Abstract
Objectives
To examine risk factors for fracture in a racially diverse cohort of healthy children in the United States.
Study design
A total of 1,470 healthy children, ages 6–17 years, underwent yearly evaluations of height, weight, body mass index, skeletal age, sexual maturation, calcium intake, physical activity levels, and dual-energy x-ray absorptiometry (DXA) bone and fat measurements for up to 6 years. Fracture information was obtained at each annual visit, and risk factors for fracture were examined using the time-dependent Cox proportional hazards model.
Results
The overall fracture incidence was 0.034 fractures per person-year with 212 children reporting a total of 257 fractures. Being white (hazard ratio [HR]=2.1), male (HR=1.8), and having skeletal age of 10–14 years (HR=2.2) were the strongest risk factors for fracture (all P≤0.001). Increased sports participation (HR=1.4), lower body fat percentage (HR=0.97), and previous fracture in white females (HR=2.1) were also significant risk factors (all P≤0.04). Overall, fracture risk decreased with higher DXA Z-scores, except in white males who had increased fracture risk with higher DXA Z-scores (HR=1.7, P<0.001).
Conclusions
Boys and girls of European descent had double the fracture risk of children from other backgrounds, suggesting that the genetic predisposition to fractures seen in elderly adults also manifests in children.
Keywords: bone mass, bone density, pediatric fracture, teenagers, adolescents, race, ethnicity
The incidence of fractures is substantially lower in black than in white elderly adults (1, 2), but whether the variant effect of race on fracture risk is also present during childhood is not established. Only two studies have prospectively examined the influence of race on pediatric fractures. A study of English children (3, 4) suggested a higher fracture rate in whites compared with non-whites, although statistical significance was not established. A study of South African children found the rate of fractures for white children to be almost twice that of children of African ancestry (5, 6). The purpose of this prospective, multi-center study was to examine race/ethnicity as a risk factor for fracture in a large, diverse cohort of children and adolescents from different regions across the United States (U.S.).
METHODS
The Bone Mineral Density in Childhood Study (BMDCS) is a multi-center longitudinal study examining bone accretion in a racially diverse cohort of 1,554 healthy boys and girls, ages 6 to 17 years, recruited from July 2002 to November 2003 at five medical centers in the U.S. (7). Consent and assent were obtained from parents/guardians and participants following approved IRB protocols. 84 children who did not return for follow-up were excluded from the analysis. Thus, 1,470 subjects, representing 95% of the original cohort, were the basis for the current study.
Detailed information about the study participants and procedures have been published previously (7). The BMDCS cohort is comprised of healthy children with height, weight and body mass index (BMI) between the 3rd and 97th percentile and no previous or current conditions that might affect bone acquisition. Children ≤10 years old with a history of more than one fracture or >10 years old with more than two prior fractures were excluded. Of 2,888 potential participants, 1,334 were ineligible, but only 23 (1.7%) were excluded based on the fracture criteria. Race and ethnicity were determined using National Institutes of Health definitions based on the parents’ self-identification. Participants whose parents were not of the same race and ethnicity were categorized based on race and ethnicity of the participant’s mother.
Participants were evaluated annually for 6 years. Height, weight, and BMI Z-scores for sex and age were calculated using normative data from the National Health and Nutrition Examination Survey (NHANES). All subjects underwent a physical examination by a pediatrician or pediatric endocrinologist to determine their stage of sexual maturation based upon Tanner stages (8). Skeletal maturity was assessed by pediatric radiologists on the basis of roentgenograms of the left hand and wrist according to the method of Greulich and Pyle (9). On the same day, dietary and physical activity questionnaires and DXA measures were obtained.
Dietary and Physical Activity Questionnaires
Dietary calcium intake was estimated using a semi-quantitative food frequency questionnaire (FFQ) developed by Block Dietary Data Systems (Berkeley, CA). The FFQ asked about the frequency and amount of intake in the last week of forty-five food and beverage items. Calcium intake was calculated using an automated computer analysis program.
Information on physical activity was obtained using an expanded version of the physical activity questionnaire originally validated by Slemenda (10). Subjects were asked about the hours/week they spent doing over 40 different physical and sedentary activities.
Bone Densitometry
Whole body, anterior-posterior (AP) lumbar spine, non-dominant forearm, and left proximal femur DXA scans were performed using Hologic, Inc. (Bedford, MA) bone densitometers (QDR4500A, QDR4500W, and Delphi A models) and analyzed using pediatric software (Hologic version 12.3). Scanner calibration was assessed as described previously (7). The precision errors for BMD and BMC were <1% for the spine phantom and <2.5% for the whole body phantom. Standard and height-adjusted DXA Z-scores were calculated using non-black normative data for sex and age reported previously (7, 11, 12). The percent body fat was calculated from the whole body DXA data as % body fat = fat mass/(fat mass + lean mass + bone mass).
