Abstract
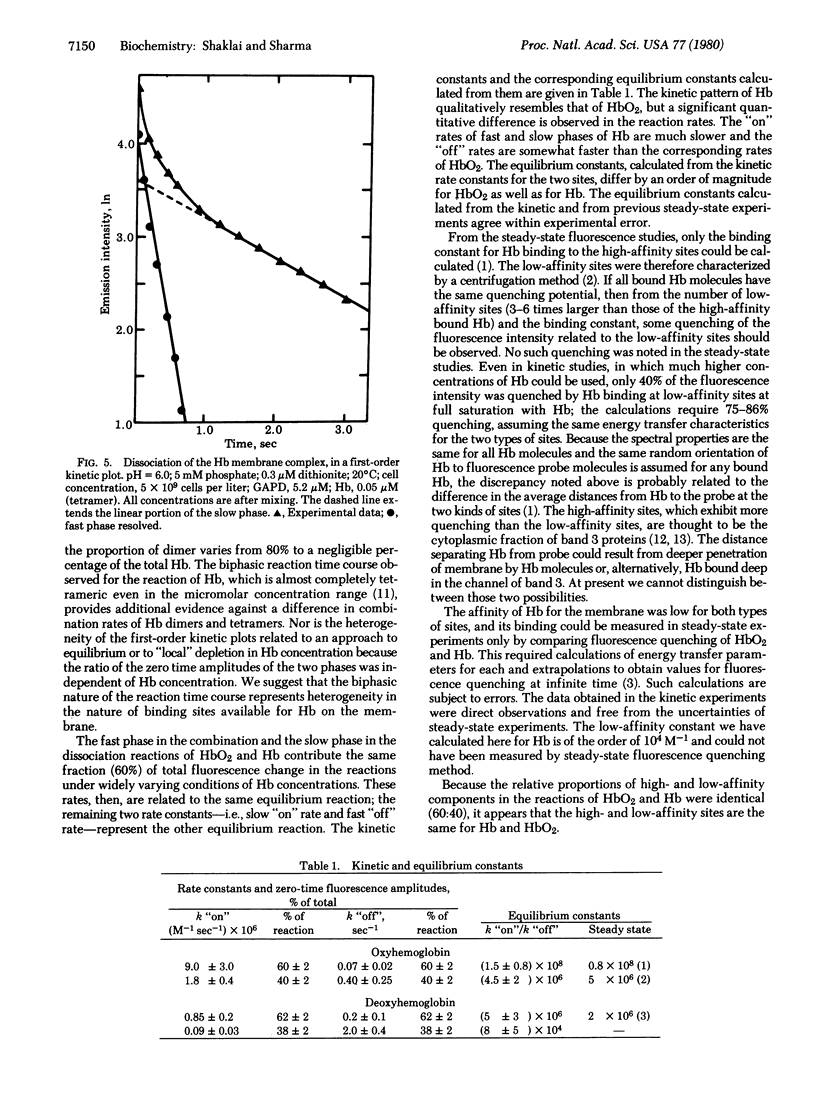
Changes in fluorescence intensity of a membrane-embedded probe were used to study the kinetics of binding of oxy- and deoxyhemoglobin to erythrocyte membranes. For these studies, stopped-flow fluorimetric techniques were utilized. Both binding and dissociation of hemoglobin from membranes followed heterogeneous first-order kinetics. The rate constants for binding of oxyhemoglobin were about 10 times larger than those of deoxyhemoglobin; the dissociation rate constants of oxyhemoglobin were about one-quarter those of the unliganded form. The results are discussed in light of the steady-state binding constants previously derived for both oxy- and deoxyhemoglobin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coin J. T., Olson J. S. The rate of oxygen uptake by human red blood cells. J Biol Chem. 1979 Feb 25;254(4):1178–1190. [PubMed] [Google Scholar]
- DANDLIKER W. B., FOX J. B., Jr The coenzyme content of rabbit muscle D-glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1956 Aug;221(2):1005–1017. [PubMed] [Google Scholar]
- Ip S. H., Johnson M. L., Ackers G. K. Kinetics of deoxyhemoglobin subunit dissociation determined by haptoglobin binding: estimation of the equilibrium constant from forward and reverse rates. Biochemistry. 1976 Feb 10;15(3):654–660. doi: 10.1021/bi00648a032. [DOI] [PubMed] [Google Scholar]
- Kant J. A., Steck T. L. Specificity in the association of glyceraldehyde 3-phosphate dehydrogenase with isolated human erythrocyte membranes. J Biol Chem. 1973 Dec 25;248(24):8457–8464. [PubMed] [Google Scholar]
- Marchesi S. L., Steers E., Marchesi V. T., Tillack T. W. Physical and chemical properties of a protein isolated from red cell membranes. Biochemistry. 1970 Jan 6;9(1):50–57. doi: 10.1021/bi00803a007. [DOI] [PubMed] [Google Scholar]
- McDaniel C. F., Kirtley M. E., Tanner M. J. The interaction of glyceraldehyde 3-phosphate dehydrogenase with human erythrocyte membranes. J Biol Chem. 1974 Oct 25;249(20):6478–6485. [PubMed] [Google Scholar]
- Salhany J. M., Cordes K. A., Gaines E. D. Light-scattering measurements of hemoglobin binding to the erythrocyte membrane. Evidence for transmembrane effects related to a disulfonic stilbene binding to band 3. Biochemistry. 1980 Apr 1;19(7):1447–1454. doi: 10.1021/bi00548a028. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Shaklai N. Functional properties of human hemoglobin bound to the erythrocyte membrane. Biochemistry. 1979 Mar 6;18(5):893–899. doi: 10.1021/bi00572a025. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Abrahami H. The interaction of deoxyhemoglobin with the red cell membrane. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1105–1112. doi: 10.1016/0006-291x(80)91586-7. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Yguerabide J., Ranney H. M. Classification and localization of hemoglobin binding sites on the red blood cell membrane. Biochemistry. 1977 Dec 13;16(25):5593–5597. doi: 10.1021/bi00644a032. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Yguerabide J., Ranney H. M. Interaction of hemoglobin with red blood cell membranes as shown by a fluorescent chromophore. Biochemistry. 1977 Dec 13;16(25):5585–5592. doi: 10.1021/bi00644a031. [DOI] [PubMed] [Google Scholar]