Abstract
Objective
To validate King’s College Hospital criteria (KCHC) in children with non-acetaminophen (APAP) induced pediatric acute liver failure (PALF) and to determine whether re-optimizing the KCHC would improve predictive accuracy.
Study design
We utilized the PALF study group database. Primary outcomes were survival without liver transplantation (LT) versus death at 21 days following enrollment. Classification and Regression Tree (CART) analysis was used to determine if modification of KCHC parameters would improve classification of death versus survival.
Results
Among 163 patients who met KCHC, 54 patients (33.1%) died within 21 days. Sensitivity of KCHC in this cohort was significantly lower than in the original study (61% vs 91%, p=0.002), and specificity did not differ significantly. The positive predictive value (PPV) and negative predictive value (NPV) of KCHC for this cohort was 33% and 88% respectively. CART analysis yielded the following optimized parameters to predict death: grade 2–4 encephalopathy, international normalized ratio >4.02 and total bilirubin >2.02 mg/dL. These parameters did not improve PPV, but NPV was significantly better (88% vs. 92%, p<0.0001).
Conclusions
KCHC does not reliably predict death in PALF. With a PPV of 33%, twice as many participants who met KCHC recovered spontaneously than died, indicating that using KCHC may cause over utilization of LT. Re-optimized cutpoints for KCHC parameters improved NPV, but not PPV. Parameters beyond the KCHC should be evaluated to create a predictive model for PALF.
Keywords: coagulopathy, prognosis, hepatic encephalopathy
Pediatric acute liver failure (PALF) is a clinical syndrome of severe liver injury, occurring in children with no prior history of liver disease. It is a life-threatening illness, which accounts for 10–13% of all pediatric liver transplants 1–3. In the pre-transplant era, survival following PALF occurred in only 29% of patients 4. With improvement in supportive therapy and liver transplantation (LT), PALF survival has increased to 31–36% in those not transplanted and to between 55–60% in those transplanted 5–7.
Although LT in PALF has improved short term survival, long-term survival is poor compared with other indications for transplantation 8. Furthermore, the ongoing shortage of viable organs heightens the need to ensure proper organ allocation 5–7, 9. Organ transplantation of children who may have recovered spontaneously would subject them to unnecessarily life-long immunosuppression, including risk of post-surgical complications and future graft failure 10–12. Therefore, methods are needed to distinguish patients who require transplantation for survival, from those who will recover with supportive care.
Several scoring systems are available to predict mortality in non-transplanted acute liver failure (ALF) patients. The King’s College Hospital criteria (KCHC), formulated in 1989, are the most extensively studied and widely applied 13. The original derivation cohort for this model consisted of 588 patients, both children and adults in the pre-LT era. The positive predictive value (PPV) for mortality in non-APAP induced ALF was 97%, indicating a high risk of death if meeting the criteria 14. Subsequent studies, performed primarily with adults, have demonstrated similar findings, with PPV ranging from 80–96% and negative predictive value (NPV) ranging from 42–82% 15–21. As PALF differs from adult ALF in several aspects including definition, presentation, etiology and outcome 22–24, the KCHC may not be applicable to assess prognosis in PALF.
By utilizing the PALF Study Group database, we intend to validate the KCHC in a large cohort of children with non-APAP induced PALF and determine whether redefining the KCHC parameters would increase the predictive accuracy of this model.
Methods
Data for this study came from the PALF Study Group consisting of 20 pediatric liver transplant sites, 17 within the United States, 1 in Canada, and 2 in the United Kingdom.
Following informed consent from a parent or legal guardian, demographic, clinical and laboratory information were recorded daily for 7 days, starting on the date of enrollment into the study. Diagnostic evaluation and medical management were consistent with the standard of care at each site. Primary outcome measures determined at 21 days after entry into the study included death or survival with native organ. The National Institutes of Health provided a Certificate of Confidentiality to the study and IRB approval was secured at each site before patient enrollment.
