Abstract
Vitis vinifera scions are commonly grafted onto rootstocks of other grape species to influence scion vigour and provide resistance to soil-borne pests and abiotic stress; however, the mechanisms by which rootstocks affect scion physiology remain unknown. This study characterized the hydraulic physiology of Vitis rootstocks that vary in vigour classification by investigating aquaporin (VvPIP) gene expression, fine-root hydraulic conductivity (Lp r), % aquaporin contribution to Lp r, scion transpiration, and the size of root systems. Expression of several VvPIP genes was consistently greater in higher-vigour rootstocks under favourable growing conditions in a variety of media and in root tips compared to mature fine roots. Similar to VvPIP expression patterns, fine-root Lp r and % aquaporin contribution to Lp r determined under both osmotic (Lp r Osm) and hydrostatic (Lp r Hyd) pressure gradients were consistently greater in high-vigour rootstocks. Interestingly, the % aquaporin contribution was nearly identical for Lp r Osm and Lp r Hyd even though a hydrostatic gradient would induce a predominant flow across the apoplastic pathway. In common scion greenhouse experiments, leaf area-specific transpiration (E) and total leaf area increased with rootstock vigour and were positively correlated with fine-root Lp r. These results suggest that increased canopy water demands for scion grafted onto high-vigour rootstocks are matched by adjustments in root-system hydraulic conductivity through the combination of fine-root Lp r and increased root surface area.
Key words: fine-root hydraulics, grapevines, PIPs, plasma-membrane intrinsic proteins, root aquaporins, rootstocks
Introduction
Perennial fruit crop scions are often grafted onto rootstocks for a variety of reasons, which include providing resistance to soil-borne pests, conferring resistance to water deficit and extreme soil types, and controlling growth of the scion (e.g. Pongrácz, 1983). Grafting affords some grower control over important agronomic traits and provides flexibility in growing a particular scion across diverse soil and environmental conditions. Grapevine rootstocks are commonly characterized according to vigour characteristics conferred to the scion (i.e. biomass accumulation and yield), which influence winegrape quality (Cortell et al., 2005, 2007, 2008) and are known to alter scion gas exchange and water use efficiency (Candolfi-Vasconcelos et al., 1994). Despite their common usage in agriculture, the mechanisms through which rootstocks affect scion vigour and resistance to abiotic stress are not fully understood or ambiguous across crop systems.
For some perennial crop species, altered scion vigour has been linked to differences in hydraulic parameters of the root system. Greater whole-root-system hydraulic conductance has been documented in vigour-inducing rootstocks of apple, peach, olive, and kiwi (Atkinson et al., 2003; Clearwater et al., 2004; Nardini et al., 2006; Solari et al., 2006). Most of these studies measured whole root systems using a high-pressure–flow meter and/or the evaporative flux method, and related results to the size of the root system (i.e., biomass or surface area). Other studies have compared the vascular anatomy between low- and high-vigour rootstocks of cherry and peach trees: several of these studies demonstrated positive correlations between calculated hydraulic conductance (based on xylem vessel diameters) and vigour (Olmstead et al., 2006a, b; Goncalves et al., 2007), while another found a negative correlation using a ratio of the phloem and xylem areas (Iwanami et al., 2009).
Despite these recent efforts to assess the role of root-system hydraulics, the contribution of fine-root hydraulic properties to known differences among rootstocks has not been deeply studied. This is surprising since radial water absorption across fine roots constitutes a large proportion of the total resistance and is known to be limiting to water uptake in the root system (Frensch and Steudle, 1989; Steudle, 2001; Steudle and Meshcheryakov, 1996). Radial water uptake across fine roots occurs via the apoplastic (flow within the cell walls) and the cell-to-cell (C–C) pathways (Steudle, 2001). Because cell membranes must be crossed in the C–C pathway, transport efficiency of this pathway is thought to be affected by the activity, density, and location of aquaporins (i.e. water-specific protein channels embedded in cell membranes) (e.g. Knipfer and Fricke, 2010). Much work over the last decade and a half has demonstrated the role of aquaporins in affecting root hydraulic properties (Javot and Maurel, 2002), yet little work has addressed whether inherent differences in aquaporin activity contribute to differences in stress resistance and vigour potential among rootstocks for perennial crop species.
Recent studies utilizing molecular tools have demonstrated the importance of aquaporins to plant vigour and water relations of herbaceous species. For transgenic tobacco growing under favourable conditions, constitutive overexpression of Arabidopsis AtPIP1b increased transpiration rates and plant vigour (Aharon et al., 2003), while antisense suppression of tobacco NtAQP1 resulted in decreased root hydraulic conductance but had negligible effects on transpiration (Siefritz et al., 2002). More recently Sade et al. (2010) demonstrated that constitutively overexpressing NtAQP1 in Arabidopsis and tomato plants can enhance transpiration, photosynthesis, and shoot growth rates under favourable growing conditions. Lovisolo et al. (2007) conducted one of the only studies to assess links between aquaporin gene expression and rootstock effects in perennial woody plants and found a positive correlation between aquaporin expression and root-specific hydraulic conductance (i.e. per gram of root dryweight), which was actually higher in olive dwarfing rootstocks. However, whole-root-system hydraulic conductance was greater in high-vigour plants due to greater root biomass (Lovisolo et al., 2007). This same research group found differential aquaporin activity, measured by mercurial inhibition, under drought conditions for grapevine rootstocks derived from varied Vitis species parentage (Lovisolo et al., 2008).
Given the link between aquaporins and root hydraulic conductance, and that aquaporins respond to many of the factors used to differentiate grapevine rootstocks (i.e. water deficit, salt, anoxia), this study investigated their role in establishing differences among commercially available Vitis rootstocks under favourable (i.e. non-stressed) growing conditions. The inherent differences in aquaporin gene expression (i.e. plasma-membrane intrinsic proteins, PIPs), fine-root hydraulic conductivity (Lp r), and % aquaporin contribution to Lp r between these rootstocks were characterized and the data with differences in root and shoot biomass and scion transpiration were paired.
Materials and methods
Plant material and growth conditions
Bare-root, bench-grafted grapevines were obtained from commercial nurseries in California and used in all greenhouse experiments. A common Vitis vinifera cv. Cabernet Sauvignon scion was grafted onto the following rootstocks: 110R, 140R, 1103P, 5BB, SO4, 101-14Mgt, and 420A. These rootstocks were chosen based on their common usage in commercial vineyards, their varying species parentage, and their differential vigour and abiotic stress tolerance. Rootstocks 110R, 140R and 1103P (all with Vitis berlandieri × Vitis rupestris parentage) are commonly characterized as high vigour and drought resistant, rootstocks 5BB and SO4 (both with V. berlandieri × Vitis riparia parentage) are characterized as moderate vigour and drought intolerant, and rootstocks 101-14Mgt (V. riparia × V. rupestris parentage) and 420A (V. berlandieri × V. riparia parentage) are characterized as low-to-moderate vigour and drought intolerant (Pongrácz, 1983). The vigour classification put forth by Pongrácz (1983) has been validated recently by a multiyear and multisite rootstock trial in California (see data summarized briefly in the Results section below), and serves as the basis for the classifications in the figures and throughout the manuscript.
Vines were acquired as dormant material and stored on wood chips at approximately 7 °C until use in the experiments. Prior to planting, all plants were rinsed with tap water, washed in a 10% sodium hyperchlorite solution, and re-rinsed in a succession of water baths. All buds were removed from the stems. Stems were then dipped into a low-temperature vat of melted wax for approximately 2 seconds and cooled in an ice bath to set the wax. Care was taken to prevent wax from getting onto the roots. Vines were potted into either soil or a hydroponics system (see below) within 24h of washing/waxing and were grown in a greenhouse.
