Abstract
Allylisothiocyanate (AITC) is a dietary component with possible anti-cancer effects, though much information about AITC and cancer has been obtained from cell studies. To investigate the effect of AITC on DNA integrity in vivo, a crossover study was conducted. Adults (n=46) consumed AITC, AITC-rich vegetables (mustard and cabbage), or a control treatment with a controlled diet for 10 days each. On day 11, volunteers provided blood and urine before and after consuming treatments. Volunteers were characterized for genotype for GSTM1 and GSTT1 (glutathione S-transferases) and XPD (DNA repair). DNA integrity in peripheral blood mononuclear cells (PBMCs) was assessed by single cell gel electrophoresis. Urine was analyzed for 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxodG) and creatinine. Ten day intake of neither AITC nor mustard/cabbage(M/C) resulted in statistically significant differences in DNA strand breaks (LS mean % DNA in tail ± SEM: 4.8 ± 0.6 for control, 5.7 ± 0.7 for AITC, 5.3 ± 0.6 for M/C) or urinary 8-oxodG (LS mean µg 8-oxodG/g creatinine ± SEM: 2.95 ± 0.09 for control, 2.88 ± 0.09 for AITC, 3.06 ± 0.09 for M/C). Both AITC and M/C increased DNA strand breaks 3h post-consumption (LS mean % DNA in tail ± SEM: 3.2 ± 0.7 for control, 8.3 ± 1.7 for AITC, 8.0 ± 1.7 for M/C), and this difference disappeared at 6h (4.2 ± 0.9 for control, 5.7 ± 1.2 for AITC, 5.5 ± 1.2 for M/C). Genotypes for GSTM1, GSTT1, and XPD were not associated with treatment effects. In summary, DNA damage appeared to be induced in the short term by AITC and AITC-rich products, but that damage disappeared quickly, and neither AITC nor AITC-rich products affected DNA base excision repair.
Keywords: sinigrin, glucosinolate, COMET, SCGE
Introduction
Allylisothiocyanate (AITC) is a dietary component with potentially important anti-cancer effects. AITC is derived from the glucosinolatesinigrin, which is found in some Brassica vegetables, including cabbage, mustard, Brussels sprouts, kale, and cauliflower. Isothiocyanatesare produced from glucosinolates when plant material is injured, thus releasing the catalyzing enzyme myrosinase to convert glucosinolates to isothiocyanates. Also known as mustard oil, AITC is additionally found in the food supply as a food additive, giving a pungent flavor to mayonnaise products, horseradish spreads, salad dressings, and other spreads and sauces.
There is a substantial body of evidence suggesting that components of Brassica vegetables have anticarcinogenic activities. Epidemiological evidence suggests that inclusion of Brassica vegetables in the diet leads to lower cancer risk [1–9]. Animal studies have demonstrated that components of Brassica vegetables or their metabolites can inhibit tumor growth in transgenic or xenograft models [10–13], and animal and cell studies have suggested that intake of Brassica vegetables or components can positively alter physiologic processes associated with cancer, such as induction of Phase II enzymes or apoptosis [14–22]. While there is much evidence that AITC has anticarcinogenic activity, much of the evidence is from in vitro investigations. Because in vitro models do not reflect in vivo digestion, metabolism, intracellular and extracellular exposure levels, certain aspects of physiological signaling, etc., it is important to complement in vitro studies with in vivo studies to gain a fuller understanding of potential for compounds to be anticarcinogenic.
Isothiocyanates are metabolized via the mercapturic acid pathway, being conjugated with glutathione by glutathione S-transferase [23]. Of the isoforms of glutathione S-transferase, GSTM1 is a particularly good catalyst for isothiocyanates [24, 25]. It thus follows that individuals with a deletion in the gene for GSTM1 may have higher circulating concentrations of isothiocyanates than individuals with fully functional GSTM1 [26] (though this has not been clearly determined [27, 28]), and genotype for GSTM1 may influence response to intake of Brassica vegetables or their components. Proteomic analysis has supported that GSTM1 genotype influences serum peptide response to Brassica vegetables [29]. In addition, because the gene for GSTT1 can also suffer from a deletion, this gene has also been of interest with respect to isothiocyanate metabolism and cancer risk [30–32]. Supporting the importance of GSTT1 genotype in response to Brassica vegetables, GSTT1 genotype has been found to influence the association between Brassica intake and risk of myocardial infarction [33].