Fracture Follow-up
At each visit, the participants were asked “During the past year, has the child broken any bones?” If the answer was “yes”, they were asked “Which bone(s)?”, “How did it happen?”, “Did the child have an X-ray?”, “Did the child have splints?”, “Did the child have a cast?”, and “Did it interfere with usual weight bearing activities or sports?” Beyond detailed questioning, no attempt was made to verify self-reported fractures using radiology reports because it is often unsuccessful (3).
Fractures were categorized as low, medium, or high impact. Low impact fractures were those occurring during a low impact collision or fall from low height comparable with the subject’s height. Medium impact fractures were those associated with sports, fighting, bicycling, skating, roller blading, or physical education classes. High impact fractures were those caused by an automobile or scooter accident, fall from high height, or other significant trauma.
Statistical Analyses
Statistical analyses were conducted using Stata (version 12.0; StataCorp LP, College Station, TX). Initial analysis was performed using descriptive statistics, simple comparisons through t-tests and chi-square tests, and unadjusted Kaplan Meier survivorship analysis. The main analysis then used the time-dependent Cox proportional hazards model to identify risk factors for first fracture. Univariate analysis was performed first, followed by investigation of interactions and multivariable analysis. Interactions were examined by testing for the significance of 2 and 3-way interaction terms involving the risk factors identified as significant in the univariate analysis. As measurements and fracture information were obtained annually, the data were easily formatted for a time-dependent Cox proportional hazards survivorship analysis.
Risk factors from the visit prior to fracture were examined, including sex, race/ethnicity, chronological age, skeletal age, sexual maturation, Z-scores for height, weight, and BMI, body fat percentage, days per week participating in organized sports or dance, hours per week watching television (TV), using a computer, or playing video games, calcium intake, and DXA Z-scores for bone mineral content (BMC) and bone mineral density (BMD) of the whole body less head (WB), anterior-posterior (AP) spine, hip total, hip neck, and 1/3 forearm. Chronological and skeletal ages were examined as squared terms because fracture rates increase through early adolescence and decrease thereafter; they were also examined using age groups (<10 years, ≥10 years and <15 years, ≥15 years) following the study of Khosla et al (13). Hazard ratios (HR) for prospectively reported fractures were examined, along with 95% confidence intervals for the HRs.
Subgroup analysis was also performed grouping the subjects based on their fracture history at baseline. 1,193 subjects had no fractures prior to baseline, 241 had one or two fractures, and 36 had an unknown fracture history.
RESULTS
The characteristics of the study sample are described in Table I. The 693 non-whites included 342 Blacks, 247 Hispanics, 104 Asians, and 84 other. The mean length of follow-up was 5.0 ± 1.5 years with the majority of subjects (908/1470, 62%) completing 6 years. A total of 212 subjects reported 257 fractures; 176 subjects sustained a single fracture incident, 30 sustained two fracture incidents, and 6 sustained three or more fracture incidents. The average age at first fracture was 13.7 ± 2.9 years.
Table 1.
Baseline characteristics of the study subjects
| White (N=693) | Non-White (N=777) | P-value | |
|---|---|---|---|
| Sex | |||
| Male | 344 (50%) | 382 (49%) | 0.86 |
| Female | 349 (50%) | 395 (51%) | |
|
| |||
| Anthropometric Characteristics | |||
| Chronological Age (yr) | 10.8 (3.1) | 11.1 (3.0) | 0.13 |
| Skeletal age (yr) | 10.8 (3.4) | 11.4 (3.4) | <0.001 |
| Height Z-score | 0.2 (0.8) | 0.1 (0.8) | .45 |
| Weight Z-score | 0.3 (0.8) | 0.4 (0.8) | 0.005 |
| BMI Z-score | 0.2 (0.8) | 0.4 (0.8) | <0.001 |
| Sexual maturation | |||
| Tanner 1 | 332 (48%) | 312 (40%) | 0.002 |
| Tanner 2 | 100 (14%) | 94 (12%) | |
| Tanner 3 | 69 (10%) | 80 (10%) | |
| Tanner 4 | 82 (12%) | 114 (15%) | |
| Tanner 5 | 110 (16%) | 176 (23%) | |
| % body fat | 22.5 (6.1) | 22.8 (6.9) | 0.44 |
|
| |||
| Physical and Dietary Characteristics | |||
| Sports participation (days/week) | 1.8 (1.7) | 1.4 (1.8) | <0.001 |
| TV/computer/video games (hours/week) | 38 (13) | 45 (16) | <0.001 |
| Calcium intake (mg/day) | 967 (491) | 890 (620) | 0.009 |
|
| |||
| Fracture History | |||
| Positive | 150 (22%) | 91 (12%) | <0.001 |
| Negative | 526 (77%) | 667 (86%) | |
| Unknown | 17 (2%) | 19 (2%) | |
|
| |||
| DXA Z-score | |||
| Whole body BMC | 0.2 (1.0) | 0.3 (1.0) | 0.01 |
| Whole body BMD | 0.1 (1.0) | 0.4 (1.0) | <0.001 |
| AP spine BMC | 0.0 (1.0) | 0.1 (1.0) | 0.20 |
| AP spine BMD | 0.0 (1.0) | 0.2 (1.0) | <0.001 |
| Hip total BMC | 0.2 (1.0) | 0.4 (1.0) | <0.001 |
| Hip total BMD | 0.1 (1.0) | 0.4 (1.1) | <0.001 |
| Hip neck BMC | 0.1 (0.9) | 0.2 (1.0) | 0.002 |
| Hip neck BMD | 0.0 (0.9) | 0.4 (1.1) | <0.001 |
| 1/3 forearm BMC | −0.1 (1.0) | 0.4 (1.1) | <0.001 |
| 1/3 forearm BMD | −0.1 (1.0) | 0.4 (1.0) | <0.001 |
Continuous variables are shown as mean (standard deviation).
