Abstract
Objective
To evaluate the existing literature on psychological, social, and behavioral aspects of chronic hepatitis C viral (HCV) infection and antiviral treatment; provide the state of the behavioral science in areas that currently hinder HCV-related health outcomes; and make recommendations for areas in which clinical psychology can make significant contributions.
Methods
The extant literature on HCV and antiviral therapy was reviewed as related to biopsychosocial factors such as mental health, substance/alcohol use, quality of life, coping, stigma, racial disparities, side effects, treatment adherence, integrated care, and psychological interventions.
Results
For reasons that have not been well elucidated, individuals infected with HCV experience psychological and somatic problems and report poor health-related quality of life. Preexisting conditions, including poor mental health and alcohol/substance use, can interfere with access to and successful completion of HCV treatment. Perceived stigma is highly prevalent and associated with psychological distress. Racial disparities exist for HCV prevalence, treatment uptake, and treatment success. During HCV treatment, patients experience exacerbation of symptoms, treatment side effects, and poorer quality of life, making it difficult to complete treatment. Despite pharmacological advances in HCV treatment, improvements in clinical and public health outcomes have not been realized. The reasons for this lack of impact are multifactorial, but include suboptimal referral and access to care for many patients, treatment-related side effects, treatment nonadherence, and lack of empirically-based approaches.
Conclusions
Biomedical advances in HCV and antiviral treatment have created a fertile field in which psychologists are uniquely positioned to make important contributions to HCV management and treatment.
Keywords: Interferon, Psychosocial, Coping, Adherence, Multidisciplinary
Overview
Chronic hepatitis C viral (HCV) infection is a global health epidemic that has gained recent attention from national and global health organizations (Institute of Medicine, 2010; U.S.DHHS, 2011). HCV slowly attacks the liver, advancing to cirrhosis or liver cancer in 20% of cases, and leads to significant mortality. HCV is transmitted through exposure to contaminated blood, with 60% of cases acquired via injection drug use or blood transfusions administered before 1992; less frequently, transmission occurs through occupational exposure, hemodialysis, and vertical transmission, and rarely through sexual contact (Alter et al., 1999; Armstrong et al., 2006). The interplay amongst several biopsychosocial factors, antiviral treatment, and health outcomes is extremely complex and warrants attention from clinical psychologists. This article reviews evidence for the role that psychological, social, and behavioral factors play in HCV health outcomes and the implications this has for future research initiatives to optimize HCV care and treatment. As a narrative review, and not a systematic or meta-analytic review, limitations such as the potential for bias are noted.
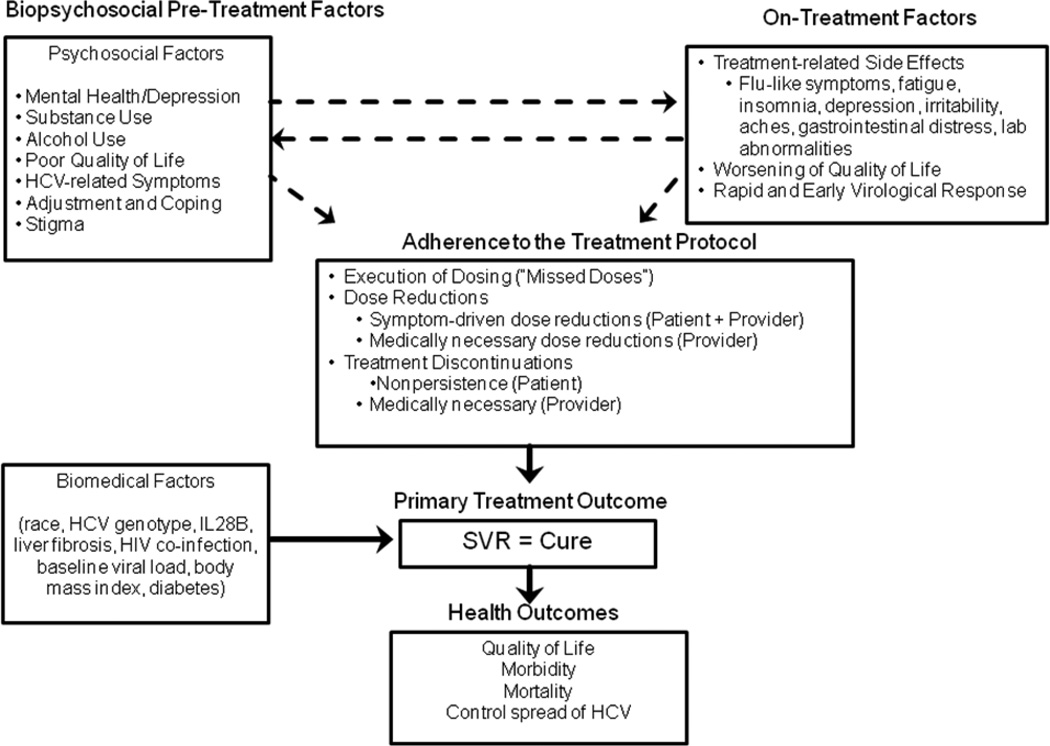
Figure 1 provides an overview of biopsychosocial factors that, if present before and during treatment, may affect HCV outcomes. A high prevalence of premorbid psychological conditions and alcohol/substance use often exist for years prior to HCV diagnosis. Due to these conditions, patients are often deferred from HCV treatment due to concerns that treatment will worsen preexisting psychological conditions or that such preexisting comorbidities will interfere with treatment adherence. Some individuals with HCV report multiple pre-treatment somatic and psychological symptoms, including fatigue, insomnia, depression, and bodily pain.
Figure 1.
HCV and Antiviral Treatment Conceptual Model
Patients often struggle to adjust to and cope with HCV, HCV-related stigma, and a lower health-related quality of life (HrQOL) relative to the general population (Bondini et al., 2007; Foster, Goldin, & Thomas, 1998; Spiegel et al., 2005). Clinical observations suggest a bi-directional relationship between the existence of biopsychosocial factors before treatment and the emergence of side effects and lower HrQOL during treatment. Biopsychosocial factors may influence the ability to cope with treatment side effects, while such side effects exacerbate preexisting symptoms and further impair precarious health. Moreover, protease inhibitor medications, recently approved to treat HCV, require patients to follow an intensive dosing schedule that can lead to viral resistance if not followed closely. Preexisting and treatment-induced conditions can hinder adherence to the treatment protocol: patients may miss doses, require dose reductions, or discontinue treatment prematurely, each of which impedes virologic cure. Conversely, eradicating HCV improves HrQOL, work productivity, disease control, and reduces morbidity and mortality (Ikegami et al., 2009; McHutchison et al., 2001; Spiegel et al., 2005; U.S.DHHS, 2011).
Empirically-based practices are needed to help patients access and complete this potentially life-saving treatment. Researchers with expertise in mental health, alcohol/substance use, coping skills, HrQOL, stigma, and health behavior change can improve knowledge of the complex interplay between biopsychosocial factors and HCV health outcomes. Such knowledge can inform development and evaluation of interventions to enhance coping skills, disease- and side-effect management, and medication adherence to improve health outcomes. To delineate these potential contributions, implications for psychosocial and behavioral research which are likely to produce significant gains in HCV outcomes, are provided throughout and at the end of the paper.
HCV Epidemiology and Impact on Public Health
Chronic HCV is a global public-health problem affecting 130 million people worldwide, including over 3.2 million Americans (Armstrong et al., 2006). HCV-related cirrhosis and liver cancer lead to 10,000 deaths each year in the U.S., and are the leading indications for liver transplant (Shepard, Finelli, & Alter, 2005). HCV also causes extrahepatic conditions, such as autoimmune syndromes of the kidneys, skin, mouth, eyes, and musculoskeletal system. A fourfold increase in the prevalence of HCV complications by 2030 is expected due to aging of a large number of infected Baby Boomers (Davis, Alter, El-Serag, Poynard, & Jennings, 2010). These projections suggest that HCV will lead to significant public health and economic burdens over the next 20 years unless more individuals successfully undergo antiviral treatment.
Antiviral Treatment for HCV
The goal of antiviral therapy is to permanently eradicate HCV. A person is considered cured if they achieve a sustained virologic response (SVR), defined as undetectable HCV RNA six months post-treatment (Ghany, Strader, Thomas, & Seeff, 2009). As Figure 1 illustrates, achieving SVR is the primary outcome of treatment and is associated with improvements in HrQOL (vitality, social functioning, health distress, HCV-specific distress), fatigue, work functioning and productivity, reduced liver inflammation, prevention of disease progression, improved survival, and control of the spread of HCV (Backus et al., 2011; Ikegami et al., 2009; McHutchison et al., 2001; Spiegel et al., 2005; World Health Organization, 1999).
Interferon-based antiviral regimens have been approved for HCV for 15 years. Until 2011 the treatment of choice was a combination of injectable pegylated interferon (IFN) and oral ribavirin (RBV) for 24 or 48 weeks, depending on genotype (i.e., viral strain). IFN is an endogenous proinflammatory cytokine that is naturally produced by the body in response to infection and inflammation; when given exogenously, it mimics the body’s activation of the immune system. Proinflammatory cytokines, including those naturally occurring as well as used in medical treatments, are associated with neuropsychiatric symptoms in humans and sickness behavior in animals(Raison, Demetrashvili, Capuron, & Miller, 2005b). During treatment for HCV, patients self-inject IFN weekly and take RBV tablets twice daily. Patients infected with genotype 1 HCV (75%) required 48 weeks of treatment for a 40–50% chance of SVR, while patients infected with genotype 2 or 3, required 24 weeks of treatment for a 70–85% chance of SVR (Fried et al., 2002).
In 2011, two protease inhibitors were approved for use in combination with IFN/RBV for patients with genotype 1. This “triple therapy” regimen boasts SVR rates of 68–75%, a substantial improvement over SVR rates during IFN/RBV therapy (Jacobson et al., 2011; Poordad et al., 2011). Moreover, treatment duration may be decreased from 48 to 24 weeks. Like the protease inhibitors introduced for HIV 15 years ago, triple therapy presents patients with new challenges for medication adherence (i.e., increased toxicity, more frequent dosing, and development of viral resistance under conditions of suboptimal adherence). Triple therapy is the current standard of care for patients with genotype 1 and will remain as such for several years.
Since approval of triple therapy last year, new oral drugs, including “IFN-free” multi-drug regimens, have been in development. In the future, such regimens may hopefully lead to curing more people, although as we have learned from the fight against HIV, behavioral, psychosocial, and environmental factors may ultimately be the issues that thwart worldwide obliteration of HCV.