To investigate the potential of dietary AITC to influence DNA integrity and repair, we have designed a human feeding study in which volunteers consumed AITC or vegetable products containing AITC, then provided blood and urine samples for assessment of DNA damage and repair. Mustard was chosen as a treatment food due to its high concentration of AITC. Cabbage was chosen as an additional treatment food because previous studies have shown that cabbage can contain high amounts of AITC-precursor sinigrin [34], though the actual sinigrin content of cabbage can be highly variable [34, 35]. Study volunteers were evaluated for genotype for GSTM1 and GSTT1, since those genotypes have been associated with different responses to Brassica vegetables. In addition, because our primary outcome was DNA integrity and repair, volunteers were also evaluated for polymorphisms in the XPD gene, which codes for a DNA repair enzyme.
Materials and Methods
Study design, diet, and treatments
The study protocol was approved by the MedStar Health Research Institute (Hyattsville, MD) and written, informed consent was obtained from each study subject. A 3-period 3-treatment randomized crossover design was used. Each period lasted 11 days and there was a 17-day washout between periods. The study was designed to test the effect of daily consumption of AITC or Brassica vegetables which provide AITC as well as the effect of an acute bolus dose. To test the effect of daily consumption of AITC or Brassica vegetables which provide AITC, volunteers consumed treatments for 10 days and provided fasting blood and urine samples on day 11. To test the acute effect of AITC or Brassica vegetables which provide AITC, volunteers consumed a bolus dose of treatment on the 11th morning of the study period, and provided blood and urine samples at 3 and 6 hours post consumption. The basal diet consisted of adequate protein, approximately 35% of calories from fat, and 3 servings of fruits and vegetables per day, to be in accord with average intakes in the United States [36, 37]. The basal diet excluded foods from the Brassicaceae plant family such as broccoli, Brussels sprouts, cabbage, cauliflower, kale, mustard, and wasabi, and prepared foods containing AITC.
The three treatments consisted of a control treatment, a mustard/cabbage (M/C) treatment, and an AITC treatment. On days 1 through 10 participants consumed their dietary treatment with dinner. On the morning of day 11, participants consumed their treatment with a slice of bread and a slice of cheese. The control treatment was 20 grams of a homemade AITC-free mayonnaise. The M/C treatment was 150 g of homogenized green cabbage (including 50 g of water), 30 g of Grey Poupon Country Dijon Mustard (Kraft Foods, Chicago, IL), and 20 g of AITC-free mayonnaise. Prior to the start of the study, the cabbage was homogenized with a commercial blender at a ratio of 2 g of cabbage to 1 g of water and mixed into a single batch. Aliquots of 150 g were frozen at −20 °C until used in the study. The AITC treatment consisted of 114.7 µmol per person of food-grade AITC (Sigma-Aldrich, St. Louis, MO, product no. W203408) incorporated into the AITC-free mayonnaise. The quantity of AITC used in the AITC treatment was chosen to match that measured in the M/C dose prior to the start of the study.
Allylisothiocyanate content in homogenized green cabbage and Grey Poupon Country Dijon Mustard was measured by a method adapted from Rouzaud et al. [38]. Two g of homogenized green cabbage were combined with 4 ml of methylene chloride and 25 µl of 100 mM benzyl isothiocyanate as an internal standard in a 9-ml screw-top glass centrifuge tube. The homogenate was vortex-mixed for 5 sec, centrifuged at 2500 g for 5 min, and the methylene chloride extract was removed by Pasteur pipette to a 10-ml glass tube. Homogenates were extracted twice and the extracts were combined. Extracts were concentrated to a volume of 0.8 ml under nitrogen gas and filtered by centrifugation with 0.2-µm nylon filters (Alltech Associates, Deerfield, IL). Allylisothiocyanate concentrations in the samples were measured by gas chromatography-mass spectrometry (GC-MS). The column was a DB-5MS capillary column (Agilent Technologies, Santa Clara, CA; length, 30 m; diameter 0.25 mm, film thickness, 0.25 µm). Helium at 1.0 ml·min−1 was used as the carrier gas. One µl of extract was injected. The oven temperature was held at 55 °C for 3 min, then increased by 6 °C·min−1 to 160 °C. Allylisothiocyanate was identified by comparison of mass spectra of sample peaks to the mass spectrum of an authentic standard (Sigma-Aldrich, product no. 377430). The response factor of AITC was measured under the same analytical conditions as the samples and was determined to be 2.59. Extraction of AITC from mustard was performed by combining 0.5 g of mustard, 4 ml of water, 4 ml of methylene chloride, and 100 µl of 5-mM benzyl isothiocyanate. The samples were vortex-mixed for 5 sec and centrifuged at 2500 g for 5 min. The supernatant was transferred to a 10-ml glass tube and the pellet was extracted with 3 ml of methylene chloride. The supernatants from the two extractions were then pooled, concentrated, filtered and measured by GC-MS in the same way as the cabbage homogenate. The AITC dose per person in the M/C treatment consisted of 114.39 µmol of AITC from the mustard and 0.34 µmol from the cabbage (114.7 µmol/d total). Allylisothiocyanate concentrations in cabbage and mustard were also measured at the conclusion of the study, and the food-grade AITC was characterized by GC-MS.
