Abstract
1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the active form of vitamin D, exerts potent effects on several tissues including cells of the immune system, where it affects T cell activation, differentiation and migration. The circulating, inactive form of vitamin D, 25(OH)D3, is generally used as an indication of “vitamin D status”. However, utilization of this precursor depends on its uptake by cells and subsequent conversion by the enzyme 25(OH)D3-1α-hydroxylase (CYP27B1) into active 1,25(OH)2D3. Using human T cells, we now show that addition of inactive 25(OH)D3 is sufficient to alter T cell responses only when dendritic cells (DCs) are present. Mechanistically, CYP27B1 is induced in DCs upon maturation with LPS or upon T cell contact resulting in the generation and release of 1,25(OH)2D3 which subsequently affects T cell responses. In most tissues, vitamin D binding protein (DBP) acts as a carrier to enhance the utilization of vitamin D. However, we show that DBP modulates T cell responses by restricting the availability of inactive 25(OH)D3 to DC. These data indicate that the level of “free” 25(OH)D3 available to DCs determines the inflammatory/regulatory balance of ensuing T cell responses.
Introduction
Besides its longstanding association with calcium regulation and bone density, more widespread physiological roles for vitamin D are now acknowledged. This is consistent with the broad distribution of the vitamin D receptor throughout the body1 and the association of low vitamin D status with numerous diseases in epidemiological studies2. Interestingly, low vitamin D appears to increase the risk of immune related diseases, including multiple sclerosis3,4, rheumatoid arthritis (RA)5, type 1 diabetes6 and inflammatory bowel disease7. Thus, vitamin D appears to have an important immune-regulatory function, which may have implications for the treatment of many conditions. Consistent with this, vitamin D supplementation in mouse models of autoimmunity has been demonstrated to have prophylactic and therapeutic benefit8-13. In-vitro studies have also identified profound immunological effects of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the biologically active form of vitamin D, including inhibited maturation of antigen presenting cells (APCs), which involves down-regulation of MHC and co-stimulatory molecules14-16. In addition, we and others have shown that 1,25(OH)2D3 can act directly upon CD4+ T cells, suppressing cytokines such as IFNγ, IL-17, IL-21 and IL-22, whilst enhancing the regulatory markers FoxP3, CTLA-4 and IL-1017-21. Although these findings demonstrate the ability of active 1,25(OH)2D3 to directly regulate T cell responses, tight homeostatic mechanisms operate in-vivo to maintain serum 1,25(OH)2D3 at approximately 0.1nM2, which is somewhat below the effective concentration of 1,25(OH)2D3 seen in in-vitro studies. It is therefore likely, that the level of 1,25(OH)2D3 circulating systemically is insufficient to promote anti-inflammatory effects in-vivo. Furthermore, serum 1,25(OH)2D3 concentration does not tend to vary greatly between individuals, where even those regarded vitamin D deficient can show relatively normal 1,25(OH)2D3 levels2. Thus, local production of active 1,25(OH)2D3 from its inactive precursor is likely to be important in order for vitamin D to contribute to immune regulation in-vivo.
Generation of active vitamin D from its inactive precursor, 25(OH)D3, which is the predominant form of vitamin D in the circulation, is carried out by the mitochondrial cytochrome P450 enzyme, 25(OH)D3-1α-hydroxylase (CYP27B1). Whilst most is known about the activity of this enzyme in the kidneys and bone, wider expression of CYP27B1 is observed22,23, including its expression by macrophages and DCs24. Indeed, high levels of 1,25(OH)2D3 are found in sarcoidosis patients resulting from CYP27B1 activity in disease-associated macrophages25. Although the ability of DCs and macrophages to convert 25(OH)D3 to 1,25(OH)2D3 is clear, whether sufficient 1,25(OH)2D3 is generated to subsequently affect T cell function, is not known. Furthermore, other factors in serum influence the bioavailability of both 25(OH)D3 and 1,25(OH)2D3 to immune cells. In this respect vitamin D binding protein (DBP) is of particular interest, since the majority of 25(OH)D3 and 1,25(OH)2D3 is transported by this protein in serum. Indeed, DBP can facilitate uptake of 25(OH)D3 by epithelial cells of the kidney proximal tubule26, mammary cells27 and osteoblasts28 that express the DBP transporter proteins megalin and cubulin. However, the impact of DBP on 25(OH)D3 uptake by immune cells is less clear. Furthermore, the DBP gene is polymorphic, and three common variants termed GC1S, GC1F and GC2 are found that appear to have different functional properties, including varying binding affinities for vitamin D29,30. Interestingly, DBP polymorphisms have also shown association with inflammatory disorders, including chronic obstructive pulmonary disease (COPD), tuberculosis (TB), Graves’ disease, RA and diabetes31-33. Thus, 25(OH)D3 status as well as DBP genotype and serum DBP concentration might modulate immune responses.
We have therefore investigated the effect of local conversion of inactive 25(OH)D3 to active 1,25(OH)2D3 by DCs on subsequent T cell responses. We observed that 1,25(OH)2D3 was readily produced by DCs upon maturation at a level sufficient to promote an anti-inflammatory T cell phenotype, exemplified by high CTLA-4 and decreased expression of IL-17, IFNγ and IL-21. In contrast, T cells alone utilised 25(OH)D3 poorly, consistent with their limited expression of CYP27B1. The magnitude of the effect resulting from DC conversion of 25(OH)D3 was dependent on the concentration of available 25(OH)D3 and was observed within a physiologically relevant range. Importantly, addition of DBP suppressed the impact of 25(OH)D3 on T cell responses depending upon its variant and concentration. Together these data suggest that 25(OH)D3 can be locally converted to 1,25(OH)2D3 by DC and that the 1,25(OH)2D3 generated becomes available to T cells. This indicates that the outcomes of T cell responses to antigen are influenced by the bioavailability of 25(OH)D3 to DC, which in turn is affected by DBP concentration and genetic variant.
Materials and Methods
Cell isolation and cell culture
PBMCs were isolated by Ficoll gradient centrifugation from fresh leukocyte reduction system cones (provided by The National Blood Service, Birmingham, UK) or from healthy donors following informed consent in line with institutional and ethical committee approval. Similarly, samples from RA patients were obtained following informed consent in line with institutional and ethical committee approval. Isolated cells were washed twice with PBS and twice with MACS buffer (0.5% BSA, 2mM EDTA in PBS) before re-suspension at 1 × 108cells/ml for magnetic separation. For CD4+CD25− isolation, CD4+ T cells were first enriched using a negative selection antibody cocktail (StemCell Technologies). CD25+CD4+ cells were subsequently removed by incubation with anti-CD25 microbeads (Miltenyi Biotech) and magnetic column separation. CD4+CD25− T cells were re-suspended in CellGro® SCGM serum free medium supplemented with 50U/ml penicillin and streptomycin. Monocytes were also isolated by negative selection using an antibody selection cocktail (StemCell Technologies). For culture to DCs, monocytes were re-suspended at 2 × 106/ml in RPMI 1640 (Life Technologies/Invitrogen) supplemented with 10% FBS, 50U/ml penicillin and streptomycin, 200μM glutamine (Life Technologies/Invitrogen) and treated with GM-CSF (800 U/ml, PeproTech) and IL-4 (500U/ml Peprotech). 1 × 106 cells were plated per well of a 24 well plate. At two days, cells were supplemented with 500μL medium containing GM-CSF and IL-4. At seven days, DCs were used in T cell stimulation assays or treated with cytokines: IFNγ (10ng/ml, Peprotech), IL-1β (10ng/ml, Peprotech), TNFα (100U/ml, Sigma), IL-15 (10ng/ml, R & D Systems) with or without anti-CD40 (10μg/ml, clone S2C6, Mabtech). When collecting DC culture supernatants for 1,25(OH)2D3 concentration measurement or supernatant transfer studies, cells were cultured in CellGro® serum free medium. All cells were cultured at 37°C, 95% humidity and 5% CO2.