Categorical variables are N (%).
Comparisons between whites and non-whites were performed using Student t-tests for continuous variables and chi-squared tests for categorical variables.
The overall fracture incidence was 0.034 fractures per person-year (257 fractures over 7,472 person-years of follow-up). Most fractures involved the upper extremity (165/257, 64%) or lower extremity (72/257, 28%) (Table II; available at www.jpeds.com). Most occurred during medium impact sports activities (185/257, 72%); only 15% (38/257) were low impact injuries. The most common sports involved were basketball (35/185, 19%), football (27/185, 15%), soccer (21/185, 11%), and skating/skiing (30/185, 16%). There were no significant differences in the distribution of location or cause of fracture between sexes or racial/ethnic groups (all Ps > 0.10).
Table 2.
Descriptive results for all fractures
| Male | Female | Total | |
|---|---|---|---|
| First Fracture (212 of 1470 subjects) | |||
| Fracture site | |||
| Upper extremity (hand, wrist, forearm, elbow, humerus, clavicle) | 87 (66.4%) | 46 (56.8%) | 133 (62.7%) |
| Lower extremity (foot, ankle, leg, pelvis) | 35 (26.7%) | 29 (35.8%) | 64 (30.2%) |
| Other (nose, ribs, jaw, other) | 9 (6.9%) | 6 (7.4%) | 15 (7.1%) |
| Cause of fracture | |||
| Low impact collision or fall | 20 (15.3%) | 12 (14.8%) | 32 (15.1%) |
| Medium impact sports injuries | 95 (72.5%) | 56 (69.1%) | 151 (71.2%) |
| High impact including motor vehicle accidents | 16 (12.2%) | 11 (13.6%) | 27 (12.7%) |
| Unknown | 0 (0.0%) | 2 (2.5%) | 2 (0.9%) |
|
| |||
| Subsequent Fractures (45 in 36 subjects) | |||
| Fracture site | |||
| Upper extremity (hand, wrist, forearm, elbow, humerus, clavicle) | 21 (67.7%) | 11 (78.6%)* | 32 (71.1%) |
| Lower extremity (foot, ankle, leg, pelvis) | 6 (19.4%) | 2 (14/3%) | 8 (17.8%) |
| Other (nose, ribs, jaw, other) | 4 (12.9%) | 1 (7.1%) | 5 (11.1%) |
| Cause of fracture | |||
| Low impact collision or fall | 5 (16.1%) | 1 (7.1%) | 6 (13.3%) |
| Medium impact sports injuries | 22 (71.0%) | 12 (85.7%) | 34 (75.6%) |
| High impact including motor vehicle accidents | 4 (12.9%) | 1 (7.1%) | 5 (11.1%) |
1 subject also fractured lower extremity
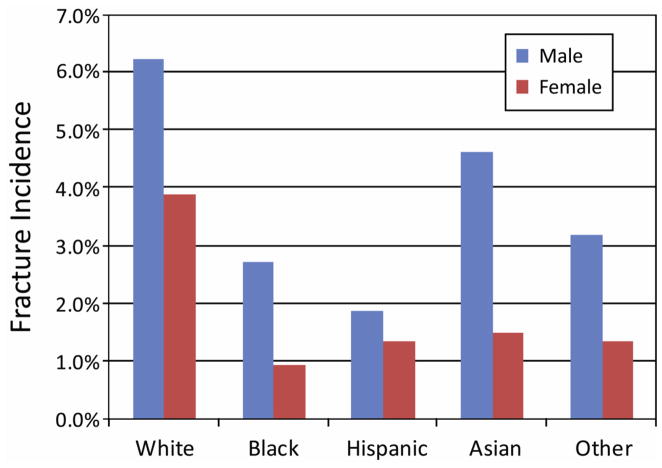
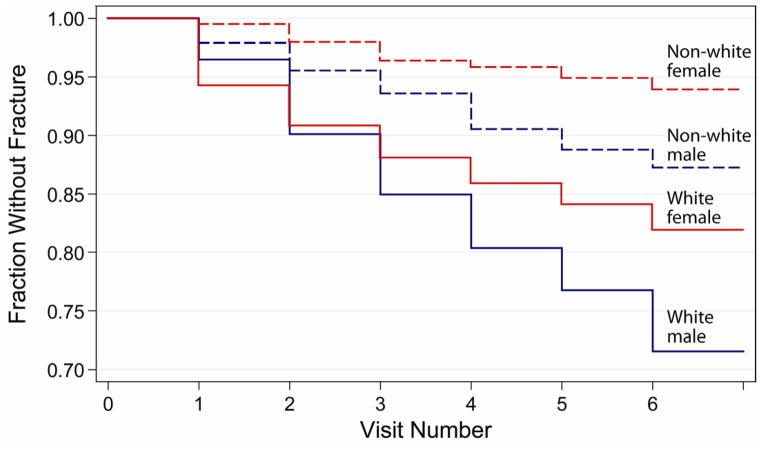
Fracture rates were higher in males than in females regardless of ancestry (Figure 1; available at www.jpeds.com). Fracture rates were also higher, regardless of sex, for whites compared with all other racial/ethnic groups (Figure 1). Survivorship plots indicate that whites had a higher prevalence of fracture at all time points compared with non-whites regardless of sex (Figure 2); remarkably, white girls experienced a higher fracture rate than non-white boys.