Patients from birth through 18 years of age were eligible for enrollment if they met the following entry criteria for the PALF study: (1) children with no known evidence of chronic liver disease, (2) evidence of acute liver injury within 8 weeks of disease onset, and (3) hepatic-based coagulopathy defined as a prothrombin time (PT) ≥ 15 seconds or international normalized ratio (INR) ≥ 1.5 not corrected by vitamin K in the presence of clinical hepatic encephalopathy (HE) or a PT ≥20 seconds or INR ≥ 2.0 regardless of the presence or absence of clinical HE 22.
Patients with APAP induced PALF were excluded from our analysis because spontaneous recovery from APAP induced liver failure is significantly higher than in non-APAP induced PALF 23. In our cohort specifically, of 111 patients with APAP induced PALF, 3 of them (2.7%) died and 5 (4.5%) underwent LT. The remaining patients survived with full recovery of their native liver. Therefore we believed the study should focus on non-APAP induced liver failure, where survival is lower and more patients are considered for LT.
Patients who underwent LT were also excluded from analysis. Our primary aim was to validate KCHC in predicting the specific outcomes of death with native the liver or spontaneous recovery in PALF. LT is an intervention that interrupts the natural course of PALF, and it is impossible to know the patient’s natural history with certainty after LT. To validate the original KCHC analysis performed on patients in the pre-LT era, we used a cohort which represents an uninterrupted natural history of PALF to determine if KCHC reliably predicts death.
Patients were categorized as meeting KCHC if they fulfilled the parameters defined in Table I. In the original analysis, patients who met KCHC were likely to die, and those who did not meet KCHC were likely to survive. Determination of whether patients in our cohort did or did not meet KCHC was based on data recorded on the day of enrollment into the PALF Study Group database.
Table 1.
King’s College Hospital Criteria for predicting mortality in acute liver failure
| Non-Acetaminophen induced ALF |
|---|
| Prothrombin time >100 s (INR > 6.5) |
| OR |
| any 3 of the following (irrespective of grade of encephalopathy): |
| • Age <10 or >40 years |
| • Etiology: non-A/non-B hepatitis, drug-induced |
| • Duration of jaundice to hepatic encephalopathy (HE) >7 days |
| • Prothrombin time >50 (INR > 3.5) |
| • Serum bilirubin >300 ìmol/L |
Statistical Analysis
Continuous variables were expressed as a mean +/− standard deviation. Categorical variables were expressed as a percentage. Prognostic value of the KCHC was determined by calculating the sensitivity, specificity, PPV and NPV. Comparison of sensitivity, specificity, and PPV to the findings from the original KCH paper was performed using tests for differences of proportions. A p-value of less than 0.05 was considered significant.
Classification and Regression Tree (CART) analysis was used to determine whether different cutpoints of KCHC components led to better classification for 21-day mortality versus survival without LT. The PPV and NPV were compared between the KCHC and those derived from CART, using McNemar test.
Results
A total of 895 participants were enrolled in the PALF Study Group database from 1999 to 2009. Among this group, 111 patients were diagnosed with APAP induced PALF, and 784 had non-APAP induced liver failure. Of those with non-APAP induced PALF, 110 (14.0%) died, 262 (33.4%) underwent transplantation, and 412 (52.6%) were alive with their native liver at 21 days following enrollment. This yielded a 522 patient study cohort where the natural history of PALF independent of liver transplantation could be assessed. The mean age was 2.7 years, with 36.8% of patients < 1 year. Males comprised 52.3% of the cohort. Approximately 69% of the participants were Caucasian.
The majority of cases of PALF were secondary to an indeterminate cause (43.1%). A total of 390 (74.7%) patients were transferred from another hospital to a PALF Study Group site, with a median time of 2.0 days from admission to transfer and 3.0 days from admission to enrollment into the database. Overall demographic and outcomes characteristics are listed in Table II.
Table 2.
