Abstract
Current models of autoimmunity suggest that delayed clearance of apoptotic cells leads to the presentation of apoptotic antigens in the context of inflammatory signals, with resultant autoimmunity. These models implicitly assume that, in contrast to early apoptotic cells (that retain membrane integrity), late apoptotic cells (with compromised membranes) act like necrotic cells (which also lack intact membranes), possibly because of the release of proinflammatory intracellular contents. We showed previously that early apoptotic and necrotic cells induce distinct mitogen-activated protein kinase modules in macrophages with which they interact. Exposure to apoptotic cells led to nearly complete inhibition of both basal and macrophage colony-stimulating factor-induced ERK1/2 by macrophages. In contrast, necrotic cells induced ERK1/2. We show here that apoptotic cells also strongly induced both c-Jun N-terminal kinase and p38, whereas necrotic cells had no detectable effect on c-Jun N-terminal kinase and p38. We also compared the signaling events induced in macrophages by exposure to early apoptotic cells, late apoptotic cells, and necrotic cells. The signaling events induced by late apoptotic cells were identical to and just as potent as those induced by early apoptotic cells. Thus, apoptotic cells are functionally equivalent throughout the cell death process, irrespective of membrane integrity. Moreover, the effects of both early and late apoptotic cells on signaling were dominant over those of necrotic cells. These data show that apoptotic cells do not become proinflammatory upon the loss of membrane integrity and are inconsistent with the notion that delayed clearance alone can lead to autoimmunity.
Apoptosis is an active energy-dependent process that generally occurs without inflammation or injury to surrounding tissues (1). Apoptotic cells express surface markers that permit their rapid recognition and ingestion by phagocytes (2, 3). Moreover, the cell membrane of cells undergoing apoptosis remains intact until relatively late (1). Thus, the vast majority of cells dying by apoptosis are cleared by phagocytes while their cell membranes are still intact and before they can release their potentially inflammatory intracellular contents.
In this view, the noninflammatory behavior of apoptotic cells is essentially passive in that inflammation is avoided by rapid and efficient clearance of apoptotic cells. In fact, apoptotic cells are also actively anti-inflammatory (4, 5). For example, the uptake of apoptotic cells actively inhibits the release of proinflammatory mediators such as interleukin 1 and tumor necrosis factor-α by macrophages (mφ)2 (4–6). This contrasts with the effect of necrotic cell uptake, which may lead to mφ activation and the release of proinflammatory cytokines (7). Based on this differential response to apoptotic versus necrotic cells, antigens derived from cells dying by these two distinct processes are thought to have opposite effects on the activation of T cells (8). The proinflammatory effects of necrotic cells may act as a “danger signal” and provide the costimulation required for T cell activation and immunity. In contrast, early apoptotic cells, being anti-inflammatory, are unlikely to activate T cells and may even be tolerogenic (9).
The effect of late apoptotic cells (apoptotic cells that have lost membrane integrity), also referred to as post-apoptotic or secondarily necrotic cells, is more uncertain (10). According to many current models, late apoptotic cells should behave like necrotic cells, because of the release of potentially inflammatory intracellular contents (11). Indeed, there is a prevailing belief that delayed or reduced clearance of apoptotic cells leads to the presentation of apoptotic antigen in the context of inflammatory signals, with resultant autoimmunity (9, 12, 13). This view is supported by studies in mice in which genetic deficiencies of any number of gene products (for example, C1q (14), the MER receptor tyrosine kinase (15), or MFG-E8 (16)) give rise both to delayed clearance of apoptotic cells and to the development of systemic autoimmune disease.
This apparent linkage may not reflect a causal relationship between altered clearance and autoimmunity. As we and others have shown, the recognition and/or uptake of apoptotic cells is accompanied by intracellular signal transduction (17, 18). For example, although necrotic cells activate the mitogen-activated protein kinase (MAPK) elements extracellular signal-regulated kinase (ERK) 1 and 2, apoptotic cells strongly inhibit ERK1/2 activity (17). We hypothesize that it is the absence of appropriate apoptotic cell-induced signaling events, rather than delayed apoptotic cell clearance per se, that is responsible for the loss of tolerance and the development of autoimmunity in these mutant mice. A strong prediction of our hypothesis is that signaling events triggered by late apoptotic cells should resemble those of early apoptotic cells and not necrotic cells.
In accord with previous work, we show here that early apoptotic and necrotic cells induce distinct MAPK modules. Consistent with our hypothesis, and in contradiction to the delayed clearance model of auto-immunity, we find that the signaling events induced in mφ by late apoptotic cells are identical to those induced by early apoptotic cells. Furthermore, our data demonstrate that both early and late apoptotic cells are dominant over necrotic cells with respect to MAPK signaling. Taken together, these data imply that delayed clearance of apoptotic cells, in and of itself, is unlikely to cause autoimmunity and suggest that the signaling events induced by apoptotic cells, whether early or late, may play a critical role in the maintenance of immune tolerance.
EXPERIMENTAL PROCEDURES
Materials
Unless otherwise stated, all of the chemicals were obtained from Sigma, Invitrogen, or Fisher.
Antibodies
Antibodies detecting Rsk were affinity-purified polyclonal rabbit IgG recognizing either the active Thr359–Ser363-phospho-rylated form of Rsk (major reactivity to Rsk1 with cross-reactivity to Rsk3) or total Rsk irrespective of its state of phosphorylation (Cell Signaling Technology, Beverly, MA). All of the antibodies detecting the active or total forms of ERK (ERK1 and ERK2), JNK (JNK1 and JNK2), and p38 (p38α, p38γ, and p38δ) were affinity-purified polyclonal rabbit IgG (Promega, Madison, WI). Anti-active ERK1/2 detects the dually phosphorylated Thr183-Glu-Tyr185 region (pTEpY) from the catalytic core of the active ERK kinase. Anti-active JNK1/2 detects the dually phosphorylated Thr183-Pro-Tyr185 region (pTPpY) derived from the catalytic core of the active form of the JNK kinase. Anti-active p38 detects the dually phosphorylated Thr180-Gly-Tyr182 region (pTGpY) derived from the catalytic core of the active form of the p38 kinase.