Figure 1.
Fracture incidence by race/ethnicity and sex
Figure 2.
Unadjusted survivorship analysis of first fracture by sex and race (white vs. non-white)
Time-Dependent Univariate Cox Analysis (unadjusted for other covariates)
As expected, males had a significantly higher fracture risk than females (Table III). Fracture risk was also higher for whites compared with all other racial groups. This was true when all non-whites were considered together and when each group (Black, Hispanic, Asian, Other) was considered separately (HRs: 1.9 to 3.1; P ≤ 0.05). There were no significant differences in fracture risk among the non-white racial/ethnic groups (HRs: 0.6 to 1.3; P > 0.18).
Table 3.
Results of univariate analysis using the time-dependent Cox proportional hazards model for first fracture
| Risk Factor (univariate) | HR | SE | 95% CI | P-value |
|---|---|---|---|---|
| Male | 1.74 | 0.25 | 1.32, 2.29 | <0.001 |
| White race | 2.67 | 0.40 | 1.99, 3.57 | <0.001 |
| Chronological age 10–14 yr | 1.78 | 0.25 | 1.35, 2.35 | <0.001 |
| Skeletal age 10–14 yr | 2.17 | 0.30 | 1.65, 2.85 | <0.001 |
| Puberty (Tanner 2–4) | 1.74 | 0.24 | 1.32, 2.29 | <0.001 |
| Height Z-score | 1.12 | 0.09 | 0.95, 1.31 | 0.17 |
| Weight Z-score | 0.89 | 0.07 | 0.75, 1.04 | 0.14 |
| BMI Z-score | 0.86 | 0.07 | 0.74, 1.00 | 0.06 |
| % body fat | 0.96 | 0.01 | 0.94, 0.98 | <0.001 |
| Sports participation (days/wk) | 1.12 | 0.04 | 1.05, 1.20 | 0.001 |
| TV/computer/video games (hours/wk) | 0.99 | 0.006 | 0.98, 1.00 | 0.12 |
| Calcium intake (g/day) | 1.26 | 0.14 | 1.01, 1.57 | 0.04 |
| Fracture prior to baseline | 1.70 | 0.27 | 1.24, 2.32 | 0.001 |
|
| ||||
| DXA Z-score, whole body BMC | 0.98 | 0.07 | 0.86, 1.12 | 0.78 |
| DXA Z-score, whole body BMD | 0.98 | 0.06 | 0.86, 1.11 | 0.73 |
| DXA Z-score, spine BMC | 0.98 | 0.07 | 0.86, 1.12 | 0.75 |
| DXA Z-score, spine BMD | 0.89 | 0.06 | 0.78, 1.01 | 0.08 |
| DXA Z-score, hip total BMC | 1.01 | 0.07 | 0.88, 1.15 | 0.94 |
| DXA Z-score, hip total BMD | 0.89 | 0.06 | 0.78, 1.01 | 0.08 |
| DXA Z-score, hip neck BMC | 0.98 | 0.07 | 0.86, 1.12 | 0.78 |
| DXA Z-score, hip neck BMD | 0.91 | 0.06 | 0.81, 1.03 | 0.16 |
| DXA Z-score, 1/3 radius BMC | 0.86 | 0.06 | 0.76, 0.98 | 0.02 |
| DXA Z-score, 1/3 radius BMD | 0.81 | 0.05 | 0.71, 0.92 | 0.001 |
Fracture risk was related to chronological and skeletal age, with the greatest fracture risk observed in 10–14 year olds (Table III). Similarly, fracture risk was higher during puberty (Tanner 2–4) than in prepubertal children (Tanner 1) or sexually mature teenagers (Tanner 5).