Demographic and outcomes characteristics of the study cohort (non-transplanted) and transplanted patients
| Study Cohort (n = 522) | Transplanted Patients (n = 262) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Male | 273 | 52.3 | 149 | 56.9 |
| Female | 249 | 47.7 | 113 | 43.1 |
| Ethnicity | ||||
| Not Hispanic or Latino | 421 | 80.7 | 205 | 78.2 |
| Hispanic or Latino | 101 | 19.3 | 57 | 21.8 |
| Race | ||||
| Caucasian | 355 | 68.9 | 187 | 71.4 |
| Non-white | 167 | 31.1 | 57 | 21.8 |
| Age (years) | ||||
| Mean | 2.7 | 5.5 | ||
| Range | 0.0 – 17.9 | 0.0–17.9 | ||
| <1 year | 192 | 36.8 | 48 | 18.3 |
| 1–2.9 years | 78 | 14.9 | 42 | 16.0 |
| 3–9.9 years | 120 | 23.0 | 95 | 36.3 |
| 10–17.9 years | 132 | 25.3 | 77 | 29.4 |
| Final Diagnosis | ||||
| Indeterminate | 225 | 43.1 | 179 | 68.3 |
| Budd-Chiari | 2 | 0.4 | 1 | 0.4 |
| Hemophagocytic Syndrome | 17 | 3.3 | 2 | 0.8 |
| Ischemia | 29 | 5.6 | 1 | 0.4 |
| Neonatal Iron Storage | 17 | 3.3 | 5 | 1.9 |
| Veno-occlusive disease | 10 | 1.9 | 1 | 0.4 |
| Wilson’s disease | 11 | 2.1 | 23 | 8.8 |
| Metabolic disorder | 49 | 9.4 | 8 | 3.1 |
| Viral hepatitis | 55 | 10.5 | 12 | 4.6 |
| Autoimmune hepatitis | 45 | 8.6 | 15 | 5.7 |
| Drug-induced | 19 | 3.6 | 6 | 2.3 |
| Mushroom toxicity | 2 | 0.4 | 2 | 0.8 |
| Other | 25 | 4.8 | 4 | 1.5 |
| Outcomes | ||||
| 21-day mortality | 110 | 21.1 | ||
| Recovery | 412 | 78.9 | ||
KCHC Validation
Of 522 participants, 163 (31.2%) met the KCHC criteria that would predict death for non-acetaminophen induced PALF, 289 (56.1%) did not meet the criteria, and 70 (13.4%) had insufficient data. The time interval between the onset of jaundice to encephalopathy was missing in 132 patients, because the information was not recorded or they had no clinical evidence of encephalopathy on admission. Of the 163 patients who met KCHC, 28 of them (17.1%) had an INR > 6.5. The remainder fulfilled the criteria by being positive for 3 of the 5 remaining parameters. Patients who met KCHC were younger (median age 1.0 years vs 4.6 years), with a greater percentage less than 1 year old (49.7 vs 29.8%). There were no significant differences in sex or ethnicity between those who did and did not meet KCHC.
Among those who met KCHC, 54 patients (33.1%) died within 21 days, and 109 patients (66.9%) survived. In the group who did not meet KCHC, 34 (11.8%) had died and 255 (88.2%) survived. The sensitivity of applying the KCHC to our cohort was significantly lower than that found in the original KCH cohort (61% vs 91%, p=0.002), but specificity did not differ significantly (70% vs 90%, p=0.17). When determining the PPV and NPV of this model, we found that 33% of patients who met the criteria died (PPV), and 88% of those not meeting criteria had survived (NPV). The PPV of 33% in our cohort was significantly lower than that of the original KCH study (96%). We did not compare NPV as the initial KCH study did not determine NPV for their cohort.
