Abstract
Increasing age is the most robust predictor of greater malignancy and treatment resistance in human gliomas. However, the adverse association of clinical course with aging is rarely considered in animal glioma models, impeding delineation of the relative importance of organismal versus progenitor cell aging in the genesis of glioma malignancy. To address this limitation, we implanted transformed neural stem/progenitor cells (NSPCs), the presumed cells of glioma origin, from 3 and 18month old mice into 3 and 20-month host animals. Transplantation with progenitors from older animals resulted in significantly shorter (p ≤ 0.0001) median survival in both 3month (37.5 vs 83 days) and 20-month (38 vs 67 days) hosts, indicating that age-dependent changes intrinsic to NSPCs rather than host animal age accounted for greater malignancy. Subsequent analyses revealed that increased invasiveness, genomic instability, resistance to therapeutic agents and tolerance to hypoxic stress accompanied aging in transformed NSPCs. Greater tolerance to hypoxia in older progenitor cells, as evidenced by elevated HIF-1 promoter reporter activity and hypoxia response gene (HRG) expression, mirror the upregulation of HRGs in cohorts of older vs younger glioma patients revealed by analysis of gene expression databases, suggesting that differential response to hypoxic stress may underlie age-dependent differences in invasion, genomic instability and treatment resistance. Our study provides strong evidence that progenitor cell aging is responsible for promoting the hallmarks of age-dependent glioma malignancy and that consideration of progenitor aging will facilitate development of physiologically and clinically relevant animal models of human gliomas.
Keywords: aging, glioma, neural stem and progenitor cells, malignancy, syngeneic model
Introduction
Adult human malignant gliomas are incurable and little progress has been realized in patient survival in over 30 years. Development of more efficacious therapeutic approaches is limited by the lack of experimental models that accurately reflect glioma biology and clinical behavior. The most robust predictor of glioma malignancy, treatment response and patient survival is age at diagnosis (Curran et al. 1993; CBTRUS 2009). Remarkably, little is known about the mechanisms by which age alters glioma biology. To model the effects of aging on glioma malignancy, the effect of age on factors intrinsic to the host (e.g., brain microenvironment) as well as on factors intrinsic to neural stem/progenitor cells (NSPCs; e.g., genomic instability), the cells of glioma origin, must be considered. To date, the relative impact of aging on host versus NSPCs on glioma development has not been determined in animal glioma models. A better understanding of how age in host and progenitor cells promotes the development of adverse clinical features could stimulate the development of more relevant animal models and identify mechanisms responsible for age-dependent worsening of malignancy in human gliomas.
To address this limitation, we examined the relative impact of aging in progenitor cells and host organism separately by implanting NSPCs from 3 and 18month mice oncogenically transformed ex vivo into syngeneic 3mo and 20mo host animals. Our results showed that aging in NSPCs, but not the host organism, significantly shortened host survival, indicating that age-dependent changes intrinsic to progenitor cells had a profound influence on malignant potential. The greater malignancy of transformed 18mo NSPCs was accompanied by increased invasion, genomic damage, resistance to radiation and alkylating agents and tolerance of hypoxic stress. The potential clinical relevance of hypoxic responses was revealed by our analysis of gene expression databases, which showed enhanced expression of hypoxia response genes in older cohorts of human glioma patients. Our findings validate incorporating aging in glioma animal models and demonstrate the potential of such models to identify potential mechanisms responsible for age-dependence of malignancy in human gliomas.
Results
The age of progenitor cells rather than host animals determines glioma malignancy
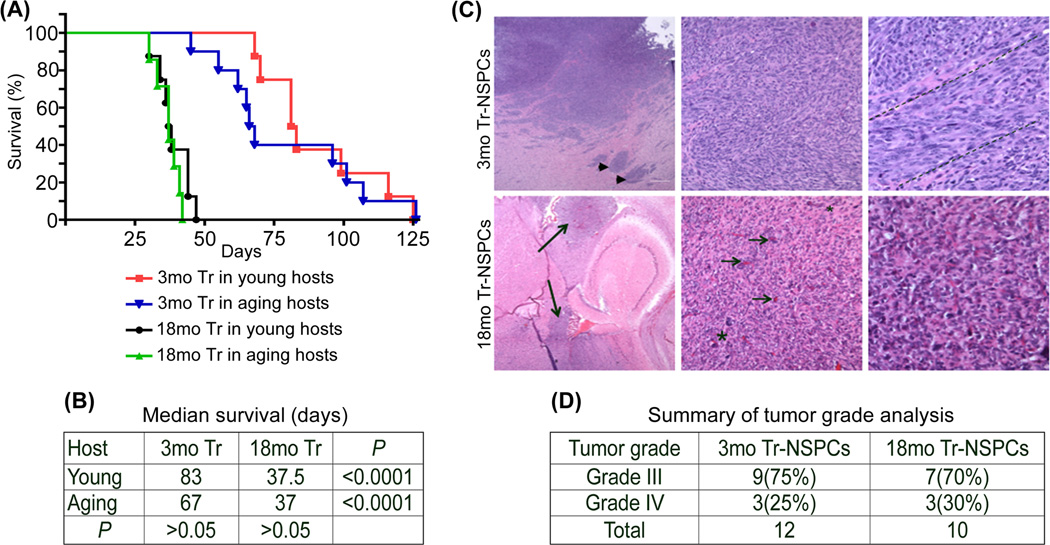
We previously reported that increased age of transformed NSPCs (Tr-NSPCs) resulted in markedly shorted survival of 4-6mo hosts (Mikheev et al. 2009). Here we address the question of whether progenitor cell or host animal age is the predominant determinant of malignancy by comparing survival of 3mo and 20 mo syngeneic mouse hosts implanted with either 3mo or 18mo Tr-NSPCs. Large differences were observed in median survival that corresponded to the age of the Tr-NSPC (hereafter referred to as NSPC age), but not the age of the hosts (Fig. 1A,B). The median survival of young 3mo hosts implanted with 3mo or 18mo Tr-NSPCs was 87 and 37.5 days, respectively (p=0.0001) while median survival of aged 20 mo hosts implanted with 3mo or 18mo Tr-NSPCs was 67 and 37 days, respectively (p=0.0001). In contrast, median survival between young and aged hosts implanted with same-aged Tr-NSPCs did not differ significantly (p=0.53 and 0.31 for 3mo or 18moTr-NSPCs, respectively; Figs. 1A,B).
Figure 1. Progenitor cell age is a predominant determinant of malignant phenotype.
Equal numbers of transformed NSPCs isolated from 3 or 18mo mouse forebrains (3mo Tr-NSPCs (Tr) and 18mo Tr-NSPCs, respectively) were implanted into the brains of 3 and 20 mo syngeneic mice to determine the effect of progenitor cell and host age on survival (A and B) and tumor histology and grade (C). Survival curves (A) for each progenitor cell and host animal age combination revealed that 18mo Tr-NSPCs significantly shortened survival in both 3mo and 20 mo hosts while host age had no significant effect on survival (B). (C) Representative H&E histology of 3mo Tr-NSPC (top row) and 18mo Tr-NSPCs (bottom row) tumors at 4× (left), 20× (middle) and 40× (right) (enlarged image Fig. 1S). Features of multinodular (arrowheads) and fascicular growth (dashed lines) were common in 3mo Tr-NSPC generated tumors while multifocal diffuse invasion (long arrows), increased microvasculature (short arrows) and diffusely invasive growth (gradual transition shown from larger to smaller asterisk) in 18mo Tr-NSPC generated tumors (summary Table 1S). (D) Summary of tumor grade analysis.