Biopsychosocial Pre-Treatment Factors
As Figure 1 depicts, psychosocial factors before treatment have a bi-directional relationship with on-treatment factors, and can also directly affect adherence to the treatment protocol, which, in turn, affects SVR. Several biomedical factors also directly affect SVR.
Premorbid Mental Health and Substance and Alcohol Use Issues
Mental health and substance/alcohol abuse represent the most significant patient-level barriers to optimal HCV care. Premorbid psychiatric and substance use disorders are highly prevalent in the HCV population, with a 40% one-month prevalence and greater than 80% lifetime prevalence (Dwight et al., 2000; El-Serag, Kunik, Richardson, & Rabeneck, 2002). These conditions can adversely affect diagnosis, referral, eligibility, and treatment outcomes (Ho et al., 2001; Leutscher et al., 2010; Volk, 2010). Patients with these conditions are less likely to engage in, or have less access to, routine healthcare where HCV screening and diagnosis occur (Evon et al., 2010). Those with active psychiatric and addiction disorders may be less likely to be referred to a specialist at diagnosis (Zickmund, Brown, & Bielefeldt, 2007). Even if such referrals are issued, these comorbidities are the most common reasons HCV specialists defer treatment (Evon et al., 2007). While several barriers to HCV care exist, improving mental health and reducing alcohol/substance use are fundamental paths through which psychological interventions can enhance HCV health outcomes. While barriers to HCV treatment are being removed, practice guidelines would gain considerably more traction if HCV care was integrated with mental health/substance use protocols (Evon et al., 2011). Healthcare reform, and support from funding agencies and insurance companies, is needed to support the practice and evaluation of empirically-based integrated care approaches.
Premorbid mental health issues
The broad term “mental health” is deliberately used here to encompass psychiatric disorders, as well as the frequently observed emotional distress of HCV patients, which cannot be fully appreciated by a mere diagnosis. Though accurate psychiatric diagnoses are also important, significant emotional distress observed by providers in the clinical setting is just as likely to influence decisions regarding treatment candidacy.
Patients with HCV not on treatment report many emotional disturbances, including depressive symptoms (70%) and irritability (74%) (Lang et al., 2006). A quarter of HCV patients meet diagnostic criteria for current major depression, 36% meet criteria for lifetime depression, and up to 70% report some level of depression (Dwight et al., 2000; Golden, O'Dwyer, & Conroy, 2005; Raison et al., 2005b). Anxiety, irritability, and hypomania have not been studied as systematically, but are important to identify and stabilize before treatment to prevent management challenges that can result in terminating treatment (Lotrich, Ferrell, Rabinovitz, & Pollock, 2010; Rifai, 2006; Russo et al., 2005).
Based on concerns that IFN treatment for HCV could worsen psychiatric symptoms, initial treatment guidelines discouraged treatment for patients with unstable psychological conditions. However, based on ample evidence, the guidelines were eventually modified to indicate that treatment “can be safely administered, provided there is comprehensive pretreatment psychiatric assessment, a risk benefit analysis, and provisions for ongoing follow-up of neuropsychiatric symptoms during antiviral therapy by a multidisciplinary team” (Ghany et al., 2009, p. 1362). Clearly, the development and evaluation of multidisciplinary treatment protocols are needed to achieve these practice guidelines.
Premorbid substance use
Many individuals with HCV have lifetime histories of substance use disorders (36– 66%) (Edlin, 2002). Intravenous drug use is the primary mode of acquisition, with 70–90% of current injection drug users estimated to be infected (Edlin, 2002; Sulkowski & Thomas, 2005). Observational studies find that illicit drug users can safely undergo and adhere to the treatment regimen and achieve good SVR rates, as long as treatment is delivered within multidisciplinary or integrated care settings (e.g., methadone clinics) (Dore et al., 2010; Grebely et al., 2010; Sylvestre et al., 2004; Sylvestre & Clements, 2007; Zanini, Covolo, Donato, & Lanzini, 2010). Notably, a meta-analysis found that multidisciplinary teams were more efficacious when psychologists (as opposed to psychiatrists or addiction specialists) were part of the team(Zanini et al., 2010). This finding highlights the need to identify the specific strategies employed by multidisciplinary teams that are associated with treatment success.
Current HCV guidelines encourage careful consideration of the risks and benefits of treating active illicit drug users, and state that ideally “HCV management is integrated with addiction treatment and delivered by multidisciplinary teams, including experienced drug abuse and psychiatric counseling services” (Ghany et al., 2009, p. 1361). In theory, the guidelines do not view active drug use as an absolute contraindication to treatment; however, in reality, very few active drug users undergo HCV treatment. Patient characteristics (e.g., low perceived severity) and social factors (e.g., unstable housing) are associated with nontreatment of drug users (Lally, Montstream-Quas, Tanaka, Tedeschi, & Morrow, 2008; Mehta et al., 2007). Provider-level beliefs and attitudes likely play an important role in treatment decision-making, but have yet to be investigated.
Several gaps in current knowledge require the attention of behavioral and social scientists. Studies have not distinguished outcomes among subgroups of drug users, even among those who inject drugs, snort cocaine, or smoke marijuana. Moreover, specific patient or drug use characteristics (e.g., type or frequency) that predict poor treatment outcomes have rarely been identified (Sylvestre & Clements, 2007). Finally, the efficacy of multidisciplinary treatment models or psychological treatments for HCV+ drugs users have not been formally tested in clinical trial methodology; therefore, no empirically-based protocols exist. As such, practice guidelines and evidence-based, tailored strategies for different subgroups are not available to guide the selection and management of reasonable treatment candidates. These gaps in the literature result in nontreatment of a vast majority of individuals, many of whom may be capable of safely adhering to the treatment regimen. Recent studies indicate that providing HCV treatment to injection drug users is highly cost-effective; however a lack of multidisciplinary treatment infrastructure to engage and treat these individuals contributes to nontreatment(Martin et al., 2012). Extensive healthcare and policy reform, including innovative treatment delivery protcols, will be needed to expand treatment to those with addiction comorbidities.
Premorbid alcohol use
Alcohol use and HCV often coexist, with a prevalence of HCV up to 30 times higher in persons with alcohol use disorders (Anand et al., 2006; Dieperink et al., 2010; Singal & Anand, 2007). Excess alcohol is an independent risk factor for development and progression of liver disease, works synergistically with HCV to advance liver disease, and allows HCV to replicate more rapidly (Nishiguchi et al., 1991; Poynard, Bedossa, & Opolon, 1997; Romero-Gomez et al., 2001; Singal & Anand, 2007; Wiley, McCarthy, Breidi, McCarthy, & Layden, 1998). Excess alcohol consumption during treatment is associated with lower SVR rates owing to immunosuppression or nonadherence (Anand et al., 2006; Singal & Anand, 2007).
The amount of alcohol that is safe for persons with HCV remains controversial. Moderate to heavy daily consumption is widely considered unsafe, is associated with liver damage, and reduces treatment efficacy (Ghany et al., 2009; Pessione et al., 1998; Westin et al., 2002). However, mild alcohol use (<30 grams/day) may not be associated with advancing liver disease or worse SVR rates (Anand et al., 2006; Bruggmann, Dampz, Gerlach, Kravecz, & Falcato, 2010; Cheung et al., 2011). Hence, recommendations for alcohol abuse counseling for heavy alcohol consumers are empirically-driven, but those for lighter drinkers are not, until more data are obtained. The lack of data and the consequent vagueness of practice guidelines leads to idiosyncratic physician decisions and mixed messages for patients (Blixen et al., 2008). Interventions to reduce excess alcohol consumption are needed. Brief physician-delivered protocols or co-location of addiction counselors in liver clinics show promise (Dieperink et al., 2010; Proeschold-Bell et al., 2011), but efficacy and cost-effectiveness have not been evaluated.
Health-related Quality of Life and HCV-related Symptoms
HCV is associated with significant reductions in patients’ health-related quality of life (HrQOL) compared to the general population, and even to patients with hepatitis B (Bondini et al., 2007; Foster et al., 1998; Spiegel et al., 2005). Most studies have used the SF-36 health survey to measure functional health status, defined as the extent to which patients can perform normal daily activities without interference from health problems(McHutchison et al., 2001; Spiegel et al., 2005; Ware, Jr., Bayliss, Mannocchia, & Davis, 1999). Studies generally find that the largest mean differences between HCV patients and the general population are on vitality, role-physical, role-emotional, and general health scales, as well as on the mental composite score (Spiegel et al., 2005). The etiology of HrQOL disparities is complex and not well understood. HrQOL is not related to liver disease severity (except in those with cirrhosis); however it has been associated with HCV-related symptoms (e.g., fatigue, depression), comorbid conditions, perceived stigma, or knowledge of having HCV (Dan et al., 2006; Fontana et al., 2005; Foster et al., 1998; Kallman et al., 2007; Lang et al., 2006; Younossi, Kallman, & Kincaid, 2007; Zickmund, Ho, Masuda, Ippolito, & LaBrecque, 2003). The effect of drug abuse history on HrQOL is equivocal (Foster et al., 1998). Fortunately, eradication of HCV through antiviral therapy is associated with improvements in HrQOL (e.g., vitality, social functioning, health distress, work functioning, and productivity)(McHutchison et al., 2001; Ware, Jr. et al., 1999).
HCV-related Stigma
Societal stigma has been defined as a “mark” that is deeply discrediting and ruins “the marked person’s normal identity” (Goffman, 1963). Societal stigma can exist for many human conditions, but infectious diseases (e.g., HIV), mental illness, drug addiction, and alcoholism carry particularly strong societal stigma (Paterson, Backmund, Hirsch, & Yim, 2007). By association, HCV has great potential to be a highly stigmatized illness.
The HIV Stigma Framework provides a conceptual model that can be applied to HCV to understand how individuals experience stigma and the mechanisms by which societal stigma affects HCV outcomes (Earnshaw & Chaudoir, 2009). The model suggests that societal stigma elicits stigma mechanisms, which, in turn, can have deleterious outcomes. Stigma mechanisms are the ways in which individuals react psychologically to knowledge that they possess the “mark” and include: (a) enacted stigma (experience of prejudice and discrimination); (b) anticipated stigma (expectations of future prejudice and discrimination); and (c) internalized stigma (endorsement of negative beliefs and feelings about themselves). These stigma mechanisms, in turn, may negatively affect psychological, behavioral, and social outcomes.