Redefining KCHC parameters
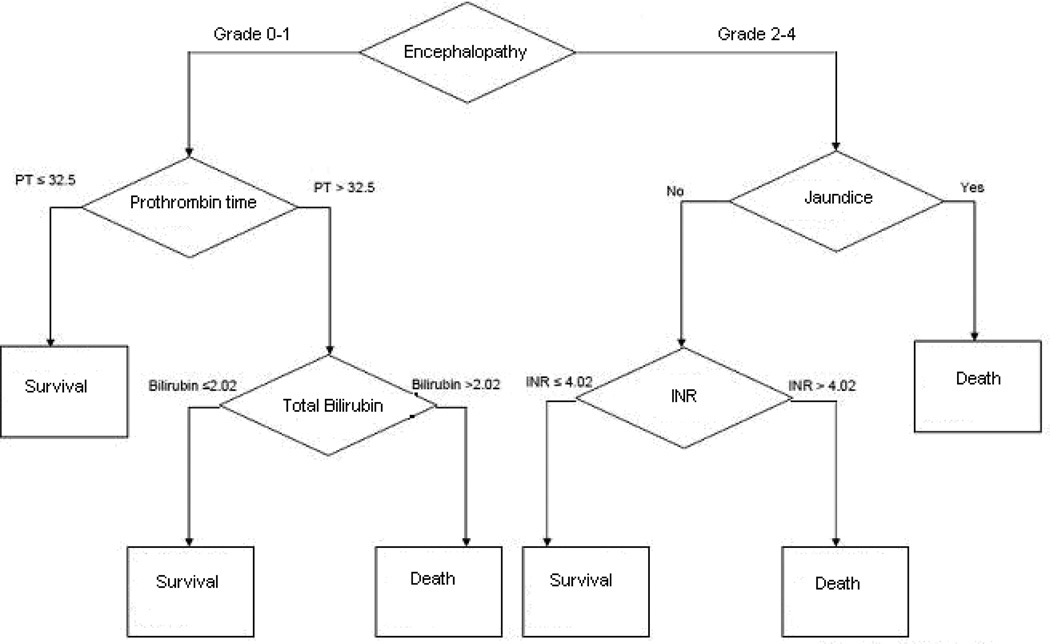
Utilizing classification and regression tree (CART) analysis, a decision tree was created to assess prognosis (survival versus mortality) utilizing optimized cut-points from the original KCHC parameters (Figure; available at www.jpeds.com). Based on our analysis, mortality was predicted based on meeting one of the following criteria: (1) grade 2–4 encephalopathy with jaundice; grade 2–4 encephalopathy with INR>4.02; and (3) grade 0–1 encephalopathy with prothrombin time>32.5s and total bilirubin >2.02 mg/dL.
Figure.
Redefined KCHC parameters to predict survival versus mortality
The redefined parameters improved PPV from 33% to 50% (p=0.20), and NPV from 88 to 92% (p<0.0001) (Figure).
Discussion
In the era prior to LT, the natural history of pediatric acute liver failure (PALF) was either death or survival with recovery of liver function. Advances in critical care management and liver transplantation have significantly improved outcome 23, 25. However, it is essential to assure that models utilized in the decision making process are accurate. The majority of studies validating the KCHC were performed in adults 17–21.
Our study utilized a patient cohort that best represents an uninterrupted natural history of PALF in the liver transplantation era, to explore the prognostic ability of the KCHC. Prior studies validating prognostic models in ALF/PALF often combine death and LT as one outcome. Combining death and LT will increase the sample size for analysis, but falsely assumes that the outcomes of LT and death are similar. We know patients with PALF who were listed for LT and would have undergone LT had an allograft been available. Some died on the waiting list, and others survived with their native liver and were removed from the liver transplant list. We can only assume the LT cohort is composed of children who would have lived or died had LT not interrupted the natural course of their disease. Therefore, the outcome of LT and death are not equivalent and should not be combined into one outcome, in an analysis to construct a model to predict death in PALF.
Our findings show that the KCHC does not reliably predict that a child with non-APAP acute liver failure is likely to die if the criteria are met (e.g. low sensitivity and low PPV), but is much more likely to predict spontaneous survival at 21-days if the criteria are not met (e.g. high specificity and PPV). These findings indicate that the KCHC may be useful to determine which patients will ultimately survive, and that one should consider continued supportive care rather than early LT for those patients who do not meet KCH criteria. However, reliance on the KCHC to predict death may lead to an unnecessary LT in a child who may survive without LT. With a PPV of 33%, up to two-thirds of patients who meet criteria are classified as likely to die and, thus, in need of an urgent LT would have survived to 21 days with supportive care. Use of re-optimized cut-points led to improvement in PPV to 50% and NPV to 92%. Although the PPV increased, a value at 50% implies that up to half of the patients who meet criteria may be at unnecessary risk to receive a LT.