Analysis performed on ten 18mo and twelve 3mo Tr-NSPCs generated tumors did not identify any relationship between host age and the distribution of tumor grade or histologic features (Figures 1C, 1S and Table S1). Further, the proportions of tumors with histological features of human WHO grade III and IV gliomas were similar for 18mo Tr-NSPCs (3 grade IV, 7 grade III) and 3mo Tr-NSPCs (3 grade IV and 9 grade III) (See Fig. 1C, Table.1S). By contrast, age-related differences in histologic features existed for presence of large/small hemorrhages in 18mo Tr-NSPC tumors (p=0.043) and fascicular growth patterns in 3moTr-NSPC tumors (p=0.008) while the increased frequency of high or medium vs low levels of local tumor cell infiltration of 18m Tr-NSPC tumors was nearly significant (p=0.052). Differences were also noted in frequency of multinodular growth (70% vs 25%) and large vs medium/small tumor areas (30% vs 67%) in 18mo Tr-NSPC vs 3mo Tr-NSPC tumors, respectively, but these did not reach statistical significance (p=0.084 and 0.087, respectively). Together these data suggested that the enhanced malignancy of “older” gliomas in this model is largely attributable to age-related properties of the tumor cells of origin as opposed to age-related influences of the host. In light of these findings, we next examined how aging in NSPCs influenced specific features of malignancy relevant to human gliomas.
Cell invasiveness increases with Tr-NSPC age
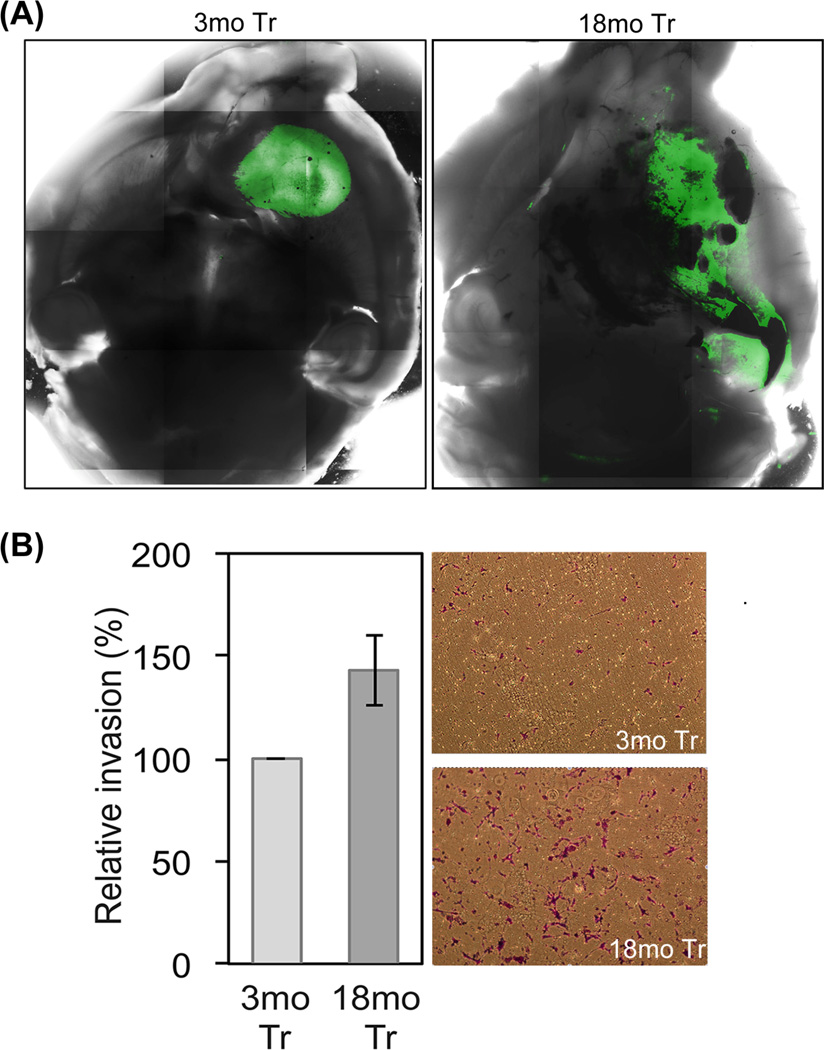
Invasion is a hallmark of increased human glioma malignancy (Palfi et al. 2004).. To determine whether progenitor cell aging enhances diffuse invasion as well as local invasion as identified in histologic analysis, we compared global growth patterns of tumors derived from equal numbers of viable 3mo and 18mo Tr-NSPCs stably labeled with GFP and implanted into 3mo host brains. Images reconstructed from whole brain laser scanning confocal microscopy demonstrated that 3mo Tr-NSPCs grew as cohesive, relatively uniform tumor masses (Fig. 2A) while, tumors derived from 18mo Tr-NSPCs were highly invasive with distant multi-focal and contiguous growth through white matter tracts (Fig. 2A). In addition, tumor cells were detected in olfactory bulb, brain stem and frequently formed contralateral tumor foci (not shown). While histologic examination of tumor borders in Figure 1C suggested that both 3mo and 18mo Tr-NSPCs are locally invasive, this more global technique of analysis indicates that only the older progenitors are capable of long range and more diffuse patterns of invasion and strongly indicate that age of Tr-NSPCs is a major determinant of tumor invasiveness. To address whether differential invasiveness is intrinsic to the age of the NSPCs, we compared the invasion of 3mo and 18mo Tr-NSPCs through matrigel-coated filters in vitro. As shown in Figure 2B, the number of cells penetrating the filter 20 hr after plating was 42% greater for 18mo Tr-NSPCs and these cells were more likely to fully penetrate and spread out on the filter. These results indicate that enhanced invasive potential in our experimental model is an age-dependent characteristic intrinsic to progenitor cells that reproduces a key feature of human glioma malignancy.
Figure 2. Tumor invasiveness increases with progenitor cell age.
(A) Representative comparisons of maximum point projection whole brain images of tumor growth patterns derived from compiled laser scanning confocal microscopy. (A) Left panel, 3mo Tr-NSPCs formed compact tumors with only local invasion (not seen at this power; see Fig. 1C); right panel, 18mo Tr-NSPCs formed tumors that showed pronounced distant invasion as well as areas of hemorrhage, both features of greater malignancy. (B) The number of cells that invaded through a matrigel coated filter was 42% greater for 18mo compared to 3mo Tr-NSPCs. Representative filters used for quantitation of invasion shown.