Grapevines were grown either hydroponically in soil, fritted clay, or a continuous re-circulating drip system (RDS) modelled after Wheatley et al. (2009). For hydroponics, ten RDS systems were used, each system consisting of eight pots (2.83 l each). Waxed vines were planted in treepots (TPOT1, Stuewe and Sons, Tangent, OR, USA), and the pots were filled with lightweight expanded clay aggregate pebbles (General Hydroponics, Sebastopol, CA, USA). Holes in the bottoms of the treepots allowed adequate drainage of hydroponic solution. The treepots were distributed evenly in a basin covered by a square piece (0.34 m2) of polyisocyanurate insulation (R-Matte Plus 3- thickness 1.9cm, Rmax, Dallas, TX, USA) perforated with eight square holes to hold the treepots. A drainage hole at the bottom of each basin was fitted with a barbed tub outlet fitting (National Garden Wholesale, Vancouver, WA, USA) to which a flexible black tubing drain line was attached. The tubing drained into a 113-l plastic reservoir (Newell Rubbermaid, Sandy Springs, GA, USA) filled with modified Hoagland’s solution. A 1.32 kl h–1 Supreme Mag Drive utility pump (Danner Manufacturing, Central Islip, NY, USA) pumped the solution up from each reservoir into two multi-outlet Maverick Head drip manifolds (DIG Irrigation, Vista, CA, USA) per basin that distributed the Hoagland’s solution between eight 7.6 l h–1 drip emitters (DIG Irrigation). Two drip emitters were placed in each pot 5cm below the pebble surface and continually supplied the hydroponic solution to the grapevines through the drip emitters. As the re-circulating solution drained from the basins, it dripped into the reservoir facilitating adequate aeration of the solution. All RDS systems were set up in a greenhouse with set temperatures between 20–28 °C. The modified Hoagland’s solution consisted of 2mM Ca(NO3)2, 3mM KNO3, 1.25mM KH2PO4, 1.5mM MgSO4, 100 µM Na2SiO3, 40 µM H3BO3, 9 µM MnSO4, 4 µM ZnSO4, 4 µM CuSO4, 0.10 µM H2MoO4, and 100 µM NaFeDTPA. pH was adjusted to approximately 5.8 using H3PO4 and KOH. Solution in each reservoir was changed in all RDS systems as needed based upon total volume losses due to transpiration and evaporation. To minimize evaporative losses from each RDS and reduce algae growth, heavy-duty aluminum foil was used to cover the reservoirs and all treepots.
For soil and fritted clay, four of the rootstocks described above (420A, 101-14Mgt, 1103P, and 110R) were grafted with a common scion (described above) and grown in either a modified UC soil mix (peat/sand/redwood compost 1:1:1, with 2.44kg m–2 dolomite lime) or fritted clay in 4.3 l pots under similar greenhouse conditions. These vines were irrigated regularly and fertilized weekly. Vines from this experimental setup were also used to evaluate rootstock vegetative growth in terms of leaf area and root biomass and were compared to results from recent field rootstock trials conducted across several years on multiple sites across grape growing regions of California.
Root sampling
Root sampling occurred between 10:00 and 10:30 a.m., and samples were returned to the laboratory within 20min of harvesting. Vines were carefully removed from pots, growing media was carefully washed from the roots, and pruning razor blades were used to cut healthy fine roots from the root mass under water. All portions of the root system sampled for Lp r included an intact root tip ensuring a measurement of the radial contribution to Lp r. Roots were transferred to the lab in nutrient solution and experiments were carried out immediately. A sub-sample of fine roots for gene expression analyses were placed into 5.0ml cryogenic vials and frozen immediately using liquid N2. Frozen samples were taken to the lab in a transport dewar and stored in a –80 °C freezer until analysed. The remaining vine and root system was dried for a minimum of 48h at 90 °C for biomass measurements.
For root tip and mature root analyses roots were first dissected and then immediately frozen using liquid N2 as described above. The root tip section consisted of the first 2cm of the root tip while mature root portions were comprised of root sections 10–20cm proximal to the tip from which all lateral roots were removed.
Aquaporin gene expression
Gene expression analyses were carried out according to Choat et al. (2009). In short, total RNA was extracted, treated with DNase, and reverse transcribed following the methods described by Castellarin et al. (2007). Quantitative real-time PCR was carried out in an ABI PRISM 7700 sequence detector (Applied Biosystems). Each reaction (20 µl) contained 1mM of each primer, 5 µl of 1:400 or 1:4,000 diluted cDNA, and 10 µl of Power SYBR Green Master Mix (Applied Biosystems). Thermal cycling conditions were 95 °C for 10min followed by 40 cycles of 95 °C for 30 s, 56 °C for 30 s, and 60 °C for 60 s. Both cDNA dilutions were run in duplicate. For rootstock studies gene transcripts were normalized to VvUbiquitin1 (TC32075, Institute for Genomic Research database) by comparing the cycle threshold (CT) of the target gene with that of VvUbiquitin1 (Bogs et al., 2005) via the comparative CT method. Gene expression was expressed as mean and SE calculated over all biological replicates. For the determination of VvUbiquitin1 variation expression was quantified absolutely using genomic DNA standards (Yun et al., 2006; Gambetta et al., 2010). Primer pair sequences for the VvPIP isogenes and VvUbiquitin1 can be found in Supplementary Table S1 (available at JXB online) and all primer pairs were validated by isolating and sequencing their PCR products to confirm identity. Aquaporin gene expression was quantified in fine roots across rootstocks relative to VvUbiquitin1, which provided a stable reference and when expression was absolutely quantified its CV was 3.6%.