There are several reasons why the KCHC may differ in accuracy between adults and children. First, HE is difficult to assess in children and may not even become clinically apparent in PALF 1. This finding has actually led to revision of the definition of ALF in children to exclude HE as a necessary criterion, if the patient has severe, uncorrectable, liver-based coagulopathy 22. Secondly, the KCH also relies on etiology of ALF in determining prognosis. However, the etiology of ALF in children differs greatly from that in adults. In children, 50% of the cases are from an indeterminate cause making it a less discriminate independent variable 22, 23. Furthermore, several etiologies of PALF are rarely or not reported in adult ALF, including metabolic defects and enzyme defects within mitochondria and infectious agents such as EBV, CMV, parvovirus, enteroviruses and herpes viruses 26–28. Finally, none of the 29 children in the original KCHC study were less than age 1 or had metabolic induced liver failure 14.
Ciocca et al also evaluated the KCHC using a cohort entirely of children with PALF. In their retrospective review of 210 patients, they found a PPV and NPV of 96% and 82%, respectively, in predicting mortality and a PPV and NPV of 95% and 82%, in predicting LT 19. Although these findings indicate better prognostic accuracy than ours, there are two important differences between these studies. First, the majority of their patients (61%) had PALF secondary to hepatitis A, and the majority of our patients (45.1%) had PALF secondary to an indeterminate cause 19. This distinction is important as it is easier characterize the natural history of hepatitis A induced PALF, which has a common set of clinical signs and symptoms, due to a single, underlying etiology. In contrast, indeterminate PALF has a number of potential causes including congenital metabolic defects, infections which may not be diagnosed, or medications/toxins which may not be detected, all of which have different clinical presentations 22, 23. In addition, the study by Ciocca et al excluded those younger than 1 year of age, and, in our cohort, 27.4% of the patients were less than 1 year old 19. Exclusion of this patient subset can alter the results of a study validating the KCH model, by eliminating those with the highest percentage of PALF secondary to inborn errors of metabolism, and by excluding those in whom it is most difficult to characterize HE. Given these differences in study design, the findings of the study by Ciocca et al may not be applicable to the broad and heterogeneous population of PALF patients represented by our cohort.
The primary limitation of our study was that information was recorded beginning on the date of enrollment into the study, as opposed to the date of admission. It is possible that certain laboratory values may have improved compared with on admission, due to supportive management. In particular, the INR is subject to improvement with administration of fresh frozen plasma or vitamin K. Therefore it is possible that some patients who would have fulfilled KCHC, based on having an elevated INR on admission, may have been incorrectly categorized due to improvement in the INR at the time of study enrollment. Additional limitations of the study include reliance on variables such as grade of encephalopathy and presence of jaundice, which are based on the subjective opinion of the physician, and incorporating prothrombin time into the reoptimized model because prothrombin time may vary among different centers29.
In summary, given the shortage of donor organs and the life-altering nature of liver transplantation, we need an accurate model to determine prognosis in PALF patients. The KCHC does have utility in predicting survival in both its original form and with redefined cutpoints. However, it cannot be relied upon to predict death, as it may lead to over-utilization of LT in patients who would otherwise survive despite meeting criteria. Though redefining the KCHC parameters improves the PPV, unnecessary transplantation may still occur in half of the patients who meet KCH criteria. We recommend further investigation with incorporation of a dynamic set of parameters into future models, as it is unlikely that an algorithm from a single time point will sufficiently predict survival or mortality.
Table 3.