A review of 21 mostly qualitative studies on HCV-related stigma suggests that 22–100% of study participants perceived stigma related to HCV (Paterson et al., 2007). Moreover, most authors concluded that this stigma is due to association with drug use, alcoholism, HIV, sexually transmitted diseases, and sexual promiscuity (Butt, 2008; Butt, Paterson, & McGuinness, 2008; Sgorbini, O'Brien, & Jackson, 2009; Zickmund et al., 2003). Importantly, while patients report HCV-related stigma during interactions with healthcare workers, family, and co-workers (Butt, 2008; Paterson et al., 2007; Richmond, Dunning, & Desmond, 2004), only one study has evaluated actual stigmatization toward people with HCV(van de Mortel, 2002).
Evidence from HCV stigma studies are consistent with the HIV Stigma Framework, which purports that enacted, anticipated, and internalized stigma are mechanisms by which societal stigma affects outcomes. For example, anticipated HCV stigma leads to frequent worry about HCV “being discovered” (Schafer, Scheurlen, Felten, & Kraus, 2005). Up to 25% of patients report nondisclosure of HCV to friends, and 55% report nondisclosure to physicians. Patients also report internalized stigma such as shame (66%) and insecurity (63%), and fear of contaminating others leads to unnecessary behaviors like not sharing drinking cups or food, and inhibiting dating or sexual activity (Zacks et al., 2006). Finally, stigma is associated with poorer psychological well-being (i.e., depression, anxiety, anger, difficulty coping, feelings of loss of control) (Golden, Conroy, O'Dwyer, Golden, & Hardouin, 2006; Zacks et al., 2006; Zickmund et al., 2003). Future studies would benefit from applying theoretical models like the HIV Stigma Framework to investigate moderators, mediators, and consequences of HCV-related stigma.
Appraisal of and Coping with HCV
As a stigmatized disease with psychological and social ramifications and an uncertain prognosis, HCV has the potential to be an extremely stressful illness. According to Lazarus and Folkman’s cognitive appraisal theory, individuals diagnosed with HCV would first assess the threat or harm of HCV (primary appraisal), and then the availability and effectiveness of internal and external resources to aid coping (secondary appraisal) (Lazarus & Folkman, 1984).
Very few substantive articles address coping with HCV. Although roughly 25 articles exist, most are case studies, editorials, or qualitative studies that are not amenable to statistical analysis and interpretation. Eight cross-sectional survey studies have described elements of appraisal or coping, but were ancillary to primary study aims. Nevertheless, broad themes that emerge from existing data can serve to generate hypotheses for more methodologically rigorous studies in HCV coping.
The extant literature suggests that individuals diagnosed with HCV appraise the illness as quite threatening (Blacklaws et al., 2009; Grassi et al., 2002; Sgorbini et al., 2009; Sutton & Treloar, 2007). In one study (N=185), patients appraised HCV as a “74” out of 100 on a disease severity scale (Constant et al., 2005). The appraisal of HCV may be moderated by several variables that require further scrutiny: (a) age (e.g., older age may be associated with worse illness perceptions); (b) history of injection drug use may be associated with less perceived threat; (c) personality traits (e.g., sense of coherence and attentional style are associated with coping styles); (d) gender (e.g., women may appraise HCV as more threatening due to fear of transmission to family); and (e) co-infection (e.g., men who have sex with men may view HCV as more threatening than HIV due to stigma and may be less willing to disclose HCV status) (Erim et al., 2010; Grundy & Beeching, 2004; Miller, Rodoletz, Mangan, Schroeder, & Sedlacek, 1996; Owen, 2008).
Patients with HCV may employ a variety of coping strategies. One study (N=264) found that the most used coping strategies were behaviorally-oriented (70%), followed by engagement in medical care (62%), and finally by cognitive and religious coping strategies (34%) (Stoller et al., 2009). People who coped behaviorally reported changing diets (44%), activity-rest cycles (17%), and hygiene practices (12%). Cognitive strategies included acceptance, compartmentalization, and downward social comparisons. Other studies report use of prayer and forms of relaxation (Kinder, 2009; Richmond, Bailey, Jr., McHutchison, & Muir, 2010). Coping strategies may be moderated by a number of factors. Older patients may be more likely to use religious coping; patients recently diagnosed with HCV may engage in more active, problem-solving coping compared to those diagnosed longer ago; and those with more advanced liver disease may use less adaptive coping than those with less advanced disease (Kraus, Schafer, Csef, Scheurlen, & Faller, 2000; Miller et al., 1996). With the exception of one quantitative study, no studies have examined the relationship between coping strategies and psychosocial outcomes (Richmond et al., 2010). As such, quantitative studies, especially those utilizing reliable and validated psychological instruments to strengthen internal validity, are needed.
Our clinical experience mirrors findings that patients actively cope by making positive lifestyle changes. In addition to quitting alcohol and drugs, it is common for patients to eat healthier, use herbal supplements, quit smoking, increase activity, and reprioritize life goals (Paterson, Butt, McGuinness, & Moffat, 2006; Sgorbini et al., 2009; Sutton & Treloar, 2007). As such, receiving a diagnosis of HCV and preparing for treatment may thus serve as potent “teachable moments”—naturally occurring health events that motivate people to make positive lifestyle changes (McBride, Emmons, & Lipkus, 2003).
Racial Disparities in HCV Prevalence, Treatment Uptake and Treatment Efficacy
It is important for clinical psychologists working with HCV patients to appreciate significant racial health disparities that exist between African Americans (AAs) and Caucasian Americans (CAs). HCV is 2–3 times more prevalent among AAs (3.2%) compared to CAs (1.5%), yet AAs are much less likely to be referred for specialty HCV care (32% vs. 71%, respectively) (Alter et al., 1999; Armstrong et al., 2006; Kanwal et al., 2007; Kramer et al., 2010; Trooskin et al., 2007). Even in clinical drug trials which provide medical treatment at no expense, less than 10% of participants are AA (Fried et al., 2002; McHutchison et al., 2009b; McHutchison et al., 2009a). While preexisting medical conditions (e.g., neutropenia, diabetes, renal insufficiency) may partially explain why AAs are excluded from drug trials (Melia et al., 2011), psychosocial factors may also influence racial differences in screening, referral, and treatment uptake, including those at the patient (e.g., health beliefs, medical mistrust), provider (e.g., knowledge, experience) and societal (e.g., access to care, insurance status) levels, but these have rarely been explored (Kanwal et al., 2007; Kramer et al., 2010). Moreover, preexisting medical conditions that exclude AAs from treatment (e.g., diabetes) are modifiable through behavioral changes which could, in turn, enhance HCV treatment eligibility and success for AAs.
Most important, AAs who undergo HCV treatment have much lower rates of SVR (20–28%) compared to CAs (40–55%) (Conjeevaram et al., 2006; Fried et al., 2002; Muir et al., 2011). Recently, the genetic polymorphism IL28B, a marker of IFN sensitivity, was found to explain about half of the racial differences in SVR (Ge et al., 2009; Thompson et al., 2010). Other variables that have been examined—educational status, body mass index, diabetes, and medication adherence—do not account for racial disparities in SVR, and more data are needed (Conjeevaram et al., 2006; Howell et al., 2008).
Health psychologists who care for individuals infected with HCV need to be aware of these disparities to help patients process health beliefs, make informed decisions about treatment, and cope with the disappointment associated with less favorable cure rates for many AAs.
Biomedical Factors
As shown in Figure 1, several biomedical factors are directly associated with treatment outcomes and thus are important for both patients and providers to consider (Conjeevaram et al., 2006; Conjeevaram et al., 2007; Thompson et al., 2010). While several of these variables are immutable, others such as body mass index and diabetes control, are modifiable through health behavior change. Collectively, these biomedical factors may account for over 50% of the variance in treatment outcomes (Ge et al., 2009). Psychosocial factors that could account for some of the remaining variance have not been well studied. Health psychologists working closely with HCV providers can help educate patients about chances of cure, carefully weigh the risks of treatment (e.g., worsening of HrQOL, depression, risk of relapse) with potential benefits, and assist in making lifestyle modifications to increase the odds of being cured.
Another biomedical factor that warrants brief discussion is co-infection with HIV and hepatitis A and B, which can complicate treatment and health outcomes (Ghany et al., 2009). More than 200,000 patients with HIV are also co-infected with HCV (Brau et al., 2002; Singal & Anand, 2009). Liver disease has emerged as a leading cause of HIV-related mortality (Soriano et al., 2011). Antiviral therapy is also less effective in HCV/HIV patients (20–38%) compared to HCV-infected patients (54%), possibly due to higher rates of side effects and premature discontinuations (Cooper, Giordano, Mackie, & Mills, 2010; Singal & Anand, 2009; Sulkowski et al., 2004). HCV treatment uptake is also quite low (10–38%) in HCV/HIV patients, perhaps due to concerns about additional side effects and the challenge of juggling multiple medications (Wagner et al., 2009). These patient concerns could be reviewed in psychologist-patient interactions or through HCV/HIV support or peer-led groups (Grebely et al., 2010).
On-Treatment Factors
Treatment-related Side Effects
IFN-based treatment is well known to induce flu-like, neuropsychiatric, gastrointestinal and other treatment-related side effects: fatigue (55%), headaches (50%), bodily pain (45%), depressive symptoms (20–40%), insomnia (38%), and irritability (26%) (Bonaccorso et al., 2001; Fried, 2002; Fried et al., 2002). Depression is the most concerning side effect due to the high incidence of premorbid depression, potential for worsening during treatment, and though rare, has been associated with suicide during treatment (Dieperink et al., 2004). Depression is also one of the leading causes of treatment discontinuations and dose reductions (Fried, 2002; Fried et al., 2002; McHutchison, Bacon, & Owens, 2007). Medically-necessary treatment discontinuations also occur for anemia, neutropenia, and other medical conditions (Fried et al., 2002).