Study participants consumed only foods provided by the Beltsville Human Nutrition Research Center (BHNRC). Breakfast and dinner on week days were consumed in the BHNRC dining room, and lunches and weekend meals were packed for carry-out. Participants were instructed to eat all foods and only foods provided to them, with the exception of coffee, tea, and diet soda. Coffee and tea intake was limited to 2 cups per day, and intake of diet soda was not limited. Consumption of coffee, tea, and soda were recorded by study participants. Study participants were asked to abstain from vitamin and mineral supplements beginning two weeks prior to the study and continuing for the duration of the study.
Study participants
Participants were recruited from the Beltsville, MD area by advertisements in local newspapers. Exclusion criteria were pregnancy, lactation, a history of kidney, liver, gastrointestinal, or metabolic disease, a history of cancer, tobacco use during the 6 months prior to the study, or the use of antibiotics or herbal supplements during the month prior to the study, and were determined by self-reporting by potential study participants. A pre-study medical screening assessed the general health of potential participants. Fifty-two participants, ages 40 to 79 y, were selected based on sex and GSTM1, GSTT1, and XPD genotypes. Three study participants did not begin the study due to family illness or unknown reasons, 2 participants dropped from the study during the first period due to dislike of the food, and 1 participant was removed from the study during the first period because she was taking a vitamin supplement. Forty-six participants completed the study (Table 1).
Table 1.
Physical characteristics of study participants1.
| Participants who completed the study (n=46) |
Participants selected for the comet assay (n=20) |
|||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| Age | 56.0 | 1.5 | 53.9 | 2.4 |
| BMI | 29.1 | 0.9 | 28.3 | 0.9 |
| Number | % of Total | Number | % of Total | |
| Sex | ||||
| Female | 34 | 73.9 | 12 | 60 |
| Male | 12 | 26.1 | 8 | 40 |
| Ethnicity | ||||
| African-American | 14 | 30.4 | 5 | 25 |
| Asian | 1 | 2.2 | 1 | 5 |
| Caucasian | 30 | 65.2 | 14 | 70 |
| Hispanic | 1 | 2.2 | 0 | 0 |
| Genotype | ||||
| XPD Codon 312 | ||||
| Asn/Asn | 4 | 8.7 | 4 | 20 |
| Asp/Asn | 22 | 47.8 | 14 | 70 |
| Asp/Asp | 20 | 43.5 | 2 | 10 |
| XPD Codon 751 | ||||
| Gln/Gln | 9 | 19.6 | 7 | 35 |
| Lys/Gln | 26 | 56.5 | 13 | 65 |
| Lys/Lys | 11 | 23.9 | 0 | 0 |
| GSTM1 | ||||
| GSTM1-null | 19 | 41.3 | 9 | 45 |
| GSTM1+ | 27 | 58.7 | 11 | 55 |
| GSTT1 | ||||
| GSTT1-null | 9 | 19.6 | 1 | 5 |
| GSTT1+ | 37 | 80.4 | 19 | 95 |
Forty-six participants completed the study. For twenty of those participants, PBMCs were collected on the final day of each treatment period immediately before treatment consumption and at 3 and 6 hours after consumption, and PBMC samples were subsequently assessed for DNA strand breaks by the comet assay.
All 46 participants were included in the measurement of DNA strand breaks by the single cell gel electrophoresis (SCGE, comet) assay in peripheral blood mononuclear cells (PBMCs) collected prior to breakfast on day 1 and prior to treatment consumption on day 11. Of these 46 participants, 20 were selected for measurement of DNA strand breaks by the comet assay in PBMCs collected immediately prior to treatment consumption and at 3 and 6 hours after consumption on day 11 (Table 1). We hypothesized that participants with single nucleotide polymorphisms (SNPs) in the DNA repair geneXPD at codon 312 (Asp→Asn) and codon (Lys→Gln) would be most likely to respond to the dietary interventions as measured by the comet assay. Therefore we chose participants with either or both SNPs for measurement of response to the acute treatment challenge. The number of participants was determined to be the maximum for which the PBMC isolation procedure could be conducted given available personnel.