CD4+CD25− T cell stimulation
T cells were cultured with DCs at a 4:1 ratio in the presence of anti-CD3 (clone OKT3, 0.5μg/ml) or stimulated with anti-CD3/anti-CD28 coated beads (Life Technologies/Invitrogen) at 1:4 bead:cell ratio. All stimulations were prepared in CellGro® SCGM serum free medium. Where Foxp3 was measured cultures were supplemented with TGFβ (1ng/ml, R and D Systems) and IL-2 (200U/ml, Peprotech). Where cytokines were measured bead stimulations were supplemented with IL-1β (10ng/ml, Peprotech), IL-6 (20ng/ml, Immunotools), IL-23 (10ng/ml, R & D systems), TGFβ (1ng/ml, R & D Systems). Where indicated, cultures were additionally supplemented with various concentrations of 1,25(OH)2D3 (a gift from Dr. L. Binderup (Leo Pharma, Ballerup, Denmark), 25(OH)D3 (Sigma), purified mixed human vitamin D binding protein (Calbiochem) as indicated or 2.5% plasma. For transwell studies, 24 well, low density 0.4μm inserts (BD Pharmingen) were used. 2.5 × 105 DCs were plated beneath the insert and 1 × 106 T cells cultured above the insert. In transwell B, DCs and T cells were co-cultured above the insert at a ratio of 1 DC: 5 T cells. Anti-CD3 was added at 0.5μg/ml.
Flow cytometry
Total CTLA-4 and FoxP3 expression was measured at four days post stimulation. Staining was performed according to the manufacturer’s instructions using a FoxP3 staining kit (ebiosciences). For cytokine detection, cells were cultured for five days prior to analysis. They were then re-stimulated for six hours with PMA (50ng/ml) and ionomycin (1μM) in the presence of brefeldin A during the last four hours. Cells were subsequently fixed with 3% PFA and permeabilised with 0.1% saponin-PBS. Antibody staining was performed for 30 minutes at room temperature in the presence of 2% goat serum. Following one saponin wash and two PBS washes, cells were re-suspended in PBS and analysed by flow cytometry. For surface stains on live cells, cells were stained on ice in 2% goat serum – PBS for 30 minutes before being collected live or fixed with 1.5% PFA. Cells were acquired on a Dako cyan flow cytometer and data analysed using FlowJo Software (Tree Star). Total cell counts were determined by adding a fixed number of counting beads to the sample immediately prior to collection. The fraction of counting beads collected was then used to convert the number of CD3+CD4+ T cells collected to the number in the stimulation well, the total content of which had been collected for analysis. All antibodies were purchased from ebioscience or BD Biosciences.
Real time PCR analysis
Total RNA was extracted using the TRIzol method (Life Technologies/Invitrogen). 0.5μg was reverse transcribed with random hexamers using TaqMan reverse transcription reagents (Life Technologies/Applied Biosystems). Quantitative real-time PCR for CYP27B1 and 18SrRNA was then performed on Applied Biosystems 7500 or 7900 machines using assays on demand from Applied Biosystems (18S rRNA, 4319413E; CYP27B1, Hs00168017_m1). Amplification of cDNAs involved incubation at 50°C, 2 min and 95°C, 10 min followed by 40 cycles of 95°C, 15 secs and 60°C, 1 min. CYP27B1 mRNA expression was then calculated relative to 18SrRNA. For RT PCR, Go Taq Polymerase (Promega) was used with the following primer sets: Megalin: (Forward) TGGCCATCGATTGGGCTGCTT, (Reverse) TGGTTGGGTCCCCCTCGCAT; Cubulin: (Forward) GCGGCTTCACTGCTTCCTA, (Reverse) GAGTGATGGTGTGCCCTTGT; β actin: (Forward) CATCACCATTGGCAATGAGC, (Reverse) CGATCCACACGGAGTACTTG. cDNAs were amplified under the following conditions: initial denaturation at 95°C, 3 min followed by 35 cycles of 95°C, 1 min; 53°C, 45 secs; 72°C, 45 secs and a final extension at 72°C for 5 mins.
Chronic obstructive pulmonary disease (COPD) plasma samples
Plasma samples were taken from the West Midlands COPD collection, which had been set up to study COPD phenotypes and progression in relation to data from biological samples. Ethical approval had been granted by the local ethics committee and all patients had given informed consent. DNA was extracted, quantified and genotyped for SNPs (rs7041 and rs4588) in the DBP gene that encode variants GC2, GC1F and GC1S, using methods described previously34.
DBP, 1,25(OH)2D3 and 25(OH)D3 measurements
DBP concentrations were measured by ELISA using a commercially available kit (Immunodiagnostik, Bensham, Germany). Total 1,25(OH)2D3 was measured by enzyme immunoassay (University of East and Anglia) and 25(OH)D3 by liquid chromatography-mass spectrometry (University of East Anglia). When collecting DC and T cell culture supernatants for 25(OH)D3 and 1,25(OH)2D3 analysis, DCs were re-suspended at 112,500 cells/ml and T cells at 450,000 cells/ml. DCs were matured with LPS (1ug/ml) and T cells stimulated with anti-CD3/anti-CD28 beads at 1 bead:4 T cells. Cultures were treated with 25(OH)D3 at the indicated concentration with or without DBP (20ug/ml) and incubated for 24 hours. Because vitamin D species were found to degrade to approximately one third of initial levels before measurement in the absence of DBP (fig. S1) spontaneous degradation was accounted for by multiplying values of 1,25(OH)2D3 and 25(OH)D3 three fold. DBP was added at the end of the experiment to all cultures as a protectant and all samples were stored at −80C before analysis.
Statistical Analysis
GraphPad Prism3 software was used for statistical analysis. P values were calculated using non-parametric Wilcoxon or Man-Whitney tests as appropriate. P value ranges are indicated in figures as follows: * = P<0.05, ** = P<0.01, *** = P<0.001.
Results
Modulation of T cell responses by 25(OH)D3 supplementation requires the presence of antigen presenting cells
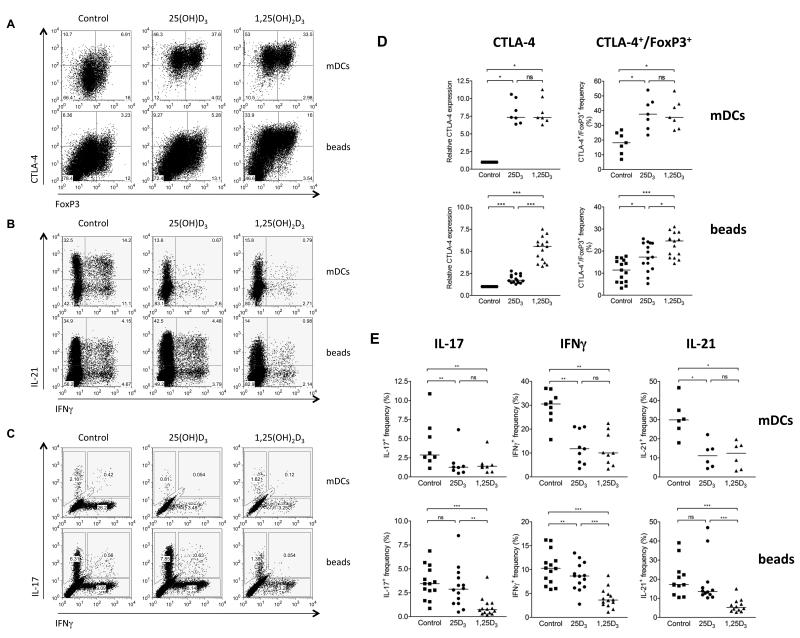
We previously demonstrated that active 1,25(OH)2D3 could directly modify the outcome of T cell stimulation20. However, since systemic levels of 1,25(OH)2D3 in vivo are potentially too low to influence T cell responses, we sought to determine whether sufficient 1,25(OH)2D3 could be locally generated from inactive 25(OH)D3 during immune activation. To test this concept we stimulated CD4+CD25− T cells with mature dendritic cells (mDCs) in the presence of inactive 25(OH)D3 and measured the effect on responding T cells. As shown in figure 1A upper panels and table Si, expression of the regulatory protein CTLA-4 and the frequency and absolute number of FoxP3+CTLA-4+ cells was strongly enhanced by addition of 25(OH)D3. By contrast both the frequency and absolute number of cells producing inflammatory cytokines was markedly suppressed by 25(OH)D3 (fig. 1B, 1C and table Si). Importantly, the magnitude of response was similar to 1,25(OH)2D3 supplementation, suggesting that efficient conversion of 25(OH)D3 had occurred. By contrast, when T cells were stimulated in the absence of APCs, (by using anti-CD3/anti-CD28 coated beads) we observed only a modest induction of CTLA-4 and little or no suppression of IFNγ, IL-17 and IL-21 by 25(OH)D3 (fig. 1A, B and C-lower panels and table Sii). The addition of active 1,25(OH)2D3 to bead-stimulated T cells was however still effective in modifying CTLA-4 and cytokine expression, indicating that bead stimulated cells were responsive to 1,25(OH)2D3, in the absence of APCs (fig. 1A, B and C-lower panels and table Sii). These findings were reproduced using a number of different blood donors (fig. 1D and E) and indicated that the presence of DCs was required for T cell responses to be effectively modified by 25(OH)D3.