Genomic instability increases with age in normal and transformed NSPCs
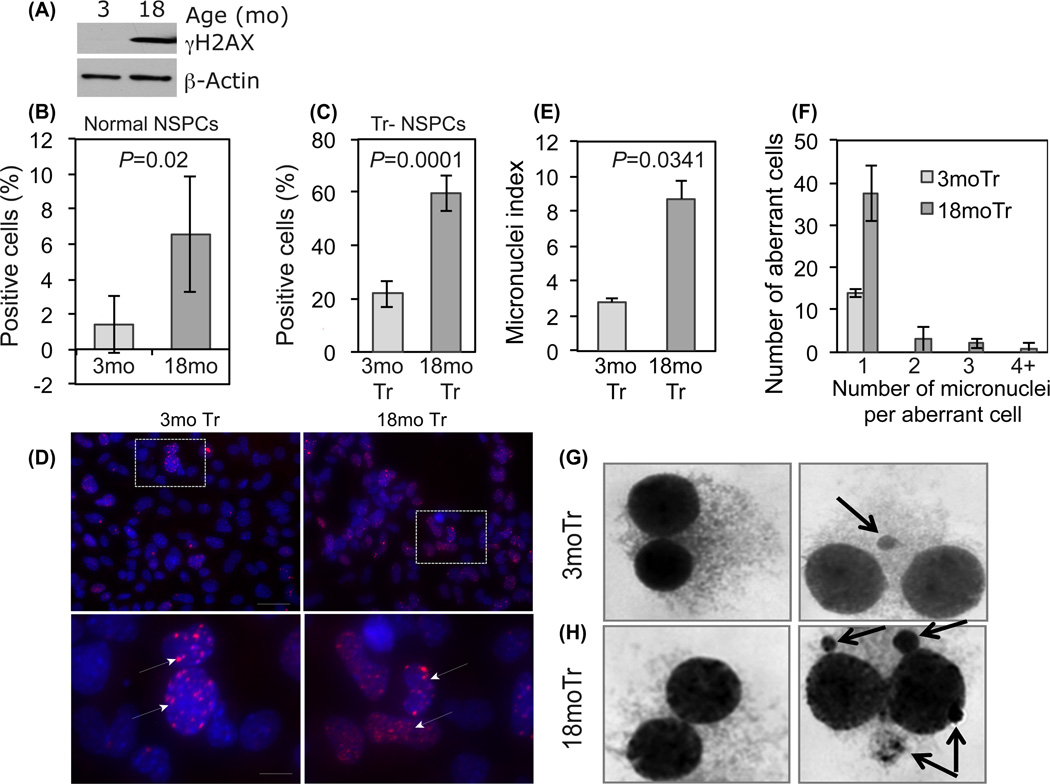
Genomic instability is a common feature of all cancers that promotes tumor malignancy and treatment resistance through selection for adaptive mutations (Walters et al. 2011). A previous study identified increased mutations in normal NSPCs from older mice, indicating that genomic instability accompanies aging (Bailey et al. 2004). Here we seek evidence for age-dependent genomic instability in normal NSPCs and Tr-NSPCs by quantifying two markers of genomic instability, γH2AX expression and micronuclei formation, indicative of mutagenic DNA strand breaks and gross chromosomal aberrations, respectively (Sedelnikova et al. 2004; Fenech et al. 2011). As shown in Figure 3A, 3mo NSPCs lacked detectable γH2AX protein in contrast to the robust levels detected in 18mo NSPCs. In accord, the fraction of NSPC nuclei containing γH2AX was markedly increased in 18mo NSPCs compared with 3mo NSPCs (6.6% vs 1.4%; p=0.02, Fig. 3B). Similar age-dependent differences were also noted for micronuclei counts in non-transformed NSPCs (Fig. 2S). Although transformation greatly increased the absolute degrees of genomic instability, age dependent disparities noted in normal cells persisted. The fraction of Tr-NSPCs cells with γH2AX foci was 60% vs 22% respectively for 18mo Tr-NSPCs and 3mo Tr-NSPCs (p=0.0001, Fig. 3C,D) and as shown in Fig 3E the fraction of cells with micronuclei was also significantly greater in 18mo Tr-NSPCs (8.7% vs 2.8%; p=0.035). The mean number of micronuclei per aberrant binucleate cell was also greater in 18 compared to 3moTr NSPCs (1.23 vs 1.0, respectively; p=0.045; Fig.3F). Of note, in the aberrant 3moTr NSPCs, no cells had more than one micronucleus. In contrast, 12/78 (15%) of aberrant 18moTr NSPC's contained multiple (up to 8) micronuclei (Fig.3G, H). Consistent with increased γH2AX foci and micronuclei formation in aged transformed NSPCs, cytogenetic analysis revealed significant increases in the mean number and percentage of 18mo Tr-NSPCs with chromatid breaks (Fig. 3S). In toto, these results strongly suggest that increased genomic instability is associated with aging in both normal and transformed NSPCs.
Figure 3. Age dependent activation of γH2AX expression and increased micronuclei formation.
(A) Western blot showing differential γH2AX protein content in normal 3 and 18mo NSPCs. (B) Quantification of γH2AX immunopositivity in cultured normal 3mo and 18mo NSPCs. (C) Quantification of γH2AX foci in 3mo and 18mo Tr-NSPCs. (D) Representative photomicrographs demonstrating detection of γH2AX foci in 3mo and 18mo Tr-NSPCs. Bottom row- higher magnification of areas designated above (arrows indicate foci). (E) Percentage of binucleate cells containing micronuclei (“Micronuclei index”) was significantly greater in 18mo compared to 3mo Tr-NSPCs (8.7% vs 2.8%, p=0.035) [pooled from a 1000 cells counted for each age in two separate experiments]. (F) Frequency of single or multiple micronuclei in aberrant binucleate cells by age. (G, H) Appearance of binucleate cells without micronuclei (left panel) or with micronuclei (right panel, arrows) for 3mo and 18mo Tr-NSPCs, respectively. Multiple micronuclei (right sided panel of 3H) were only detected in 18mo Tr-NSPCs.
Resistance to clinically utilized genotoxic agents increases with NSPC age
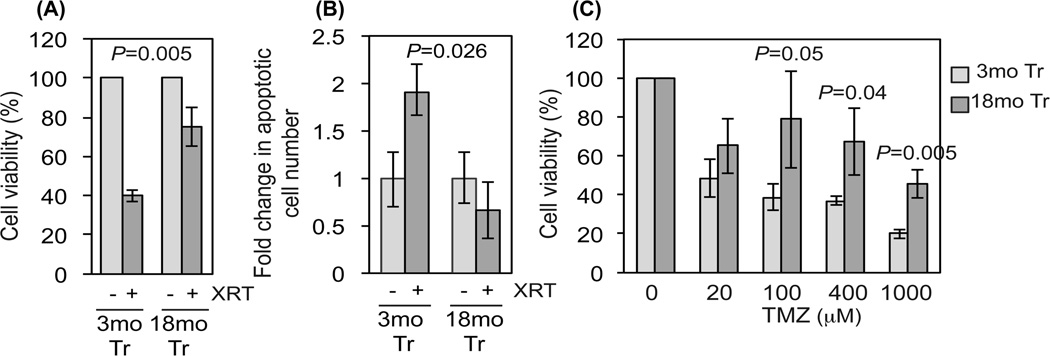
Increased patient age inversely correlates with response to radiation and alkylating agent-based chemotherapy (Rosenblum et al. 1982; Barker et al. 2001; Stupp et al. 2009). With this in mind, we assayed the sensitivity of 3mo and 18mo Tr-NSPCs to radiation and the methylating agent temozolomide (TMZ), both standard components of post-operative glioma therapy (Mrugala & Chamberlain 2008). As shown in Figure 4A, irradiation with 20 Gy γ-rays, a near maximal singlefraction stereotactic radiation dose, produced 40% survival in 3mo Tr-NSPCs compared to 80% survival in 18mo Tr-NSPCs (p=0.005). The radiation-induced reduction in survival in 3mo Tr-NSPCs was accompanied by a significant 1.9-fold increase in the number of apoptotic (i.e., Annnexin V expressing) cells (p=0.026), while no change in apoptotic cell fraction was detected in 18mo Tr-NSPCs (Fig. 4B). These results indicate that 18mo Tr-NSPCs are intrinsically more radioresistant than the 3mo Tr-NSPCs. Similarly, 3mo Tr-NSP Cs were significantly more sensitive than 18mo Tr-NSPCs to TMZ at doses greater than 100 µM (Fig. 4C). Of note, these differences in response to genotoxic stress were not reflected in differential activity of O6-methylguanine-DNA methyltransferase (MGMT), the sole mammalian repair activity that removes O6-methylguanine from DNA (Silber et al. 2012) or Ape1,the major mammalian abasic site endonuclease central to repair of abasic sites and single-strand breaks containing oxidized deoxyribose moieties (Abbotts & Madhusudan 2010). Together, these results demonstrate an age-dependent increase in Tr-NSPC resistance to clinically utilized genotoxic agents that mimics the worse clinical outcome that accompanies aging in human gliomas based on mechanisms other than differential MGMT or Ape1 activity.
Figure 4. Resistance to genotoxic stress in Tr-NSPCs increases with age.