Across studies involving Vitis, there is confusion when integrating genomic and cDNA for a number of VvPIP isogenes making it difficult to resolve if multiple extremely closely related cDNAs represent allelic variants, true isogenes, or possibly the same gene (in the case of partial cDNAs). This is due to the high level of conservation among PIPs at the DNA level (Shelden et al., 2009) and the high level of heterozygosity present among V. vinifera cultivars (Myles et al., 2011). To account for these issues, all available VvPIP gene sequences (Shelden et al., 2009) were clustered (unpublished data) and primer pairs were designed to amplify all related gene sequences within a given cluster of extremely closely related isogene/allelic variants by designing primer sequences across regions of perfect homology. This is especially important regarding PIP1-2 and PIP1-4 which are 98% identical at the DNA level, and PIP1-3 and PIP1-5 which are 96% identical at the DNA level (Shelden et al., 2009). Therefore, this study has reported expression levels as PIP1-2:1-4 and VvPIP1-3:1-5 for these putative isogenes/allelic variants.
Fine-root Lp r
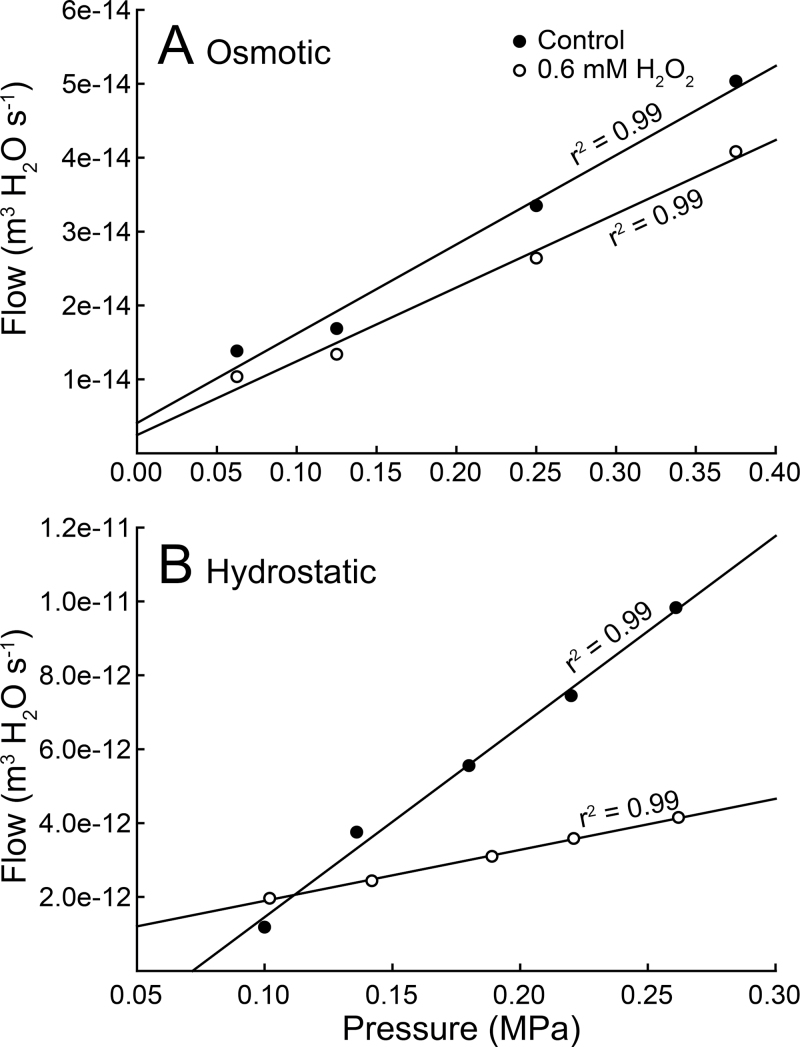
Hydraulic conductivity (Lp r) was measured in fine roots using two different methods depending on the driving force used. For experiments using a hydrostatic pressure gradient, a meniscus tracking method similar to that described by Choat et al. (2009) was used with modifications to the apparatus based upon feasibility differences in applying pressure to fine roots versus berries (Supplementary Fig. S1). Healthy, unbranched fine roots (including tip) were excised under water (using a fresh razor blade). Fine roots were fed through the cylinder of a hard, plastic luer fitting (polypropylene 3.2mm 200 series, Value Plastics, Fort Collins, CO, USA) such that the upper ~3mm of the segment (i.e. the downstream end of the segment) was left exposed above the fitting. The root was sealed into the luer fitting using non-toxic, dental impression polymer (Pentron Clinical Technologies, LLC, Wallingford, CT, USA) to prevent compression of the tissue (similar to details described in McElrone et al., 2007). Seals were tested for each sample prior to taking measurements. Samples were then connected to additional luer connectors attached to plastic tubing fed through the lid of a pressure chamber (Soil Moisture Equipment Corp, Santa Barbara, CA, USA) and submersed in diH2O inside the pressure chamber. The tube protruding from the lid was connected to a Swagelok reducing union that also held a microcapillary (i.d. = 20 µm) that was used to measure outflow from the sample by tracking the movement of a meniscus at the air–water interface. The first hydrostatic experiments used a single pressure of 0.2MPa to determine fine-root Lp r, which was confirmed to be in the linear range (see corresponding results in Fig. 5). A follow-up experiment utilized two of the rootstocks with consistently divergent vigour ratings and VvPIP gene expression (420A and 110R) to determine pressure–flow relationships in both hydrostatic and osmotic gradients, and to confirm accuracy of the single pressure measurements in the first experiment. For the hydrostatic measurements, a range of pressures was used (0.1–0.3MPa, in a minimum of four 0.03–0.05MPa pressure steps; see Fig. 5). Baseline Lp r values were first obtained with the root submersed in diH2O, and then measurements were repeated with 0.6mM H2O2 for aquaporin chemical inhibition. Hydrogen peroxide based solutions have been used effectively as inhibitors of aquaporin activity, while providing lower toxicity than mercuric chloride (HgCl2) (Henzler et al., 2004; Ye and Steudle, 2006; McElrone et al., 2007). The distance travelled by the meniscus was recorded every 60 s to calculate a volumetric flow rate. Lp r (m s–1 MPa–1) was calculated using the following equation: Lp r = (Qv/P) × (1/A), where Qv is the volumetric flow rate (m3 s–1), P (MPa) is the pressure applied to the root, and A (m2) is the surface area of a cylinder calculated from fine-root segment length and radius (North and Nobel, 1991). Upon completion of Lp r measurements, roots were scanned using an Epson 1640 scanner (Seiko Epson Corporation, Nagano, Japan) and then analysed using WinRHIZO version 2003a (Régent Instruments, Quebec, Canada) to obtain data regarding root length, surface area, and diameter. The WinRHIZO program and this scanning method have been determined to be an accurate and effective method of obtaining root data (Himmelbauer et al., 2004). Root biomass was tightly correlated with root surface area regardless of rootstock (r 2 = 0.78, P < 0.001). Root surface area was determined from root biomass according to this relationship.
Fig. 5.
Examples of osmotic (A) and hydrostatic (B) pressure–flow relationships: typical examples across a range of pressures for both control (filled circles) and 0.6mM H2O2 treated (open circles) 110R roots.
For experiments using an osmotic pressure gradient, healthy unbranched fine roots (including tip) were excised under water (using a fresh razor blade) and glued into a 500-µm diameter glass capillary. The capillary and root were fed into a custom-made chamber (Supplementary Fig. S1) in which the solution could be changed and flow through the root was quantified via the movement of the meniscus in the capillary. Roots were equilibrated for at least 1h in diH2O followed by measurements of flow in sucrose solutions of various osmotic strengths (0, 0.125, 0.25, 0.5MPa). In some cases measurements were replicated on the same root using both sucrose and mannitol solutions of equal osmotic strengths with no difference in the resulting Lp r. The root was allowed to equilibrate in each solution for at least 30 minutes and flows were stable. Lp r was determined as the slope of the pressure–flow relationship across at least four different osmotic pressures (e.g. Fig. 5). Aquaporin inhibition was then carried out immediately on the same root as described above.