Many treatment-related side effects may be amenable to treatment by psychological interventions in three ways. First, early somatic/neurovegetative symptoms (fatigue, insomnia, aches and pains, headaches) seem to precede and predict changes in cognitive-affective depressive symptoms occurring later in treatment (Robaeys et al., 2007; Wichers, Koek, Robaeys, Praamstra, & Maes, 2005). Thus, early psychological interventions aimed at coping with somatic symptoms may reduce the development of full-blown major depression later in treatment. Second, because many patients report preexisting symptoms that are exacerbated during treatment, psychological interventions before HCV treatment may improve symptom management before and during treatment, leading to better outcomes. Finally, our clinical experience suggests that patients complain of mild to moderate symptom clusters (e.g., fatigue-insomnia-depression-bodily pain) that take an insidious toll on life functioning, as opposed to one severe side effect. This clinical observation is consistent with studies in other medical populations that have observed a pain-depression-fatigue symptom cluster and has been associated with elevations in neuroendocrine markers (Thornton, Andersen, & Blakely, 2010). The recent interest in symptom clustering associated with hyperarousal of the sympathetic nervous system and hypothalamic-pituitary-adrenal axis dovetails nicely with psychoneuroimmunological research in HCV exploring interferon’s role as a proinflammatory cytokine in induction of the inflammatory response system and manifestation of sickness behavior by patients during HCV treatment(Charlton, 2000; Maes, 2011; Raison et al., 2005a; Wichers & Maes, 2002). These findings suggest that pre-treatment interventions aimed at stress management and coping with somatic symptoms could improve psychological and physiological indices of stress, leading to better treatment outcomes.
Worsening of Health-related Quality of Life
HrQOL deteriorates during HCV treatment for reasons that are multifactorial but largely related to treatment-related side effects and poor health status (Younossi et al., 2007). HrQOL may also diminish during treatment for reasons unrelated to side effects. Undergoing HCV treatment can conjure feelings of guilt and shame or the idea that treatment is penance for past indiscretions (Zacks et al., 2006). Patients may attempt to keep treatment a secret from others, which leads to social isolation, or may experience enacted stigma upon disclosure to others (Sgorbini et al., 2009). Patients report difficulties relating to their partners and fulfilling parental duties (Sgorbini et al., 2009). Treatment may cause significant financial burden due to missed work, insurance bills, and travel expenses related to obtaining treatment in a specialty clinic, which could be hours away. Finally, patients tire of the intensive pill burden, injectable medications, and rigorous appointment schedules. These psychosocial factors may contribute to worsening HrQOL during treatment.
Rapid or Early Virological Response
Two robust predictors of SVR are rapid virological response (undetectable virus at treatment week 4) and early virological response (undetectable virus at week 12) (Idrees & Riazuddin, 2009; Rodriguez-Torres et al., 2010; Yu, Wang, Sun, Li, & Li, 2007). Viral load is measured at early clinic visits to aid HCV providers in determining likelihood of cure. Medication adherence is essential throughout treatment, but particularly so during the first 12 weeks of treatment in order to achieve these responses (Lo, III et al., 2009). Health psychologists can assist in psychoeducation about medication adherence, development of adherence strategies, and helping patients cope with the disappointment of not achieving early virological response.
Adherence to the Treatment Protocol
As Figure 1 illustrates, biopsychosocial factors before and during treatment have the potential to reduce adherence to the treatment protocol, which, in turn, lower chances for SVR. “Adherence to the treatment protocol” has historically been defined as the actual amount of medication taken compared to the prescribed amount, for the full duration of treatment. Patients who are maintained on greater than 80% of IFN and 80% of RBV for greater than 80% of the treatment duration have greater probability of achieving SVR (McHutchison et al., 2002). Unfortunately, this 80/80/80 standard does not permit us to differentiate between protocol deviations due to patient behaviors and decisions, and those which are medically-necessary due to serious adverse events and safety concerns. Distinguishing between these deviations would allow us to identify the greatest threats to treatment efficacy and guide the development of precise interventions to overcome them.
Execution of Dosing
How well patients take their HCV medications as prescribed is critical to the success of treatment, but has rarely been the primary outcome of interest in HCV studies. However, studies of patients’ medication-taking behaviors will likely burgeon in the next few years with approval of triple therapy. Dosing protease inhibitors 3–4 times a day, eight hours apart, with 20 grams of fat is such a cumbersome dosing regimen that proper dosing may only occur 51–65% of the time (Claxton, Cramer, & Pierce, 2001).
Execution of dosing is defined as “the extent to which patient actual dosing behavior corresponds to the ideal dosing regimen,” and the term “missed doses” is commonly used to describe the discrepancy between actual and ideal dosing (Urquhart & Vrijens, 2005). The few HCV studies to report on execution of dosing suggest that patients have problems taking their medications, the amount of medication missed increases over time, and may be enough to impede virologic response and SVR (Alam, Stainbrook, Cecil, & Kistler, 2010; Cacoub et al., 2008; Conjeevaram et al., 2006; Fumaz et al., 2007; Lo, III et al., 2009; Smith et al., 2007; Weiss et al., 2008).
Understanding the role of psychological and behavioral factors that affect dosing execution will become paramount, so that interventions can be developed to optimize SVR. Toward this end, execution of dosing should be a primary outcome of treatment studies and should be measured prospectively using objective methods. Patients at risk for missing doses need to be identified. Virtually no studies exist on predictors of missed doses in HCV, so extrapolation from HIV studies seems reasonable. Predictors of medication adherence to HIV regimens operate at the patient (e.g., beliefs about treatment, self-efficacy, depression, substance use, socioeconomic status, cognitive dysfunction), regimen (e.g., number and timing of doses, side effects), provider (e.g., communication skills), and social (e.g., social norms, support) levels (Golin et al., 2002). These same variables may predict missed doses during HCV treatment.
Dose reductions and premature treatment discontinuations
Dose reductions are sometimes necessary to mitigate severe treatment-related side effects but can compromise treatment efficacy. Symptom-driven dose reductions, although generally initiated by the provider, are dose reductions that occur to manage patients’ intolerance of side effects or emotional disturbances that are problematic but not life-threatening (e.g., irritability, tearfulness). Medically-necessary dose reductions initiated by the provider are often out of the patient’s control (e.g., lab abnormalities, virological nonresponse, medical disorders) and should not be counted as acts of patient nonadherence.
There are also situations where treatment is discontinued prematurely, owing to a combination of patient behaviors, patient decisions, and provider decisions. Persistence is a patient-level factor, defined as the total length of time a patient takes a medication, from first dose to last dose (Urquhart & Vrijens, 2005; Vrijens, Vincze, Kristanto, Urquhart, & Burnier, 2008). All noncompliant behaviors or decisions to prematurely stop the regimen that are judged to be under the patients’ control are deemed acts of nonpersistence: choosing to drop out; being lost to follow-up; not complying with physician recommendations; and discontinuing treatment early due to intolerance of unpleasant, but not medically dangerous side effects. In contrast, medically-necessary treatment discontinuations are due to safety issues, are out of the patient’s control, and occur to manage lab abnormalities or medical conditions.
Psychological and Behavioral Intervention Research in HCV Psychological Interventions to Cope with HCV and Treatment-related Side Effects
The management of HCV treatment side effects has been almost exclusively under the purview of medical intervention, which is appropriate for the management of laboratory abnormalities or other medically severe adverse side effects. However, many treatment discontinuations and dose reductions may be due more to patients’ inability to tolerate symptoms that interfere with life functioning. Antidepressant medications have been the most readily available therapeutic option for HCV specialists to manage their patients’ emotional distress. However, the evidence demonstrating the efficacy of antidepressants in ameliorating IFN-induced depression is equivocal (ez-Quevedo et al., 2011; Kraus et al., 2008). Pharmacotherapy also has limitations (e.g., patient reluctance due to side effects, addiction history, liver toxicity) and does not build self-efficacy or coping skills to empower patients to self-manage symptoms.
As with other diseases, health psychologists can play an important role in attenuating decrements in HrQOL, as well as contribute to multidisciplinary disease management. Given that patients experience preexisting clusters of somatic/neurovegetative symptoms in the early weeks of treatment (e.g., fatigue, insomnia, pain, headache) that lead to reduced HrQOL and worsening depression later in treatment, multi-component psychological interventions that treat multiple concurrent symptoms and improve overall coping skills may be highly beneficial. Empirically-proven psychological treatments for other disease symptoms and treatment side effects exist and can be adapted for HCV treatment (Andersen et al., 2004; Antoni, Ironson, & Schneiderman, 2007; Given et al., 2004; Groessl et al., 2011; Safren et al., 2009).
Several intervention frameworks hold promise for adaptation to HCV. Based on a preexisting self-management intervention grounded in social cognitive learning theory, a recent clinical trial found improvements in HCV knowledge, self-efficacy, and vitality/energy in veterans not on treatment who participated in a 6-week self-management group, compared to those who did not (Groessl et al., 2011). Given that many patients cannot or choose not to undergo HCV treatment, self-management programs that facilitate problem-solving skills, day-to-day management of illness, and enhancing social support may be particularly useful for individuals learning to cope with chronic liver disease. Another suitable intervention may be the Cognitive Behavioral Treatment for Depression and Adherence (CBT-AD) model, which combines traditional CBT to treat depression with motivational interviewing and problem-solving skills targeting medication adherence (Safren et al., 2009). The CBT-AD was delivered in individual sessions with HIV+ persons and has been shown to reduce depression post-treatment and 12 months later in patients with refractory depression. Interventions can also be guided by the Cognitive Behavioral Stress Management (CBSM) intervention and the Biobehavioral Intervention for Cancer Stress and Disease (Andersen et al., 2004; Antoni et al., 2007). The CBSM is a 10-session group-based intervention focused on stress reduction and relaxation kills, decreasing negative mood, assertiveness and communication training, and anger management. The Biobehavioral Intervention is also group-based and includes strategies to reduce stress (e.g., progressive muscle relaxation), improve mood (e.g., positive coping, problem-solving), alter health behaviors (diet, exercise, smoking cessation), and maintain treatment adherence (Andersen et al., 2004). The group-based delivery system of these interventions makes them more cost-effective and likely to ameliorate some of the social isolation commonly reported by HCV patients. Given multiple similarities between HCV patients and those with HIV or cancer undergoing medical treatments, these frameworks may be well-suited for adaptation for HCV interventions.
Active ingredients of other behavioral medicine protocols may need to be incorporated into multi-component interventions to specifically target insomnia, headache, and pain. Stimulus control, sleep hygiene, sleep restriction strategies and constructive worry techniques could target insomnia (Edinger et al., 2009). Identification and management of triggers using daily diaries may reduce headaches (Nicholson, Buse, Andrasik, & Lipton, 2011). Flu-like symptoms and join pain may respond to specific pain coping skills like establishing activity-rest cycles and learning attention diversion (Keefe et al., 1990). Helping patients acquire the skills to manage early somatic side effects may preserve HrQOL and thwart the development of major depression.