Figure 1. Inactive 25(OH)D3 promotes an anti-inflammatory phenotype only when T cells are stimulated by dendritic cells.
CD4+CD25− T cells were stimulated with LPS-matured dendritic cells (mDCs) plus anti-CD3 (top rows) or anti-CD3/CD28 coated beads (beads) (bottom rows) in the presence of carrier control, 50nM 25(OH)D3 or 10nM 1,25(OH)2D3. Following stimulation, cells were gated on CD3, and co-stained for A) CTLA-4 and FoxP3, B) IL-21 and IFNγ or C) IL-17 and IFNγ and analysed by flow cytometry. Representative data are shown in A-C and data from multiple experiments in D and E. Horizontal lines indicate median values. Significance was tested by a two-tailed Wilcoxon matched pairs test.
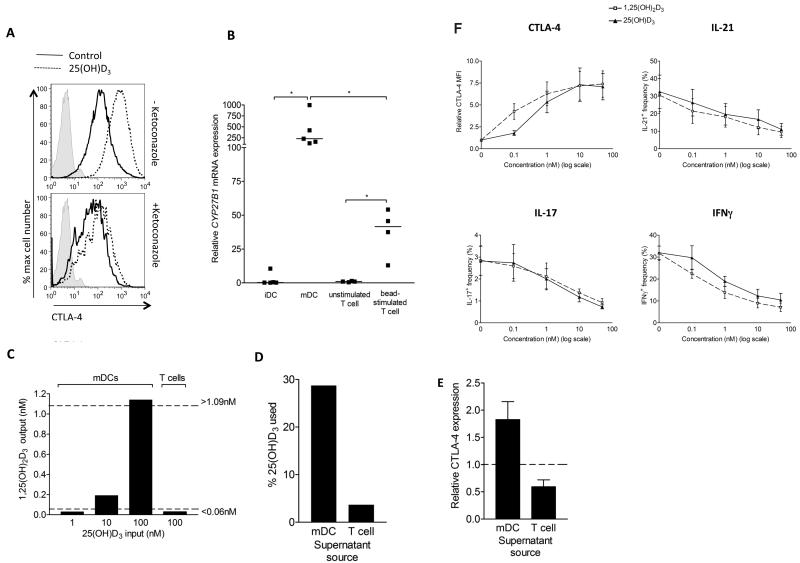
To test whether the apparent responses to 1,25(OH)2D3 in our DC driven assays might involve CYP27B1 activity, we treated these stimulations with the CYP27B1 inhibitor, ketoconazole. This revealed that T cell induction of CTLA-4 upon 25(OH)D3 supplementation was suppressed by ketoconazole supporting a role for CYP27B1 activity (fig. 2A). Moreover, whilst CYP27B1 mRNA expression is reported in both DCs and T cells24,35-37 their relative expression has not been formally compared. Thus, to examine whether the lack of T cell response to 25(OH)D3 in the absence of DCs could relate to levels of the converting enzyme, we compared CYP27B1 mRNA expression in T cells and DCs before and after stimulation. This revealed that CYP27B1 mRNA was low in unstimulated T cells and immature DC (iDC) and that DC maturation with LPS strongly up-regulated CYP27B1 mRNA (fig. 2B). By contrast, CYP27B1 mRNA in T cells upon stimulation was significantly lower, supporting the hypothesis that relative to DCs, T cells may be poor convertors of 25(OH)D3.
Figure 2. CYP27B1 mRNA expression correlates with 25(OH)D3 conversion activity.
A) CD4+CD25− T cells were stimulated for four days with mDCs in the presence or absence of ketoconazole and total CTLA-4 expression in CD25+ T cells measured by flow cytometry. B) CYP27B1 mRNA expression was measured in immature (iDCs), LPS-matured dendritic cells (mDCs) and in un-stimulated and bead stimulated T cells by quantitative real-time PCR. Levels were normalised to 18S rRNA and are plotted relative to the unstimulated T cell control. Horizontal lines indicate median expression and significance was tested by two-tailed Mann Whitney tests. C,D) LPS treated DCs (mDC) and bead stimulated CD4+CD25− T cells were cultured for 24 hours in the presence of 25(OH)D3 at indicated concentrations and the concentration of 1,25(OH)2D3 measured at the end of the assay (C). Dotted lines indicate the sensitivity range of the assay. The percentage of 25(OH)D3 used (D) was calculated by normalising to the level in the medium alone control. Data are from a single experiment representative of two performed. E) Supernatants from 25(OH)D3-treated mDC or bead stimulated T cell cultures were used to supplement bead-driven T cell stimulations. CTLA-4 expression was compared to the level in the presence of control medium. F). CD4+CD25− T cells were stimulated with mature dendritic cells (mDCs) plus anti-CD3 in the presence of 25(OH)D3 or 1,25(OH)2D3 at the concentrations shown. Following stimulation, cells were stained for CD3, CTLA-4, IL-17, IFNγ or IL-21 and analysed by flow cytometry. Mean values from five experiments are plotted. Bars indicate SEM.
We next examined the 25(OH)D3 conversion by mDCs and anti-CD3/CD28 stimulated T cells by measuring the concentrations of 1,25(OH)2D3 and 25(OH)D3 in the supernatants of cultures supplemented with 25(OH)D3 for 24 hour. As shown in figure 2C, 1,25(OH)2D3 could be detected in 25(OH)D3 supplemented DC cultures but was below the detection limit in T cell cultures even at 100nM of 25(OH)D3. Correspondingly, we found that approximately 30% of available 25(OH)D3 substrate was utilised by mDCs, whilst only 4% was used by bead stimulated T cells (fig. 2D). These measurements give a useful estimate of the relative difference in conversion efficiency by DCs and T cells. Moreover, supernatants from 25(OH)D3 supplemented DC cultures could induce CTLA-4 expression when transferred to bead stimulated T cells indicating they contained functionally significant levels of 1,25(OH)2D3 (fig. 2E). Interestingly, parallel titration studies for 25(OH)D3 and 1,25(OH)2D3 (fig. 2F) revealed similar responses at equivalent concentrations of 25(OH)D3 or 1,25(OH)2D3, thereby reinforcing the idea of effective local conversion by the DC.