Cell Culture
Primary cultures of bone marrow (BM)-derived mφ were prepared as previously described (19). Briefly, BM cells were expressed from the femur and tibia of BALB/c or C57BL/6 mice. After centrifugation at 500 × g, red blood cells were lysed by resuspending the cell pellet in Tris/NH4Cl lysis buffer (9:1 mixture of 0.16 M NH4Cl, pH 7.2, and 0.17 M Tris base, pH 7.2) at 37 °C for 5 min. The cells were then washed three times in RPMI 1640 and plated in 100-mm tissue culture plates at 5 × 106 cells/dish in R.10 medium (RPMI 1640 plus 10% FBS, with 2 mM L-glutamine, 5 mM HEPES, 100 units/ml penicillin, and 100 μg/ml streptomycin) containing 15% L929 cell conditioned medium (LCM) as a source of macrophage-colony stimulating factor (M-CSF). After 3 days of incubation, additional R.10 plus 15% LCM was added. After an additional 3 days, when BM mφ were ~90% confluent, the medium was removed and replaced with fresh R.10 plus 15% LCM. After 24 h, to make the cells quiescent for use in signaling studies, the medium was replaced with R.0 (R.10 medium minus FBS) without LCM for 48 h.
The Jurkat human T cell leukemia line (American Type Culture Collection, TIB-152, Manassas, VA) and the conditionally immortalized mouse kidney proximal tubule epithelial cell line (BUMPT (Boston University mouse proximal tubule cells)) (20) were used as a source of apoptotic and necrotic cells. Jurkat cells were maintained in RPMI 1640 containing 10% FBS, 1 mM sodium pyruvate, 2 mM L-glutamine, 10 mM HEPES, and 100 units/ml penicillin-streptomycin. BUMPT cells were maintained in high glucose Dulbecco’s modified Eagle’s medium containing 10% FBS, 2 mM L-glutamine, 10 mM HEPES, 100 units/ml penicillin-streptomycin, and 10 units/ml γ-interferon.
Preparation of Apoptotic, Necrotic, and Late Apoptotic Cells and Latex Beads
Apoptosis of Jurkat cells was induced by transferring cells to R.0 containing 0.5% fatty acid-free bovine serum albumin and 1 μg/ml staurosporine. Apoptosis was produced by exposure to stauro-sporine for 3 h, followed by washing and resuspension in fresh medium plus 0.5% fatty acid-free bovine serum albumin. Early apoptotic cells were used after incubation for an additional 2 h, and late apoptotic cells were used after overnight incubation for an additional 18 h. Apoptosis of BUMPT cells was similarly induced, except that staurosporine was added to FBS-free medium, and the cells were detached by the addition of 5 mM EDTA after exposure to staurosporine. Necrosis of both Jurkat and BUMPT cells was induced by washing cells three times in FBS-free medium and then heating cells suspended in the appropriate FBS-free medium to 65 °C for 40 min. Latex beads (1.15 μm) obtained from Seradyne (Indianapolis, IN) were used in control studies as a neutral phagocytic stimulus in place of apoptotic or necrotic cells.
Induction of apoptosis and necrosis was confirmed by flow cytometry. Viable cells were defined as cells that were both propidium iodide (PI)- and annexin V-negative. Early apoptotic cells were defined as PI-negative cells with annexin V staining and decreased cell size. Necrotic cells were defined as PI-positive cells of normal or increased cell size. Late apoptotic cells were defined as PI-positive cells with annexin V staining and decreased cell size. The loss of membrane integrity in necrosis and late apoptosis was also confirmed by Trypan blue staining. By these criteria, early apoptotic cell preparations contained ~85% early apoptotic and ~15% late apoptotic cells. Necrotic cell preparations contained ~95% necrotic cells. Late apoptotic cell preparations contained ~95% late apoptotic cells. In all preparations, viable cells, defined as PI-negative cells of normal size without annexin V staining, comprised <5% of the total cell population.
Western Blot Analysis
The cells were lysed in 50 mM HEPES, pH 7.5, containing 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM phenylmeth-ylsulfonyl fluoride, and 200 μM orthovanadate. The lysates were centrifuged at 10,000 × g for 10 min at 4 °C, and the supernatants were stored at −70 °C. Samples standardized for protein content by the bicincho-ninic acid protein assay (Pierce) were separated by SDS-PAGE using 4–20% acrylamide gels (Novex Tris-glycine gels; Invitrogen). Protein was transferred to polyvinylidene difluoride membranes and probed with antibody according to the manufacturer’s instructions.
RESULTS
Apoptotic and Necrotic Cells Have Divergent Effects on the ERK1/2 Pathway
For apoptotic and necrotic cells to have differential effects on immune function, the signaling events induced by exposure to these two types of dead cells must be distinct. In accord with this notion, we have previously shown that apoptotic and necrotic cells have opposite effects on ERK1/2 signaling (17). It should be noted that the necrotic cells used in our studies, although lacking membrane integrity, are intact cells and have a normal nuclear morphology, as assessed by light microscopy and Hoechst staining (17). Although exposure to necrotic cells activated ERK1/2 in primary cultures of BM mφ, as indicated by phosphorylation at the catalytic core on Thr183 and Tyr185, exposure to apoptotic cells inhibited both basal and M-CSF-induced ERK1/2 activity (17).