Rootstock and aquaporin inhibition effects on whole-vine transpiration
To measure the effects of rootstock on whole-vine transpiration, vines with a common Cabernet Sauvignon scion were grafted onto the four rootstocks described above. These vines were grown in 4.3 l pots under greenhouse conditions, watered to saturation once daily, fertilized with quarter-strength modified Hoagland’s solution weekly.
Vines were watered to saturation at the start of each day with either diH2O or a 35mM H2O2 solution, allowed to drain, and sealed in plastic bags up to the base of the stem. Each bagged, potted plant was weighed every 2h on a laboratory scale (EA150CE-1, Sartorius, Goettingen, Germany). Bags were removed after the final weighing of the day at 8 p.m. and all vines were watered with diH2O. This protocol was repeated for multiple days, and midday water use for each rootstock was determined from this data. At the end of the experiment, leaf area was determined for each vine using a LI-3000C leaf area meter (Licor Biosciences, Lincoln, NE, USA), and midday transpiration rates of the whole vine were calculated on a leaf area basis.
Statistical analysis
All statistical analyses were carried out using SAS software (SAS Institute, http://www.sas.com). ANOVAs were carried out by both rootstock and treatment (control and inhibited) and Tukey’s HSD was used for means separation when ANOVA results were significant.
Results
Rootstock effects on vine growth
Under the experimental conditions of this study, the vine growth parameters were consistent with commonly used vigour ratings for the study rootstocks (Table 1). Leaf area and/or root biomass were significantly greater in the high-vigour rootstocks (1103P and 110R) compared to those from rootstocks that are commonly considered low-to-moderate vigour (420A and 101-14Mgt) (P < 0.05; Table 1). These results are consistent with a recent summary of field rootstock trials from California; across five sites and three scion varieties pruning weights of scions grown on 1103P and 110R rootstocks were significantly greater by an average 43% (range of 25–70%) compared to 420A and 101-14Mgt rootstocks across sites (P < 0.05) (JA Wolpert, personal communication). There were also very few differences between rootstocks within a given vigour rating (i.e. 110R vs. 1103P) in these field trials.
Table 1.
Leaf area and root weights of grape rootstocks tested in this study. Value are mean ± SE. Different superscript letters within a row indicate significant differences among rootstocks (n = 12; P < 0.05).
| Rootstock | ||||
|---|---|---|---|---|
| 420A | 101-14Mgt | 1103P | 110R | |
| Leaf area (cm2) | 2343±96a,b | 2111±130a | 2609±67b | 2574±198b |
| Root dryweight (g) | 13.6±1.1a | 12.6±2.1a | 18.3±2.2a,b | 23.2±2.5b |
| Root freshweight (g) | 57.0±3.4a | 56.0±5.6a | 62.8±7.4a,b | 72.2±3.7b |
Aquaporin gene expression
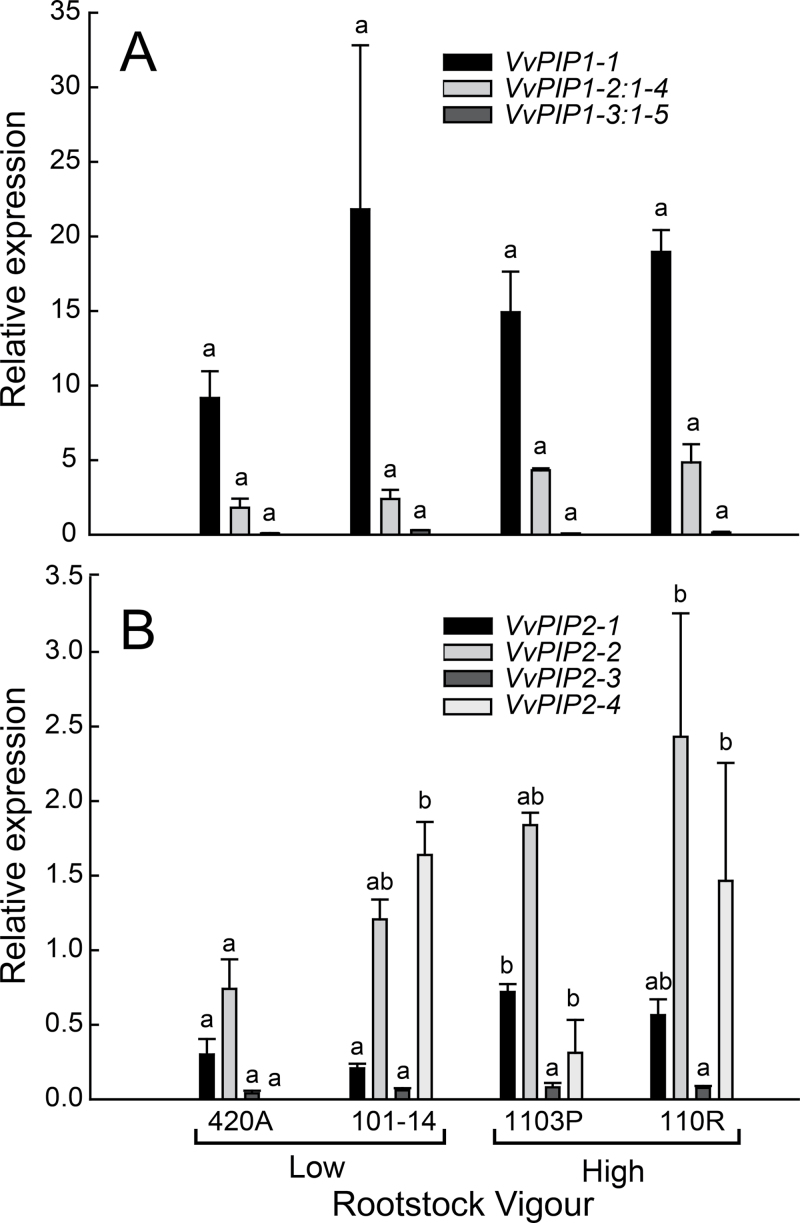
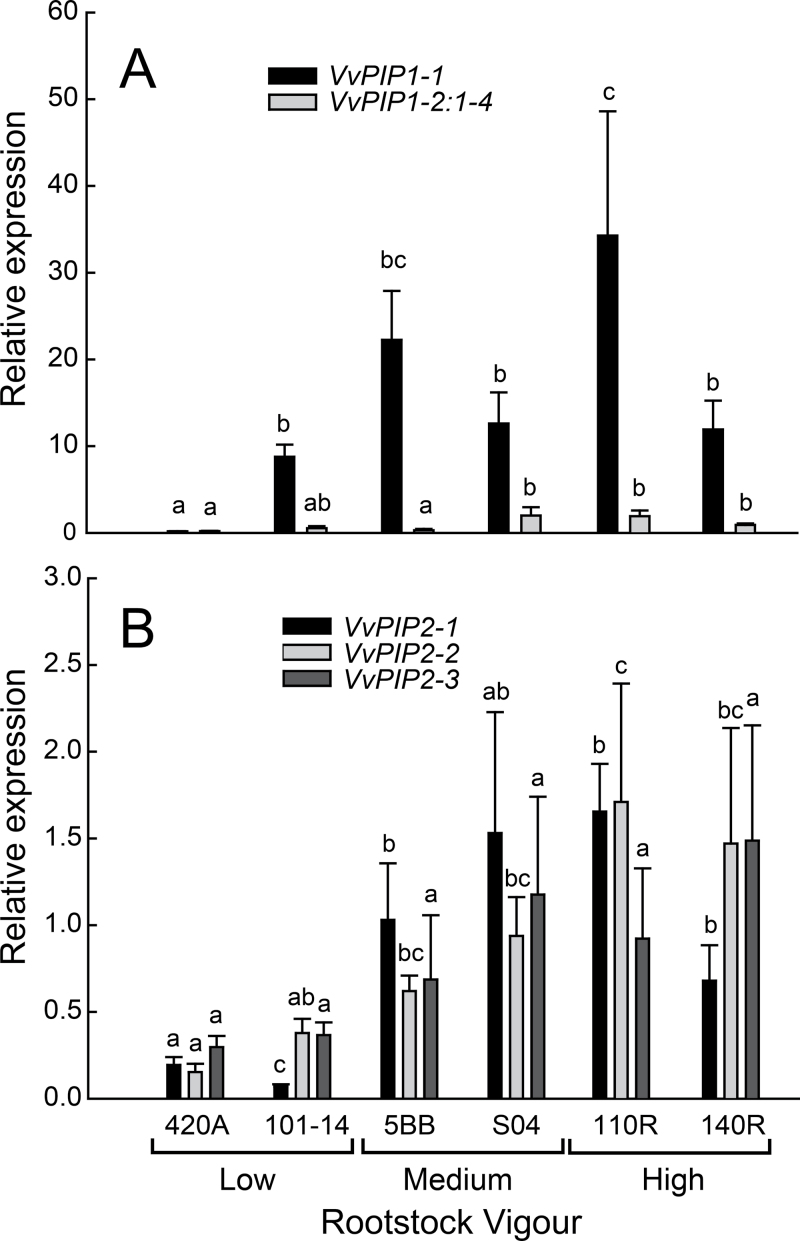
Under favourable growing conditions, expression of several aquaporin genes varied significantly between rootstocks and some were greater for higher-vigour rootstocks regardless of the growing media (Figs. 1 and 2). VvPIP1–1 was the most prominently expressed aquaporin isogene with levels at least 3-fold greater than that of VvPIP1-2:1-4 and at least 5-fold greater than any VvPIP2 family member (Figs. 1 and 2). VvPIP1-3: 1-5 was expressed at extremely low levels in soil (Fig. 1A) and was undetectable in hydroponically grown roots (Fig. 2A). The expression of many VvPIP2 family members were greater in high-vigour rootstocks (Figs. 1B and 2B). Expression of both VvPIP2-1 and VvPIP2-2 increased with vigour in both soil and hydroponics (Figs. 1B and 2B). VvPIP2-3 followed this same general trend with increased expression in higher-vigour rootstocks when plants were grown in hydroponics (Fig. 2B), but in soil VvPIP2-3 consistently had the lowest expression of all isogenes (Fig. 1B). VvPIP2-4 was variably expressed in all rootstocks, but was undetected in the lowest-vigour rootstock 420A (Fig. 1B).
Fig. 1.
Individual (greyscale) relative expression (relative to VvUbiquitin1) for VvPIP1 (A) and VvPIP2 (B) gene families in various rootstocks grown in soil media. Bars represent mean ± SE and different letters indicate significant differences between rootstocks for each isogene (n = 3; P < 0.05).
Fig. 2.
Individual (grayscale) relative expression for the (A) VvPIP1 and (B) VvPIP2 gene families for various grapevine rootstocks grown in a re-circulating drip hydroponic system. Due to lack of adequate sample tissue at the time of sample repeat analysis, VvPIP2-4 expression was not assessed in hydroponically grown roots. Bars represent mean ± SE and different letters indicate significant differences between rootstocks for each isogene (n = 4; P < 0.05).
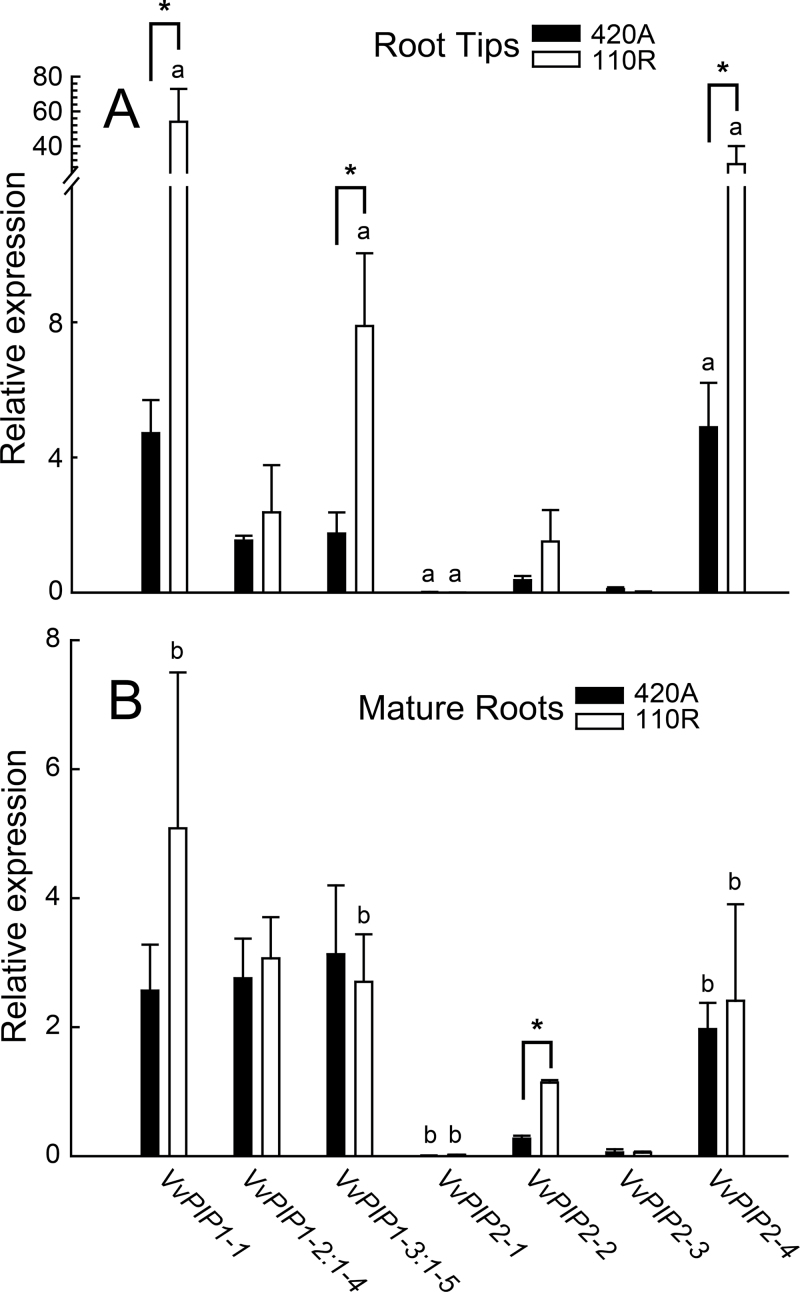
Root tips (apical 2cm of the fine root) typically had greater levels of VvPIP expression than mature roots (10–20cm back from the tip), and this pattern was even more dramatic in 110R compared to 420A (i.e. tips are more different than mature roots for 110R, whereas expression was much more consistent along the length of the root for 420A) (Fig. 3). Interestingly, there were few significant differences between the rootstocks within the mature root zone, suggesting that any differential aquaporin physiology between rootstocks would be realized almost exclusively in the root tip. Even though aquaporin expression was dominant in the root tip, which represents a small portion of the total root absorptive area, aquaporins still contributed significantly to the Lp r of the fine roots (see results below).