Activated T cells can induce CYP27B1 expression and 1,25(OH)2D3 generation in dendritic cells
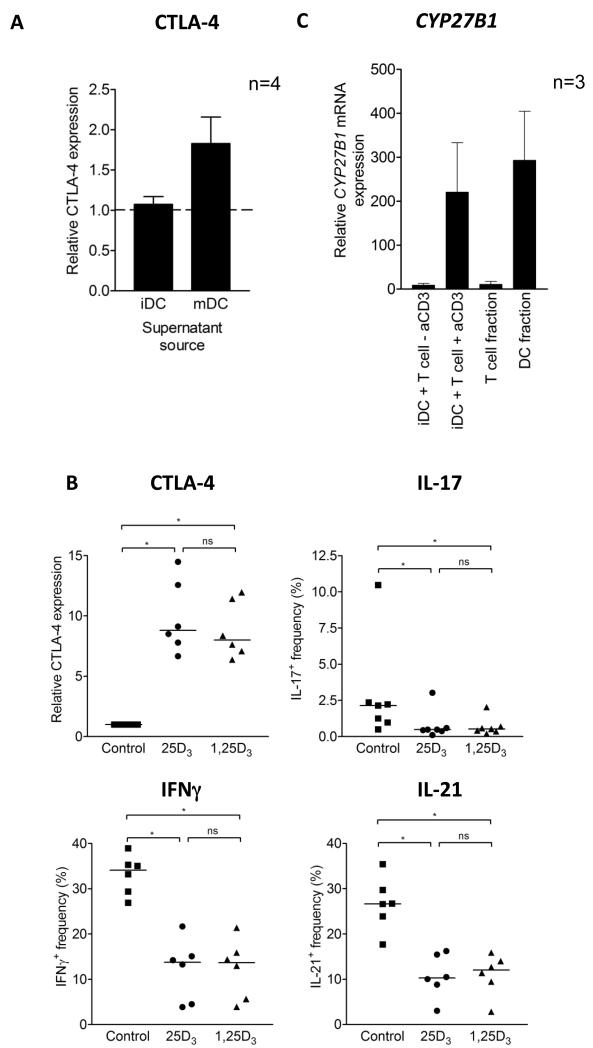
Induction of CYP27B1 in response to LPS might be an important mechanism during an innate immune response to microbes, however we were also interested in whether 1,25(OH)2D3 generation could occur in the absence of this DC maturation signal. To investigate this, we stimulated T cells using iDCs, which expressed low levels of CYP27B1 mRNA. We observed that, in isolation, iDC were not able to generate sufficient 1,25(OH)2D3 to influence CTLA-4 expression (fig. 3A). Surprisingly, however, following T cell activation by iDC, changes in CTLA-4, IL-17, IFNγ and IL-21 were found to be equivalent for both 25(OH)D3 and 1,25(OH)2D3-supplemented stimulations (fig. 3B). This suggested that efficient 25(OH)D3 conversion still occurred and that during the stimulation process CYP27B1 was induced. In support of this, we detected high CYP27B1 mRNA expression in co-cultures of T cells and DCs (fig. 3C). Importantly, CYP27B1 mRNA was induced only in the presence of both anti-CD3 and T cells, suggesting the need to activate T cells in order to stimulate CYP27B1 expression. To confirm which cells expressed CYP27B1, we sorted T cells and DCs after 20 hours of stimulation and measured CYP27B1 mRNA expression in the two fractions. As shown in figure 3C, CYP27B1 mRNA was highly enriched in the DC fraction indicating that activated T cells could induce CYP27B1 in DCs and again emphasising the relative lack of CYP27B1 expression in T cells. For comparison, we also tested the ability of APCs other than cultured DC to generate functionally useful amounts of 1,25(OH)2D3. This showed efficient 25(OH)D3 conversion when stimulating CD4+ CD25− T cells with purified CD14+ monocytes (fig. S2A) or when culturing un-manipulated PBMC cultures that contained both CD14+ and CD14− CD11c+ cells with anti-CD3. Furthermore, mononuclear cells isolated from the blood and synovial fluid of RA patients were also able to convert 25(OH)D3 (fig. S2B).
Figure 3. T cell activation promotes CYP27B1 expression in dendritic cells, leading to 25(OH)D3 conversion and an anti-inflammatory T cell phenotype.
A) Supernatants from cultures of 25(OH)D3-treated immature DCs (iDCs) or mature DCs (mDCs) were used to supplement bead-stimulated T cell cultures. At four days, CTLA-4 expression was measured and compared to the level attained when T cells were stimulated in control medium. B) CD4+CD25− T cells were stimulated with iDCs plus anti-CD3 in the presence of control, 50nM 25(OH)D3 or 10nM 1,25(OH)2D3. Following stimulation, cells were stained for CD3, co-stained for CTLA-4, IL-17, IFNγ or IL-21 and measured by flow cytometry. C) At 20 hours, CYP27B1 mRNA was measured in cultures containing iDCs and T cells (+/− anti-CD3) and in purified T cell and DC fractions isolated from these cultures by cell sorting.
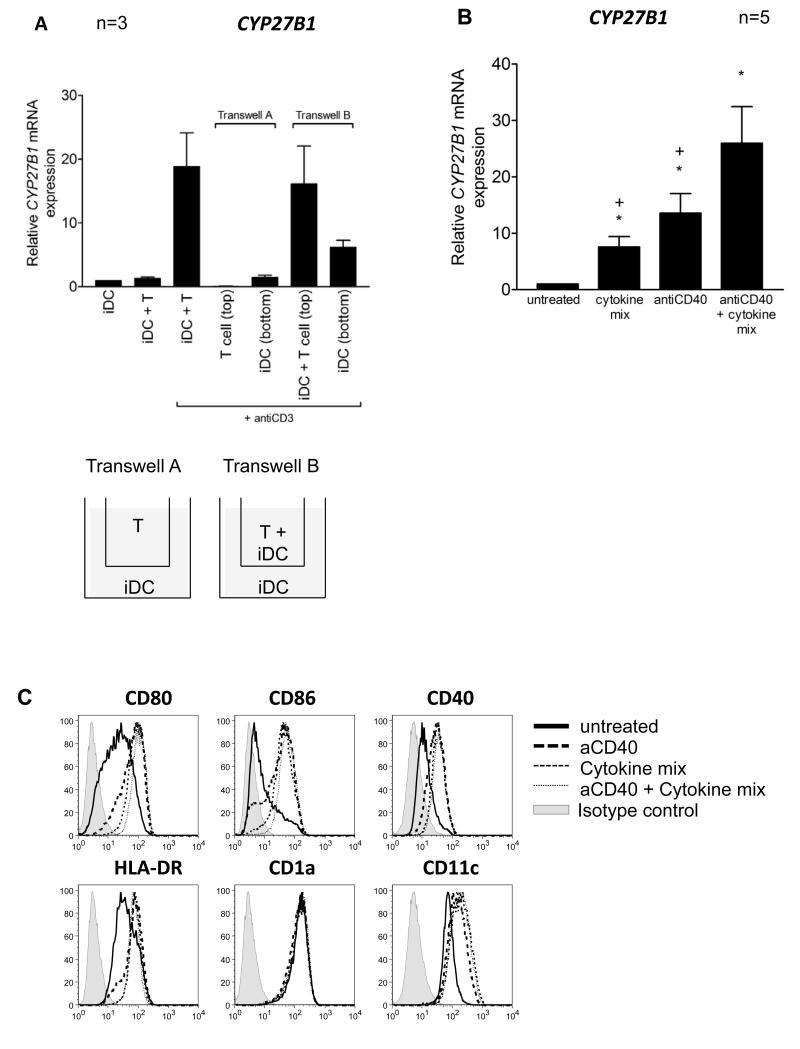
To distinguish whether cell contact or soluble factors were required for upregulation of CYP27B1 in iDC driven stimulations, we used a transwell approach. As shown in figure 4A, CYP27B1 was not induced in iDCs that were separated from anti-CD3 treated T cells (transwell A). In contrast, soluble factors from wells containing DC and activated T cells permitted some induction of CYP27B1 in DCs. However, the greatest induction of CYP27B1 occurred in DCs that were in direct contact with activated T cells (transwell B). Together these data suggest the importance of surface molecule engagement for CYP27B1 induction in DCs. Consistent with these data, we found that treating DCs with a range of pro-inflammatory cytokines (IFNγ, IL-1β, TNFα and IL-15) reported to influence CYP27B1 expression in other cell types38-44, gave a small increase in the expression of CYP27B1 mRNA (fig. 4B). Similarly, an agonistic antibody to CD40 also promoted CYP27B1 mRNA expression by the DC. Notably, however, anti-CD40 used in combination with inflammatory cytokines, resulted in the highest induction (fig. 4B). These treatments were also found to up-regulate several markers associated with DC maturation (fig.4C). Thus, together, these data indicate that maturation of DCs during co-culture with activated T cells permits efficient induction of CYP27B1 in the DC and can be driven by surface engagement with CD40 and the release of inflammatory cytokines.