Although there was a trend towards greater fracture risk for taller, thinner subjects (higher height Z-score and lower weight and BMI Z-scores), the relationships between fracture risk and anthropometric measures did not reach statistical significance (Table III). However, fracture risk increased with lower body fat percentage and greater sports participation. Time spent watching TV, using a computer, and playing video games was not significantly related to fracture risk. Fracture risk was higher with greater calcium intake, apparently due to sex differences, because calcium intake is not a significant risk factor once sex is taken into account (P = 0.20).
Having a fracture prior to baseline was a significant risk factor for a subsequent fracture. A history of previous fracture was a stronger risk factor for females (HR: 2.4; 95% CI: 1.5 to 3.9; P < 0.001) than for males (HR: 1.3; 95% CI: 0.8 to 2.0; P = 0.23) and for whites (HR: 1.6; 95% CI 1.1 to 2.3; P = 0.01) compared with non-whites (HR: 1.2; 95% CI: 0.6 to 2.4; P = 0.67).
There was a general trend of reduced fracture risk with increased DXA Z-scores, although the relationship was only statistically significant for radius BMC and BMD (Table III). Similar results were found for height-adjusted (data not shown) and standard (non-adjusted) Z-scores.
Interactions
Testing for interactions between sex, race (white vs. other), puberty, chronological age, skeletal age, previous fracture, sports participation, body fat percentage, and DXA Z-scores revealed two significant interactions. The first involved sex, white race, and fracture history. A positive fracture history significantly increased fracture risk in white females (HR = 2.4; 95% CI: 1.4 to 4.0; P = 0.001), but not in males or non-white females (HRs: 0.8 to 1.3; P > 0.57). The second significant interaction involved sex, white race, and DXA Z-scores. For white males, fracture risk was generally greater with higher DXA Z-scores (HR > 1). In contrast, for all other groups (females and non-white males) fracture risk was generally lower with higher DXA Z-scores (HR < 1). To account for these interactions, white*female*previous_fracture and white*male*DXA Z-score terms were included in the subsequent multivariable analysis.
Time-Dependent Multivariable Cox Analysis
Multivariable analysis using the time-dependent Cox proportional hazards model indicated that white ancestry and male sex were among the strongest risk factors for first fracture (Table IV). Chronological age, skeletal age, and puberty were also significant risk factors when entered independently (HRs: 1.7 to 2.3; P < 0.001), but because of their close relationship only chronological age was included in the final model. Greater sports participation and lower body fat percentage were significantly associated with increased fracture risk. In contrast, neither TV/computer use (HR: 0.99; 95% CI: 0.98 to 1.01; P = 0.36), height, weight, and BMI Z-scores (HRs: 0.8 to 1.1; all Ps > 0.39), nor calcium intake were significant risk factors.
Table 4.
Prediction of risk of first fracture using the time-dependent multivariable Cox proportional hazards model for entire cohort, subjects with no fractures prior to baseline, and subjects with a positive fracture history prior to baseline
| Entire cohort (N=1,470)
| ||||
|---|---|---|---|---|
| Risk Factor | HR | SE | 95% CI | P-value |
| White race | 2.18 | 0.34 | 1.60, 2.97 | <0.001 |
| Male | 1.71 | 0.33 | 1.17, 2.49 | 0005 |
| Age 10–14 yr | 1.96 | 0.29 | 1.47, 2.61 | <0.001 |
| Body fat % | 0.97 | 0.02 | 0.94, 1.00 | 0.02 |
| Sports ≥4 days/wk | 1.35 | 0.21 | 1.00, 1.84 | 0.05 |
| White * Female * Previous Fracture | 1.84 | 0.61 | 0.96, 3.54 | 0.07 |
| DXA Z-score, 1/3 radius BMD† | 0.74 | 0.07 | 0.62, 0.88 | 0.001 |
| White * Male * 1/3 radius BMD Z-score† | 1.66 | 0.22 | 1.27, 2.17 | <0.001 |
| BMI Z-score | 1.09 | 0.12 | 0.88, 1.36 | 0.43 |
| Calcium intake (g/day) | 1.10 | 0.23 | 0.72, 1.67 | 0.66 |
| Fracture prior to baseline | 1.02 | 0.13 | 0.79, 1.32 | 0.87 |
| Subjects with no fractures prior to baseline (N=1,193)
| ||||
|---|---|---|---|---|
| Risk Factor | HR | SE | 95% CI | P-value |
