Abstract
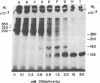
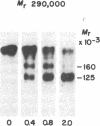
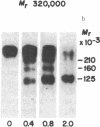
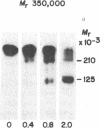
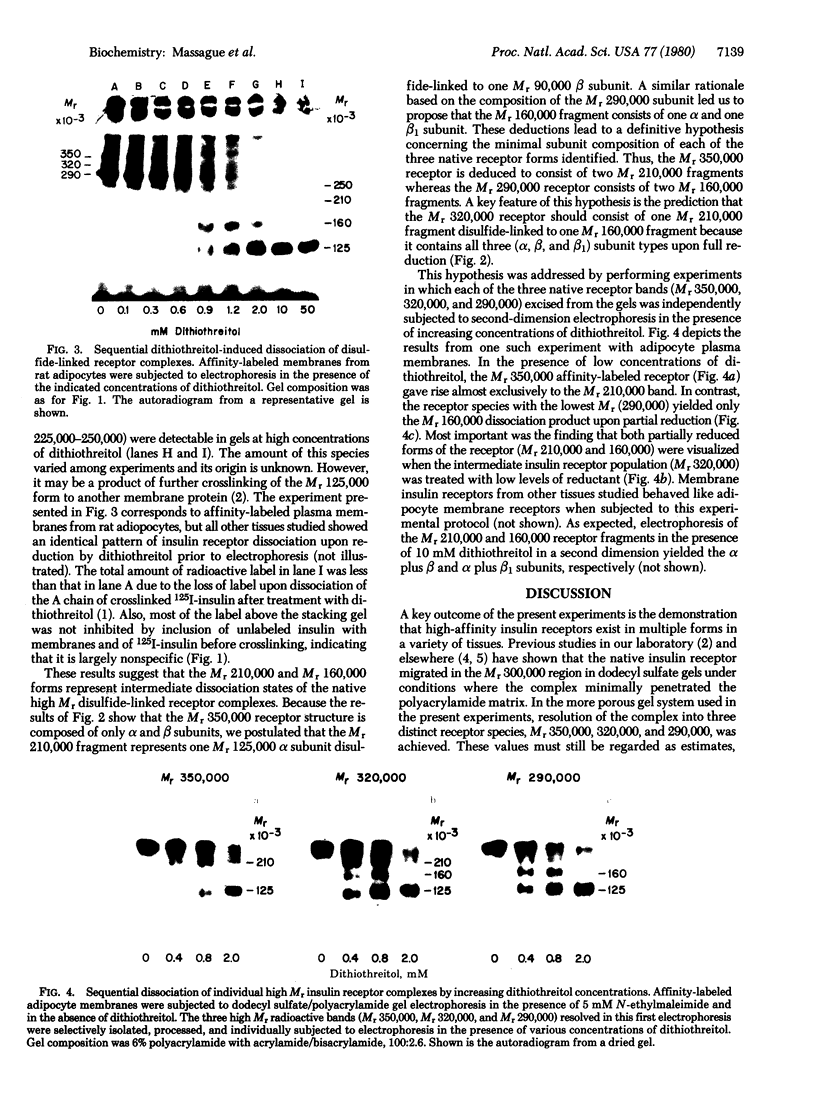
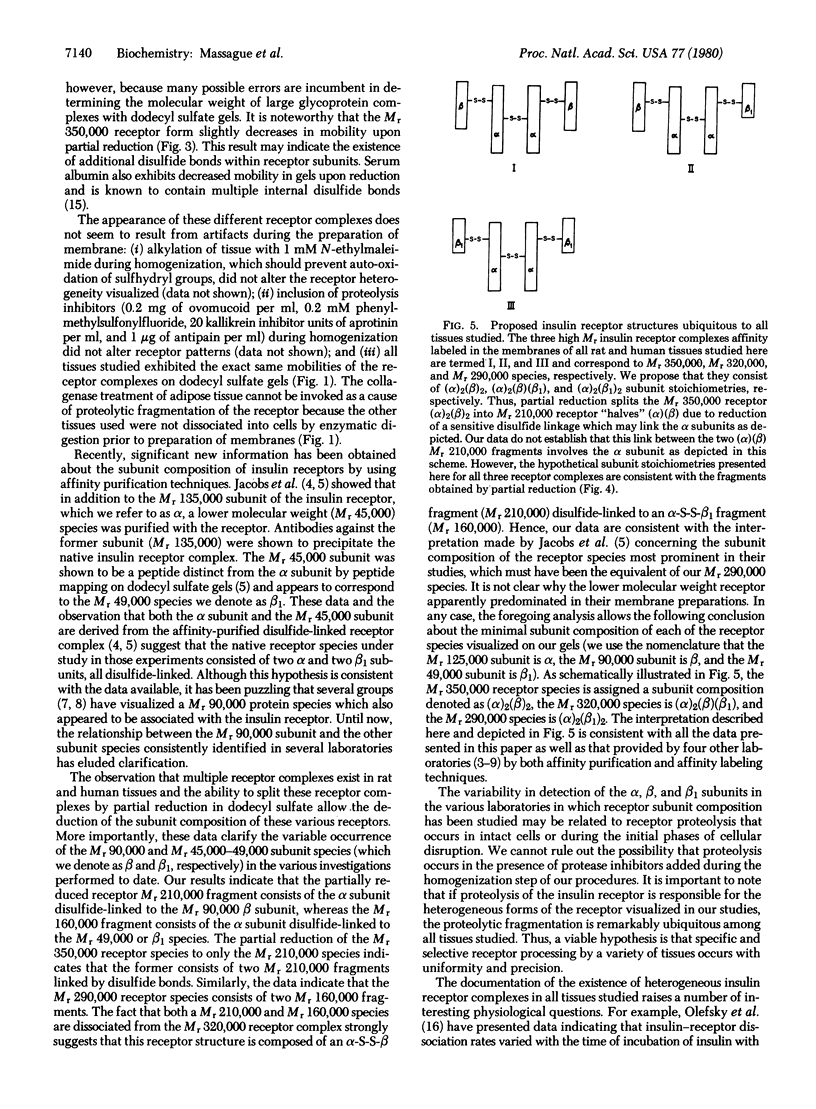
Plasma membrane insulin receptors, affinity labeled by covalent crosslinking to receptor-bound 125I-labeled insulin, are shown to appear as a heterogeneous population of three major disulfide-linked complexes (Mr 350,000, 320,000, and 290,000) upon electrophoresis in highly porous dodecyl sulfate/polyacrylamide gels in the absence of reductant. This pattern is consistent in all rat and human tissues that were analyzed. Upon reduction of disulfide bonds, each of these receptor structures is dissociated in two successive steps. Low concentrations of dithiothreitol promote a first step of disulfide bond reduction in which the Mr 350,000 species splits into a Mr 210,000 form and the Mr 290,000 species splits into a Mr 160,000 form. In contrast, both the Mr 210,000 and Mr 160,000 receptor fragments are generated from the native Mr 320,000 species upon partial reduction, indicating an asymmetrical structure. The second step of receptor reduction occurs upon treatment of the native disulfide-linked receptor complexes with high concentrations of dithiothreitol. Under these conditions, the Mr 350,000 receptor yields a Mr 125,000 subunit, denoted as alpha, and a Mr 90,000 subunit, denoted as beta, whereas the Mr 290,000 receptor dissociates into the alpha subunit and a Mr 49,000 subunit, denoted as beta 1. The Mr 320,000 receptor band is found to consist of alpha, beta, and beta 1 subunits upon complete reduction. The partially reduced Mr 210,000 receptor fragment is composed of the alpha subunit disulfide-linked to the beta subunit, whereas the Mr 160,000 species consists of the alpha subunit disulfide-linked to the beta 1 subunit. Thus, the stoichiometry of the three ubiquitous native insulin receptor structures of Mr 350,000, 320,000, and 290,000 are (alpha) 2 (beta) 2, (alpha) 2 (beta) (beta 1), and (alpha) 2 (beta 1) 2, respectively.