Fig. 3.
Individual VvPIP isogene expression in root tips (A) and mature roots (B) of 420A (black) and 110R (white). Bars represent mean ± SE and asterisks indicate significant differences between rootstocks for a given isogene (n = 3–5; P < 0.05). Bars labelled with different letters between panels A and B represent significant differences within a rootstock between root tips and mature roots for a given isogene (n = 3–5; P < 0.05).
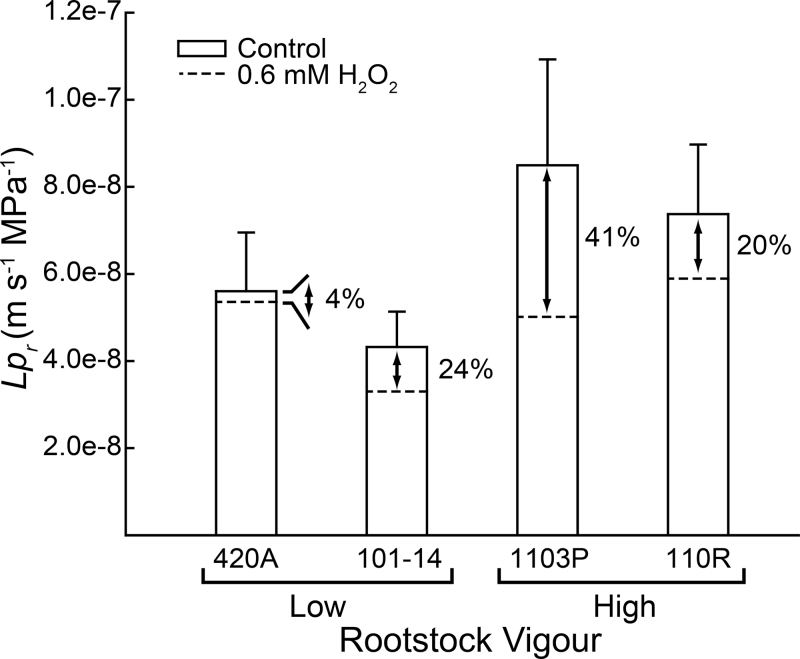
Root Lp r
Hydrostatically driven fine-root Lp r was originally measured on four rootstocks of varying vigour (Fig. 4). Average Lp r Hyd of fine roots was approximately 60% greater in high-vigour rootstocks, although the differences were not significant (P > 0.05) due to high variability across roots. Lp r Hyd for all rootstocks was reduced in response to chemical inhibition, with the greatest absolute reduction observed in 1103P and 110R. Differences in mean Lp r Hyd among high- and low-vigour rootstocks were reduced with aquaporin inhibition (Fig. 4A). 420A had the lowest level of inhibition, which corresponded with the consistently low expression levels of the VvPIP isogenes (Figs. 1 and 2).
Fig. 4.
Hydrostatic fine-root hydraulic conductivity (Lp r Hyd) across rootstocks. Bars represent mean native fine-root Lp r Hyd and dashed lines represent fine-root Lp r Hyd after inhibition. The percentage reduction in fine-root Lp r caused by the inhibition is shown to the right of each bar. Values are mean ± SE (n≥7).
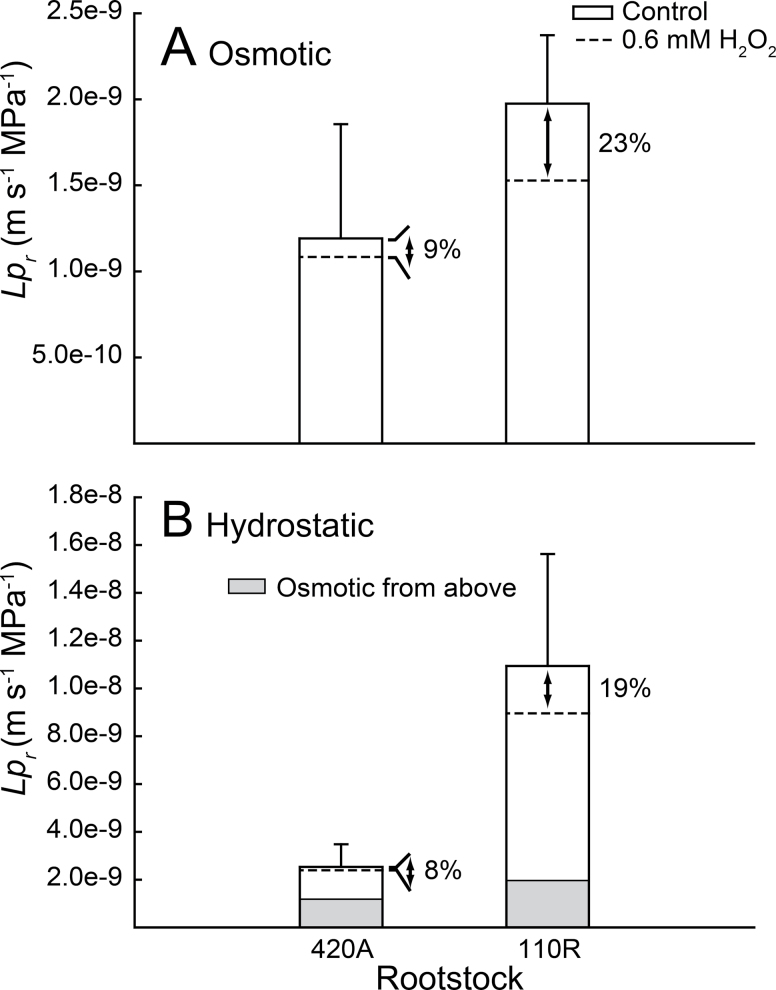
Due to their consistently divergent patterns of VvPIP gene expression, rootstocks 420A and 110R were used in follow-up experiments to assess aquaporin contribution to fine-root Lp r under both osmotic (Lp r Osm) and hydrostatic (Lp r Hyd) pressure gradients (Figs. 5 and 6). Pressure and flow were linearly related over a broad range of pressures for both osmotic and hydrostatic gradients, and aquaporin inhibition decreased Lp r while the pressure–flow relationships remained linearly related (Fig. 5). Values of Lp r and % aquaporin contribution to Lp r were similar when a single pressure or range of pressures was used for the measurements (Figs. 4 and 6). Lp r Hyd was greater than Lp r Osm for both rootstocks, but the difference was only 2-fold greater for 420A, while it was ~5-fold greater for 110R (Fig. 6B; compare shaded regions to full bar). Both Lp r Osm and Lp r Hyd were lower in the low-vigour rootstock 420A, but differences were not significantly different (P > 0.05). The decrease in Lp r Osm and Lp r Hyd due to aquaporin inhibition was equivalent within each rootstock (i.e. the % decrease was similar regardless of whether flow was driven osmotically or hydrostatically), but 110R roots exhibited a significantly greater reduction (~20%) in Lp r compared to ~9% in 420A (P < 0.02) under both conditions (Fig. 6).
Fig. 6.
Fine-root hydraulic conductivity (Lp r) of 420A and 110R rootstocks for osmotically (A) or hydrostatically (B) produced pressure gradients. Bars represent mean native fine-root Lp r (i.e. uninhibited) and dashed lines represent fine-root Lp r after chemical inhibition. For hydrostatic data, the grey portion of the bar represents the osmotic data from (A) for reference. The percentage reduction in fine-root Lp r caused by the inhibition is shown to the right of each bar. Values are mean ± SE and different letters indicate significant differences between rootstocks (n≥3; P < 0.05).