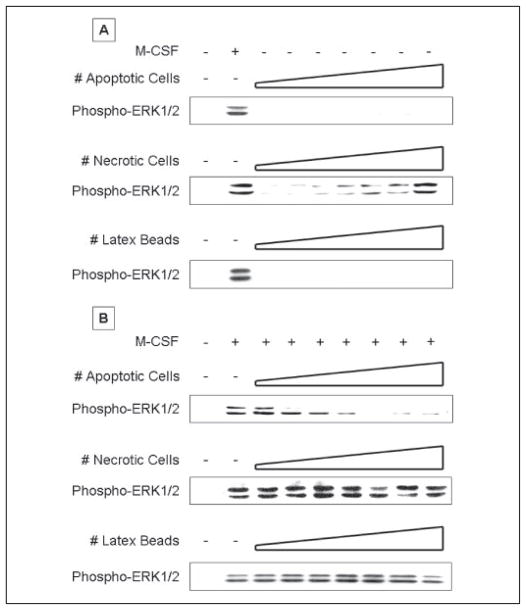
To examine this differential behavior in greater detail, we determined the concentration dependence of the ERK1/2 response to apoptotic and necrotic cells (Fig. 1). Primary cultures of BM mφ were grown to confluence and rendered quiescent by culture for 48 h in FBS-free medium (R.0). Quiescent mφ showed minimal levels of basal active phosphorylated ERK1/2. Stimulation with M-CSF for 15 min induced nearly maximal levels of phosphorylated ERK1/2. In the absence of M-CSF, exposure to early apoptotic BUMPT cells induced no phosphorylated ERK1/2 and, in fact, in most experiments inhibited basal ERK1/2 phosphorylation. In contrast, exposure to necrotic BUMPT cells for 15 min activated ERK1/2 in a dose-dependent manner. Induction was first evident at a necrotic cell to mφ ratio of ~1:32, reached half-maximal levels at a ratio of 1:4 to 1:2, and matched the M-CSF response at a ratio of 1:1. Latex beads, a neutral phagocytic stimulus, had no effect on ERK1/2 phosphorylation. Apoptotic and necrotic cells alone contributed no activated ERK1/2 in these experiments (Ref. 17 and data not shown).
FIGURE 1. Apoptotic cells inhibit whereas necrotic cells activate ERK1/2 in bone marrow-derived macrophages.
Serum-starved BM-derived macrophages were stimulated with increasing numbers of apoptotic cells, necrotic cells, or latex beads alone for 15 min (A) or stimulated with M-CSF for 15 min following a 30-min pre-exposure to apoptotic cells, necrotic cells, or latex beads (B). Apoptotic or necrotic BUMPT cells were added to macrophages at a cell to macrophage ratio ranging from 1:64 to 1:1 in 2-fold increments, as indicated by the schematic diagram of increasing cell ratio. Nonadherent cells were removed by rinsing, and macrophage lysates were probed with anti-active ERK1/2 antibody. Equal loading was confirmed by Ponceau S staining of blotted proteins as well as probing for total ERK1/2 (supplemental Fig. S1). Apoptotic and necrotic cells alone contributed no active ERK1/2 in these experiments.
We next determined the effect that prior exposure to these stimuli for 30 min had on subsequent activation of ERK1/2 by M-CSF (Fig. 1B). Strikingly, prior exposure to early apoptotic cells strongly inhibited M-CSF-induced phosphorylation of ERK1/2. Inhibition by early apoptotic cells occurred in a dose-dependent manner. Inhibition was first evident at an apoptotic cell to mφ ratio of 1:32, reached half-maximal levels at a ratio of 1:16, and was virtually complete at a ratio of 1:4. In contrast, prior exposure to necrotic cells slightly increased the level of phosphorylated ERK1/2 seen after 15 min of stimulation with M-CSF. Thus, the responses to early apoptotic and necrotic cells were not only opposite in direction but also different in concentration dependence. Early apoptotic cells were more potent stimuli, achieving a half-maximal effect at an ~4-fold lower ratio of 1:16, as compared with a ratio of 1:4 for necrotic cells. Latex beads had no effect on ERK1/2 phosphorylation in response to M-CSF.
To confirm these effects on ERK1/2 activation, we examined the state of phosphorylation of the ribosomal kinase Rsk, an immediate downstream target of ERK1/2. We specifically looked at the ERK1/2 phosphorylation site Ser573 on the activation loop of Rsk (21). As shown in Fig. 2, the pattern of phosphorylation for Rsk in response to early apoptotic cells, necrotic cells, and latex beads closely paralleled that for phosphorylation of ERK1/2. Early apoptotic cells strongly inhibited Rsk phosphorylation in response to M-CSF, whereas necrotic cells alone induced dose-dependent phosphorylation of Rsk.
FIGURE 2. Apoptotic cells inhibit whereas necrotic cells activate p90Rsk, a downstream substrate of ERK1/2, in BM macrophages.
Serum-starved BM macrophages were stimulated with increasing numbers of apoptotic cells, necrotic cells, or latex beads alone for 15 min (A) or stimulated with M-CSF for 15 min following a 30-min pre-exposure to apoptotic cells, necrotic cells, or latex beads (B). Apoptotic or necrotic BUMPT cells were added to macrophages at a cell to macrophage ratio ranging from 1:64 to 1:1 in 2-fold increments, as indicated by the schematic diagram of increasing cell ratio. Non-adherent cells were removed by rinsing, and macrophage lysates were probed with anti-active phospho-p90Rsk antibody. Equal loading was confirmed by Ponceau S staining of blotted proteins as well as probing for total p90Rsk (supplemental Fig. S2). Apo-ptotic and necrotic cells alone contributed no active p90Rsk in these experiments.
To ensure that the total protein (irrespective of phosphorylation) was equivalent on all blots and to ensure that decreased phosphorylation of ERK1/2 and p90Rsk following exposure to apoptotic cells cannot be attributed to dilution of intact macrophage proteins by phagocytosed proteins derived from apoptotic cells, we performed Ponceau S staining (not shown), and we probed lysates for total ERK1/2, total p90Rsk, total p38, and total JNK1/2 (supplemental figures). As shown in supplemental Figs. S1 and S2, the levels of total ERK1/2 and total p90Rsk were equivalent and not affected by exposure to apoptotic cells. This equivalence of total levels of ERK1/2 and p90Rsk, despite exposure to apoptotic cells, implies that phagocytosed material from apoptotic cells represents a minor fraction of macrophage cell lysates. Similarly, exposure to necrotic cells had no effect on the expression of total ERK1/2 and total p90Rsk. It should also be noted that dilutional effects are not a concern in the case of necrotic cells, because they activated ERK1/2 and p90Rsk. Moreover, as shown below, exposure to apoptotic cells not only inhibited ERK1/2 and p90Rsk but also induced the activation of JNK and p38 (Fig. 3). Because the same lysates were probed for all four kinases, it is unlikely that a dilutional artifact can explain such divergent signaling events.
