leaf senescence and impairs phosphorus remobilization New Phytologist(in press, doi: 10.1111/nph.12006) [DOI] [PubMed] [Google Scholar]
- Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T. 2007. Root tip contact with low-phosphate media reprograms plant root architecture Nature Genetics 39 792–796 [DOI] [PubMed] [Google Scholar]
- Tarafdar JC, Claassen N. 1988. Organic phosphorus-compounds as a phosphorus source for higher-plants through the activity of phosphatases produced by plant-roots and microorganisms Biology and Fertility of Soils 5 308–312 [Google Scholar]
- Ticconi CA, Abel S. 2004. Short on phosphate: plant surveillance and countermeasures Trends in Plant Science 9 548–555 [DOI] [PubMed] [Google Scholar]
- Tran HT, Qian W, Hurley BA, She Y, Wang D, Plaxton WC. 2010. a Biochemical and molecular characterization of AtPAP12 and AtPAP26: the predominant purple acid phosphatase isozymes secreted by phosphate-starved Arabidopsis thaliana Plant Cell and Environment 33 1789–1803 [DOI] [PubMed] [Google Scholar]
- Tran HT, Hurley BA, Plaxton WC. 2010. b Feeding hungry plants: the role of purple acid phosphatases in phosphate nutrition Plant Science 179 14–27 [Google Scholar]
- Veljanovski V, Vanderbeld B, Knowles VL, Snedden WA, Plaxton WC. 2006. Biochemical and molecular characterization of AtPAP26, a vacuolar purple acid phosphatase up-regulated in phosphate-deprived Arabidopsis suspension cells and seedlings Plant Physiology 142 1282–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li Z, Qian W, et al. 2011. The Arabidopsis purple acid phosphatase AtPAP10 is predominantly associated with the root surface and plays an important role in plant tolerance to phosphate limitation Plant Physiology 157 1283–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasaki J, Kojima S, Maruyama H, Haase S, Osaki M, Kandeler E. 2008. Localization of acid phosphatase activities in the roots of white lupin plants grown under phosphorus-deficient conditions Soil Science and Plant Nutrition 54 95–102 [Google Scholar]
- Wasaki J, Maruyama H, Tanaka M, Yamamura T, Dateki H, Shinano T, Susumu I, Osaki M. 2009. Overexpression of the LASAP2 gene for secretory acid phosphatase in white lupin improves the phosphorus uptake and growth of tobacco plants Soil Science and Plant Nutrition 55 107–113 [Google Scholar]
- Wasaki J, Omura M, Ando M, Dateki H, Shinano T, Osaki M, Ito H, Matsui H, Tadano T. 2000. Molecular cloning and root specific expression of secretory acid phosphatase from phosphate deficient lupin (Lupinus albus L.) Soil Science and Plant Nutrition 46 427–437 [Google Scholar]
- Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO. 2001. Phosphate availability regulates root system architecture in Arabidopsis Plant Physiology 126 875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Harrison M, Wang ZY. 2006. Cloning and characterization of a novel purple acid phosphatase gene (MtPAP1) from Medicago truncatula Barrel Medic Journal of Integrative Plant Biology 48 204–211 [Google Scholar]
- Zhang C, McManus MT. 2000. Identification and characterisation of two distinct acid phosphatases in cell walls of roots of white clover Plant Physiology and Biochemistry 38 259–270 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.