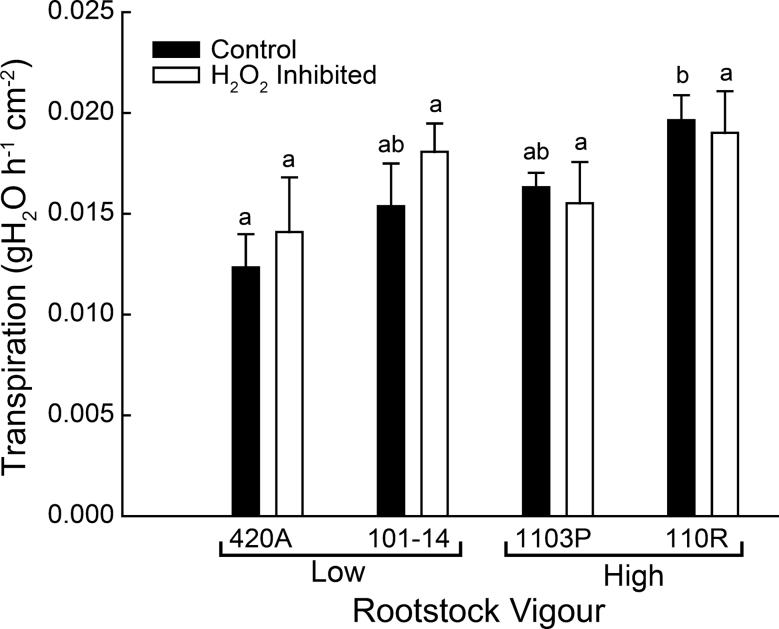
Whole-plant relationships
Whole-vine water use was positively correlated with rootstock vigour; 110R had significantly greater transpiration per unit leaf area than 420A (Fig. 7). No significant effect of aquaporin chemical inhibition on transpiration was found for any of the rootstocks, but differences between the rootstocks disappeared when the root systems were treated with the inhibitor.
Fig. 7.
Midday transpiration for whole vines grafted on to each of four rootstocks. Leaf area was determined for each vine at the end of the experiment and used to scale measurements per unit leaf area. Data represent the mean ± SE and different letters indicate significant differences between rootstocks (n ≥5; P < 0.05). There were no significant differences in transpiration between control and H2O2 inhibited (P = 0.60).
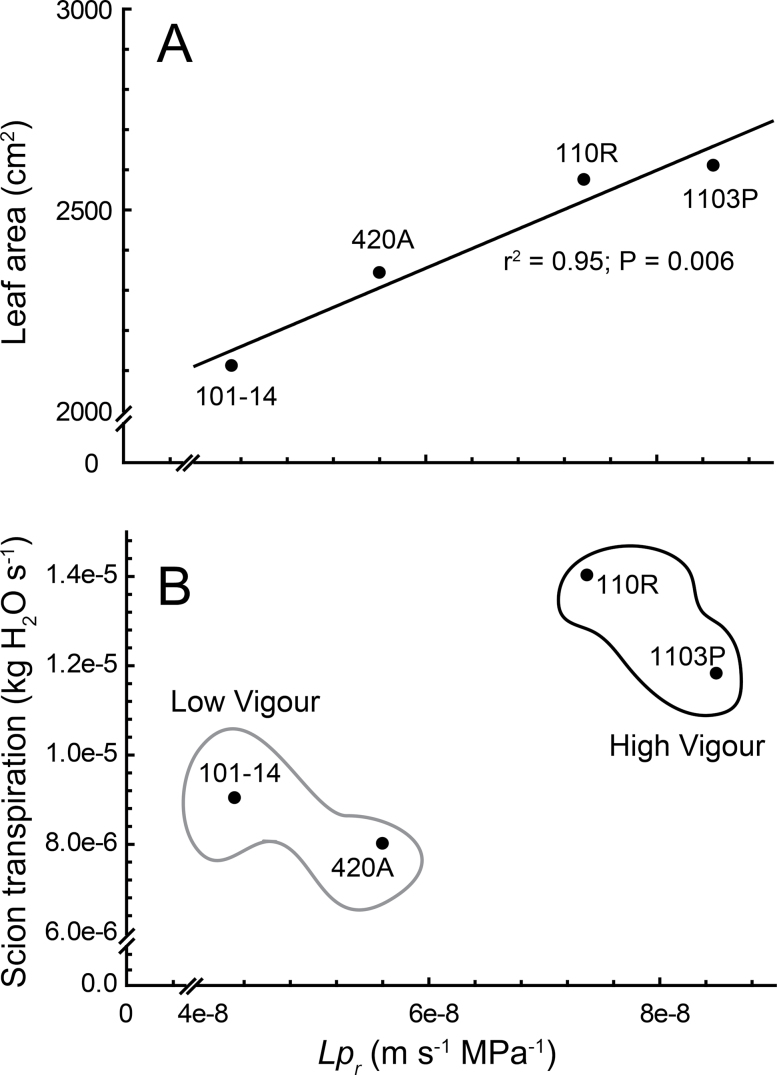
In order to relate differences in fine-root Lp r to canopy water demands, this study calculated correlations among several parameters. Fine-root Lp r was positively correlated with leaf area and whole-vine transpiration, respectively (Fig. 8). Fine-root Lp r increased by 60% on average for high-vigour rootstocks compared to their low-vigour counterparts (Fig. 8A).
Fig. 8.
Relationships of fine-root Lp r Hyd with leaf area (A) and whole-plant water use (B) across the four rootstocks.
Discussion
The Vitis rootstocks with varying vigour classifications studied here exhibit significantly different patterns of VvPIP expression under favourable conditions in a variety of growth media; VvPIP expression was consistently greater in high-vigour rootstocks. Similarly, fine-root Lp r and % aquaporin contribution to Lp r determined under both osmotic (Lp r Osm) and hydrostatic (Lp r Hyd) pressure gradients were consistently greater in high-vigour rootstocks. Leaf area-specific transpiration and total leaf area increased with rootstock vigour and were positively correlated with fine-root Lp r. These results suggest that differences among rootstocks results in part from the differences in individual fine-root Lp r (influenced by VvPIP gene expression and activity).
Variable aquaporin expression and activity across rootstocks
Since the C–C pathway plays a prominent role in radial water absorption across fine roots (Steudle, 2001), it follows that aquaporins would contribute to hydraulic differences among rootstocks. In this study, high-vigour rootstocks had greater expression of several VvPIP isogenes and an average Lp r that was approximately 60% greater than low-vigour rootstocks, although Lp r was so variable that differences were not significant. Evidence from multiple studies on transgenic systems is mixed regarding the linkage between aquaporin expression, root Lp r, and vigour. NtAQP1 antisense lines of tobacco exhibited reduced root Lp r yet no differences in plant growth (Siefritz et al., 2002). Others found that constitutive overexpression of AtPIP1b in tobacco resulted in increased shoot biomass, but the role root hydraulics was not investigated (Aharon et al., 2003). Several studies using Arabidopsis found that various aquaporin antisense and knockout lines exhibited decreases in Lp r with no change in shoot biomass (Kaldenhoff et al., 1998; Javot and Maurel, 2002; Martre et al., 2002). These studies and the results reported here suggest that varied aquaporin expression may be involved in altering the growth potential of the scion in ways other than or in addition to directly altering hydraulic conductance consistently across the root system.
In this study, the % reduction in Lp r due to aquaporin inhibition was correlated with VvPIP expression and varied among rootstocks, ranging from just 4% in 420A to 40% in 1103P. This range suggests that in general the apoplastic pathway dominates radial transport in fine roots of grapevine under favourable conditions. The magnitude of aquaporin contribution to Lp r is highly variable across species, ranging from 20–90%, with higher values typically reported for herbaceous species (reviewed in Javot and Maurel, 2002). The reductions in Lp r found here are similar in magnitude to the 5–45% reductions in root-system Lp r when aquaporins were inhibited with mercuric chloride across several Vitis rootstocks under water deficit (Lovisolo et al., 2008). Studies in Arabidopsis utilizing antisense and mutant lines have found similar results with contributions ranging from 20–60% (Martre et al., 2002; Javot et al., 2003; Postaire et al., 2010).