Figure 4. Contact between activated T cells and immature dendritic cells promotes CYP27B1 upregulation.
A) CYP27B1 mRNA expression was measured in immature dendritic cell (iDC) and T cell cultures set up in combinations as shown. In transwell systems, anti-CD3 was present in both the top and bottom chambers. B) IDCs were treated with cytokines (IFNγ, IL-1β, TNFα and IL-15), anti-CD40 alone or antiCD40 with cytokines for 20 hours and their expression of CYP27B1 monitored by qPCR. Mean expression relative to the level in untreated DCs is graphed. Error bars indicate SEM. Wilcoxon matched pair tests were used to test significance. Significant differences (P<0.05) compared to untreated or antiCD40 with cytokines are indicated by stars and crosses respectively. C) Flow cytometric analysis of DC markers: CD80, CD86, CD40, HLA-DR, CD1a and CD11c following treatment as described in B. Data are from one experiment representative of n≥3.
Vitamin D Binding Protein affects T cell responses to dendritic cells
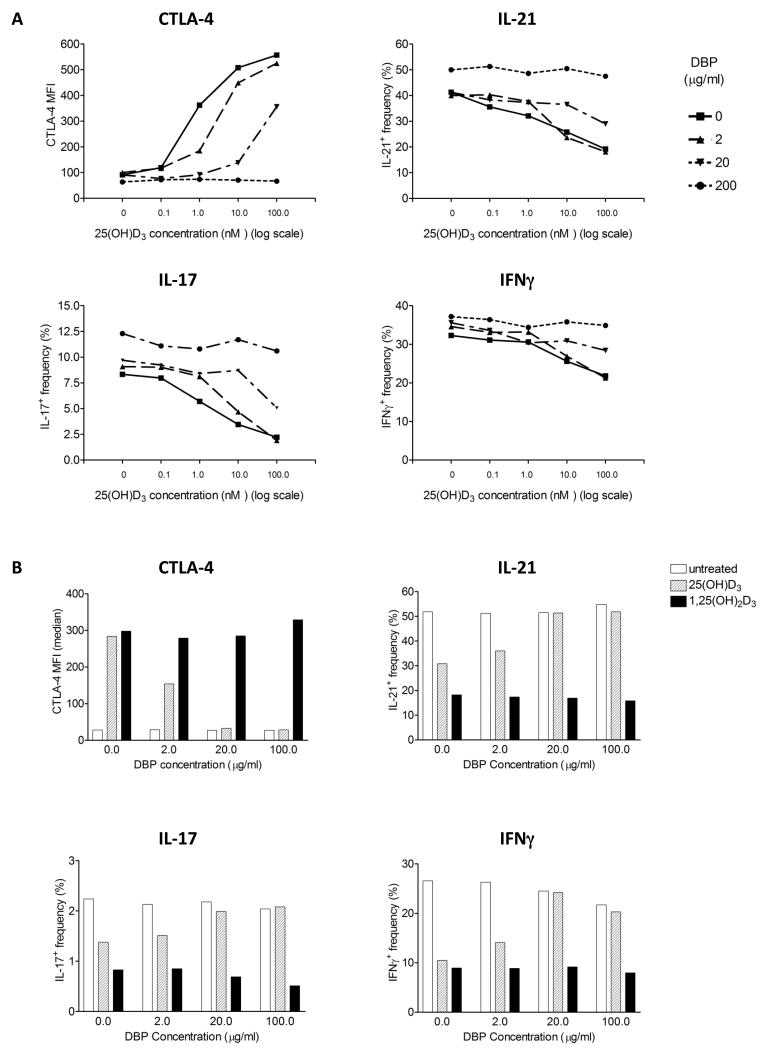
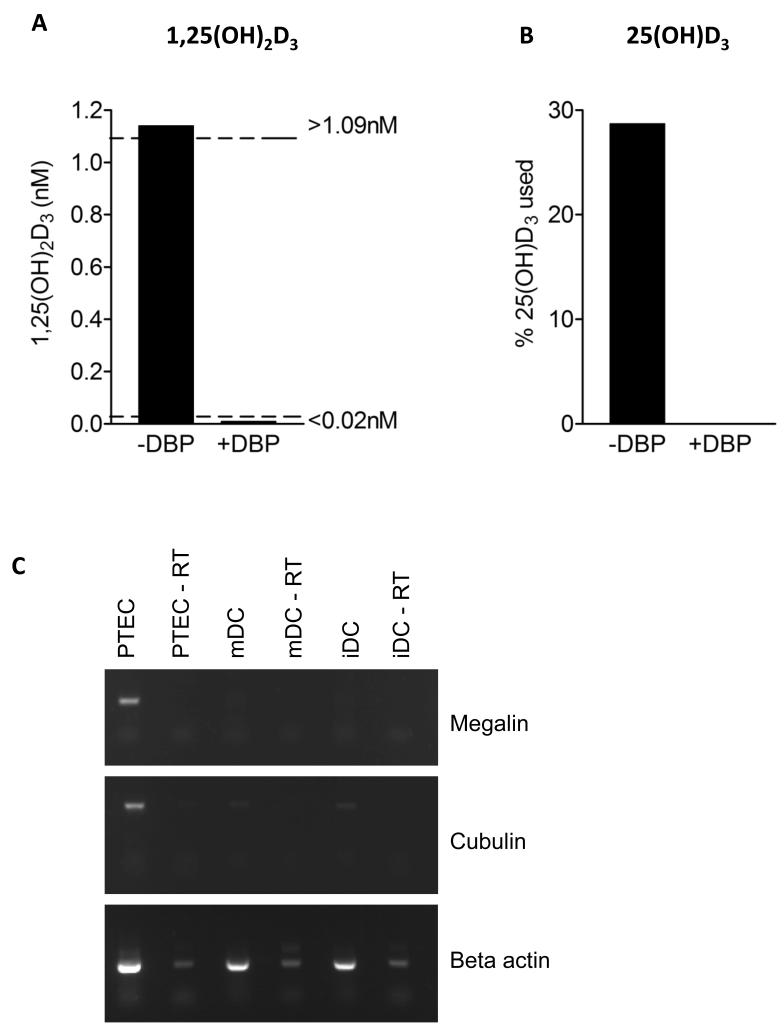
Given that most circulating 25(OH)D3 is bound to vitamin D binding protein (DBP), which facilitates its uptake by cells, we determined the impact of DBP on T cell responses. Surprisingly, as shown in figure 5A, addition of purified DBP suppressed T cell responses to 25(OH)D3 in a concentration dependent manner. Interestingly, responses to 1,25(OH)2D3 were much less affected (fig. 5B), suggesting that DBP might preferentially restrict 25(OH)D3 uptake by DCs but have less impact on T cell response to 1,25(OH)2D3. Consistent with the effect of DBP on T cell phenotype in response to 25(OH)D3, we were also unable to detect generation of 1,25(OH)2D3 in the presence of DBP (fig. 6A). Furthermore 25(OH)D3 did not decrease from input levels in these cultures suggesting lack of availability to DC (fig. 6B). We considered that this inhibition of conversion might be due to absence of the DBP transporter proteins, megalin or cubulin in DCs. Accordingly, we could not detect mRNA for megalin or cubulin in DCs in contrast to robust expression in proximal tubule epithelial cell lines (fig. 6C). Overall, these data suggest a role for DBP in controlling the availability of 25(OH)D3 for immune regulation.