Integrated, Multidisciplinary Approaches for HCV Care
The Action Plan for Combating the Silent Epidemic of Viral Hepatitis consistently emphasizes the pivotal role of integrated, multidisciplinary approaches to optimize HCV care for patients with substance use and mental health problems (U.S.DHHS, 2011). Integrated models of care are broadly defined as clinical strategies or interventions “intended to improve the coordination of care and communication among caregivers, streamline protocols for movement across the care system, co-locate services, or deploy fully integrated service teams” (Willenbring, 2005) p. S228). Prior studies highlight a few promising models worthy of further investigation. For instance, when HCV treatment is provided within integrated methadone maintenance and infectious disease clinics, illicit drug users are more able to access care, complete treatment, and achieve good SVR rates (Grebely et al., 2010; Sylvestre et al., 2004; Sylvestre & Clements, 2007). Co-locating mental health/addiction specialists within liver clinics is also a viable option. We demonstrated that co-location of a psychologist employing case management and motivational interviewing strategies with patients deferred due to active mental health and substance use issues, improved eligibility for HCV treatment. Participants in the intervention condition were twice as likely (42%) to be approved for treatment by providers compared to those who received usual care (18%)(Evon et al., 2011).
With these ideas being echoed recently by other global and federal healthcare organizations, a major paradigm shift in HCV care is anticipated that will place greater focus and resources on the development, implementation, and evaluation of team-based disease management of HCV. Psychologists’ considerable expertise and knowledge of the behavioral and social sciences are needed at the table. In particular, psychologists can bring their knowledge of implementation sciences as well as expertise in designing, conducting, and evaluating protocols to program development. Guided by useful approaches such as Intervention Mapping (Barthlomew, Parcel, & Kok, 2011), psychologists have much to offer those seeking new and better ways to care for patients with HCV.
Adherence Interventions
Only three observational studies and no clinical trials of HCV adherence interventions have been published (Cacoub et al., 2008; Marino et al., 2009; Sylvestre & Clements, 2007). Patients (N=674) who received “therapeutic education” (support documents, educational material, individual sessions) had higher rates of dosing IFN (78%) and RBV (70%) compared to patients who did not receive education (69% and 56%, respectively) (Cacoub et al., 2008). In another study, 50 patients who received ongoing pharmacist-delivered “psychoeducation” (pharmacological and behavioral side effect management, education, support) had dosing rates of 85.7% (Marino et al., 2009). Finally, 68% of patients receiving HCV treatment in a multidisciplinary peer-assisted methadone clinic were described as medically adherent (Sylvestre & Clements, 2007). These studies provide relevant observations about the usefulness of psychoeducation, pharmacist-delivered education, and multidisciplinary teams. However, no conclusions can be made regarding the efficacy of these interventions due to lack of control groups, and the putative active ingredients of these interventions were not measured. Finally, adherence enhancement interventions for HCV should consider using novel measurement technology and intervention delivery systems that are being tested in other medical populations, such as smart-phone technology, interactive voice response technology, and smart-phone video-capturing of medication-taking behaviors (Hoffman et al., 2009; Schroder, Johnson, & Wiebe, 2007; Swendeman & Rotheram-Borus, 2010).
Research Implications: Where Can Psychology Contribute?
As highlighted throughout this review and exemplified below, clinical psychologists are well-positioned (a) to shed light on covariates related to HCV health and treatment outcomes to enhance knowledge and to inform clinical practice guidelines and intervention development; and (b) to lead the development, implementation, and evaluation of interventions and programs that will allow clinical scientists to establish evidence-based practices and improve access to HCV care and treatment outcomes.
Where there is insufficient knowledge of psychosocial factors (e.g., alcohol use, coping styles, stigma, reasons for missing doses) associated with HCV health and treatment outcomes in order to inform intervention development or clinical practice, there is a compelling need for rigorous observational, cross-sectional, and prospective studies. For instance, improving the quantitative measurement of alcohol consumption, particularly at the mild to moderate levels of use, and associating consumption levels with liver disease severity, progression, and treatment outcomes is greatly needed to establish evidence-based clinical guidelines and tailor interventions for specific cohorts of drinkers. Similarly, the impact of illicit substance use behaviors (i.e., drug type, frequency, duration, quantity) on treatment outcomes is also needed to inform clinical practice, such as helping providers select reasonable treatment candidates. As healthcare providers’ beliefs and attitudes about treating patients with co-occurring alcohol or drug use partly determine who gains access to HCV treatment, understanding provider beliefs and attitudes could inform strategies to improve adherence to national guidelines and empirically-based best practices. Finally, it behooves researchers to measure and evaluate separately factors that decrease adherence to treatment protocols, and to distinguish between medically-necessary and patient-driven factors. Deviations from the protocol that threaten treatment efficacy may be related to different patient characteristics (moderators), occur for different reasons (mechanisms), have different clinical consequences, and therefore will require markedly different intervention tactics.
Where there is a clear and pressing need or sufficient evidence to support intervention research, psychologists can lead the development and evaluation of interventions to improve HCV health and treatment outcomes. Consistent with recent federal agency recommendations, examining integrated and/or multidisciplinary disease management models for patients with comorbid mental health, alcohol, and drug addiction is drastically needed (U.S.DHHS, 2011). In clinical practices where integrated care models are less feasible, programs that train medical providers to deliver brief screening and interventions to reduce alcohol and drug use are being called for (U.S.DHHS, 2011). Studies testing interventions to enhance adherence to HCV treatment regimens and optimize dose-taking behaviors, with an emphasis on identifying mechanisms and use of cutting-edge web-based, telehealth, and smart-phone technology for measurement and intervention delivery, can point to new directions to improve health outcomes.
Psychological interventions to cope with HCV disease and treatment-related side effects are needed. Delivery of a skill-building period prior to initiating HCV treatment, followed by maintenance sessions during treatment, may be more effective in stabilizing premorbid HCV symptoms and improving tolerance of treatment-related side effects. Cognitive and behavioral strategies should target somatic side effects first (e.g., fatigue, insomnia, pain) to stymie transition into later cognitive/affective depressive symptoms and worsening of HrQOL (Robaeys et al., 2007; Wichers et al., 2005). Group-based interventions may be ideal to reduce social isolation, minimize stigma, and build peer support; these nonspecific therapeutic group processes may enhance intervention outcomes. Finally, studies should explore the effects of psychological interventions on physiological markers (e.g., immune functioning, virological response), as well as the cost-effectiveness and sustainability of such interventions.
Concluding Comments
Optimal care for individuals with HCV will entail knowledge of and interventions for infectious disease prevention, screening, management of comorbid psychological and addiction disorders, HrQOL, racial disparities, stigma, access to care, treatment adherence, and disease- and treatment-related side effects. Clinical psychologists can greatly contribute to having an impact on HCV care and treatment in real-world settings by engaging in multidisciplinary team science and clinical practice aimed at reducing the individual, public, and economic burden of HCV. To have such an impact, clinical psychologists need to forge new partnerships with gastroenterologists and hepatologists, many of whom may be unfamiliar with the many ways that clinical health psychologists and behavioral medicine experts can greatly enhance patient care through evidence-based practices. While medical providers may be keenly aware of the inherent psychosocial and behavioral challenges that interfere with optimal HCV management and treatment, they may not realize that clinical psychologists are also behavioral and social scientists, equipped to help them and their patients to better manage symptoms and treatment side effects, cope with stigma, stress, and mental health issues, and make requisite lifestyle changes. Clinical psychologists are encouraged to consider expansion and application of their expertise to the field of hepatology, where only a few from our discipline are currently represented, to develop a new interdisciplinary field of psycho-hepatology which can more broadly focus on biopsychosocial aspects of chronic liver diseases.
Acknowledgements
We would like to thank Drs. Jason Bonner, Suzanne Lechner, Rebecca Shelby, Tamara Somers, Laura Porter, Jeffrey Greeson, Becky White, Jennifer Loftis, Alan Christensen, and the reviewers for their thoughtful feedback during the writing of this manuscript.
Funding Support: This manuscript was supported, in part, by National Institutes of Health Award Number K23DK089004-02 (Evon); a mentoring grant (K24 DK066144-01;Fried), and a Center for Aids Research grant (P30-AI50410; Golin).
Dr. Evon has received research grant support from Roche/Genetech and has served as an ad hoc consultant for Vertex Pharmaceuticals. Dr. Fried has had research grant funding and/or has served as an ad hoc consultant for Roche/Genetech, Vertex, Merck, Tibotec, GlaxoSmithkline, Gilead, Janssen, Novartis, Abbott, Bristol Myers Squibb.
Footnotes
Potential Competing Interests: Drs. Golin and Keefe have nothing to disclose.