GSTM1 and GSTT1 genotyping
Genotyping was conducted at the Bionomics Research and Technology Center of the Environmental & Occupational Health Sciences Research Institute (Piscataway, NJ). A polymerase chain reaction (PCR) method was used to detect the presence (heterozygous or homozygous for the presence of the gene) or absence (homozygous gene deletion) of the GSTM1 and GSTT1 genes in genomic DNA isolated from whole blood. The 15-µl reaction volume consisted of 2 µl of DNA, 0.2 mM dNTPs, 3 µl of PCR buffer, 8.7 µl of DNAse-free water, 0.2 µM each of the forward and reverse primers, and 0.1 µl (5 U/µl) of PromegaTaq polymerase (Madison, WI). For GSTM1, the forward primer was 5' -GAACTCCCTGAAAAGCTAAAG and the reverse primer was 5' -GTTGGGCTCAAATATACGGTGG. For GSTT1, the forward primer was 5' - TTCCTTACTGGTCCTCACATC and the reverse primer was 5' -TCACCGGATCATGGCCAGCA.
The PCR thermal cycling protocol was the same for genotyping of GSTM1 and GSTT1. Initial denaturation was at 94 °C for 2 min followed by 40 cycles of 94 °C for 30 s, 51 °C for 30 s, 72 °C for 30 s, and 1 cycle of 72 °C for 5 min. DNA products were electrophoresed on a 2% agarose gel. Participants who were heterozygous or homozygous for GSTM1 had a 240-bp PCR fragment whereas individuals who were GSTM1-null did not have this fragment. GSTT1+ individuals had a 490-bp PCR fragment and GSTT1-null individuals did not.
XPD single nucleotide polymorphism (SNP) genotyping
Genotyping was conducted at the Bionomics Research and Technology Center of the Environmental & Occupational Health Sciences Research Institute (Piscataway, NJ). Potential study participants were genotyped for XPD SNPs at codon 312 (Asp→Asn) and codon 751 (Lys→Gln) by TaqMan PCR allelic discrimination assays. Five-µl reaction volumes were used consisting of 2.5 µl of master mix (Life Technologies, Carlsbad, CA), 2 µl of DNA, 0.25 µl of DNAse-free water, and 0.25 µl of assay mix containing 900 nM of primers and probes. The design strand for XPD312 was the reverse strand. Primers were 5' -CCCACCTGGCCAACCC (forward) and 5' -CTGCGAGGAGACGCTATCAG (reverse), and the probes were 5' – CACTTCGTTGGGCAGC (reporter was VIC for labeling the sequence corresponding to Asn) and 5' -CTTCGTCGGGCAGC (reporter was FAM for labeling the sequence corresponding to Asp). For XPD751, the design strand was the forward strand. The forward primer was 5' -ACCCGCCCCACTCAGA and the reverse primer was 5' -GCCTGGAGCAGCTAGAATCAG. The probes were 5' -CTATCCTCTGCAGCGTC (VIC for labeling the sequence corresponding to Gln) and 5'–TCTATCCTCTTCAGCGTC (FAM for labeling the sequence corresponding to Asp). Thermal cycling was performed with an ABI 7900 sequence detection system (Life Technologies) as follows: initial activation of taq polymerase was at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The final step was the allelic discrimination endpoint plate read which segregated the genotypes being tested.
Peripheral blood mononuclear cell collection and isolation
To test for the daily treatment effect on DNA strand breaks in PBMCs, fasting blood samples were collected in 10-ml Vacutainer tubes containing EDTA (Beckton Dickinson, Franklin Lakes, NJ) prior to breakfast on days 1 and 11. The acute challenge treatment effect was tested on samples that were collected immediately prior to treatment consumption and at 3 and 6 hours after consumption on day 11. Blood was manipulated at room temperature and PBMCs were isolated within one hour of phlebotomy. Whole blood was diluted to a final volume of 20 ml with RPMI-1640 culture medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Invitrogen). Diluted blood was divided between two tubes and each aliquot was layered over 10 ml of Ficoll (GE Healthcare, Waukesha, WI). The tubes were centrifuged for 30 minutes at 700 g and 20°C. PBMCs, floating as a band at the Ficoll interface, were recovered and washed twice by centrifugation in RPMI-1640+10% FBS. Cell pellets were resuspended in 8 ml of ice cold freeze medium (50% FBS, 40% RPMI-1640, 10% dimethyl sulfoxide) [39]. Cell samples, held on ice, were aliquotted among five 1.5-ml cryovials and transferred to foam boxes for overnight temperature stratification at −80°C prior to storage in liquid nitrogen. An aliquot of each cell sample was evaluated by trypan blue staining. All samples had 85% or higher viability.