Figure 5. Vitamin D binding protein suppresses 25(OH)D3 driven anti-inflammatory T cell responses.
A) CD4+CD25− T cells were stimulated with mature dendritic cells (mDCs) plus anti-CD3 in the presence of 25(OH)D3 across the range 0-100nM. Stimulations were cross titrated with purified human vitamin D binding protein (DBP) at the concentrations shown. Following stimulation, CD3+ cells were analysed for CTLA-4, IL-17, IFNγ and IL-21 expression by flow cytometry. Data are from one experiment representative of two performed. B) CTLA-4, IL-21, IL-17 and IFNγ expression was measured by flow cytometry after CD4+CD25− T cells were stimulated with DCs in the presence of 10nM 25(OH)D3 or 10nM 1,25(OH)2D3 and purified human DBP at the concentrations shown.
Figure 6. Vitamin D binding protein inhibits availability of 25(OH)D3 to DCs.
A,B) LPS treated DCs (mDC) or medium alone were incubated for 24 hours in the presence of 100nM 25(OH)D3 with or without 20μg/ml DBP and the endpoint concentration of 1,25(OH)2D3 measured (A). Dotted lines indicate the sensitivity range of the assay. The percentage of 25(OH)D3 used during the assay (B) was calculated by normalising to the level in the medium alone control. C) RT-PCR analysis of megalin, cubulin and β actin expression in proximal tubule kidney epithelial cells (PTEC), mDCs and iDCs. −RT indicates without reverse transcriptase. Data are from a single experiment representative of three performed.
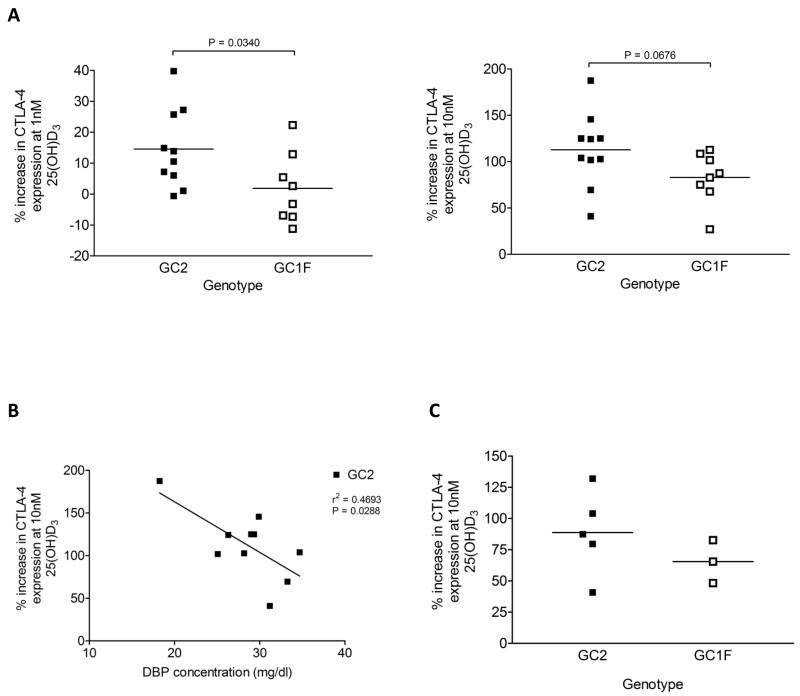
The three most common DBP variants, GC1S, GC1F and GC2, have different abilities to bind 25(OH)D3 and 1,25(OH)2D3, their Kd values for 25(OH)D3 being 0.9nM, 1.7nM and 2.8nM respectively, whilst those for 1,25(OH)2D3 are 56nM, 160nM and 240nM respectively29. We therefore considered the extent to which the immune effects of vitamin D might be dependent upon DBP variants. We carried out mDC-driven T cell stimulations in the presence of plasma from individuals with at least one GC2 (low affinity) or one GC1F (high affinity) allele but not both and compared responses to 25(OH)D3 supplementation. Plasma samples were taken from a COPD cohort, for which data relating to DBP genotype, DBP concentration and vitamin D status were already available. The percentage increase in CTLA-4 expression at either 1nM or 10nM 25(OH)D3 relative to baseline suggested a weaker response by the high affinity GC1F group (fig. 7A). This observation was consistent with the stronger 25(OH)D3 binding GC1F variant reducing 25(OH)D3 availability to the DC compared to the weaker binding GC2 species. Using our GC2 patients who showed the widest spread of plasma DBP concentration (18-30mg/dl), we also investigated whether variation in plasma DBP concentration could influence T cell responses to vitamin D. As shown in figure 7B, DBP concentration inversely correlated with percentage increase in CTLA-4 expression. Therefore, both DBP binding affinity and level of expression appear to influence responses to 25(OH)D3 supplementation. Similar trends for DBP variant were seen for a smaller cohort of healthy individuals (fig. 7C), supporting the possibility that DBP might influence the availability of 25(OH)D3 to APCs and subsequently 1,25(OH)2D3 to T cells.
Figure 7. Vitamin D binding protein genotype influences the magnitude of T cell responses to 25(OH)D3 supplementation.
CD4+ CD25− T cells were stimulated with mature dendritic cells for four days at increasing 25(OH)D3 concentrations and supplemented with: 2.5% plasma from COPD patients with at least one GC2 or one GC1F allele but not both (A and B) or 2.5% plasma from healthy donors with at least one GC2 or one GC1F allele but not both (C). CTLA-4 expression was measured by flow cytometry and plotted as percentage increase in expression at the given 25(OH)D3 concentration relative to 0nM 25(OH)D3. Horizontal lines show mean values. Significance between GC2 and GC1F groups was tested by two-tailed Mann Whitney tests (A and C) and linear regression analysis used to test association between CTLA-4 induction and DBP concentration (B).
Discussion
A number of studies now support a function for 1,25(OH)2D3 on immune cells such as monocytes, dendritic cells (DCs) and T cells. Whilst active 1,25(OH)2D3 can directly influence T cells that express the VDR following activation, it is unlikely that circulating levels of 1,25(OH)2D3 are sufficient to have such an impact. It is known that antigen presenting cells (APCs) such as macrophages, monocytes and DC can upregulate the enzymatic machinery necessary to activate 25(OH)D322,24,42,45. Furthermore, they can respond to the 1,25(OH)2D3 they generate in an autocrine fashion as indicated by upregulation of the VDR target genes, cathelicidin and the vitamin D inactivating enzyme, CYP24A145,46, as well as reduced antigen presentation24 or enhanced anti-microbial activity42. Thus, APCs may also be able to generate and locally secrete sufficient 1,25(OH)2D3 to directly influence their cognate T cell partners in a paracrine manner. However, such functional interplay relating to vitamin D metabolism between T cells and DC has not been extensively studied. We approached this question using a previously characterised model where exogenously added 1,25(OH)2D3 was found to have an impact on T cell responses, resulting in the downregulation of pro-inflammatory cytokines and the upregulation of the regulatory proteins CTLA-4 and Foxp320. Using these changes in T cell phenotype as a readout for the availability of 1,25(OH)2D3, we observed that T cell responses in the presence of 25(OH)D3 were virtually identical to those in the presence of active 1,25(OH)2D3 as long as DCs were present. The effectiveness of DC as a source of local 1,25(OH)2D3 correlated with their robust expression of CYP27B1 converting enzyme upon maturation with LPS. In contrast, T cells alone only modestly expressed this enzyme. Other studies have reported CYP27B1 expression in T cells35-37. However, whilst these indicate that T cells could metabolise 25(OH)D3 few functional readouts have so far been reported. In addition, the concentrations of 25(OH)D3 required for any effects appear to exceed those expected in-vivo36,37. By contrast, we have shown that the level of CYP27B1 activity in T cells is in fact relatively low compared to DCs and enzyme activity is insufficient to markedly influence their phenotype as measured by expression of a range of cytokines and regulatory associated molecules across physiologically relevant concentrations of 25(OH)D3.