Even though there were consistent differences in the expression of VvPIP genes in bulk fine-root tissue among rootstocks, the localized patterns of expression and varying permeability in different tissue and cell types likely play important roles in regulating water uptake (e.g. Knipfer et al., 2011). Large differences in VvPIP expression were found along the length of the root with greater expression in root tips. This finding is consistent with previous studies that reported peak mRNA and/or protein levels of PIPs in root tips of tobacco, grapevines, and barley, respectively (Otto and Kaldenhoff, 2000; Vandeleur et al., 2009; Knipfer et al., 2011). Despite historical literature demonstrating that unsuberized fine-root tips constitute a small proportion of total root system and that significant water uptake can occur in older suberized portions of roots (Kramer and Bullock, 1966; Chung and Kramer, 1975; Macfall et al., 1990), contemporary studies often imply that water uptake occurs only where fine-root tips proliferate. In fact, Queen (1967) measured the relative permeability of various portions of Concord grapevine root systems and found that the terminal end of the root (~8cm) was 65–545-times less permeable to water than any other part of the current season roots (measured incrementally to ~20cm back from the root tip and included partially suberized tissue). The current season roots were also only 5-times more permeable than the heavily suberized roots from previous growing seasons (Queen, 1967). Given the potential for substantial water uptake in older portions of grapevine roots, one would expect consistent PIP expression along the root length if aquaporins were playing a consistent role in increasing root permeability. Higher PIP expression and activity in root tips of woody plants may actually play an analogous role to patterns seen in barley roots, an herbaceous species (e.g. Knipfer et al., 2011). Instead of altering bulk permeability of the entire root-system, peak aquaporin expression in the differentiation and elongation zones of the root tip may play an important role in influencing the rate of growth and architecture of new root tissue. This is consistent with the hypothesis that the predominance of aquaporin localization in vascular tissues, and especially phloem tissues, suggests a role in source–sink relationships (Schaffner, 1998; Suga et al., 2003; Fraysse et al., 2005). Concentrated expression of aquaporins in the root tip could also enable the advancing tissue to better sense and handle changing spatial and temporal conditions of the soil (Eapen et al., 2005).
In this study, expression of several VvPIP2 isogenes was significantly greater in high-vigour rootstocks when compared to the low-vigour rootstock 420A. However, there is overlap in expression patterns between the high-vigour rootstocks and 101-14, a low-vigour rootstock. Several studies on other plant species have shown that expression of PIP2 isoforms results in greater membrane water permeability in Xenopus oocytes compared with PIP1 isoforms, which are often hydraulically inactive/non-functional (Yamada et al., 1995; Chaumont et al., 2000; Dordas et al., 2000; Vandeleur et al., 2009). However, co-expression of particular PIP1 and PIP2 isoforms can increase membrane hydraulic permeability above levels measured with the expression of those genes alone (Fetter et al., 2004; Alleva et al., 2010); a similar pattern was found for VvPIPs of grapevines (Vandeleur et al., 2009). Therefore, the current study suggests that differences in VvPIP1 expression could play a large role in controlling hydraulic permeability through VvPIP1-VvPIP2 interactions as suggested by Vandeleur et al. (2009). Fine-scale details of VvPIP co-regulation may play a larger functional role under water deficit conditions when the contribution of the C–C pathway to radial transport increases due to the formation of apoplastic barriers (Vandeleur et al., 2009). More work is needed in this area to determine the interactive role of VvPIPs in controlling cellular water relations, particularly in the root tip under varying conditions.
This study found that Lp r Hyd was much greater than Lp r Osm for both low-vigour 420A and high-vigour 110R rootstocks. This finding is consistent with expectations based on the parallel pathways of the composite transport model (Steudle, 2001), where the apoplast has a lower resistance and would predominate during high flow conditions driving by transpiration. However, Lp r Hyd and Lp r Osm for a given rootstock exhibited similar %reductions in flow under aquaporin inhibition- implying there is still substantial flow through the C–C pathway even under a hydrostatic driving force. Roots provide a complex anatomical context for radial water movement, where the pathways exist in parallel in the cortex and stele but movement is presumably restricted to the C–C pathway through the endodermis (Knipfer and Fricke, 2010). In fine roots, including both growing and differentiated root portions, the anatomical context is further complicated since the growing root portions often lack a developed Casparian band or other apoplastic barriers.
Vigour and root hydraulic conductance
For several perennial crop species, altered scion vigour has been linked to differences in hydraulic parameters of the root system. The hydraulic capacity of a root system to deliver water to the scion can be brought about by increases in Lp r (per root surface area or per biomass), and/or whole-root-system surface area. Lovisolo et al. (2007) showed that lower whole-root-system hydraulic conductance of olive dwarfing rootstocks resulted primarily from decreased root-system biomass. Low whole-root-system hydraulic conductance found in low-vigour rootstocks of peach was associated with less fine-root surface area quantified as length per unit root dryweight (Solari et al., 2006). In a recent study of drought resistance, Alsina et al. (2011) found that grapevines grafted onto 1103P rootstock (high vigour) exhibited greater whole-root-system hydraulic conductance compared to 101-14 (low vigour) resulting from continued growth at greater depth during the warmer and drier summer months.
Several studies suggest that whole-root-system hydraulic conductance is positively correlated with vigour (Nardini et al., 2006; Solari et al., 2006; Clearwater et al., 2004; Lovisolo et al., 2007), but the correlation of fine-root Lp r (per root surface area or per biomass) appears much more variable. In the current study, root surface area-specific Lp r was positively correlated with leaf area and canopy water demands, a pattern similar to those demonstrated in deep fine roots accessed via caves (McElrone et al., 2007) and in apple using root hydraulic conductance per length (Atkinson et al., 2003). However, Clearwater et al. (2004) found a positive correlation between whole-root-system hydraulic conductance and vigour despite root hydraulic conductance per amount of leaf area being greatest in low-vigour rootstocks. The current analysis of results presented in Solari et al. (2006) demonstrated that when whole-root-system hydraulic conductance was scaled per unit root dryweight it was also greater for dwarfing rootstocks. Likewise, Lovisolo et al. (2007) found the same relationship in olive (using values scaled per unit root dryweight) and the authors concluded that higher aquaporin expression found in the dwarfing rootstock was responsible for the increased hydraulic conductance.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Table S1. Primer pair sequences used in this study.
Supplementary Fig. S1. Experimental set-up for the determination of Lp r with osmotic and hydrostatic pressure gradients.
Acknowledgements
The authors would like to acknowledge funding from the USDA-ARS CRIS research project 5306-21220-004-00 and grants from the American Vineyard Foundation to A.J.M. and the California Grape Rootstock Improvement Commission to K.F. and M.A.W. They are grateful to T. Knipfer for his critical input into the manuscript, K. Hoover for help with root sampling and molecular analyses, and J. Wolpert and M. Anderson for providing access to rootstock trial data for comparisons.