(A) Comparison of cell viability assayed by reduction of Alamar Blue in 3mo and transformed 18mo Tr-NSPCs 48 hr after irradiation with 20 Gy of 137Cs γ-rays. (B) Activation of apoptosis assessed by FACS analysis of Annexin V immunoreactivity in 3mo and transformed 18mo Tr NSPCs 48 hr after irradiation with 20 Gy of 137Cs γ-rays. (C) Viability measured as reduction of Alamar Blue in 3mo and 18mo Tr-NSPCs incubated for 24 with the alkylating agent temozolomide (TMZ). Each data point represents an average of 6 replicates.
Proliferation, growth and resistance to hypoxia increase with NSPC age
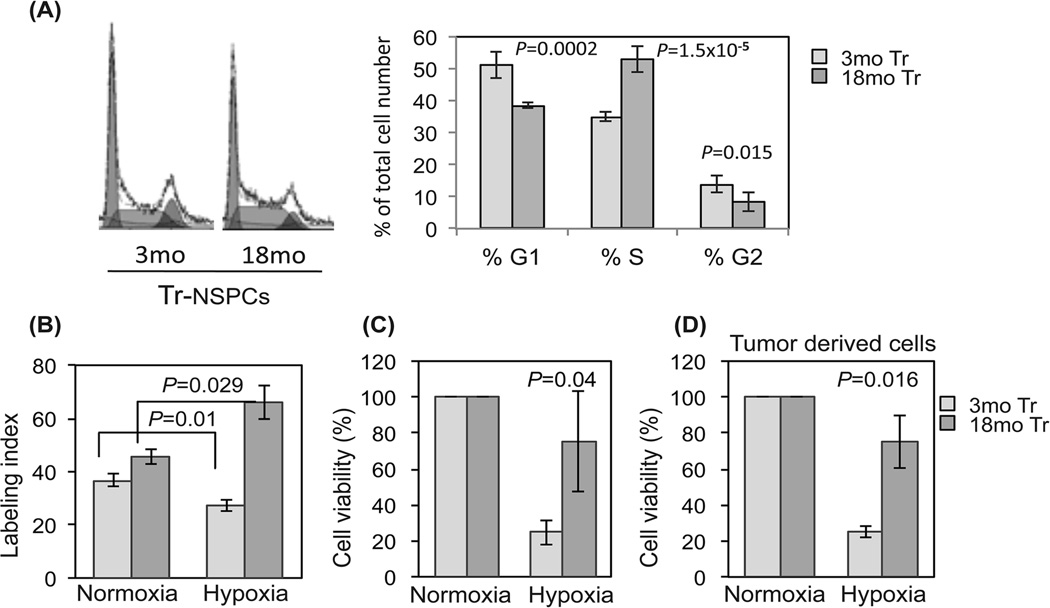
We previously reported an association between increased Ki-67 labeling index and shorter survival of 18mo vs 3mo Tr-NSPC derived tumors in same-aged hosts (Mikheev et al. 2009). To determine whether these in vivo differences are intrinsic to transformed cell age we analyzed cell cycle characteristics and cell culture doubling times. Based on FACS analysis, the %S-phase fraction of 18mo vs 3mo Tr-NSPCs was significantly increased (53% vs 35% respectively; p=0.000015) while the %G1 was higher in 3mo Tr-NSPCs (51% vs 39%; p=0.002). Cell doubling times for 18mo and 3mo Tr-NSPCs were 19.4 vs 23.3 hours, respectively (data not shown). Unlike proliferation and cell growth, no significant age-dependent differences were noted in neural differentiation potential (Figure 4S).
Intra-tumoral hypoxia strongly influences glioma proliferation, invasion and treatment response (Jensen 2009). Therefore, we quantified age-dependent differences in BrdU incorporation and cell survival in response to hypoxic stress. BrdU incorporation at normoxia was slightly higher in 18mo vs 3mo Tr-NSPCs (46% vs 37% Fig. 5B) although this difference did not reach statistical significance. After incubation for 48 hr at 1% O2 while the BrdU labeling index of 3mo Tr-NSPCs decreased significantly from 37% to 27% (p=0.01) while the BrdU labeling index increased in 18mo Tr-NSPCs from 46% to 66% (p=0.029; Fig. 5B). With prolonged hypoxic stress (7 days at 1% O2) only 24.7% of 3mo Tr-NSPCs were viable (compared to normoxic controls) (p=0.04) compared to 75.4% of 18mo Tr-NSPCs (Fig. 5C). Likewise, the difference in survival of stem-like cells isolated from tumors generated by 18mo Tr-NSPCs vs 3mo Tr-NSPCs was nearly identical to the parental transformed cells prior to implantation (70% vs 32%; p=0.016; Fig. 5D). These data indicate that differential tolerance to hypoxic stress distinguishes young and aged Tr-NSPCs, both in vitro and in vivo, and suggests that the resistance to hypoxia that characterizes increasing malignancy in human gliomas is promoted by age-related changes intrinsic to progenitor cells.
Figure 5. Resistance to hypoxia in Tr-NSPCs increases with age.
(A) Quantitation of histograms (left) from FACS demonstrated an increased S-phase fraction of 18mo Tr-NSPCs cultured at normoxia. (B) BrdU incorporation in 3mo and 18mo Tr-NSPCs cultured for 48 hours in normoxia (21% O2) or hypoxia (1% O2). (C,D) Cell viability after exposure to chronic hypoxia (7days in 1% O2) assayed by reduction of Alamar Blue of (C) 3mo and 18mo Tr-NSPCs and (D) tumor derived glioma stem-like cells isolated from tumors formed from 3mo and 18mo Tr-NSPCs. Cell viability in hypoxia was normalized to cell counts at 21% O2.
Tr-NSPC aging recapitulates age-dependent changes in response to hypoxia observed in human gliomas
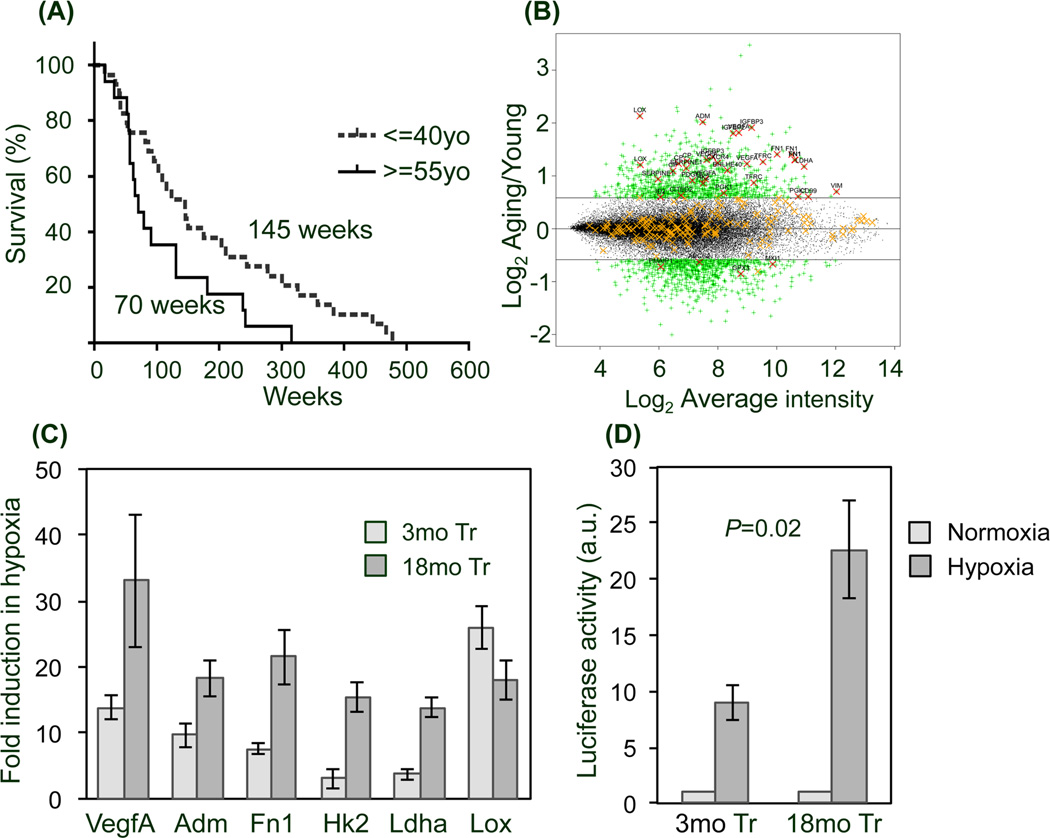
To explore the potential clinical relevance of age-dependent responses to hypoxic stress, we examined the expression of HIF-1-regulated hypoxia response genes (HRGs) in age-defined cohorts of human malignant glioma patients extracted from a public database (Phillips et al. 2006). The 87 HRGs used for this analysis have HIF-1 response elements and validated responsiveness to hypoxia (Table 2S) (Benita et al. 2009). The median survivals of the age-defined cohorts of grade III and IV malignant gliomas (≤ 40 vs ≥ 55 years old) were significantly different (145 weeks vs 70 weeks, respectively; p=0.036, Fig. 6A). Strikingly, among 23 differentially expressed HRGs, 19 were upregulated in the older cohort indicating an age-related upregulation of HRGs (Fig. 6B and Table 1). To eliminate the influence of lower grade tumors (15/29 grade III in young cohort vs only 2/15 grade III in aged) we repeated the analysis considering only grade IV GBM patients and found a similar upregulation in HRG expression in the aged patient cohort (Table 1). Our analysis indicates that increased expression of genes that promote resistance to hypoxia accompanies older age and might contribute to greater malignancy in human gliomas.