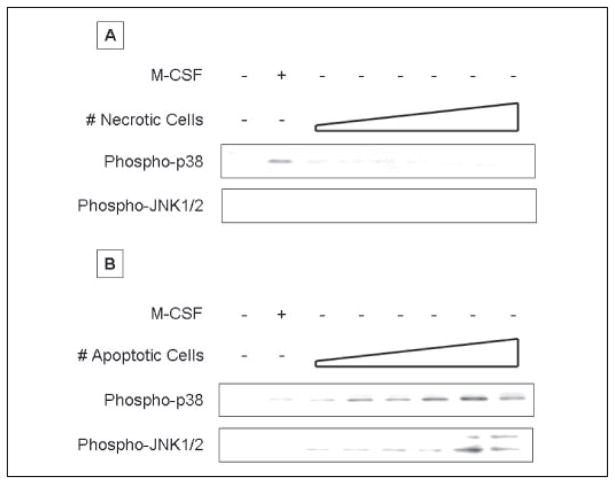
FIGURE 3. Apoptotic cell activate p38 and JNK, whereas necrotic cells have no discernible effect on p38 and JNK.
Serum-starved BM macrophages were exposed to increasing numbers of apoptotic (A) or necrotic (B) Jurkat cells for 15 min. Apoptotic or necrotic cells were added to macrophages at a cell to macrophage ratio ranging from 1:64 to 1:1 in 2-fold increments. Nonadherent cells were removed by rinsing, and macrophage lysates were probed with anti-active p38 or anti-active JNK antibody. Equal loading was confirmed by Ponceau S staining of blotted proteins as well as probing for total p38 (supplemental Fig. S3) or JNK (not shown). Apoptotic and necrotic cells alone contributed no active p38 and JNK in these experiments.
We observed identical results with early apoptotic and necrotic cells derived from human Jurkat cells (data not shown). Together with our previous results (17), we note that these effects on ERK1/2 are characteristic of early apoptotic and necrotic cells, regardless of the species or tissue type of the dead cells. We have observed similar effects on ERK1/2 activation with all of the following: human Jurkat T cells, murine renal epithelial BUMPT cells, and primary murine splenocytes and thymocytes. The ability of apoptotic cells to inhibit ERK1/2 activation is also independent of the particular suicidal stimulus with which death is induced (e.g. staurosporine, high dose hydrocortisone, γ-irradiation, and ultraviolet irradiation) (Ref. 17 and data not shown). Moreover, these effects of early apoptotic and necrotic cells on ERK1/2 are not limited to mφ but extend also to nonprofessional phagocytes, because early apoptotic cells inhibited epidermal growth factor-induced ERK1/2 phosphorylation in murine embryonic fibroblasts (and data not shown).3 We conclude that early apoptotic and necrotic cells, without restriction to species or stimulus, have characteristic and divergent effects on the phosphorylation and activation of ERK1/2.
Apoptotic and Necrotic Cells Also Have Differential Effects on Other MAPK Modules
We examined the effects of early apoptotic and necrotic cells on two additional MAPK modules, JNK1/2 and p38 (Fig. 3 and supplemental Fig. S3). Quiescent BM mφ had undetectable levels of phosphorylated JNK1/2 and p38. M-CSF weakly induced phosphorylation of p38 and had no effect on JNK1/2. In contrast to their inhibitory effect on ERK1/2 activity, early apoptotic cells induced dose-dependent phosphorylation of both JNK1/2 and p38. The concentration dependences of the JNK1/2 and p38 responses to early apoptotic cells were similar to that of the ERK1/2 response. Although the JNK and p38 modules are activated in response to a wide variety of stresses and inflammatory stimuli (23), exposure to necrotic cells had no detectable effect on these two pathways, either alone (Fig. 3 and supplemental Fig. S3) or administered prior to M-CSF stimulation (data not shown). Latex beads, like necrotic cells, had no effect on the levels of phosphorylated JNK1/2 and p38 (data not shown). These data extend our observations of the divergent patterns of the effects of early apoptotic and necrotic cells on the three MAPK modules.
The Effects of Apoptotic Cells Are Dominant over Those of Necrotic Cells
The fact that early apoptotic and necrotic cells have divergent effects on the ERK1/2, JNK1/2, and p38 modules presents a definitive setting with which to determine the effect of mixtures of these two stimuli on mφ signaling events. Implicit in the delayed clearance model of autoimmunity is the assumption that the proinflammatory effects of necrotic cells, like those hypothesized for late apoptotic cells, should dominate over those of early apoptotic cells.
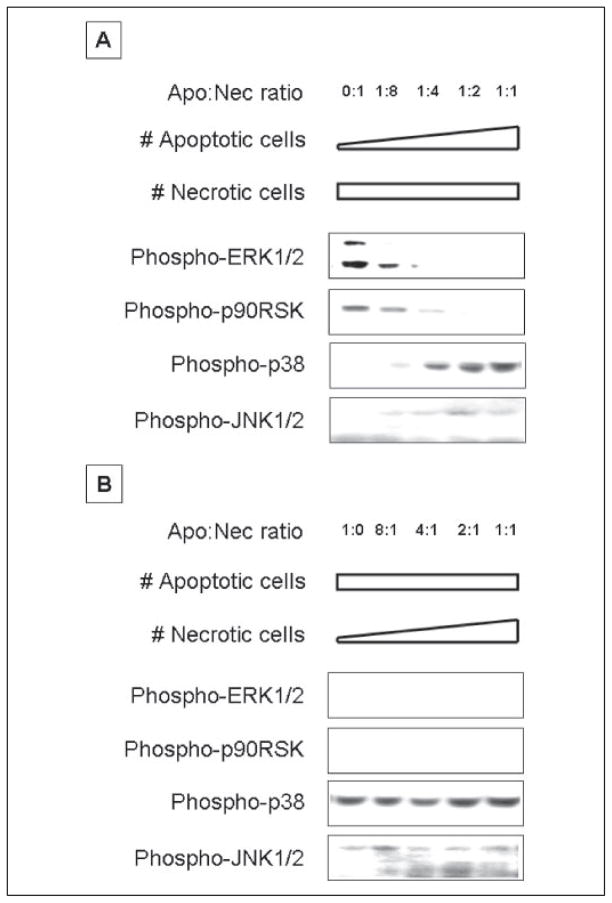
We tested this in two complementary sets of experiments. In the first (Fig. 4A and supplemental Fig. S4A), we incubated BM mφ with a fixed number of necrotic cells (inducing maximal phosphorylation of ERK1/2; first lane in Fig. 4A), together with a graded number of apoptotic cells, the ratio of apoptotic to necrotic cells ranging from 1:8 to 1:1. In the second (Fig. 4B and supplemental Fig. S4B), we added a fixed number of apoptotic cells (inducing maximal inhibition of ERK1/2 phosphorylation; first lane in Fig. 4B), together with a graded number of necrotic cells, the ratio of apoptotic to necrotic cells ranging from 8:1 to 1:1. Remarkably, in both sets of experiments, and with respect to each MAPK module examined, the effects of apoptotic cells were strongly dominant over those of necrotic cells. For example, when apoptotic cells were present at an optimal number for inhibiting ERK1/2 phosphorylation, no number of necrotic cells was able to even minimally counter the apoptotic cell signal (Fig. 4B and supplemental Fig. S4B). Similarly, despite an optimal number of necrotic cells for induction of ERK1/2 phosphorylation, 1 apoptotic cell/4 necrotic cells was able to overcome completely the necrotic cell effect (Fig. 4A and supplemental Fig. S4A). This last result is especially noteworthy in that a suboptimal number of apoptotic cells (with respect to M-CSF-induced ERK1/2 phosphorylation) still was able to completely inhibit the response to an optimal number of necrotic cells.