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Cuatrecasas P. The subunit structure of rat liver insulin receptor. Antibodies directed against the insulin-binding subunit. J Biol Chem. 1980 Jul 25;255(14):6937–6940. [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Shechter Y., Bissell K., Cuatrecasas P. Purification and properties of insulin receptors from rat liver membranes. Biochem Biophys Res Commun. 1977 Aug 8;77(3):981–988. doi: 10.1016/s0006-291x(77)80074-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang U., Kahn C. R., Harrison L. C. Subunit structure of the insulin receptor of the human lymphocyte. Biochemistry. 1980 Jan 8;19(1):64–70. doi: 10.1021/bi00542a010. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Kobayashi M., Chang H. Interactions between insulin and its receptors after the initial binding event. Functional heterogeneity and relationships to insulin degradation. Diabetes. 1979 May;28(5):460–471. doi: 10.2337/diab.28.5.460. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Baron M. D., Jones R. H., Sönksen P. H. Photoreactive insulin analogues used to characterise the insulin receptor. Biochem Biophys Res Commun. 1980 Jan 29;92(2):492–498. doi: 10.1016/0006-291x(80)90360-5. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor of rat adiopocyte plasma membrane. J Biol Chem. 1978 Mar 25;253(6):1743–1745. [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor proteins of liver plasma membrane preparations. Biochemistry. 1980 Jan 8;19(1):70–76. doi: 10.1021/bi00542a011. [DOI] [PubMed] [Google Scholar]