Figure 6. Increased hypoxia response gene (HRG) expression is associated with increased malignancy in older glioma patients and is replicated in older Tr-NSPCs.
(A) Kaplan-Meier survival analysis from a public database (Phillips et al. 2006) demonstrates shorter median survival of older (≥ 55yo) vs younger (≤ 40yo) malignant glioma patients (70 vs 145 weeks; p=0.036). (B) Scatter plot demonstrating that nearly all HRGs (adapted from (Benita et al. 2009)) are upregulated in aging vs young patient cohorts (enlarged image Fig 5S, summary in Table 1). The relative expression of HRGs is shown as log2 of old/young plotted against log2 average intensity. HRG probe sets with significant differences (i.e., > 1.5× expression change, p<0.05) are indicated by a red “X” as opposed to those lacking significance (orange “X”). (C) Expression of selected HRGs upregulated in older gliomas were quantified by qRT-PCR in 3mo and 18mo Tr-NSPCs after 48 hr exposure to hypoxia (1% O2). Fold elevation is relative to cells grown in 21% O2. (D) Activation of a HIF-1a luciferase reporter gene is greater in 18mo than 3mo Tr-NSPCs exposed to 1% O2 for 8 hr.
Table 1.
Age-dependent differential expression of hypoxia response genes in malignant glioma patients
| Probeset ID | Fold change (old vs young)* |
P.Value | Gene Symbol$ |
|---|---|---|---|
| 215446_s_at | 4.39 | 4.8E-05 | LOX |
| 202912_at | 4.05 | 9.0E-05 | ADM |
| 210095_s_at | 3.76 | 1.7E-04 | IGFBP3 |
| 210512_s_at | 3.53 | 1.2E-03 | VEGFA |
| 202718_at | 3.49 | 4.2E-03 | IGFBP2 |
| 212464_s_at | 2.65 | 4.7E-05 | FN1 |
| 204846_at | 2.42 | 9.5E-04 | CP |
| 208691_at | 2.40 | 7.6E-05 | TFRC |
| 217028_at | 2.37 | 2.9E-03 | CXCR4 |
| 200650_s_at | 2.26 | 3.2E-05 | LDHA |
| 202627_s_at | 2.21 | 1.9E-03 | SERPINE1 |
| 201170_s_at | 2.15 | 5.6E-04 | BHLHE40 |
| 205463_s_at | 1.88 | 2.8E-03 | PDGFA |
| 202934_at | 1.81 | 4.7E-03 | HK2 |
| 201426_s_at | 1.63 | 5.9E-03 | VIM |
| 227068_at | 1.60 | 8.4E-03 | PGK1 |
| 209357_at | 1.55 | 1.2E-02 | CITED2 |
| 201029_s_at | 1.52 | 3.4E-03 | CD99 |
| 213931_at | 1.51 | 1.1E-02 | ID2 |
| 209735_at | −1.55 | 3.9E-02 | ABCG2 |
| 202364_at | −1.59 | 6.0E-04 | MXI1 |
| 204285_s_at | −1.65 | 3.6E-02 | PMAIP1 |
| 214091_s_at | −1.81 | 4.1E-02 | GPX3 |
| Unique to grade IV GBM | |||
| 203939_at | 1.70 | 3.37E-02 | NT5E |
| 222847_s_at | 1.50 | 1.35E-02 | EGLN3 |
Age >=55 vs<=40 years old (p<0.05, cut off difference ≥1.5 fold)
-Genes with age dependent differential expression when combined grade III and IV are included in the analysis. Genes shown in bold also showed age dependent regulation when only grade IV tumors were included in the analysis.
We next examined the effect of hypoxia on expression in 3mo and 18mo Tr-NSPCs of a sub-set of HRGs found to be upregulated in malignant gliomas from patients ≥ 55 years old (i.e., VEGFA, FN1, HK2, LDHA, ADM and LOX). As shown in Figure 6C, incubation at 1% O2 for 48 hours increased expression of all genes from 3- to 33-fold in 3mo and 18mo Tr-NSPCs. Notably, the relative fold-changes in expression for all genes except LOX were 2- to 5-fold higher in 18mo than in 3mo Tr-NSPCs (Fig. 6C). Since HRGs can be regulated through HIF-1-independent mechanisms, we determined whether the age-dependent differences in HRG expression reflected different levels of HIF-1 activity in 3moTr and 18moTr NSPCs. As illustrated in Figure 6D, after incubation for 8 hours in 1% O2, normalized luciferase reporter activity was approximately 2.4-fold higher in 18mo than in 3mo Tr-NSPCs (22-fold vs 9-fold; p=0.02). These data indicate that hypoxic stress recapitulates in Tr-NSPCs age-dependent changes in HRG expression associated with greater malignancy observed in human gliomas and provide additional evidence that age-related changes intrinsic to glioma progenitor cells play a predominant role in determining tumor malignancy.
DISCUSSION
It has long been appreciated that aging is strongly associated with increasing malignancy in human gliomas (Curran et al. 1993; Ohgaki & Kleihues 2005; CBTRUS 2009). The underlying mechanisms by which age promotes aggressive clinical behavior in the most common primary brain tumors, however, remain to be elucidated. The paucity of animal models that replicate the age-dependence of glioma malignancy greatly limits investigation of aging mechanisms that promote a more aggressive phenotype. Contrary to clinical experience, early rodent studies demonstrated increased animal survival with age after glioma induction from carcinogen or oncogenic virus exposure (Perese 1964; Copeland & Bigner 1977). By contrast, recent studies using syngeneic implants of established glioma cell lines made landmark observations that agerelated decreases in immune competency (Wheeler et al. 2003) and recruitment of endogenous neural progenitors (Glass et al. 2005) contributed to decreased survival in older hosts. While these seminal studies provided new insight into the role of host factors in age-dependent malignancy, many questions remained. In particular, the relative impact of aging in glioma cells of origin vs the host has been heretofore untested.