Outcomes data for individual King’s College Hospital Criteria parameters applied to PALF cohort
| King’s College Hospital Criteria Parameter | Total (n=522) |
Met criteria | Death* | PPV | NPV | SENSITIVITY | SPECIFICITY |
|---|---|---|---|---|---|---|---|
| 1.a) INR > 6.5 or prothrombin time > 100 s | 521 | n=28 | n=8 | 29% | 80% | 7% | 95% |
| 1.b) INR > 3.5 or prothrombin time > 50 s | 521 | n=119 | n=45 | 38% | 84% | 41% | 82% |
| 2.a) Age < 10 years old | 522 | n=390 | n=85 | 22% | 81% | 77% | 26% |
| Age < 1 year old | 522 | n=192 | n=49 | 26% | 82% | 45% | 65% |
| 2.b) Etiology not hepatitis A or B (can be indeterminate) |
522 | n=515 | n=110 | 21% | 100% | 100% | 2% |
| Duration of jaundice to encephalopathy 2.c) > 7 days |
390 | n=22 | n=7 | 32% | 86% | 12% | 95% |
| 2.d) Serum bilirubin > 17.6 mg/dl | 502 | n=100 | n=26 | 26% | 80% | 25% | 81% |
| Number meeting KCH criteria: (INR > 6.5 or prothrombin time > 100 s) OR meet any of three criteria (1.b, 2.a, 2.b, 2.c, 2.d) Number not meeting criteria Number unknown whether criteria were met |
452 | n=163 n=289 n=70 |
n=54 | 33% | 88% | 61% | 70% |
Death among those who met each individual criteria.
Acknowledgments
Supported by National Institutes of Health (U01-DK072146-05, UL1 RR02501-04, UL1 RR024131, UL1 RR024153) and the General Clinical Research Centers Program, National Center for Research Resources, NIH (MO1 RR00069 and MO1 RR08084).
Abbreviations
- PALF
pediatric acute liver failure
- LT
liver transplantation
- KCHC
King’s College Hospital criteria
- APAP
acetaminophen
- PPV
positive predictive value
- NPV
negative predictive value
- CART
classification and regression tree
- INR
international normalized ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Durand P, Debray D, Mandel R, Baujard C, Branchereau S, Gauthier F, et al. Acute liver failure in infancy: a 14-year experience of a pediatric liver transplantation center. J Pediatr. 2001;139:871–876. doi: 10.1067/mpd.2001.119989. [DOI] [PubMed] [Google Scholar]
- 2.Russell GJ, Fitzgerald JF, Clark JH. Fulminant hepatic failure. J Pediatr. 1987;111:313–319. doi: 10.1016/s0022-3476(87)80446-8. [DOI] [PubMed] [Google Scholar]
- 3.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 4.Psacharopoulos HT, Mowat AP, Davies M, Portmann B, Silk DB, Williams R. Fulminant hepatic failure in childhood: an analysis of 31 cases. Arch Dis Child. 1980;55:252–258. doi: 10.1136/adc.55.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devictor D, Desplanques L, Debray D, Ozier Y, Dubousset AM, Valayer J, et al. Emergency liver transplantation for fulminant liver failure in infants and children. Hepatology. 1992;16:1156–1162. [PubMed] [Google Scholar]
- 6.Ee LC, Shepherd RW, Cleghorn GJ, Lewindon PJ, Fawcett J, Strong RW, et al. Acute liver failure in children: A regional experience. J Paediatr Child Health. 2003;39:107–110. doi: 10.1046/j.1440-1754.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 7.Rivera-Penera T, Moreno J, Skaff C, McDiarmid S, Vargas J, Ament ME. Delayed encephalopathy in fulminant hepatic failure in the pediatric population and the role of liver transplantation. J Pediatr Gastroenterol Nutr. 1997;24:128–134. doi: 10.1097/00005176-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Soltys KA, Mazariegos GV, Squires RH, Sindhi RK, Anand R. Late graft loss or death in pediatric liver transplantation: an analysis of the SPLIT database. Am J Transplant. 2007;7:2165–2171. doi: 10.1111/j.1600-6143.2007.01893.x. [DOI] [PubMed] [Google Scholar]
- 9.Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures with acetaminophen: hepatoxicity after multiple doses in children. J Pediatr. 1998;132:22–27. doi: 10.1016/s0022-3476(98)70479-2. [DOI] [PubMed] [Google Scholar]