Given the importance of DC as a source of the CYP27B1 converting enzyme, the control of its expression is of interest. In line with the findings of others24,43, we observed that bacterial LPS, which signals through TLR4, could induce strong CYP27B1 mRNA expression in DCs. Induction of CYP27B1 by APCs in response to gram positive TLR2/1 ligands has also been observed42,43,47. Thus, up regulation of CYP27B1 in APCs appears a central mechanism in vitamin D regulated innate immune responses. In addition to DC maturation induced by microbial products we observed that during the course of T cell activation, DCs matured sufficiently to induce functionally significant CYP27B1 expression. Whilst soluble factors from these cultures or recombinant pro-inflammatory cytokines permitted detectable CYP27B1 mRNA induction, more effective up-regulation was observed when DCs were in contact with T cells. Consistent with this observation, agonistic CD40 antibodies were also effective in stimulating CYP27B1 expression. Combined treatment with CD40 antibodies and inflammatory cytokines supported the strongest induction of CYP27B1, similar to the additive effects of cytokines and TLR signalling upon CYP27B1 expression, as shown in other studies40,42,43. Overall, our study provides further evidence that cognate interactions occurring between DCs and T cells, including CD40 ligation, promote the induction of CYP27B1 and subsequent metabolism of 25(OH)D3 at a level that is sufficient to regulate the T cell response. This mechanism could therefore be of importance in the control of immune responses under non-infectious settings, such as might occur in autoimmune responses. The data presented here demonstrate CYP27B1 activity in a range of myeloid cell populations, supporting the view that the expression of CYP27B1 could be a general feature of myeloid cell maturation. Indeed it would be of interest to ascertain how myeloid cells from different tissues or disease states vary in their levels of CYP27B1 expression and activity. Furthermore, given that retinal hydrogenase, the enzyme that activates vitamin A, has been associated with the CD103+ subclass of gut resident DCs48, variations in CYP27B1 expression with particular DC subclasses would also be of interest.
A major issue relating to the impact of vitamin D biology on immune cells concerns the concentration of its various metabolites available under physiological conditions. We have observed that titrating 25(OH)D3 across physiological levels revealed efficient conversion and T cell modulation even at concentrations as low as 1nM, although the magnitude of the response increased towards 100nM suggesting a broad dynamic range. Thus, the outcome of a T cell response that takes place in the presence of CYP27B1 expressing APCs is likely to be proportionally sensitive to the available 25(OH)D3. Such data are important, given the number of studies linking 25(OH)D3 status with autoimmune conditions8,49-52, where alteration of the inflammatory/regulatory T cell balance is likely to be involved in the pathogenesis. At present, total vitamin D is used as an indication of vitamin D status. However, a number of factors could influence its availability to target cells. An important candidate is vitamin D binding protein (DBP) since the majority of vitamin D is bound to this protein in serum. Interestingly, it has recently been suggested that DBP levels can modify the relationship between vitamin D status and bone mineral density53. Accordingly, the relationship between vitamin D status and immunological function might also be influenced by DBP, depending on the ability of immune cells to express DBP transporters. We observed that addition of DBP strongly suppressed T cell responses to 25(OH)D3, reducing the magnitude of CTLA-4 induction and inflammatory cytokine suppression. Thus, whilst DBP may bind to the surfaces of immune cells including monocytes54, neutrophils55 and lymphocytes56,57 it appears that DBP actually restricts availability to DC and consequently limits T cell responses to 25(OH)D3. This contrasts with the ability of DBP to promote uptake of 25(OH)D3 by some cell types including kidney cells26, mammary cells27 and osteoblasts28. Interestingly, we did not detect any significant inhibition by DBP on responses to 1,25(OH)D3, perhaps suggesting that the major effect of DBP is in preventing uptake of 25(OH)D3 by DCs and that 1,25(OH)2D3 may be less inhibited than 25(OH)D3 due to its lower affinity for DBP. Notably, our findings are consistent with those of Chun et al., who showed that DBP attenuated monocyte responses to vitamin D metabolites, as measured by the induction of cathelicidin and CYP24A1 mRNA46. Correale et al suggest that macrophages and CD4+ T cells can take up DBP58. Whilst their report seemingly contrasts with our finding that DBP inhibits T cell responses to 25(OH)D3, we would note that their assay could not distinguish between simple binding of DBP to cells and the utilisation of transporter proteins to effectively take up DBP. Given the multifunctional properties of DBP, however, it is likely that differences in DBP binding could have consequences on T cell behaviour even aside from effects upon 25(OH)D3 metabolism. Thus, the role of DBP in modifying responses of immune cells to vitamin D metabolites is worthy of further investigation. In our study we added purified DBP into serum free media, however, the concentrations of DBP required for effects in-vivo are also likely to be influenced by other factors in serum. For example, polyunsaturated fatty acids such as arachidonic acid can bind to DBP and reduce its affinity for both 25(OH)D3 and 1,25(OH)2D359,60. Moreover, the level of DBP within immune environments, such as lymph nodes is presently unknown. Thus, whilst it is clear that DBP can significantly affect the availability of 25(OH)D3 to DCs, the level of its impact under physiological conditions is presently uncertain. A further issue relating to the influence of DBP on T cell responses to 25(OH)D3 is its potential genetic variation, since this can affect its affinity for vitamin D metabolites29. Our initial data suggest that the stronger binding GC1F genotype more effectively limits the impact of 25(OH)D3 on CTLA-4 expression compared to the weaker binding GC2 allele. Chun et al, similarly observed that serum containing the weaker binding GC2 protein was less inhibitory than that from GC1F individuals in their monocyte assays46. Thus, together, these studies provide coherent evidence that the availability of 25(OH)D3 to cells of the immune system can be inhibited by the presence of DBP and that variation in DBP genotype might further influence the magnitude of inhibition. Furthermore, they raise the question of the extent to which total 25(OH)D3 accurately reflects the level of 25(OH)D3 that is accessible for immune regulation.
Both environmental and genetic factors impact significantly on the development of autoimmune conditions. Accordingly, understanding how environmentally regulated hormones such as vitamin D influence the immune system may be of importance. Low vitamin D status has been correlated with more active disease in autoimmune models and population studies, which is in keeping with the observation that the effects of 1,25(OH)2D3 on the adaptive immune response appear to be predominantly immunosuppressive. Of the T cell markers studied, we have found the up regulation of CTLA-4 to be particularly sensitive to 1,25(OH)2D3 which is in keeping with the presence of vitamin D binding sites close to the CTLA-locus61. Such upregulation of CTLA-4 by 1,25(OH)2D3 is likely to be of regulatory significance, since we and others have recently identified that CTLA-4 is a direct effector of T cell suppression that works by downregulating its natural ligands62-65. Furthermore, it is also clear that 1,25(OH)2D3 can target ligand downregulation on DC directly22. Thus, control of costimulatory molecules could be a key part of the emerging immunosuppressive action of 1,25(OH)2D3 on the adaptive immune system8,9.
An increasing body of evidence suggests that vitamin D can have profound effects on the immune system. The present data reveal that the outcome of T cell activation is strongly influenced by the availability of the inactive 25(OH)D3 precursor to DCs, since, upon maturation, these cells upregulate CYP27B1 and generate local 1,25(OH)2D3 at sufficient level for T cells to respond. Our data further suggest that the availability of 25(OH)D3 depends on both the serum level of inactive 25(OH)D3 and the concentration and genotype of DBP. Accordingly the level of “free vitamin D” available to the immune system might be lower than that indicated by standard measures of vitamin D status but could be clinically relevant in the control of inflammatory disease.
Supplementary Material
Acknowledgments
LEJ is funded by Arthritis Research UK, OSQ was funded by the BBSRC and TZH and ZB were funded by the Wellcome Trust.