Here we describe for the first time a syngeneic mouse glioma model that reproduced malignant features characteristic of human gliomas in older patients as evidenced by gross morphology, histology, aggressive behavior and gene expression. Importantly, our model permits separate examination of the effects of progenitor cell and organismal aging on tumor malignancy. Our findings implicate progenitor cell age as a predominant determinant of aggressive behavior in gliomas, as convincingly demonstrated in the two-fold reduction in median survival produced by 18mo compared to 3mo Tr-NSPCs when implanted into young as well as aged hosts (Fig. 1). Accordingly, our data also demonstrated age-dependent cell intrinsic increases in hallmarks of glioma malignancy, including greater invasiveness, insensitivity to radiation and temozolomide and resistance to hypoxia. Moreover, older Tr-NSPCs replicate the enhanced expression of HRGs that accompanies aging in human gliomas and display evidence of increased genomic instability, a driving force of malignant progression (Shiras et al. 2007; Walters et al. 2011), attesting to the clinical and physiological relevance of our model. In toto, our findings indicate that aging is accompanied by specific changes in glioma progenitor cells that foster malignancy and that our model has utility as a tool to investigate age-related mechanisms that drive glioma malignant progression. We are aware of only one other study where aging in presumed cells of origin was investigated (Vicente-Duenas et al.). In this study, implantation of transformed B cell precursors from 18mo compared to 3mo animals significantly reduced survival in same-aged hosts (Vicente-Duenas et al.) while host age (4mo vs 18mo) had no impact on survival after implantation of a syngeneic B-cell leukemia cell line. Therefore, using an experimental paradigm nearly identical to our prior report (Mikheev et al. 2009) Vicente-Duenas (2010) reproduced the differential effects of aging in cells of origin versus the host. Together, these results and our current and previously reported findings suggest that cell intrinsic aging may be a determinant of malignancy in cancers other than glioma.
Our data suggest that genomic instability and hypoxic stress responses may be potentially interrelated mechanisms central to aging-related gliomagenesis and malignancy. For instance, we confirmed prior observations of an aging related increase in genomic instability in normal NSPCs (Bailey et al. 2004) and extended this observation to their transformed progeny. In mouse brain, age-related genomic instability was first demonstrated as loss of heterozygosity at specific loci in 20mo but not 2mo cultured NSPCs (Bailey et al. 2004). In accord, we found increased abundance of γH2AX foci and micronuclei accompanying aging in both normal and transformed NSPCs. γH2AX foci are indicative of double-strand breaks, clastogenic DNA lesions produced by oxidative free radicals that lead to gross chromosomal alterations as evidenced in our study by micronuclei formation. Our observation of elevated numbers of γH2AX foci with age in normal and transformed NSPCs is in accord with previous reports of γH2AX in aging tissues (Sedelnikova et al. 2004), and consistent with an increased tolerance of accumulating DNA lesions produced by endogenous oxidative stress over the cell’s lifespan. The vast majority of oxidative DNA damage consists of single-strand breaks and altered or missing bases which, if unrepaired, interact with DNA replication to form double-strand breaks. Importantly, resistance to hypoxia in older progenitors is accompanied by greater proliferative potential and viability (Fig.5), implying greater tolerance to double-strand breaks and their precursors.
Regarding hypoxia, the greater tolerance to hypoxia and even increased proliferation displayed by 18mo compared to 3mo Tr-NSPCs has precedent in our earlier report of a comparable age-dependent increase in resistance to hypoxic insult in untransformed NSPCs (Stoll et al. 2011). These findings suggest that the acquisition of hypoxic phenotypes or tolerance of hypoxia in glioma may reflect intrinsic changes in normal progenitor cells that occur during aging. Greater hypoxic tolerance in 18mo Tr-NSPCs corresponded to increased HIF-1 activity (Fig. 6) which is linked to enhanced angiogenesis, tumor cell invasion, and treatment resistance in human gliomas (reviewed in (Jensen 2009)). Activation of HIF expression also fosters self-renewal, proliferation and survival in glioma stem cells in vitro (Mathieu et al.) and increases the likelihood of tumor initiation when progenitors are implanted in host animals (Li et al. 2009). The observation of greater hypoxiadriven expression of HRGs in 18mo Tr-NSPCs that are also upregulated in more malignant human gliomas from older patients (Fig. 6B) supports the relevance of differential activation of HRGs as a mechanism contributing to age-dependent glioma malignancy. Accordingly, other human glioma studies have identified a correlation between hypoxic signaling and tumor grade (Dreyfuss et al. 2009) and poor prognosis in grade IV GBM patients (Flynn et al. 2008).
Together our data indicate that aging related increases in genomic instability and hypoxic tolerance occur in both normal and transformed NSPCs. This suggests the possibility that carry over and possible amplification of these pre-existing properties from normal to transformed cells contributes to the differential malignancy related to aging. These observations also underscore a central unanswered question; namely, how might these factors interact to promote aging and malignant phenotypes in NSPCs? Tolerance of DNA damage has been suggested as a mechanism to deal with prolonged genotoxic stress and reduced DNA repair capacity in aging NSPCs (Kenyon & Gerson 2007). Therefore, in aging NSPCs, increased resistance to hypoxia may promote genomic instability leading to a “mutator phenotype” enhancing genetic diversity and malignant potential (Beckman & Loeb 2005). Conversely, genomic instability with aging may serve to select for hypoxic phenotype. For instance, by generating molecular diversity, increased genomic instability in normal or transformed NSPCs could promote selection of cells in the aging neurovascular stem cell niche or hypoxic tumor microenvironment, respectively. Of note, reduced VEGF signaling of neural stem/progenitor cells in the aging microvascular niche (Bernal & Peterson, 2011) is linked to reduced neurogenesis with aging. If retained after malignant transformation these differences in cell intrinsic hypoxic tolerance acquired during normal aging would presumably contribute to accelerated selection and tumor growth and malignancy as observed in this model.
Relevant to our findings of age-dependent increases in genomic instability, hypoxia has pleiotropic effects on DNA damage and repair and cell survival linked to genomic instability and treatment resistance (Wilson & Hay 2011) (Bristow & Hill 2008). Increased expression of HRGs that promote survival under hypoxic stress are therefore likely to confer resistance to genotoxic agents such as we observed for radiation and temozolomide (Fig. 4). In accord with this supposition is the observation that cytotoxic therapies for glioma are significantly less effective with increasing patient age (Rosenblum et al. 1982; Grant et al. 1995; Stupp et al. 2009). However, elevated expression of HRGs may afford new therapeutic targets for older glioma patients as evidenced by the finding that older GBM patients are significantly more responsive to the VEGF inhibitor bevacizumab than younger patients (Nghiemphu et al. 2009), presumably due to increased expression of the HIF1 regulated gene VEGF.
In summary, we have developed a syngeneic mouse model using transformed NSPCs that permits investigation of the relative contribution of progenitor cell and organismal aging to the development of malignancy in human gliomas. Our model recapitulated hallmark features of human glioma malignancy and demonstrated a primary importance of aging in presumed glioma progenitor cells, as opposed to the host. A caveat of our observations is that they derive from a single model system. Since aging neural phenotypes can be strain specific future studies are warranted in additional strains to validate the current findings and conclusions. Additional studies of interest would be to define the effects of additional specific transformation paradigms and progenitor cell sub-types on age-dependent malignancy. Our current data provide the basis for these future studies by dramatically demonstrating for the first time the potential importance of considering both the cell of glioma origin and host in modeling aging-dependent glioma malignancy. Such models will facilitate the delineation of mechanisms responsible for the age dependence of aggressive tumor behavior and potentially serve as clinically relevant surrogate to evaluate the efficacy of new therapies in young and old glioma patients.