FIGURE 4. Apoptotic cell-mediated signaling events are dominant over those mediated by necrotic cells mediated signaling in BM macrophages.
Serum-starved BM macrophages were pre-exposed to mixtures of apoptotic (Apo) and necrotic (Nec) BUMPT cells for 30 min. A, the number of necrotic cells was kept constant at a necrotic cell to macrophage ratio of 1:1, and the number of apoptotic cells was increased as shown. B, the number of apoptotic cells was kept constant at an apoptotic cell to macrophage ratio of 1:1, and the number of necrotic cells was increased as shown. Nonadherent cells were removed by rinsing, and macrophage lysates were probed with anti-active ERK1/2, p38, JNK, and p90Rsk antibodies. Equal loading was confirmed by Ponceau S staining of blotted proteins as well as probing for total ERK1/2, p38, JNK, or p90Rsk (supplemental Fig. S4, except for total JNK, which is not shown). Apoptotic and necrotic cells alone contributed no active ERK1/2, p38, JNK, or p90Rsk in these experiments.
Despite the Loss of Membrane Integrity, Late Apoptotic Cells Behave Equivalently to Early Apoptotic Cells
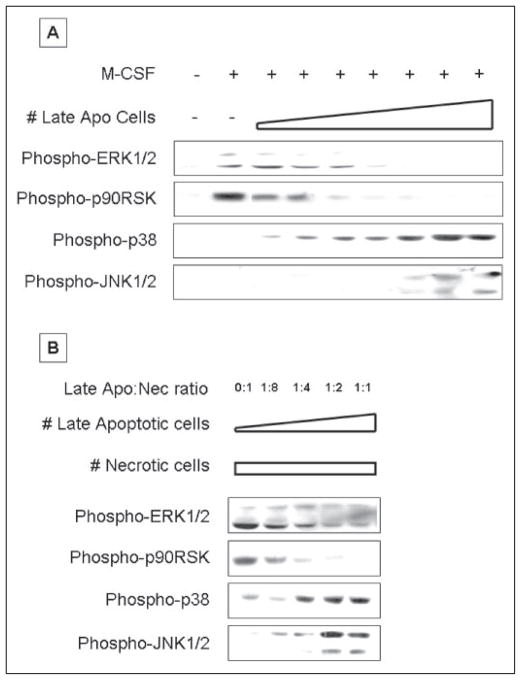
In light of these observations, we asked whether late apoptotic cells, which have lost membrane integrity, still retain the characteristic behavior of early apoptotic cells. Late apoptotic cells were generated by overnight incubation at 37 °C following a 3-h exposure to staurosporine. These cells displayed the features of late apoptosis, including reduced cell size and failure to exclude Trypan blue or PI. Late apoptotic cells were not washed and were added directly to BM mφ in their overnight culture medium, so that BM mφ were exposed not only to the late apoptotic cells themselves but also to any intracellular contents that may have been released. Strikingly, late apoptotic cells induced the same characteristic MAPK response profile, as did early apoptotic cells (Fig. 5A and supplemental Fig. S5A). Thus, late apoptotic cells inhibited M-CSF-induced ERK1/2 phosphorylation and subsequent Rsk phosphorylation and also stimulated phosphorylation of JNK1/2 and p38. Importantly, the dose dependence of late apoptotic cells was similar to that of early apoptotic cells.
FIGURE 5. Late apoptotic cells induce the same signaling events as do early apoptotic cells.
A, serum-starved BM macrophages were pre-exposed to increasing numbers of late apoptotic (Apo) BUMPT cells for 30 min and then stimulated with M-CSF for 15 min. Late apoptotic cells were added to macrophages at a cell to macrophage ratio ranging from 1:64 to 1:1 in 2-fold increments. B, serum-starved BM macrophages were pre-exposed to mixtures of apoptotic and necrotic (Nec) BUMPT cells for 30 min. The number of necrotic cells was kept constant at a necrotic cell to macrophage ratio of 1:1, and the number of apoptotic cells was increased as shown. Nonadherent cells were removed by rinsing, and macrophage lysates were probed with anti-active ERK1/2, p38, JNK, and p90Rsk antibodies. Equal loading was confirmed by Ponceau S staining of blotted proteins as well as probing for total ERK1/2, p38, JNK, or p90RSK (supplemental Fig. S5, except for total JNK, which is not shown). Apoptotic and necrotic cells alone contributed no active ERK1/2, p38, JNK, or p90Rsk in these experiments.