Reference List
- Alam I, Stainbrook T, Cecil B, Kistler KD. Enhanced adherence to HCV therapy with higher dose ribavirin formulation: final analyses from the ADHERE registry. Aliment.Pharmacol.Ther. 2010;32(4):535–542. doi: 10.1111/j.1365-2036.2010.04381.x. [DOI] [PubMed] [Google Scholar]
- Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through, 1994. N.Engl.J.Med. 1999;341(8):556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- Anand BS, Currie S, Dieperink E, Bini EJ, Shen H, Ho SB, et al. Alcohol use and treatment of hepatitis C virus: results of a national multicenter study. Gastroenterology. 2006;130(6):1607–1616. doi: 10.1053/j.gastro.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, et al. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J.Clin.Oncol. 2004;22(17):3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni M, Ironson G, Schneiderman N. Treatments That Work. New York: Oxford University Press; 2007. Cognitive-Behavioral Stress Management. [Google Scholar]
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through, 2002. Ann.Intern.Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis, C. Clin.Gastroenterol.Hepatol. 2011;9(6):509–516. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Barthlomew KL, Parcel GS, Kok G. Planning Health Promotion Programs: An Intervention Mapping Approach. John Wiley and Sons; 2011. [Google Scholar]
- Blacklaws H, Veysey H, Skinner V, Reid RS, Hawken G, Veysey M. Interferon treatment for chronic hepatitis C: a family impact study. Gastroenterol.Nurs. 2009;32(6):377–383. doi: 10.1097/SGA.0b013e3181c10759. [DOI] [PubMed] [Google Scholar]
- Blixen CE, Webster NJ, Hund AJ, Perzynski AT, Kanuch SW, Stoller EP, et al. Communicating about alcohol consumption to nonharmful drinkers with hepatitis C: patient and provider perspectives. J.Gen.Intern.Med. 2008;23(3):242–247. doi: 10.1007/s11606-007-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorso S, Puzella A, Marino V, Pasquini M, Biondi M, Artini M, et al. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res. 2001;105(1–2):45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- Bondini S, Kallman J, Dan A, Younoszai Z, Ramsey L, Nader F, et al. Health-related quality of life in patients with chronic hepatitis, B. Liver Int. 2007;27(8):1119–1125. doi: 10.1111/j.1478-3231.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- Brau N, Bini EJ, Shahidi A, Aytaman A, Xiao P, Stancic S, et al. Prevalence of hepatitis C and coinfection with HIV among United States veterans in the New York City metropolitan area. Am.J.Gastroenterol. 2002;97(8):2071–2078. doi: 10.1111/j.1572-0241.2002.05924.x. [DOI] [PubMed] [Google Scholar]
- Brothers BM, Yang HC, Strunk DR, Andersen BL. Cancer patients with major depressive disorder: testing a biobehavioral/cognitive behavior intervention. J.Consult Clin.Psychol. 2011;79(2):253–260. doi: 10.1037/a0022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggmann P, Dampz M, Gerlach T, Kravecz L, Falcato L. Treatment outcome in relation to alcohol consumption during hepatitis C therapy: an analysis of the Swiss Hepatitis C Cohort Study. Drug Alcohol Depend. 2010;110(1–2):167–171. doi: 10.1016/j.drugalcdep.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Butt G. Stigma in the context of hepatitis C: concept analysis. Journal of Advanced Nursing. 2008;62(6):712–724. doi: 10.1111/j.1365-2648.2008.04641.x. [DOI] [PubMed] [Google Scholar]
- Butt G, Paterson BL, McGuinness LK. Living with the stigma of hepatitis, C. Western Journal of Nursing Research. 2008;30(2):204–221. doi: 10.1177/0193945907302771. [DOI] [PubMed] [Google Scholar]
- Cacoub P, Ouzan D, Melin P, Lang JP, Rotily M, Fontanges T, et al. Patient education improves adherence to peg-interferon and ribavirin in chronic genotype 2 or 3 hepatitis C virus infection: a prospective, real-life, observational study. World J.Gastroenterol. 2008;14(40):6195–6203. doi: 10.3748/wjg.14.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton BG. The malaise theory of depression: Major depressive disorder is sickness behavior and antidepressants are analgesic. Medical Hypotheses. 2000;54:126–130. doi: 10.1054/mehy.1999.0986. [DOI] [PubMed] [Google Scholar]
- Cheung O, Sterling RK, Salvatori J, Williams K, Hubbard S, Luketic VA, et al. Mild alcohol consumption is not associated with increased fibrosis in patients with chronic hepatitis, C. J.Clin.Gastroenterol. 2011;45(1):76–82. doi: 10.1097/MCG.0b013e3181e12511. [DOI] [PubMed] [Google Scholar]
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin.Ther. 2001;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131(2):470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Conjeevaram HS, Kleiner DE, Everhart JE, Hoofnagle JH, Zacks S, Afdhal NH, et al. Race, insulin resistance and hepatic steatosis in chronic hepatitis, C. Hepatology. 2007;45(1):80–87. doi: 10.1002/hep.21455. [DOI] [PubMed] [Google Scholar]
- Constant A, Castera L, Quintard B, Bernard PH, De L, V, Couzigou P, et al. Psychosocial factors associated with perceived disease severity in patients with chronic hepatitis C: relationship with information sources and attentional coping styles. Psychosomatics. 2005;46(1):25–33. doi: 10.1176/appi.psy.46.1.25. [DOI] [PubMed] [Google Scholar]
- Cooper CL, Giordano C, Mackie D, Mills EJ. Equitable access to HCV care in HIV-HCV co-infection can be achieved despite barriers to health care provision. Ther.Clin.Risk Manag. 2010;6:207–212. doi: 10.2147/tcrm.s9951. 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan AA, Martin LM, Crone C, Ong JP, Farmer DW, Wise T, et al. Depression, anemia and health-related quality of life in chronic hepatitis, C. J.Hepatol. 2006;44(3):491–498. doi: 10.1016/j.jhep.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513–521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Ho SB, Heit S, Durfee JM, Thuras P, Willenbring ML. Significant reductions in drinking following brief alcohol treatment provided in a hepatitis C clinic. Psychosomatics. 2010;51(2):149–156. doi: 10.1176/appi.psy.51.2.149. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Ho SB, Tetrick L, Thuras P, Dua K, Willenbring ML. Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis, C. Gen.Hosp.Psychiatry. 2004;26(3):237–240. doi: 10.1016/j.genhosppsych.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Hellard M, Matthews GV, Grebely J, Haber PS, Petoumenos K, et al. Effective treatment of injecting drug users with recently acquired hepatitis C virus infection. Gastroenterology. 2010;138(1):123–135. doi: 10.1053/j.gastro.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwight MM, Kowdley KV, Russo JE, Ciechanowski PS, Larson AM, Katon WJ. Depression, fatigue, and functional disability in patients with chronic hepatitis, C. J.Psychosom.Res. 2000;49(5):311–317. doi: 10.1016/s0022-3999(00)00155-0. [DOI] [PubMed] [Google Scholar]
- Earnshaw VA, Chaudoir SR. From conceptualizing to measuring HIV stigma: a review of HIV stigma mechanism measures. AIDS Behav. 2009;13(6):1160–1177. doi: 10.1007/s10461-009-9593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Olsen MK, Stechuchak KM, Means MK, Lineberger MD, Kirby A, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32(4):499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin BR. Prevention and treatment of hepatitis C in injection drug users. Hepatology. 2002;36(5) Suppl 1:S210–S219. doi: 10.1053/jhep.2002.36809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, Kunik M, Richardson P, Rabeneck L. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002;123(2):476–482. doi: 10.1053/gast.2002.34750. [DOI] [PubMed] [Google Scholar]
- Erim Y, Tagay S, Beckmann M, Bein S, Cicinnati V, Beckebaum S, et al. Depression and protective factors of mental health in people with hepatitis C: a questionnaire survey. Int.J.Nurs.Stud. 2010;47(3):342–349. doi: 10.1016/j.ijnurstu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Evon DM, Simpson K, Kixmiller S, Galanko J, Dougherty K, Golin C, et al. A Randomized Controlled Trial of an Integrated Care Intervention to Increase Eligibility for Chronic Hepatitis C Treatment. Am.J.Gastroenterol. 2011;106(10):1777–1786. doi: 10.1038/ajg.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evon DM, Simpson KM, Esserman D, Verma A, Smith S, Fried MW. Barriers to accessing care in patients with chronic hepatitis C: the impact of depression. Aliment.Pharmacol.Ther. 2010;32(9):1163–1173. doi: 10.1111/j.1365-2036.2010.04460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evon DM, Verma A, Dougherty KA, Batey B, Russo M, Zacks S, et al. High deferral rates and poorer treatment outcomes for HCV patients with psychiatric and substance use comorbidities. Dig.Dis.Sci. 2007;52(11):3251–3258. doi: 10.1007/s10620-006-9669-0. [DOI] [PubMed] [Google Scholar]
- ez-Quevedo C, Masnou H, Planas R, Castellvi P, Gimenez D, Morillas RM, et al. Prophylactic treatment with escitalopram of pegylated interferon alfa-2a-induced depression in hepatitis C: a 12-week, randomized, double-blind, placebo-controlled trial. J.Clin.Psychiatry. 2011;72(4):522–528. doi: 10.4088/JCP.09m05282blu. [DOI] [PubMed] [Google Scholar]
- Fontana RJ, Bieliauskas LA, Back-Madruga C, Lindsay KL, Kronfol Z, Lok AS, et al. Cognitive function in hepatitis C patients with advanced fibrosis enrolled in the HALT-C trial. J.Hepatol. 2005;43(4):614–622. doi: 10.1016/j.jhep.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Foster G, Goldin R, Thomas H. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27(1):209–212. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5) Suppl 1:S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N.Engl.J.Med. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Fumaz CR, Munoz-Moreno JA, Ballesteros AL, Paredes R, Ferrer MJ, Salas A, et al. Influence of the type of pegylated interferon on the onset of depressive and neuropsychiatric symptoms in HIV-HCV coinfected patients. AIDS Care. 2007;19(1):138–145. doi: 10.1080/09540120600645539. [DOI] [PubMed] [Google Scholar]
- Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given C, Given B, Rahbar M, Jeon S, McCorkle R, Cimprich B, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J.Clin.Oncol. 2004;22(3):507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- Goffman E. Stigma: Notes on the Management of Spoiled Identity. Engelwood Cliffs, NJ: Prentice-Hall; 1963. [Google Scholar]