Experimental Procedures
Key experimental procedures are described below; additional details are available as supplemental information.
Isolation, propagation and transformation of NSPCs
Experiments were performed according to procedures approved by the University of Washington Institutional Animal Care and Use Committee (IACUC). Adult neural stem and progenitor cells (NSPCs) were isolated from forebrains C57BL/6 mice, propagated in serum free proliferation media (EGF and FGF supplemented) and transformed by retroviral transduction with HPV18 E6E7 viral oncogenes and Ha-RasV12 at the fourth passage as previously described (Petit et al. 2007), (Mikheev et al. 2009). Equal MOIs of each retrovirus were added to the same number of cells of each age under antibiotic selection to ensure similar efficiency of transformation. Pools of selected cells of the same passage numbers in log growth were used for syngeneic transplants.
Intracranial injection, histology and whole brain imaging
Equal numbers of transformed 3mo or 18mo NSPCs pre- labeled with EGFP were implanted into young 3mo (n=8 implanted with 3mo and 18mo Tr-NSPCs, respectively) or 20mo aging (n= 10 and 7 implanted with 3mo and 18mo Tr-NSPCs, respectively) C57Bl mice as described (Mikheev et al. 2009; Mikheeva et al. 2010). Animals were sacrificed when displaying terminal indicators (excess weight loss, moribund or neurologic signs). The interval from implant to sacrifices was recorded as overall survival. After perfusion with 4% paraformaldehyde (PFA) paraffin embedded sections of from 22 randomly selected animals (6 each from young and old hosts implanted with 3mo Tr-NSPCs and 5 each from young and old hosts implanted with 18mo Tr-NSPCs) were cut and processed for hematoxylin and eosin (H&E) staining. Tumor grade and histology features were qualitatively scored according to WHO criteria for human glioma by an experienced surgical neuropathologist (D.E.B.) blinded to the sample identity (see supplement for details of histologic analysis). Significant differences were determined as p < 0.05 using Fisher’s exact test. Global patterns of tumor growth were analyzed in brains from randomly selected three-month old hosts implanted with 3mo Tr-NSPCs (n=3) and 18mo Tr-NSPCs (n=4) cells using confocal laser scanning microscopy (Fluoview-1000) as previously described (Mikheeva et al. 2010).
Invasion Assay
Invasion assays were performed as previously described (Mikheeva et al. 2010) (Elias et al. 2005) using matrigel coated filters.
γH2AX detection
A γH2AX rabbit polyclonal antibody (#2577, Cell Signaling Technology, Inc, Danvers, MA) was used to detect H2AX protein in western bots and nuclear foci by immunocytochemistry. At least 600 cells were counted to determine the γH2AX labeling index in each of two independent experiments.
Micronucleus and cytogenetic analysis
Briefly, cells were treated for 24 hours with cytochalasin B were analyzed for the presence and number of micronuclei per binucleate cell according to published methods (Baliga et al. 2007). Cytogenetic analysis of fifty metaphase spreads was performed for each culture as previously described (Schwartz et al. 1999).
Cell cycle (FACS) analysis, cell growth and differentiation
Isolated nuclei stained with DAPI were analyzed using BD Influx cell sorter (BD Biosciences) followed by cell cycle analysis using Multicycle AV software (Phoenix Flow Systems, San Diego CA). The doubling times were calculated by counting viable cell numbers (Vi-cell, Beckman Coulter, Inc) in cultures at log-growth phase using a linear regression on the log-scale plot using the following formula doubling time= log2/slope. Differentiation potential was determined by quantifying MAP2 + cells (neurons), GFAP+ cells (astrocytes) and CNPase + cells (oligodendroglia) after growth in lineage specific differentiation media as previously described (Stoll et al. 2011) and supplemental information).
Cellular responses (viability, apoptosis and proliferation) to gentoxic (irradiation, TMZ) or hypoxic stress
After irradiation with 20Gy (1Gy/min) from a Cs137 source, cells were assayed after 48 hours for percent viability by quantitation of reduced Alamar Blue (resazurin) reagent (O'Brien et al. 2000) and percent apoptosis at 16 hours by AnnexinV-Cy5 FACS. Cell viability 24 hours after exposure to different concentrations (0, 20, 100, 400, 1000 µM) of the alkylating drug TMZ was quantified as above using Alamar blue. Proliferation in response to a 48 hour exposure to hypoxia (1% O2) was quantified by counting at least 600 cells after immunostaining with anti-BrdU antibody (Santa Cruz #32323) to obtain BrdU labeling indices. Longer-term effects of hypoxia (7 days at 1% O2) on viability were quantified using the Alamar Blue assay on both primary transformed cell cultures and cells isolated from tumors propagated using the same protocols outlined above.
Bioinformatics analysis of microarray data
The age-dependent distribution of hypoxia regulated genes (HRGs) with HIF-1 response elements (Benita et al. 2009) was analyzed in age-defined malignant glioma patients (<40 yrs; >55yrs) extracted from a public gene expression database (NCBI GEO repository GSE4271 (Phillips et al. 2006)). Survival data for each age group was used to determine differences in median survival by age while significance of age-dependent differentially regulated HRGs was defined using established statistical methods (see supplemental information for details).
Quantitative RT-PCR
RNA isolation, reverse-transcription, thermocycling and relative quantification were carried out as previously described (Mikheeva et al. 2010). A summary of primer pairs used for each gene is provided in supplemental data (Table 4S).
HIF-1 promoter reporter assay
After an 8-hour incubation in 1% O2 cells, HIF1 activity was measured in cells co-transfected with HIF-1 luciferase reporter (Bhattacharya et al. 1999) and galactosidase expression plasmid according to published protocols (Mikheev et al. 2000).
Data analysis and statistics
An unpaired Student’s t-test was used to compare labeling index (BrdU and γH2AX), viability, apoptosis, micronuclei index and HIF-1 activity. Median survival of tumor-bearing animal cohorts and of glioma patient cohorts (Phillips et al. 2006) was compared by Kaplan-Meier analysis using Prizm4 statistical software (GraphPad Software, La Jolla, CA). Differences in histologic features between age-groups were determined using Fisher’s exact test with significance defined as p<0.05.
Supplementary Material
Acknowledgements
We thank Dr. Peter Rabinovitch and Donna Prunkard for assistance with FACS analysis and Glen McDonald of the CHDD Cellular Morphology Core for his expert assistance with confocal imaging. We thank Dr. A.Kung for providing HIF-1 reporter construct. This study was supported in part by NIH grants R21AG029406, R01CA136808, the Fred Hutchinson/University of Washington Cancer Consortium CCSG pilot grant, and the Institute for Stem Cell and Regenerative Medicine Tietze Stem Cell Scientist Award (to R.C.R.), NiH grant AG038305 (to P.J.H.) and NINDS Institutional Center Core Grant to support the viral core facility in the Neuroproteomics Center at the University of Washington (NS055088).