We also tested the relationship of late apoptotic cells and necrotic cells when the two populations were cultured together with BM mφ (Fig. 5B and supplemental Fig. S5B). We found that late apoptotic cells were as effective as early apoptotic cells in inhibiting phosphorylation of ERK1/2 and Rsk in response to necrotic cells. Late apoptotic cells also induced phosphorylation of JNK1/2 and p38, irrespective of the presence or quantity of necrotic cells. We conclude that, at least with respect to modulation of ERK1/2, JNK1/2, and p38, late apoptotic cells behave identically to early apoptotic cells. Thus, regardless of membrane integrity, apoptotic cells have a characteristic effect on mφ signal transduction, at least in relation to MAPK signaling events, and this effect is strongly dominant over that of necrotic cells.
Late Apoptotic Cells Do Not Release Proinflammatory Contents
The dominance of apoptotic cells with respect to ERK1/2, JNK1/2, and p38 signaling may mask the potential release of proinflammatory intracellular contents from late apoptotic cells. As shown in Fig. 6A, we explored this possibility by separating a suspension of late apoptotic BUMPT cells (Fraction 1) into the following fractions: low speed centrifugation supernatant (apoptotic bodies, secreted molecules, and released intracellular material) (Fraction 2), low speed centrifugation pellet (dead cells and cell ghosts) (Fraction 3), high speed ultracentrifugation supernatant (soluble intracellular material) (Fraction 4), and high speed ultracentrifugation pellet (apoptotic bodies plus particulate intracellular material) (Fraction 5). Fractions 1, 2, and 3 were derived from late apoptotic cell suspensions corresponding to an apoptotic to mφ cell ratio of 1:1 (inducing maximal inhibition of ERK1/2 phosphorylation; see Fig. 5A). To control for the fact that the later fractions may contain decreased cellular material, Fractions 4 and 5 were derived from late apoptotic cell suspensions corresponding to a 4-fold increased apoptotic to mφ cell ratio of 4:1.
FIGURE 6. The released intracellular contents of late apoptotic cells do not activate ERK1/2.
A, the procedure used to separate late apoptotic and necrotic BUMPT cell suspensions into five distinct fractions is diagramed. Fractions 1–3 were derived from late apoptotic and necrotic cell suspensions corresponding to a cell to mφ ratio of 1:1, whereas Fractions 4 and 5 were derived from suspensions corresponding to a cell to mφ ratio of 4:1. B, serum-starved BM macrophages were exposed to late apoptotic cells (Fraction 1) and the 4 fractions (Fractions 2–5) from late apoptotic cell for 15 min alone or were stimulated with M-CSF for 15 min following a 30-min pre-exposure to these fractions. C, serum-starved BM macrophages were exposed similarly to necrotic cells (Fraction 1) or their fractions (Fractions 2–5) for 15 min alone. Nonadherent cells were removed by rinsing, and macrophage lysates were probed with anti-active ERK1/2 antibody. Equal loading was confirmed by Ponceau S staining of blotted proteins as well as probing for total ERK1/2 (supplemental Fig. S6). Late apoptotic and necrotic cells alone contributed no active ERK1/2, p38, JNK, or p90Rsk in these experiments.
When added to mφ as a sole stimulus, none of the fractions from late apoptotic cells, including the high speed ultracentrifugation supernatant containing the soluble intracellular contents from late apoptotic cells, induced ERK1/2 phosphorylation (Fig. 6B and supplemental Fig. S6B). When mφ were stimulated with M-CSF after prior exposure to these fractions for 30 min, inhibition of ERK1/2 phosphorylation occurred with each of the fractions except the high speed ultracentrifugation supernatant, for which we observed an undiminished ERK response. We conclude that the characteristic inhibitory activity of apoptotic cells resides in the membrane fractions derived from those cells, including apoptotic bodies and cell ghosts. We could detect no separable proinflammatory activity in any of the fractions, including the soluble intracellular contents from late apoptotic cells.
By comparison, all fractions derived from a parallel separation of necrotic cells induced ERK1/2 phosphorylation (Fig. 6C and supplemental Fig. S6C). In contrast to apoptotic cells, the proinflammatory activity associated with necrotic cells is both soluble and membrane-associated.
DISCUSSION
Our results demonstrate that apoptotic cells and necrotic cells trigger divergent signaling responses in BM mφ. These results extend previous findings indicating that recognition of apoptotic cells and necrotic cells by mφ occurs via distinct mechanisms (5) and reveal that separate recognition processes are linked to contrasting signaling outcomes. Necrotic cells activate ERK1/2, whereas apoptotic cells inhibit activated ERK1/2, regardless of the activating stimulus. We presented data demonstrating that prior interaction with apoptotic cells precludes subsequent ERK1/2 activation; apoptotic cells also can inhibit previously activated ERK1/2 (data not shown) (17). Apoptotic cells also activate JNK1/2 and p38, whereas necrotic cells have no detectable effect on these two MAPK modules. We find that the effects of apoptotic cells on signaling responses, like their effects on cytokine secretion (5), are dominant over those of necrotic cells and can be exerted even when the apoptotic cells are present in reduced numbers. It is important to note that both soluble and membrane-associated material derived from necrotic cells can mimic the effects exerted by whole necrotic cells. Strikingly, our data document that apoptotic cells retain the ability to elicit their characteristic signaling responses throughout the death process, irrespective of membrane integrity. Indeed, the effects of late apoptotic cells are identical in all respects to those of early apoptotic cells. Any intracellular contents released from late apoptotic cells are neutral and not proinflammatory.
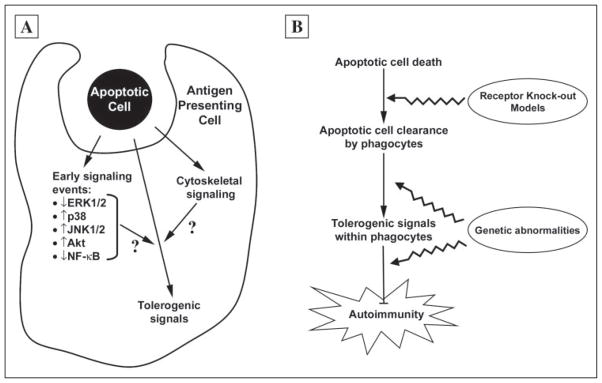
Our results may have important implications for our understanding of autoimmunity and tolerance (Fig. 7). Recent studies suggest that necrotic and apoptotic cells have opposing effects on immune homeostasis (24). Necrotic cells, by virtue of leaking their inflammatory intracellular contents, may provide the costimulation needed for activation of naive T cells and therefore may promote immunity (25). Apoptotic cells, on the other hand, being actively anti-inflammatory, do not activate T cells, and may even be tolerogenic (26). According to current models, late apoptotic cells, which have lost membrane integrity and leak their intracellular contents, should behave like necrotic cells and induce an immune response (27). Thus, delayed or impaired clearance of apoptotic cells, as observed with targeted deletion of the complement protein C1q (14) or the MER receptor tyrosine kinase (15), is thought to lead to presentation of apoptotic antigen in the context of inflammatory signals, with resultant autoimmunity (Fig. 7).