- Golden J, Conroy R, O'Dwyer A, Golden D, Hardouin J. Illness-related stigma, mood and adjustment to illness in persons with hepatitis C. Soc.Sci.Med. 2006;63(12):3188–3198. doi: 10.1016/j.socscimed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Golden J, O'Dwyer A, Conroy R. Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen.Hosp.Psychiatry. 2005;27(6):431–438. doi: 10.1016/j.genhosppsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Golin CE, Liu H, Hays RD, Miller LG, Beck CK, Ickovics J, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J.Gen.Intern.Med. 2002;17(10):756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi L, Satriano J, Serra A, Biancosino B, Zotos S, Sighinolfi L, et al. Emotional stress, psychosocial variables and coping associated with hepatitis C virus and human immunodeficiency virus infections in intravenous drug users. Psychother.Psychosom. 2002;71(6):342–349. doi: 10.1159/000065993. [DOI] [PubMed] [Google Scholar]
- Grebely J, Knight E, Genoway KA, Viljoen M, Khara M, Elliott D, et al. Optimizing assessment and treatment for hepatitis C virus infection in illicit drug users: a novel model incorporating multidisciplinary care and peer support. Eur.J.Gastroenterol.Hepatol. 2010;22(3):270–277. doi: 10.1097/meg.0b013e32832a8c4c. [DOI] [PubMed] [Google Scholar]
- Groessl EJ, Weingart KR, Stepnowsky CJ, Gifford AL, Asch SM, Ho SB. The hepatitis C self-management programme: a randomized controlled trial. J.Viral Hepat. 2011;18(5):358–368. doi: 10.1111/j.1365-2893.2010.01328.x. [DOI] [PubMed] [Google Scholar]
- Grundy G, Beeching N. Understanding social stigma in women with hepatitis, C. Nurs.Stand. 2004;19(4):35–39. doi: 10.7748/ns2004.10.19.4.35.c3720. [DOI] [PubMed] [Google Scholar]
- Ho SB, Nguyen H, Tetrick LL, Opitz GA, Basara ML, Dieperink E. Influence of psychiatric diagnoses on interferon-alpha treatment for chronic hepatitis C in a veteran population. Am.J.Gastroenterol. 2001;96(1):157–164. doi: 10.1111/j.1572-0241.2001.03468.x. [DOI] [PubMed] [Google Scholar]
- Hoffman J, Sundsmo J, Cunningham J, Vago F, Munly K, Dekker D. Mobile direct observational treatment (MDOT) findings: Implications for HIV medication compliance; 5th IAS Conference on HIV Pathogenesis and Treatment; 2009. Abstract CDD185. [Google Scholar]
- Howell CD, Dowling TC, Paul M, Wahed AS, Terrault NA, Taylor M, et al. Peginterferon pharmacokinetics in African American and Caucasian American patients with hepatitis C virus genotype 1 infection. Clin.Gastroenterol.Hepatol. 2008;6(5):575–583. doi: 10.1016/j.cgh.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrees M, Riazuddin S. A study of best positive predictors for sustained virologic response to interferon alpha plus ribavirin therapy in naive chronic hepatitis C patients. BMC Gastroenterol. 2009;20 doi: 10.1186/1471-230X-9-5. 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Taketomi A, Soejima Y, Yoshizumi T, Fukuhara T, Kotoh K, et al. The benefits of interferon treatment in patients without sustained viral response after living donor liver transplantation for hepatitis, C. Transplant.Proc. 2009;41(10):4246–4252. doi: 10.1016/j.transproceed.2009.08.070. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N.Engl.J.Med. 2011;364(25):2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- Kallman J, O'Neil MM, Larive B, Boparai N, Calabrese L, Younossi ZM. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig.Dis.Sci. 2007;52(10):2531–2539. doi: 10.1007/s10620-006-9708-x. [DOI] [PubMed] [Google Scholar]
- Kanwal F, Hoang T, Spiegel BM, Eisen S, Dominitz JA, Gifford A, et al. Predictors of treatment in patients with chronic hepatitis C infection - role of patient versus nonpatient factors. Hepatology. 2007;46(6):1741–1749. doi: 10.1002/hep.21927. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Caldwell DS, Williams DA, Gil KM, Mitchell D, Robertson C, et al. Pain coping skills training in the management of osteoarthritic knee pain: A comparative study. Behavior Therapy. 1990;21(1):49–62. [Google Scholar]
- Kinder M. The lived experience of treatment for hepatitis, C. Gastroenterol.Nurs. 2009;32(6):401–408. doi: 10.1097/SGA.0b013e3181c1497f. [DOI] [PubMed] [Google Scholar]
- Kramer JR, Kanwal F, Richardson P, Giordano TP, Petersen LA, El-Serag HB. Importance of Patient, Provider, and Facility Predictors of Hepatitis C Virus Treatment in Veterans: A National Study. Am.J.Gastroenterol. 2011;106(3):483–491. doi: 10.1038/ajg.2010.430. [DOI] [PubMed] [Google Scholar]
- Kraus M, Schafer A, Csef H, Scheurlen M, Faller H. Emotional state, coping styles, and somatic variables in patients with chronic hepatitis, C. Psychosomatics. 2000;41(5):377–384. doi: 10.1176/appi.psy.41.5.377. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Schafer A, Schottker K, Keicher C, Weissbrich B, Hofbauer I, et al. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomised, double-blind, placebo-controlled study. Gut. 2008;57(4):531–536. doi: 10.1136/gut.2007.131607. [DOI] [PubMed] [Google Scholar]
- Lally MA, Montstream-Quas SA, Tanaka S, Tedeschi SK, Morrow KM. A qualitative study among injection drug using women in Rhode Island: attitudes toward testing, treatment, and vaccination for hepatitis and HIV. AIDS Patient.Care STDS. 2008;22(1):53–64. doi: 10.1089/apc.2006.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CA, Conrad S, Garrett L, Battistutta D, Cooksley WG, Dunne MP, et al. Symptom prevalence and clustering of symptoms in people living with chronic hepatitis C infection. J.Pain Symptom.Manage. 2006;31(4):335–344. doi: 10.1016/j.jpainsymman.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- Leutscher PD, Lagging M, Buhl MR, Pedersen C, Norkrans G, Langeland N, et al. Evaluation of depression as a risk factor for treatment failure in chronic hepatitis, C. Hepatology. 2010;52(2):430–435. doi: 10.1002/hep.23699. [DOI] [PubMed] [Google Scholar]
- Lo RV, III, Amorosa VK, Localio AR, O'Flynn R, Teal V, Dorey-Stein Z, et al. Adherence to hepatitis C virus therapy and early virologic outcomes. Clin.Infect.Dis. 2009;48(2):186–193. doi: 10.1086/595685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Labile anger during interferon alfa treatment is associated with a polymorphism in tumor necrosis factor alpha. Clin.Neuropharmacol. 2010;33(4):191–197. doi: 10.1097/WNF.0b013e3181de8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2011;35(3):664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Marino EL, varez-Rubio L, Miro S, Modamio P, Banos F, Lastra CF, et al. Pharmacist intervention in treatment of patients with genotype 1 chronic hepatitis, C. J.Manag.Care Pharm. 2009;15(2):147–150. doi: 10.18553/jmcp.2009.15.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NK, Vickerman P, Miners A, Foster GR, Hutchinson SJ, Goldberg DJ, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55(1):49–57. doi: 10.1002/hep.24656. [DOI] [PubMed] [Google Scholar]
- McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ.Res. 2003;18(2):156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Bacon BR, Owens GS. Making it happen: managed care considerations in vanquishing hepatitis, C. Am.J.Manag.Care. 2007;13(Suppl 12):S327–S336. [PubMed] [Google Scholar]
- McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N.Engl.J.Med. 2009a;360(18):1827–1838. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N.Engl.J.Med. 2009b;361(6):580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis, C. Gastroenterology. 2002;123(4):1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Ware JE, Jr, Bayliss MS, Pianko S, Albrecht JK, Cort S, et al. The effects of interferon alpha-2b in combination with ribavirin on health related quality of life and work productivity. J.Hepatol. 2001;34(1):140–147. doi: 10.1016/s0168-8278(00)00026-x. [DOI] [PubMed] [Google Scholar]
- Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, et al. Limited Uptake of Hepatitis C Treatment Among Injection Drug Users. J.Community Health. 2008;33(3):126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia MT, Muir AJ, McCone J, Shiffman ML, King JW, Herrine SK, et al. Racial differences in hepatitis C treatment eligibility. Hepatology. 2011;54(1):70–78. doi: 10.1002/hep.24358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Rodoletz M, Mangan CE, Schroeder CM, Sedlacek TV. Applications of the monitoring process model to coping with severe long-term medical threats. Health Psychol. 1996;15(3):216–225. doi: 10.1037//0278-6133.15.3.216. [DOI] [PubMed] [Google Scholar]
- Muir AJ, Hu KQ, Gordon SC, Koury K, Boparai N, Noviello S, et al. Hepatitis C treatment among racial and ethnic groups in the IDEAL trial. J.Viral Hepat. 2011;18(4):e134–e143. doi: 10.1111/j.1365-2893.2010.01402.x. [DOI] [PubMed] [Google Scholar]
- Nicholson RA, Buse DC, Andrasik F, Lipton RB. Nonpharmacologic treatments for migraine and tension-type headache: how to choose and when to use. Curr.Treat.Options.Neurol. 2011;13(1):28–40. doi: 10.1007/s11940-010-0102-9. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Kuroki T, Yabusako T, Seki S, Kobayashi K, Monna T, et al. Detection of hepatitis C virus antibodies and hepatitis C virus RNA in patients with alcoholic liver disease. Hepatology. 1991;14(6):985–989. [PubMed] [Google Scholar]
- Owen G. An 'elephant in the room'? Stigma and hepatitis C transmission among HIV-positive 'serosorting' gay men. Cult.Health Sex. 2008;10(6):601–610. doi: 10.1080/13691050802061673. [DOI] [PubMed] [Google Scholar]
- Paterson BL, Backmund M, Hirsch G, Yim C. The depiction of stigmatization in research about hepatitis, C. International Journal on Drug Policy. 2007;18(5):364–373. doi: 10.1016/j.drugpo.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Paterson BL, Butt G, McGuinness L, Moffat B. The construction of hepatitis C as a chronic illness. Clin.Nurs.Res. 2006;15(3):209–224. doi: 10.1177/1054773806288569. [DOI] [PubMed] [Google Scholar]
- Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis, C. Hepatology. 1998;27(6):1717–1722. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
- Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N.Engl.J.Med. 2011;364(13):1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis, C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349(9055):825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- Proeschold-Bell RJ, Patkar AA, Naggie S, Coward L, Mannelli P, Yao J, et al. An Integrated Alcohol Abuse and Medical Treatment Model for Patients with Hepatitis C. Dig.Dis.Sci. 2011 doi: 10.1007/s10620-011-1976-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J.Clin.Psychiatry. 2005a;66(1):41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19(2):105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond J, Dunning P, Desmond P. Hepatitis C: A medical and social diagnosis. Australian Nursing Journal. 2004;73:1–3. [PubMed] [Google Scholar]