References
- Abbotts R, Madhusudan S. Human AP endonuclease 1 (APE1): from mechanistic insights to druggable target in cancer. Cancer Treat Rev. 2010;36:425–435. doi: 10.1016/j.ctrv.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Bailey KJ, Maslov AY, Pruitt SC. Accumulation of mutations and somatic selection in aging neural stem/progenitor cells. Aging Cell. 2004;3:391–397. doi: 10.1111/j.1474-9728.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- Baliga MS, Wang H, Zhuo P, Schwartz JL, Diamond AM. Selenium and GPx-1 overexpression protect mammalian cells against UV-induced DNA damage. Biol Trace Elem Res. 2007;115:227–242. doi: 10.1007/BF02685998. [DOI] [PubMed] [Google Scholar]
- Barker FG, 2nd, Chang SM, Larson DA, Sneed PK, Wara WM, Wilson CB, Prados MD. Age and radiation response in glioblastoma multiforme. Neurosurgery. 2001;49:1288–1297. doi: 10.1097/00006123-200112000-00002. discussion 1297-1288. [DOI] [PubMed] [Google Scholar]
- Beckman RA, Loeb LA. Genetic instability in cancer: theory and experiment. Semin Cancer Biol. 2005;15:423–435. doi: 10.1016/j.semcancer.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37:4587–4602. doi: 10.1093/nar/gkp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal GM, Peterson DA. Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells. Aging Cell. 2011;10:466–482. doi: 10.1111/j.1474-9726.2011.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes & development. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2005. 2009 doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland DD, Bigner DD. Influence of age at inoculation on avian oncornavirus-induced brain tumor incidence, tumor morphology, and postinoculation survival in F344 rats. Cancer Res. 1977;37:1657–1661. [PubMed] [Google Scholar]
- Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- Dreyfuss JM, Johnson MD, Park PJ. Meta-analysis of glioblastoma multiforme versus anaplastic astrocytoma identifies robust gene markers. Mol Cancer. 2009;8:71. doi: 10.1186/1476-4598-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MC, Tozer KR, Silber JR, Mikheeva S, Deng M, Morrison RS, Manning TC, Silbergeld DL, Glackin CA, Reh TA, Rostomily RC. TWIST is expressed in human gliomas and promotes invasion. Neoplasia. 2005;7:824–837. doi: 10.1593/neo.04352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M, Kirsch-Volders M, Natarajan AT, Surralles J, Crott JW, Parry J, Norppa H, Eastmond DA, Tucker JD, Thomas P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis. 2011;26:125–132. doi: 10.1093/mutage/geq052. [DOI] [PubMed] [Google Scholar]
- Flynn JR, Wang L, Gillespie DL, Stoddard GJ, Reid JK, Owens J, Ellsworth GB, Salzman KL, Kinney AY, Jensen RL. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;113:1032–1042. doi: 10.1002/cncr.23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R, Synowitz M, Kronenberg G, Walzlein J-H, Markovic DS, Wang L-P, Gast D, Kiwit J, Kempermann G, Kettenmann H. Glioblastoma-Induced Attraction of Endogenous Neural Precursor Cells Is Associated with Improved Survival. J. Neurosci. 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant R, Liang BC, Page MA, Crane DL, Greenberg HS, Junck L. Age influences chemotherapy response in astrocytomas. Neurology. 1995;45:929–933. doi: 10.1212/wnl.45.5.929. [DOI] [PubMed] [Google Scholar]
- Jensen RL. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol. 2009;92:317–335. doi: 10.1007/s11060-009-9827-2. [DOI] [PubMed] [Google Scholar]
- Kenyon J, Gerson SL. The role of DNA damage repair in aging of adult stem cells. Nucleic Acids Res. 2007;35:7557–7565. doi: 10.1093/nar/gkm1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Zhang Z, Zhou W, Wang AJ, Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, Tewari M, Liu A, Vessella R, Rostomily R, Born D, Horwitz M, Ware C, Blau CA, Cleary MA, Rich JN, Ruohola-Baker H. HIF Induces Human Embryonic Stem Cell Markers in Cancer Cells. Cancer Res. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev AM, Mikheev SA, Zhang Y, Aebersold R, Zarbl H. CArG binding factor A (CBF-A) is involved in transcriptional regulation of the rat Ha-ras promoter. Nucleic Acids Res. 2000;28:3762–3770. doi: 10.1093/nar/28.19.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev AM, Stoll EA, Mikheeva SA, Maxwell JP, Jankowski PP, Ray S, Uo T, Morrison RS, Horner PJ, Rostomily RC. A syngeneic glioma model to assess the impact of neural progenitor target cell age on tumor malignancy. Aging Cell. 2009;8:499–501. doi: 10.1111/j.1474-9726.2009.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheeva SA, Mikheev AM, Petit A, Beyer R, Oxford RG, Khorasani L, Maxwell JP, Glackin CA, Wakimoto H, Gonzalez-Herrero I, Sanchez-Garcia I, Silber JR, Horner PJ, Rostomily RC. TWIST1 promotes invasion through mesenchymal change in human glioblastoma. Mol Cancer. 2010;9:194. doi: 10.1186/1476-4598-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrugala MM, Chamberlain MC. Mechanisms of disease: temozolomide and glioblastoma--look to the future. Nat Clin Pract Oncol. 2008;5:476–486. doi: 10.1038/ncponc1155. [DOI] [PubMed] [Google Scholar]
- Nghiemphu PL, Liu W, Lee Y, Than T, Graham C, Lai A, Green RM, Pope WB, Liau LM, Mischel PS, Nelson SF, Elashoff R, Cloughesy TF. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72:1217–1222. doi: 10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European journal of biochemistry / FEBS. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- Palfi S, Swanson KR, De Bouard S, Chretien F, Oliveira R, Gherardi RK, Kros JM, Peschanski M, Christov C. Correlation of in vitro infiltration with glioma histological type in organotypic brain slices. Br J Cancer. 2004;91:745–752. doi: 10.1038/sj.bjc.6602048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perese DM. Age and Sex Factors in Experimental Brain Tumors. Surgical forum. 1964;15:429–431. [PubMed] [Google Scholar]
- Petit A, Sellers DL, Liebl DJ, Tessier-Lavigne M, Kennedy TE, Horner PJ. Adult spinal cord progenitor cells are repelled by netrin-1 in the embryonic and injured adult spinal cord. Proc Natl Acad Sci U S A. 2007;104:17837–17842. doi: 10.1073/pnas.0703240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Rosenblum ML, Gerosa M, Dougherty DV, Reese C, Barger GR, Davis RL, Levin VA, Wilson CB. Age-related chemosensitivity of stem cells from human malignant brain tumours. Lancet. 1982;1:885–887. doi: 10.1016/s0140-6736(82)92154-7. [DOI] [PubMed] [Google Scholar]
- Schwartz JL, Murnane J, Weichselbaum RR. The contribution of DNA ploidy to radiation sensitivity in human tumour cell lines. Br J Cancer. 1999;79:744–747. doi: 10.1038/sj.bjc.6690119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- Shiras A, Chettiar ST, Shepal V, Rajendran G, Prasad GR, Shastry P. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells. 2007;25:1478–1489. doi: 10.1634/stemcells.2006-0585. [DOI] [PubMed] [Google Scholar]
- Silber JR, Bobola MS, Blank A, Chamberlain MC. O(6)-Methylguanine-DNA methyltransferase in glioma therapy: Promise and problems. Biochimica et biophysica acta. 2012;1826:71–82. doi: 10.1016/j.bbcan.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll EA, Cheung W, Mikheev AM, Sweet IR, Bielas JH, Zhang J, Rostomily RC, Horner PJ. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. J Biol Chem. 2011;(44):38592–38601. doi: 10.1074/jbc.M111.252171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Vicente-Duenas C, Abollo-Jimenez F, Ruiz-Roca L, Alonso-Escudero E, Jimenez R, Cenador MB, Criado FJ, Cobaleda C, Sanchez-Garcia I. The age of the target cell affects B-cell leukaemia malignancy. Aging (Albany NY) 2:908–913. doi: 10.18632/aging.100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters DK, Wu X, Tschumper RC, Arendt BK, Huddleston PM, Henderson KJ, Dispenzieri A, Jelinek DF. Evidence for ongoing DNA damage in multiple myeloma cells as revealed by constitutive phosphorylation of H2AX. Leukemia. 2011;25:1344–1353. doi: 10.1038/leu.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler CJ, Black KL, Liu G, Ying H, Yu JS, Zhang W, Lee PK. Thymic CD8+ T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol. 2003;171:4927–4933. doi: 10.4049/jimmunol.171.9.4927. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.