FIGURE 7. Exposure of macrophages to apoptotic cells induces multiple signaling events that contribute to immune homeostasis.
A, the interaction of macrophages with apoptotic cells results in a number of signaling events. As shown in this study, apoptotic cells inhibit ERK1/2 but activate JNK1/2 and p38. We have previously shown that apoptotic cells activate the anti-apoptotic kinase Akt (17) and inhibit activation of proinflammatory transcription factors (5, 10). Recognition of apoptotic cells also leads to cytoskeletal changes that facilitate phagocytosis of apoptotic cells. Among the signaling events induced by apoptotic cells, we speculate that some play a critical role in maintaining self-tolerance by regulating the presentation of apoptotic cell-derived self-antigens in the context of major histocompatibility complex molecules and other T cell-stimulatory molecules. B, the signaling events induced by exposure to apoptotic cells may play a role in preventing autoimmunity. According to the model we suggest, loss of self-tolerance, with resultant autoimmunity, may occur through one of two fundamental mechanisms. First, impaired clearance of apoptotic cells, as may occur through targeted deletion of relevant genes, results in a decreased interaction of apoptotic cells with phagocytes and therefore a decreased amount of tolerogenic signals within the phagocyte. However, as shown in this study, persistence of apoptotic cells, with the loss of membrane integrity, does not in and of itself alter the nature of the signaling events induced in response to apoptotic cell recognition, merely its intensity, and therefore apoptotic cell persistence is unlikely itself to be the cause of autoimmunity. Alternatively, inherited or acquired abnormalities within the tolerogenic signaling pathways themselves, that are induced by apoptotic cells, may constitute a predisposing background for the development of autoimmunity.
The delayed clearance model makes several predictions that we tested directly. First, for necrotic and apoptotic cells to have opposing effects on immune function, the signaling events induced by exposure to these two types of cells must be distinct. This prediction is fulfilled, at least in terms of signaling events involving the ERK, JNK, and p38 MAPK modules. Second, the model predicts that late apoptotic cells, which have lost membrane integrity, should behave like necrotic cells. However, in sharp contrast to this prediction, late apoptotic cells were indistinguishable from early apoptotic cells in all of their effects on mφ. Late apoptotic cells inhibited basal and M-CSF-induced ERK1/2 activity, late apoptotic cells activated JNK1/2 and p38, and, as elaborated below in the third prediction of the delayed clearance model, late apoptotic cells were fully dominant over necrotic cells. Third, the model predicts that the signaling events induced by necrotic cells must be dominant over those induced by apoptotic cells. This prediction arises from the following considerations. Because apoptotic cells are continuously generated in vivo, delayed clearance of apoptotic cells will lead to the coexistence of both early and late apoptotic cells. Immunity will result only if the inflammatory signals in response to the presumed inflammatory intracellular contents of late apoptotic cells can override the anti-inflammatory effects of early apoptotic cells. Because the intracellular contents of necrotic cells should be at least as inflammatory as those of late apoptotic cells, we pitted apoptotic cells against necrotic cells. Again, in sharp contrast to the prediction of the delayed clearance model, the signaling events induced by apoptotic cells were dominant over those induced by necrotic cells. Remarkably, late apoptotic cells were just as dominant as early apoptotic cells in terms of inhibiting ERK1/2 activity and stimulating JNK1/2 and p38. Thus, in striking contrast to the predictions of the delayed clearance model, apoptotic cells appear to be functionally equivalent throughout their existence, irrespective of membrane integrity. Finally, in direct opposition to the delayed clearance model, neither cell-associated nor soluble material recovered from late apoptotic cells was proinflammatory.
Although our results argue against the delayed clearance model of autoimmunity, there is an important caveat. Our studies focused on three specific MAPK signaling cascades and may not generalize to other signal transduction pathways. It is possible that there exist other signaling pathways and/or intermediates for which the predictions of the delayed clearance model hold true and that these other pathways play a more determining role in the balance between tolerance and immunity. This seems unlikely, however, because we have also shown that late apoptotic cells mimic early apoptotic cells with respect to proinflammatory transcriptional activity (10) and inflammatory cytokine secretion (5). Moreover, even if delayed clearance contributes to the development of autoimmunity, it is not in and of itself sufficient to produce autoimmunity, because targeted deletion of CD14 or the mannose-binding lectin leads to the in vivo accumulation of apoptotic cells in the absence of autoimmunity (28, 29).