- 10.Riordan SM, Williams R. Mechanisms of hepatocyte injury, multiorgan failure, and prognostic criteria in acute liver failure. Semin Liver Dis. 2003;23:203–215. doi: 10.1055/s-2003-42639. [DOI] [PubMed] [Google Scholar]
- 11.Bucuvalas JC, Alonso E, Magee JC, Talwalkar J, Hanto D, Doo E. Improving long-term outcomes after liver transplantation in children. Am J Transplant. 2008;8:2506–2513. doi: 10.1111/j.1600-6143.2008.02432.x. [DOI] [PubMed] [Google Scholar]
- 12.Truong DQ, Bourdeaux C, Wieers G, Saussoy P, Latinne D, Reding R. The immunological monitoring of kidney and liver transplants in adult and pediatric recipients. Transpl Immunol. 2009;22:18–27. doi: 10.1016/j.trim.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 14.O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 15.Yantorno SE, Kremers WK, Ruf AE, Trentadue JJ, Podesta LG, Villamil FG. MELD is superior to King's college and Clichy's criteria to assess prognosis in fulminant hepatic failure. Liver Transpl. 2007;13:822–828. doi: 10.1002/lt.21104. [DOI] [PubMed] [Google Scholar]
- 16.Shakil AO, Kramer D, Mazariegos GV, Fung JJ, Rakela J. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6:163–169. doi: 10.1002/lt.500060218. [DOI] [PubMed] [Google Scholar]
- 17.Anand AC, Nightingale P, Neuberger JM. Early indicators of prognosis in fulminant hepatic failure: an assessment of the King's criteria. J Hepatol. 1997;26:62–68. doi: 10.1016/s0168-8278(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 18.Bernal W, Wendon J, Rela M, Heaton N, Williams R. Use and outcome of liver transplantation in acetaminophen-induced acute liver failure. Hepatology. 1998;27:1050–1055. doi: 10.1002/hep.510270421. [DOI] [PubMed] [Google Scholar]
- 19.Ciocca M, Ramonet M, Cuarterolo M, Lopez S, Cernadas C, Alvarez F. Prognostic factors in paediatric acute liver failure. Arch Dis Child. 2008;93:48–51. doi: 10.1136/adc.2006.115113. [DOI] [PubMed] [Google Scholar]
- 20.Bailey B, Amre DK, Gaudreault P. Fulminant hepatic failure secondary to acetaminophen poisoning: a systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med. 2003;31:299–305. doi: 10.1097/00003246-200301000-00048. [DOI] [PubMed] [Google Scholar]
- 21.Lebel S, Nakamachi Y, Hemming A, Verjee Z, Phillips MJ, Furuya KN. Glycine conjugation of paraaminobenzoic acid (PABA): a pilot study of a novel prognostic test in acute liver failure in children. J Pediatr Gastroenterol Nutr. 2003;36:62–71. doi: 10.1097/00005176-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Squires RH, Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Squires RH., Jr Acute liver failure in children. Semin Liver Dis. 2008;28:153–166. doi: 10.1055/s-2008-1073115. [DOI] [PubMed] [Google Scholar]
- 24.Liu E, MacKenzie T, Dobyns EL, Parikh CR, Karrer FM, Narkewicz MR, et al. Characterization of acute liver failure and development of a continuous risk of death staging system in children. J Hepatol. 2006;44:134–141. doi: 10.1016/j.jhep.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 26.Lee WS, Kelly DA, Tanner MS, Ramani P, de Ville de Goyet J, McKiernan PJ. Neonatal liver transplantation for fulminant hepatitis caused by herpes simplex virus type 2. J Pediatr Gastroenterol Nutr. 2002;35:220–223. doi: 10.1097/00005176-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Abzug MJ. Prognosis for neonates with enterovirus hepatitis and coagulopathy. Pediatr Infect Dis J. 2001;20:758–763. doi: 10.1097/00006454-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Wang SM, Liu CC, Yang YJ, Yang HB, Lin CH, Wang JR. Fatal coxsackievirus B infection in early infancy characterized by fulminant hepatitis. J Infect. 1998;37:270–273. doi: 10.1016/s0163-4453(98)92076-x. [DOI] [PubMed] [Google Scholar]
- 29.Northup PG, Sundaram V, Fallon MB, Reddy KR, Balogun RA, Sanyal AJ, et al. Hypercoagulation and thrombophilia in liver disease. J Thromb Haemost. 2008;6:2–9. doi: 10.1111/j.1538-7836.2007.02772.x. [DOI] [PubMed] [Google Scholar]