| White race | 2.10 | 0.36 | 1.51, 2.94 | <0.001 |
| Male | 1.69 | 0.35 | 1.14, 2.53 | 0.01 |
| Age 10–14 yr | 1.89 | 0.31 | 1.37, 2.61 | <0.001 |
| Body fat % | 0.96 | 0.02 | 0.93, 1.00 | 0.04 |
| Sports ≥4 days/wk | 1.18 | 0.22 | 0.82, 1.71 | 0.36 |
| DXA Z-score, 1/3 radius BMD† | 0.73 | 0.07 | 0.60, 0.89 | 0.002 |
| White * Male * 1/3 radius BMD Z-score† | 1.91 | 0.30 | 1.41, 2.60 | <0.001 |
| BMI Z-score | 1.03 | 0.13 | 0.81, 1.31 | 0.81 |
| Calcium intake (g/day) | 0.93 | 0.14 | 0.69, 1.26 | 0.64 |
| Subjects with a positive fracture history prior to baseline (N=241)
| ||||
|---|---|---|---|---|
| Risk Factor | HR | SE | 95% CI | P-value |
| White race | 3.21 | 1.27 | 1.47, 6.99 | 0.003 |
| Male | 0.87 | 0.35 | 0.40, 1.92 | 0.73 |
| Age 10–14 yr | 1.74 | 0.54 | 0.94, 3.20 | 0.08 |
| Body fat % | 0.96 | 0.03 | 0.90, 1.03 | 0.27 |
| Sports ≥4 days/wk | 1.99 | 0.59 | 1.11, 3.57 | 0.02 |
| DXA Z-score, 1/3 radius BMD† | 0.78 | 0.14 | 0.55, 1.12 | 0.18 |
| White * Male * 1/3 radius BMD Z-score† | 1.06 | 0.30 | 0.61, 1.85 | 0.83 |
| BMI Z-score | 1.32 | 0.34 | 0.80, 2.18 | 0.28 |
| Calcium intake (g/day) | 1.24 | 0.32 | 0.75, 2.05 | 0.40 |
Similar results were obtained for all other DXA measures.
In general, fracture risk decreased with higher DXA Z-scores (HRs: 0.74 to 0.89). The results were statistically significant for spine BMD, hip neck and total BMD, and radius BMC and BMD (all Ps < 0.03). However, for white males, fracture risk increased with higher DXA Z-scores (HRs for interaction: 1.3 to 1.7; P ≤ 0.10). As a subgroup, white males had a hazard ratio of 1.22 for forearm BMD, compared with 0.89 for non-white males, 0.70 for white females, and 0.51 for non-white females with similar results for the other DXA variables.
Positive fracture history was not, in general, a significant risk factor for future fracture (P > 0.68). However, white females with a positive fracture history had an increased fracture risk (P ≤ 0.07).
Subgroup Analysis of Subjects with No Fractures Prior to Baseline
Similar results were obtained when studying only the 1,193 subjects without a history of fractures at baseline. Again, white race, male sex, age of 10–14 years, and decreased body fat percentage were all strong and significant risk factors for future fracture (Table IV). Using standard or height-adjusted values, lower DXA Z-scores were associated with higher fracture risk, except in white males who had increased fracture risk with higher DXA Z-scores. The main difference in this subgroup was a lower hazard ratio for sports participation (1.2 vs. 1.4). For the subjects who were fracture free at baseline, sports participation was no longer a significant risk factor for prospective fracture (P = 0.36).
Subgroup Analysis of Subjects with Fractures Prior to Baseline
For the subjects who had fractured prior to baseline (122 male, 119 female; 150 white, 36 black, 32 Hispanic, 6 Asian, 17 other), white race was an even stronger risk factor for prospective fracture than in the overall group (HR: 3.2 vs. 2.2) (Table IV). Interestingly, sports participation also had a stronger influence on fracture risk than in the overall group (HR: 2.0 vs. 1.4). The hazard ratio for age was reduced, and male sex and body fat percentage were no longer significant. Although DXA Z-scores were no longer statistically significant, they had similar hazard ratios for their overall effect (0.78 vs. 0.74 for radius BMD) but lower hazard ratios for the white male interaction (1.06 vs. 1.66 for radius BMD).
DISCUSSION
The results of this multi-center prospective study provide strong evidence that race/ethnicity is a major determinant of fracture risk in healthy children living in the U.S. We found a much higher fracture rate in non-Hispanic white children compared with children of other ancestry. These differences were independent of chronological age, skeletal age, body mass, and degree of sexual maturation. On average, healthy boys and girls of European descent had more than twice the fracture risk of healthy children from other backgrounds; this effect was greater than the difference in fracture risk between boys and girls.