References
- 1.Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2011:1–12. doi: 10.1017/S0029665111001650. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Hanwell HE, Banwell B. Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim Biophys Acta. 2011;1812:202–212. doi: 10.1016/j.bbadis.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Mowry EM. Vitamin D: evidence for its role as a prognostic factor in multiple sclerosis. J Neurol Sci. 2011;311:19–22. doi: 10.1016/j.jns.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 5.Cutolo M, Pizzorni C, Sulli A. Vitamin D endocrine system involvement in autoimmune rheumatic diseases. Autoimmun Rev. 2011;11:84–87. doi: 10.1016/j.autrev.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Hypponen E. Vitamin D and increasing incidence of type 1 diabetes-evidence for an association? Diabetes Obes Metab. 2010;12:737–743. doi: 10.1111/j.1463-1326.2010.01211.x. [DOI] [PubMed] [Google Scholar]
- 7.Raman M, Milestone AN, Walters JR, Hart AL, Ghosh S. Vitamin D and gastrointestinal diseases: inflammatory bowel disease and colorectal cancer. Therap Adv Gastroenterol. 2011;4:49–62. doi: 10.1177/1756283X10377820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheum. 2008;4:404–412. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 9.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Cantorna MT. Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys. 2012;523:103–106. doi: 10.1016/j.abb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross HS, Nittke T, Kallay E. Colonic vitamin D metabolism: implications for the pathogenesis of inflammatory bowel disease and colorectal cancer. Mol Cell Endocrinol. 2011;347:70–79. doi: 10.1016/j.mce.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Larsson P, Mattsson L, Klareskog L, Johnsson C. A vitamin D analogue (MC 1288) has immunomodulatory properties and suppresses collagen-induced arthritis (CIA) without causing hypercalcaemia. Clin Exp Immunol. 1998;114:277–283. doi: 10.1046/j.1365-2249.1998.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayne CG, Spanier JA, Relland LM, Williams CB, Hayes CE. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41:822–832. doi: 10.1002/eji.201040632. [DOI] [PubMed] [Google Scholar]
- 14.Berer A, Stockl J, Majdic O, et al. 1,25-Dihydroxyvitamin D(3) inhibits dendritic cell differentiation and maturation in vitro. ExpHematol. 2000;28:575–583. doi: 10.1016/s0301-472x(00)00143-0. [DOI] [PubMed] [Google Scholar]
- 15.Canning MO, Grotenhuis K, de Wit H, Ruwhof C, Drexhage HA. 1-alpha,25-Dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. EurJEndocrinol. 2001;145:351–357. doi: 10.1530/eje.0.1450351. [DOI] [PubMed] [Google Scholar]
- 16.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 17.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colin EM, Asmawidjaja PS, van Hamburg JP, et al. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010;62:132–142. doi: 10.1002/art.25043. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda U, Wakita D, Ohkuri T, et al. 1alpha,25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol Lett. 2010;134:7–16. doi: 10.1016/j.imlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D(3) and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urry Z, Xystrakis E, Richards DF, et al. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J ClinInvest. 2009;119:387–398. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 23.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 24.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314–3316. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 25.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 27.Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006;136:2754–2759. doi: 10.1093/jn/136.11.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atkins GJ, Anderson PH, Findlay DM, et al. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone. 2007;40:1517–1528. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Hum Genet. 1993;92:183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 30.Wood AM, Bassford C, Webster D, et al. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax. 2011;66:205–210. doi: 10.1136/thx.2010.140921. [DOI] [PubMed] [Google Scholar]
- 31.Martineau AR, Leandro AC, Anderson ST, et al. Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J. 2010;35:1106–1112. doi: 10.1183/09031936.00087009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papiha SS, Pal B. Gc (vitamin D binding protein) subtypes in rheumatoid arthritis. Hum Genet. 1985;70:278–280. doi: 10.1007/BF00273457. [DOI] [PubMed] [Google Scholar]
- 33.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006;372:33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Wood AM, Simmonds MJ, Bayley DL, Newby PR, Gough SC, Stockley RA. The TNFalpha gene relates to clinical phenotype in alpha-1-antitrypsin deficiency. Respir Res. 2008;9:52. doi: 10.1186/1465-9921-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baeke F, Korf H, Overbergh L, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121:221–227. doi: 10.1016/j.jsbmb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 36.Correale J, Ysrraelit MCl, GaitÃin MIs. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 37.Sigmundsdottir H, Pan J, Debes GF, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 38.Bikle DD, Pillai S, Gee E, Hincenbergs M. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. 1989;124:655–660. doi: 10.1210/endo-124-2-655. [DOI] [PubMed] [Google Scholar]
- 39.Bikle DD, Pillai S, Gee E, Hincenbergs M. Tumor necrosis factor-alpha regulation of 1,25-dihydroxyvitamin D production by human keratinocytes. Endocrinology. 1991;129:33–38. doi: 10.1210/endo-129-1-33. [DOI] [PubMed] [Google Scholar]
- 40.Edfeldt K, Liu PT, Chun R, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A. 2010;107:22593–22598. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gyetko MR, Hsu CH, Wilkinson CC, Patel S, Young E. Monocyte 1 alpha-hydroxylase regulation: induction by inflammatory cytokines and suppression by dexamethasone and uremia toxin. J Leukoc Biol. 1993;54:17–22. doi: 10.1002/jlb.54.1.17. [DOI] [PubMed] [Google Scholar]
- 42.Krutzik SR, Hewison M, Liu PT, et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 44.van Driel M, Koedam M, Buurman CJ, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 45.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 46.Chun RF, Lauridsen AL, Suon L, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams JS, Ren S, Liu PT, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cantorna MT. Vitamin D and multiple sclerosis: an update. Nutr Rev. 2008;66:S135–138. doi: 10.1111/j.1753-4887.2008.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50:72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 51.Pappa HM, Gordon CM, Saslowsky TM, et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118:1950–1961. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311–319. doi: 10.1177/0148607107031004311. [DOI] [PubMed] [Google Scholar]
- 53.Powe CE, Ricciardi C, Berg AH, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLeod JF, Kowalski MA, Haddad JG. Characterization of a monoclonal antibody to human serum vitamin D binding protein (Gc globulin): recognition of an epitope hidden in membranes of circulating monocytes. Endocrinology. 1986;119:77–83. doi: 10.1210/endo-119-1-77. [DOI] [PubMed] [Google Scholar]
- 55.Kew RR, Sibug MA, Liuzzo JP, Webster RO. Localization and quantitation of the vitamin D binding protein (Gc-globulin) in human neutrophils. Blood. 1993;82:274–283. [PubMed] [Google Scholar]
- 56.Machii T, Kimura H, Ueda E, et al. Distribution of Gc protein (vitamin D binding protein) on the surfaces of normal human lymphocytes and leukemic lymphocytes. Acta Haematol. 1986;75:26–29. doi: 10.1159/000206075. [DOI] [PubMed] [Google Scholar]
- 57.Petrini M, Allegrini A, Ambrogi F, et al. Binding of GC (VDBP) to membranes of human B lymphocytes following stripping of extant protein. J Endocrinol Invest. 1995;18:630–637. doi: 10.1007/BF03349781. [DOI] [PubMed] [Google Scholar]
- 58.Correale J, Ysrraelit MC, Gaitan MI. Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol. 2010;185:4948–4958. doi: 10.4049/jimmunol.1000588. [DOI] [PubMed] [Google Scholar]
- 59.Bouillon R, Xiang DZ, Convents R, Van Baelen H. Polyunsaturated fatty acids decrease the apparent affinity of vitamin D metabolites for human vitamin D-binding protein. J Steroid Biochem Mol Biol. 1992;42:855–861. doi: 10.1016/0960-0760(92)90094-y. [DOI] [PubMed] [Google Scholar]
- 60.Calvo M, Ena JM. Relations between vitamin D and fatty acid binding properties of vitamin D-binding protein. Biochem Biophys Res Commun. 1989;163:14–17. doi: 10.1016/0006-291x(89)92091-3. [DOI] [PubMed] [Google Scholar]
- 61.Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–1360. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 64.Zheng Y, Manzotti CN, Burke F, et al. Acquisition of suppressive function by activated human CD4+ J Immunol. 2008;181:1683–1691. doi: 10.4049/jimmunol.181.3.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.