In light of these considerations, we propose that the absence of apoptotic cell-induced signaling events, rather than delayed apoptotic cell clearance per se, is the critical event leading to the loss of tolerance and the development of autoimmunity. We therefore hypothesize that the signaling pathways induced by the binding and/or uptake of apoptotic cells are critical to the maintenance of tolerance and that deficient or abnormal signaling events within these same pathways can predispose to autoimmunity. Consistent with this hypothesis, we have previously shown that mφ from prediseased mice of all the major inbred murine models of spontaneous autoimmunity, including multiple strains that develop systemic lupus erythematosus, as well as the autoimmune diabetes-prone NOD strain, have an identical apoptotic cell-dependent abnormality in the expression of multiple cytokines (22, 30–32). Affected systemic lupus erythematosus-prone strains include MRL/+, MRL/lpr, NZB, NZW, NZB/W F1, BXSB, and LG/J. No similar defect in cytokine expression can be found in 16 nonautoimmune strains, including three strains that develop type II (nonautoimmune) diabetes mellitus. Importantly, in the absence of apoptotic cells, cytokine expression by these autoimmune-prone strains is completely normal. Furthermore, cytokine expression is not the sole apoptotic cell-dependent abnormality observed in mφ from prediseased autoimmune-prone mice. mφ from NOD and the same systemic lupus erythematosus-prone strains also have a reversible defect in the activity of the cytoplasmic G protein Rho, a key regulator of the cytoskeleton, resulting in abnormalities of adhesion and cytoskeletal organization (31, 32). Again, no similar abnormalities were observed in mφ from multiple nonauto-immune control strains. These results provide strong support for our hypothesis that abnormalities in the signaling events induced by apoptotic cells may be causally related to the development of autoimmunity. The downstream consequences of these signaling events, in terms of gene transcription and phentotypic function, are the subject of ongoing investigation.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants DK59793 and HL69722 (to J. S. L.) and AG024234 (to D. S. U.), by operating grants from the Arthritis Society of Canada (to J. R.) and the Canadian Institutes of Health Research (to J. R.), and by a Young Investigator Award from the National Kidney Foundation of Illinois (to J. S. L.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
The abbreviations used are: mφ, macrophage(s); BM, bone marrow; ERK1/2, extracellular signal-regulated kinase 1 and 2; MAPK, mitogen-activated protein kinase; M-CSF, macrophage colony-stimulating factor; JNK, c-Jun N-terminal kinase; FBS, fetal bovine serum; LCM, L929 cell conditioned medium; PI, propidium iodide.
M. Cvetanovic, J. E. Mitchell, V. Patel, B. S. Avner, Y. Su, P. T. van der Saag, P. L. Witte, S. Fiore, J. S. Levine, and D. S. Ucker, submitted for publication.
References
- 1.Majno G, Joris I. Am J Pathol. 1995;14:3–15. [PMC free article] [PubMed] [Google Scholar]
- 2.Platt N, da Silva RP, Gordon S. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 3.Savill J, Fadok V. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 4.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. J Clin Investig. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocco RE, Ucker DS. Mol Biol Cell. 2001;12:919–930. doi: 10.1091/mbc.12.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voll RE, Hermann M, Roth EA, Stach C, Kalden JR. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 7.Fadok VA, Bratton DL, Guthrie L, Henson PM. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 8.Skoberne M, Beignon AS, Larsson M, Bhardwaj N. Curr Top Microbiol Immunol. 2005;289:259–292. doi: 10.1007/3-540-27320-4_12. [DOI] [PubMed] [Google Scholar]
- 9.Gaipl US, Voll RE, Sheriff A, Franz S, Kalden JR, Herrmann M. Autoimmun Rev. 2005;4:189–194. doi: 10.1016/j.autrev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Cvetanovic M, Ucker DS. J Immunol. 2004;172:880–889. doi: 10.4049/jimmunol.172.2.880. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Molinaro C, Johnson N, Casiano CA. Arthritis Rheum. 2001;44:2642–2652. doi: 10.1002/1529-0131(200111)44:11<2642::aid-art444>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, Herrmann M. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell DA, Pickering MC, Warren J, Fossati-Jimack L, Cortes-Hernandez J, Cook HT, Botto M, Walport MJ. J Immunol. 2002;168:2538–2543. doi: 10.4049/jimmunol.168.5.2538. [DOI] [PubMed] [Google Scholar]
- 15.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 17.Reddy SM, Hsiao KH, Abernethy VE, Fan H, Longacre A, Lieberthal W, Rauch J, Koh JS, Levine JS. J Immunol. 2002;169:702–713. doi: 10.4049/jimmunol.169.2.702. [DOI] [PubMed] [Google Scholar]
- 18.Giles KM, Ross K, Rossi AG, Hotchin NA, Haslett C, Dransfield I. J Immunol. 2001;167:976–986. doi: 10.4049/jimmunol.167.2.976. [DOI] [PubMed] [Google Scholar]
- 19.Levine JS, Pugh BJ, Hartwell D, Fitzpatrick JM, Marshak-Rothstein A, Beller DI. Eur J Immunol. 1993;23:2951–2958. doi: 10.1002/eji.1830231134. [DOI] [PubMed] [Google Scholar]
- 20.Sinha D, Wang Z, Ruchalski KL, Levine JS, Krishnan S, Lieberthal W, Schwartz JH, Borkan SC. Am J Physiol. 2005;288:F703–F713. doi: 10.1152/ajprenal.00189.2004. [DOI] [PubMed] [Google Scholar]
- 21.Frodin M, Gammeltoft S. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 22.Koh JS, Wang Z, Levine JS. J Immunol. 2000;165:4190–4201. doi: 10.4049/jimmunol.165.8.4190. [DOI] [PubMed] [Google Scholar]
- 23.Kyriakis JM, Avruch J. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 24.Albert ML. Nat Rev Immunol. 2004;4:223–231. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 25.Barker RN, Erwig LP, Hill KS, Devine A, Pearce WP, Rees AJ. Clin Exp Immunol. 2002;127:220–225. doi: 10.1046/j.1365-2249.2002.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hugues S, Mougneau E, Ferlin W, Jeske D, Hofman P, Homann D, Beaudoin L, Schrike C, Von Herrath M, Lehuen A, Glaichenhaus N. Immunity. 2002;16:169–181. doi: 10.1016/s1074-7613(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 27.Savill J, Dransfield I, Gregory C, Haslett C. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 28.Devitt A, Parker KG, Ogden CA, Oldreive C, Clay MF, Melville LA, Bellamy CO, Lacy-Hulbert A, Gangloff SC, Goyert SM, Gregory CD. J Cell Biol. 2004;167:1161–1170. doi: 10.1083/jcb.200410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. J Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 30.Longacre A, Koh JS, Hsiao KKH, Gilligan H, Fan H, Patel VA, Levine JS. J Leukocyte Biol. 2004;76:971–984. doi: 10.1189/jlb.0604346. [DOI] [PubMed] [Google Scholar]
- 31.Fan H, Longacre A, Meng F, Patel V, Hsiao K, Koh JS, Levine JS. J Immunol. 2004;172:4834–4843. doi: 10.4049/jimmunol.172.8.4834. [DOI] [PubMed] [Google Scholar]
- 32.Fan H, Patel VA, Longacre A, Levine JS. J Leuk Biol. 2006;79:155–165. doi: 10.1189/jlb.0705408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.