References
- Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G. 2003. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress The Plant Cell 15 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva K, Marquez M, Villarreal N, Mut P, Bustamante C, Bellati J, Martinez G, Civello M, Amodeo G. 2010. Cloning, functional characterization, and co-expression studies of a novel aquaporin (FaPIP2;1) of strawberry fruit Journal of Experimental Botany 61 3935–3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsina MM, Smart DR, Bauerle T, de Herralde F, Biel C, Stockert C, Negron C, Save R. 2011. Seasonal changes of whole root system conductance by a drought-tolerant grape root system Journal of Experimental Botany 62 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson CJ, Else MA, Taylor L, Dover CJ. 2003. Root and stem hydraulic conductivity as determinants of growth potential in grafted trees of apple (Malus pumila Mill.). Journal of Experimental Botany 54 1221–1229 [DOI] [PubMed] [Google Scholar]
- Bogs J, Downey MO, Harvey JS, Ashton AR, Tanner GJ, Robinson SP. 2005. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves Plant Physiology 139 652–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi-Vasconcelos MC, Candolfi MP, Koblet W. 1994. Retranslocation of carbon reserves from the woody storage tissues into the fruit as a response to defoliation stress during the ripening period in Vitis vinifera L Planta 192 567–573 [Google Scholar]
- Castellarin SD, Matthews MA, Di Gaspero G, Gambetta GA. 2007. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries Planta 227 101–112 [DOI] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Jung R, Chrispeels MJ. 2000. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity Plant Physiology 122 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Gambetta GA, Shackel KA, Matthews MA. 2009. Vascular function in grape berries across development and its relevance to apparent hydraulic isolation Plant Physiology 151 1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HH, Kramer PJ. 1975. Absorption of water and 32P through suberized and unsuberized roots of loblolly pine Canadian Journal of Forest Research 5 229–235 [Google Scholar]
- Clearwater MJ, Lowe RG, Hofstee BJ, Barclay C, Mandemaker AJ, Blattmann P. 2004. Hydraulic conductance and rootstock effects in grafted vines of kiwifruit Journal of Experimental Botany 55 1371–1382 [DOI] [PubMed] [Google Scholar]
- Cortell JM, Halbleib M, Gallagher AV, Righetti TL, Kennedy JA. 2005. Influence of vine vigor on grape (Vitis vinifera L. cv. Pinot noir) and wine proanthocyanidins Journal of Agricultural and Food Chemistry 53 5798–5808 [DOI] [PubMed] [Google Scholar]
- Cortell JM, Halbleib M, Gallagher AV, Righetti TL, Kennedy JA. 2007. Influence of vine vigor on grape (Vitis vinifera L. cv. Pinot Noir) anthocyanins. 1. Anthocyanin concentration and composition in fruit Journal of Agricultural and Food Chemistry 55 6575–6584 [DOI] [PubMed] [Google Scholar]
- Cortell JM, Sivertsen HK, Kennedy JA, Heymann H. 2008. Influence of vine vigor on Pinot noir fruit composition, wine chemical analysis, and wine sensory attributes American Journal of Enology and Viticulture 59 1–10 [Google Scholar]
- Dordas C, Chrispeels MJ, Brown PH. 2000. Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots Plant Physiology 124 1349–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen D, Barroso ML, Ponce G, Campos ME, Cassab GI. 2005. Hydrotropism: root growth responses to water Trends in Plant Science 10 44–50 [DOI] [PubMed] [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F. 2004. Interactions between plasma membrane aquaporins modulate their water channel activity The Plant Cell 16 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraysse LC, Wells B, McCann MC, Kjellbom P. 2005. Specific plasma membrane aquaporins of the PIP1 subfamily are expressed in sieve elements and guard cells Biology of the Cell 97 519–534 [DOI] [PubMed] [Google Scholar]
- Frensch J, Steudle E. 1989. Axial and radial hydraulic resistance to roots of maize (Zea mays L) Plant Physiology 91 719–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambetta GA, Matthews MA, Shaghasi TH, McElrone AJ, Castellarin SD. 2010. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape Planta 232 219–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves B, Correia CM, Silva AP, Bacelar EA, Santos A, Ferreira H, Moutinho-Pereira JM. 2007. Variation in xylem structure and function in roots and stems of scion-rootstock combinations of sweet cherry tree (Prunus avium L.) Trees Structure and Function 21 121–130 [Google Scholar]
- Henzler T, Ye Q, Steudle E. 2004. Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals Plant, Cell and Environment 27 1184–1195 [Google Scholar]
- Himmelbauer ML, Loiskandl W, Kastanek F. 2004. Estimating length, average diameter and surface area of roots using two different image analyses systems Plant and Soil 260 111–120 [Google Scholar]
- Iwanami H, Moriya S, Abe K. 2009. Relationships between sap flow, hydraulic conductivity, and the anatomical characteristics of stems and roots in apple rootstocks of different vigour Journal of Horticultural Science and Biotechnology 84 632–638 [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, et al. 2003. Role of a single aquaporin isoform in root water uptake The Plant Cell 15 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javot H, Maurel C. 2002. The role of aquaporins in root water uptake Annals of Botany 90 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Grote K, Zhu JJ, Zimmermann U. 1998. Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana The Plant Journal 14 121–128 [DOI] [PubMed] [Google Scholar]
- Knipfer T, Besse M, Verdeil J, Fricke W. 2011. Aquaporin-facilitated water uptake in barley (Hordeum vulgare L.) roots Journal of Experimental Botany 62 4115–4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipfer T, Fricke W. 2010. Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare) New Phytologist 187 159–170 [DOI] [PubMed] [Google Scholar]
- Kramer PJ, Bullock HC. 1966. Seasonal variations in proportions of suberized and unsuberized roots of trees in relation to absorption of water American Journal of Botany 53 200–204 [Google Scholar]
- Lovisolo C, Secchi F, Nardini A, Salleo S, Buffa R, Schubert A. 2007. Expression of PIP1 and PIP2 aquaporins is enhanced in olive dwarf genotypes and is related to root and leaf hydraulic conductance Physiologia Plantarum 130 543–551 [Google Scholar]
- Lovisolo C, Tramontini S, Flexas J, Schubert A. 2008. Mercurial inhibition of root hydraulic conductance in Vitis spp. rootstocks under water stress Environmental and Experimental Botany 63 178–182 [Google Scholar]
- Macfall JS, Johnson GA, Kramer PJ. 1990. Observation of a water-depletion region surrounding loblolly-pine roots by magnetic-resonance-imaging Proceedings of the National Academy of Sciences, USA 87 1203–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ. 2002. Plasma membrane aquaporins play a significant role during recovery from water deficit Plant Physiology 130 2101–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrone AJ, Bichler J, Pockman WT, Addington RN, Linder CR, Jackson RB. 2007. Aquaporin-mediated changes in hydraulic conductivity of deep tree roots accessed via caves Plant, Cell and Environment 30 1411–1421 [DOI] [PubMed] [Google Scholar]
- Myles S, Boyko AR, Owens CL, et al. 2011. Genetic structure and domestication history of the grape Proceedings of the National Academy of Sciences, USA 108 3530–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A, Gasco A, Raimondo F, Gortan E, Lo Gullo MA, Caruso T, Salleo S. 2006. Is rootstock-induced dwarfing in olive an effect of reduced plant hydraulic efficiency? Tree Physiology 26 1137–1144 [DOI] [PubMed] [Google Scholar]
- North GB, Nobel PS. 1991. Changes in hydraulic conductivity and anatomy caused by drying and rewetting roots of Agave deserti (Agavaceae) American Journal of Botany 78 906–915 [Google Scholar]
- Olmstead MA, Lang NS, Ewers FW, Owens SA. 2006a. Xylem vessel anatomy of sweet cherries grafted onto dwarfing and nondwarfing rootstocks Journal of the American Society for Horticultural Science 131 577–585 [Google Scholar]
- Olmstead MA, Lang NS, Lang GA, Ewers FW, Owens SA. 2006b. Examining the vascular pathway of sweet cherries grafted onto dwarfing rootstocks Hortscience 41 674–679 [Google Scholar]
- Otto B, Kaldenhoff R. 2000. Cell-specific expression of the mercury-insensitive plasma-membrane aquaporin NtAQP1 from Nicotiana tabacum Planta 211 167–172 [DOI] [PubMed] [Google Scholar]
- Pongrácz DP. Rootstocks for grape-vines. Cape Town, South Africa: David Philip Publisher; 1983. [Google Scholar]
- Postaire O, Tournaire-Roux C, Grondin A, Boursiac Y, Morillon R, Schaffner AR, Maurel C. 2010. A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis Plant Physiology 152 1418–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen WH. 1967. Radial movement of water and 32P through suberized and unsuberized roots of grape. PhD dissertation, Duke University, Durham, NC, USA
- Sade N, Gebretsadik M, Seligmann R, Schwartz A, Wallach R, Moshelion M. 2010. The role of tobacco aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress Plant Physiology 152 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner AR. 1998. Aquaporin function, structure, and expression: are there more surprises to surface in water relations? Planta 204 131–139 [DOI] [PubMed] [Google Scholar]
- Shelden MC, Howitt SM, Kaiser BN, Tyerman SD. 2009. Identification and functional characterisation of aquaporins in the grapevine, Vitis vinifera. Functional Plant Biology 36 1065–1078 [DOI] [PubMed] [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R. 2002. PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants The Plant Cell 14 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari LI, Johnson S, Dejong TM. 2006. Hydraulic conductance characteristics of peach (Prunus persica) trees on different rootstocks are related to biomass production and distribution Tree Physiology 26 1343–1350 [DOI] [PubMed] [Google Scholar]
- Steudle E. 2001. The cohesion-tension mechanism and the acquisition of water by plant roots Annual Review of Plant Physiology and Plant Molecular Biology 52 847–875 [DOI] [PubMed] [Google Scholar]
- Steudle E, Meshcheryakov AB. 1996. Hydraulic and osmotic properties of oak roots Journal of Experimental Botany 47 387–401 [Google Scholar]
- Suga S, Murai M, Kuwagata T, Maeshima M. 2003. Differences in aquaporin levels among cell types of radish and measurement of osmotic water permeability of individual protoplasts Plant and Cell Physiology 44 277–286 [DOI] [PubMed] [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD. 2009. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine Plant Physiology 149 445–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley MD, Tattersall EAR, Tillett RL, Cramer GR. 2009. An expanded clay pebble, continuous recirculating drip system for viable long-term hydroponic grapevine culture American Journal of Enology and Viticulture 60 542–549 [Google Scholar]
- Yamada S, Katsuhara M, Kelly WB, Michalowski CB, Bohnert HJ. 1995. A family of transcripts encoding water channel proteins – tissue-specific expression in the common ice plant The Plant Cell 7 1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Steudle E. 2006. Oxidative gating of water channels (aquaporins) in corn roots Plant, Cell and Environment 29 459–470 [DOI] [PubMed] [Google Scholar]
- Yun JJ, Heisler LE, Hwang II, et al. 2006. Genomic DNA functions as a universal external standard in quantitative real-time PCR Nucleic Acids Research 34 6718–6718 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.