The results of this study corroborate previous data indicating that pediatric fractures occur predominantly during physical activities such as sports and are more common in boys and during adolescence (4, 14–16). The basis for the greater incidence of fractures during adolescence is likely related to a temporary imbalance between bone resorption and apposition at a time when loading rapidly increases due to greatly increased muscle mass, strength, and activity (13, 15, 17, 18). In general, there was an inverse relationship between DXA bone measures and fracture risk. Paradoxically, the opposite occurred in white males; in this subgroup, higher DXA values were associated with higher fracture risk. Previous studies have produced inconsistent findings regarding the relationship between fractures and DXA bone measures. Clark et al observed an inverse relationship between fractures and DXA measures in a predominantly white cohort (19, 20). In contrast, Thandrayen et al observed a positive relationship between bone mass and fractures in white males, but no relationship between DXA values and fractures in other groups (6). They noted that white boys who fractured were more physically active and leaner than those who did not fracture, which was also the case in our cohort. This suggests that white boys who are more physically active develop higher bone mass, but not enough to offset the mechanical demands associated with the increased physical activity. The relationship between physical activity and fractures warrants further investigation as it depends on both activity- and site-specific influences on bone as well as changes in risk exposure.
The structural basis for the reduced fracture resistance of white children is yet to be completely defined. Prior studies using computed tomography (CT) have shown that compared with children of African descent, those of European heritage have lower cancellous bone density in the axial skeleton and smaller bone size in the appendicular skeleton (21). High-resolution CT has shown that post-menopausal Asian women have greater cortical and trabecular thickness, but smaller bone area at the radius and tibia when compared with European women (22, 23).
We did not observe any relationships between fracture risk and body habitus or calcium intake, except for a higher fracture risk in subjects with less body fat. This is in contrast to previous reports showing children who fracture to have higher body weight and/or body mass (6, 24, 25). Furthermore, although previous studies suggest that a previous fracture increases fracture risk regardless of sex (26), we found this relationship only in white girls. Our exclusion of children with a history of multiple fractures may have lessened this effect. Fracture risk may also be affected by other factors not examined in the current study, such as risk-taking behavior not captured by the questionnaires or differences in balance, proprioception, or fall mechanics. Most importantly, skeletal characteristics related to bone structure not captured by DXA are likely contributors to the variance in bone strength among children.
The strengths of this study include its use of the large, well-characterized BMDCS cohort and the length of regular follow-up for up to 6 years. The study subjects were racially and ethnically diverse and were recruited from five different geographic locations distributed across the U.S. allowing ancestry differences to be studied. Assessments were conducted annually following highly detailed and standardized procedures. The physiological changes associated with growth and development were well characterized; sexual and skeletal development were determined yearly by pediatric endocrinologists and pediatric radiologists, respectively, and all DXA measurements were analyzed at a central core facility following rigorous protocols.
The primary limitations of this study derive from its use of self-reporting to assess fractures, dietary intake, physical activity, and TV/computer/video game use. However, our results are quantitatively similar to those observed in numerous studies on the incidence of pediatric fractures. To encourage accurate reporting, subjects and families were interviewed in person during visits, and subjects were only asked to report on their current activity levels and fractures occurring since the previous visit. Although it is possible that reporting differed among races, this potential confounder is unlikely to negate the large magnitude of the effects observed in this study.
A differential effect of race on fracture risk has been recognized ever since it was noted, nearly 50 years ago, that fractures of the hip occur infrequently in elderly African Americans (1, 27, 28). Racial/ethnic disparities in fracture risk are also well-documented among other racial groups such as Asians (23, 27). It now appears that this influence is not confined to the elderly, who experience fragility fractures as bone mass declines, but is also present in childhood, when bone acquisition, physical activity, and the risk for trauma are greatest. Our results provide compelling evidence that children of European descent are much more susceptible to fractures than those of other racial/ethnic backgrounds. A deeper understanding of the structural and genetic basis of this racial disparity may ultimately contribute to the prevention of fractures throughout life.
Acknowledgments
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NO1-HD-1-3228, NO1-HD-1-3329, NO1-HD-1-3330, NO1-HD-1-3331, NO1-HD-1-3332, and NO1-HD-1-3333).
We are indebted to our collaborators in the pediatric endocrine divisions of each Bone Mineral Density in Childhood Study Clinical Center. In particular, we acknowledge Drs. Andrea Kelly, David Langdon, Thomas Moshang, Steve Willi, Lorraine Katz, Charles Stanley, and Craig Alter and the faculty in the Division of Pediatric Endocrinology at Children’s Hospital of Philadelphia; Lynda Fisher, Mitchell Geffner, Debra Jeandron, Steven Mittelman, Pisit Pitukcheewanont, and Francine Kaufman from Children’s Hospital Los Angeles; Susan Rose, Frank Biro, Peggy Stenger, Debbie Elder, and James Heubi from Cincinnati Children’s Hospital and Medical Center; Mary Horlick, Natasha Leibel, and Abeer Hassoun at Columbia University Medical Center-St. Luke’s Hospital; and Jean-Claude Desmangles from Creighton University. We also acknowledge the guidance and advice from the Data Safety and Monitoring Board members: Drs. Clifford Rosen, Ralph D’Agostino, Ingrid Holm, James Reynolds, and Reginald Tsang.