- Richmond JA, Bailey DE, Jr, McHutchison JG, Muir AJ. The use of mind-body medicine and prayer among adult patients with chronic hepatitis, C. Gastroenterol.Nurs. 2010;33(3):210–216. doi: 10.1097/SGA.0b013e3181e01a7b. [DOI] [PubMed] [Google Scholar]
- Rifai MA. Hepatitis C treatment of patients with bipolar disorder: a case series. Prim.Care Companion.J.Clin.Psychiatry. 2006;8(6):361–366. doi: 10.4088/pcc.v08n0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaeys G, De BJ, Wichers MC, Bruckers L, Nevens F, Michielsen P, et al. Early prediction of major depression in chronic hepatitis C patients during peg-interferon alpha-2b treatment by assessment of vegetative-depressive symptoms after four weeks. World J.Gastroenterol. 2007;13(43):5736–5740. doi: 10.3748/wjg.v13.i43.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Torres M, Torriani F, Rockstroh J, Depamphilis J, Carosi G, Dieterich DT. Degree of viral decline early in treatment predicts sustained virological response in HCV-HIV coinfected patients treated with peginterferon alfa-2a and ribavirin. HIV.Clin.Trials. 2010;11(1):1–10. doi: 10.1310/hct1101-1. [DOI] [PubMed] [Google Scholar]
- Romero-Gomez M, Grande L, Nogales MC, Fernandez M, Chavez M, Castro M. Intrahepatic hepatitis C virus replication is increased in patients with regular alcohol consumption. Dig.Liver Dis. 2001;33(8):698–702. doi: 10.1016/s1590-8658(01)80048-7. [DOI] [PubMed] [Google Scholar]
- Russo S, Kema IP, Haagsma EB, Boon JC, Willemse PH, den Boer JA, et al. Irritability rather than depression during interferon treatment is linked to increased tryptophan catabolism. Psychosom.Med. 2005;67(5):773–777. doi: 10.1097/01.psy.0000171193.28044.d8. [DOI] [PubMed] [Google Scholar]
- Safren SA, O'Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer A, Scheurlen M, Felten M, Kraus MR. Physician-patient relationship and disclosure behaviour in chronic hepatitis C in a group of German outpatients. Eur.J.Gastroenterol.Hepatol. 2005;17(12):1387–1394. doi: 10.1097/00042737-200512000-00019. [DOI] [PubMed] [Google Scholar]
- Schroder KE, Johnson CJ, Wiebe JS. Interactive Voice Response Technology applied to sexual behavior self-reports: a comparison of three methods. AIDS Behav. 2007;11(2):313–323. doi: 10.1007/s10461-006-9145-z. [DOI] [PubMed] [Google Scholar]
- Sgorbini M, O'Brien L, Jackson D. Living with hepatitis C and treatment: the personal experiences of patients. J.Clin.Nurs. 2009;18(16):2282–2291. doi: 10.1111/j.1365-2702.2009.02806.x. [DOI] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect.Dis. 2005;5(9):558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Singal AK, Anand BS. Management of hepatitis C virus infection in HIV/HCV co-infected patients: clinical review. World J.Gastroenterol. 2009;15(30):3713–3724. doi: 10.3748/wjg.15.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J.Clin.Gastroenterol. 2007;41(8):761–772. doi: 10.1097/MCG.0b013e3180381584. [DOI] [PubMed] [Google Scholar]
- Smith SR, Wahed AS, Kelley SS, Conjeevaram HS, Robuck PR, Fried MW. Assessing the validity of self-reported medication adherence in hepatitis C treatment. Ann.Pharmacother. 2007;41(7):1116–1123. doi: 10.1345/aph.1K024. [DOI] [PubMed] [Google Scholar]
- Soriano V, Sherman KE, Rockstroh J, Dieterich D, Back D, Sulkowski M, et al. Challenges and opportunities for hepatitis C drug development in HIV-hepatitis C virus-co-infected patients. AIDS. 2011;25(18):2197–2208. doi: 10.1097/QAD.0b013e32834bbb90. [DOI] [PubMed] [Google Scholar]
- Spiegel BM, Younossi ZM, Hays RD, Revicki D, Robbins S, Kanwal F. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41(4):790–800. doi: 10.1002/hep.20659. [DOI] [PubMed] [Google Scholar]
- Stoller EP, Webster NJ, Blixen CE, McCormick RA, Perzynski AT, Kanuch SW, et al. Lay management of chronic disease: a qualitative study of living with hepatitis C infection. Am.J.Health Behav. 2009;33(4):376–390. doi: 10.5993/ajhb.33.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski MS, Felizarta F, Smith C, Slim J, Berggren R, Goodman R, et al. Daily versus thrice-weekly interferon alfa-2b plus ribavirin for the treatment of chronic hepatitis C in HIV-infected persons: a multicenter randomized controlled trial. J.Acquir.Immune.Defic.Syndr. 2004;35(5):464–472. doi: 10.1097/00126334-200404150-00004. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Thomas DL. Epidemiology and natural history of hepatitis C virus infection in injection drug users: implications for treatment. Clin.Infect.Dis. 2005;40(Suppl 5):S263–S269. doi: 10.1086/427440. [DOI] [PubMed] [Google Scholar]
- Sutton R, Treloar C. Chronic illness experiences, clinical markers and living with hepatitis, C. J.Health Psychol. 2007;12(2):330–340. doi: 10.1177/1359105307074278. [DOI] [PubMed] [Google Scholar]
- Swendeman D, Rotheram-Borus MJ. Innovation in sexually transmitted disease and HIV prevention: internet and mobile phone delivery vehicles for global diffusion. Curr.Opin.Psychiatry. 2010;23(2):139–144. doi: 10.1097/YCO.0b013e328336656a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur.J.Gastroenterol.Hepatol. 2007;19(9):741–747. doi: 10.1097/MEG.0b013e3281bcb8d8. [DOI] [PubMed] [Google Scholar]
- Sylvestre DL, Loftis JM, Hauser P, Genser S, Cesari H, Borek N, et al. Co-occurring Hepatitis C, substance use, psychiatric illness: treatment issues and developing integrated models of care. J.Urban.Health. 2004;81(4):719–734. doi: 10.1093/jurban/jth153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139(1):120–129. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29(3):333–337. doi: 10.1037/a0018836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trooskin SB, Navarro VJ, Winn RJ, Axelrod DJ, McNeal AS, Velez M, et al. Hepatitis C risk assessment, testing and referral for treatment in urban primary care: role of race and ethnicity. World J.Gastroenterol. 2007;13(7):1074–1078. doi: 10.3748/wjg.v13.i7.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S.Department of Health & Human Services (DHHS) Combating the Silent Epidemic of Viral Hepatitis: Action Plan for th Prevention, Care & Treatment of Viral Hepatitis. 2011 [Google Scholar]
- Urquhart J, Vrijens B. New findings about patient adherence to prescribed drug dosing regimens: An introductoin to pharmionics. The European Journal of Hospital Pharmacy Science. 2005;11(5):103–106. [Google Scholar]
- van de Mortel TF. Health care workers' knowledge of hepatitis C and attitudes towards patients with hepatitis C: a pilot study. Aust.J.Adv Nurs. 2002;20(1):13–19. [PubMed] [Google Scholar]
- Volk ML. Antiviral therapy for hepatitis C: why are so few patients being treated? J.Antimicrob.Chemother. 2010;65(7):1327–1329. doi: 10.1093/jac/dkq157. [DOI] [PubMed] [Google Scholar]
- Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336(7653):1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Ryan G, Osilla KC, Bhatti L, Goetz M, Witt M. Treat early or wait and monitor? A qualitative analysis of provider hepatitis C virus treatment decision-making in the context of HIV coinfection. AIDS Patient.Care STDS. 2009;23(9):715–725. doi: 10.1089/apc.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Jr, Bayliss MS, Mannocchia M, Davis GL. Health-related quality of life in chronic hepatitis C: impact of disease and treatment response. The Interventional Therapy Group. Hepatology. 1999;30(2):550–555. doi: 10.1002/hep.510300203. [DOI] [PubMed] [Google Scholar]
- Weiss JJ, Bhatti L, Dieterich DT, Edlin BR, Fishbein DA, Goetz MB, et al. Hepatitis C patients' self-reported adherence to treatment with pegylated interferon and ribavirin. Aliment.Pharmacol.Ther. 2008;28(3):289–293. doi: 10.1111/j.1365-2036.2008.03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin J, Lagging LM, Spak F, Aires N, Svensson E, Lindh M, et al. Moderate alcohol intake increases fibrosis progression in untreated patients with hepatitis C virus infection. J.Viral Hepat. 2002;9(3):235–241. doi: 10.1046/j.1365-2893.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int.J.Neuropsychopharmacol. 2002;5(4):375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Praamstra AJ, Maes M. Early increase in vegetative symptoms predicts IFN-alpha-induced cognitive-depressive changes. Psychol.Med. 2005;35(3):433–441. doi: 10.1017/s0033291704003526. [DOI] [PubMed] [Google Scholar]
- Wiley T, McCarthy M, Breidi L, McCarthy M, Layden T. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28(3):805–809. doi: 10.1002/hep.510280330. [DOI] [PubMed] [Google Scholar]
- Willenbring ML. Integrating care for patients with infectious, psychiatric, and substance use disorders: concepts and approaches. AIDS. 2005;19(Suppl 3):S227–S237. doi: 10.1097/01.aids.0000192094.84624.c2. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global surveillance and control of hepatitis, C. Journal of Viral Hepatitis. 1999;6:35–47. [PubMed] [Google Scholar]
- Younossi Z, Kallman J, Kincaid J. The effects of HCV infection and management on health-related quality of life. Hepatology. 2007;45(3):806–816. doi: 10.1002/hep.21565. [DOI] [PubMed] [Google Scholar]
- Yu JW, Wang GQ, Sun LJ, Li XG, Li SC. Predictive value of rapid virological response and early virological response on sustained virological response in HCV patients treated with pegylated interferon alpha-2a and ribavirin. J.Gastroenterol.Hepatol. 2007;22(6):832–836. doi: 10.1111/j.1440-1746.2007.04904.x. [DOI] [PubMed] [Google Scholar]
- Zacks S, Beavers K, Theodore D, Dougherty K, Batey B, Shumaker J, et al. Social stigmatization and hepatitis C virus infection. J.Clin.Gastroenterol. 2006;40(3):220–224. doi: 10.1097/00004836-200603000-00009. [DOI] [PubMed] [Google Scholar]
- Zanini B, Covolo L, Donato F, Lanzini A. Effectiveness and tolerability of combination treatment of chronic hepatitis C in illicit drug users: meta-analysis of prospective studies. Clin.Ther. 2010;32(13):2139–2159. doi: 10.1016/S0149-2918(11)00021-X. [DOI] [PubMed] [Google Scholar]
- Zickmund S, Brown K, Bielefeldt K. A systematic review of provider knowledge of hepatitis C: is it enough for a complex disease? Dig.Dis.Sci. 2007;52(10):2550–2556. doi: 10.1007/s10620-007-9753-0. [DOI] [PubMed] [Google Scholar]
- Zickmund S, Ho EY, Masuda M, Ippolito L, LaBrecque DR. 'They Treated Me Like a Leper': Stigmatization and the Qualify of Life of Patients with Hepatitis, C. Journal of General Internal Medicine. 2003;18(10):835–844. doi: 10.1046/j.1525-1497.2003.20826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]