ABBREVIATIONS
- BMC
bone mineral content
- BMD
bone mineral density (areal)
- BMDCS
Bone Mineral Density in Childhood Study
- BMI
body mass index
- DXA
dual-energy x-ray absorptiometry
- HR
hazard ratio
Footnotes
The authors declare no conflicts of interest.
Acknowledgments available at www.jpeds.com
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson JJ, Pollitzer WS. Ethnic and genetic differences in susceptibility to osteoporotic fractures. Adv Nutr Res. 1994;9:129–49. doi: 10.1007/978-1-4757-9092-4_8. [DOI] [PubMed] [Google Scholar]
- 2.Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T, et al. Racial differences in fracture risk. Epidemiology. 1994;5:42–7. doi: 10.1097/00001648-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res. 2006;21:1489–95. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark EM, Ness AR, Tobias JH. Vigorous physical activity increases fracture risk in children irrespective of bone mass: a prospective study of the independent risk factors for fractures in healthy children. J Bone Miner Res. 2008;23:1012–22. doi: 10.1359/jbmr.080303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thandrayen K, Norris SA, Pettifor JM. Fracture rates in urban South African children of different ethnic origins: the Birth to Twenty cohort. Osteoporos Int. 2009;20:47–52. doi: 10.1007/s00198-008-0627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thandrayen K, Norris SA, Micklesfield LK, Pettifor JM. Heterogeneity of fracture pathogenesis in urban South African children: the birth to twenty cohort. J Bone Miner Res. 2011;26:2834–42. doi: 10.1002/jbmr.491. [DOI] [PubMed] [Google Scholar]
- 7.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–99. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 8.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 9.Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2. California: Stanford University Press; 1959. [Google Scholar]
- 10.Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston CC., Jr Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991;6:1227–33. doi: 10.1002/jbmr.5650061113. [DOI] [PubMed] [Google Scholar]
- 11.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96:3160–9. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–73. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khosla S, Melton LJ, 3rd, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA. 2003;290:1479–85. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 14.Rauch F, Neu C, Manz F, Schoenau E. The development of metaphyseal cortex--implications for distal radius fractures during growth. J Bone Miner Res. 2001;16:1547–55. doi: 10.1359/jbmr.2001.16.8.1547. [DOI] [PubMed] [Google Scholar]
- 15.Bailey DA, Wedge JH, McCulloch RG, Martin AD, Bernhardson SC. Epidemiology of fractures of the distal end of the radius in children as associated with growth. J Bone Joint Surg Am. 1989;71:1225–30. [PubMed] [Google Scholar]
- 16.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res. 2004;19:1976–81. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 17.Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, et al. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24:1033–42. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res. 25:1521–6. [Google Scholar]
- 19.Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res. 2008;23:173–9. doi: 10.1359/jbmr.071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117:e291–7. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilsanz V, Skaggs DL, Kovanlikaya A, Sayre J, Loro ML, Kaufman F, et al. Differential effect of race on the axial and appendicular skeletons of children. J Clin Endocrinol Metab. 1998;83:1420–7. doi: 10.1210/jcem.83.5.4765. [DOI] [PubMed] [Google Scholar]
- 22.Walker MD, Liu XS, Stein E, Zhou B, Bezati E, McMahon DJ, et al. Differences in bone microarchitecture between postmenopausal Chinese-American and white women. J Bone Miner Res. 26:1392–8. doi: 10.1002/jbmr.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XF, Seeman E. Epidemiology and structural basis of racial differences in fragility fractures in Chinese and Caucasians. Osteoporos Int. 23:411–22. doi: 10.1007/s00198-011-1739-2. [DOI] [PubMed] [Google Scholar]
- 24.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20:2090–6. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- 25.Skaggs DL, Loro ML, Pitukcheewanont P, Tolo V, Gilsanz V. Increased body weight and decreased radial cross-sectional dimensions in girls with forearm fractures. J Bone Miner Res. 2001;16:1337–42. doi: 10.1359/jbmr.2001.16.7.1337. [DOI] [PubMed] [Google Scholar]
- 26.Goulding A, Jones IE, Williams SM, Grant AM, Taylor RW, Manning PJ, et al. First fracture is associated with increased risk of new fractures during growth. J Pediatr. 2005;146:286–8. doi: 10.1016/j.jpeds.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–94. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 28.Gyepes M, Mellins HZ, Katz I. The low incidence of fracture of the hip in the negro. JAMA. 1962;181:1073–4. [Google Scholar]