Single cell gel electrophoresis (comet) assay
The laboratory in which the assay was performed had UV-attenuating film applied to the room windows and the plastic covers of the overhead fluorescent light fixtures. The observed UVA levels in the laboratory were routinely below the limits of detection measured with a digital UV power meter (model UV 1365, EDTM, Inc., Toledo, OH).
The comet assay was performed as described by Singh et al. [40] with small modifications. Peripheral blood mononuclear cells, retrieved from liquid nitrogen storage, were thawed quickly at 37 °C, washed twice by centrifugation in calcium- and magnesium-free PBS and held on ice. Cells were mixed with low melting temperature agarose (1% LMA in Ca++/Mg++ free PBS) which was maintained at a temperature of 37° C in a PTC-100 thermal cycler (MJ Research, Inc.). The cells in low melt agarose were spread across the wells of commercially prepared comet slides (Trevigen Corp.). Slides with cells in agarose were transferred to a foil covered tray and held at 4° C for one hour prior to overnight storage at 4° C in Coplin jars containing lysis buffer (10 mM Tris-HCl, pH 10, 2.5 M NaCl, 100 mM EDTA, 10% DMSO, 1% Triton X-100). DNA alkaline unwinding took place at 4°C for 20 minutes in electrophoresis buffer cooled to 4°C (300 mM NaOH, 1 mM EDTA, pH > 13). Electrophoresis was conducted in a temperature-controlled chromatography cabinet set at 4°C. The temperature inside the cabinet averaged approximately 6°C during electrophoresis. Electrophoresis was conducted at 1 volt/cm and 300 mA for 60 min. At the conclusion of electrophoresis, the slides were rinsed 3 times with ice-cold deionized water and after a brief immersion in 70% ethanol were air-dried on a slide warmer set at 37°C. Slides were placed in a light-tight slide box and stored briefly in a desiccator.
Prior to image analysis, slides were stained with SYBR Gold stain (Invitrogen) diluted 1:10,000 with 1x TE buffer (10 mM Tris-HCl+1 mM EDTA, pH 7.5) and covered with a glass cover slip for 5 min prior to scoring. Comet images were observed using an Eclipse E400 fluorescence microscope (Nikon, Tokyo, Japan) coupled to a Pulnix CCD video camera (Kinetic Imaging LTD, Merseyside, UK). The microscope and camera were in turn connected to a PC. Comet signatures of 100 randomly selected cells per slide were analyzed via Komet version 5.0 image analysis software (Kinetic Imaging LTD). The percent DNA in the head and tail, Olive tail moment, and tail length were measured. Values of the comet measures of the 100 randomly selected cells were averaged for each slide. Percent of DNA in the tail was used as the comet measure for statistical analysis because it has a linear relationship to DNA break frequency, is relatively unaffected by threshold settings, and yields the widest possible range (i.e., 0 to 100%) [41].
8-Oxo-7,8-dihydro-2’-deoxyguanosine (8-oxodG) Analysis
Concentrations of urinary 8-oxodG were performed by ESA Laboratories, Inc. (Chelmsford, MA) using a proprietary high pressure liquid chromatography method with electrochemical detection and automated column-switching as previously reported [42]. This method was validated with 3600 urine samples collected from healthy subjects and subjects with a variety of pathologies, using internal quality control and external blind testing. In a recent study, 17 laboratories including ESA Laboratories, Inc. analyzed identical test samples by electrochemical, MS, or enzyme-linked immunosorbant assay (ELISA) methods. The results of this study demonstrated good within-technique agreement and that results from the electrochemical (including the method used in our study) and MS methods were within 1 standard deviation of the consensus mean [43]. To calculate sample concentrations in our study, a standard curve was generated in each sample run with 8-oxodG concentrations of 0.5, 1.0, 2.0, 5.0, 10.0, and 20.0 µg/L. The inter-day coefficient of variation (CV) was 5.3% at 1.9 µg/L and the intra-day CV was 8.6% at 1.9 µg/L. Urinary 8-oxodG values were normalized for urinary creatinine concentrations.
Statistics
Analyses of variance of the comet data (percent tail DNA) and the 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxodG) data were performed using the MIXED procedure in SAS (ver. 9.2, SAS Institute Inc., Cary, NC). Data were tested for normality with the Shapiro-Wilk statistic and by inspection of stem-leaf plots and normal probability plots of residuals. Because the data were strongly skewed, the logit-transformation was applied to the comet data.
To account for the serial correlation of repeated measures on the same experimental unit (participant), covariance structures were fit to the data. The MIXED procedure was used to generate Akaike Information Criteria (AIC) and Bayesian Information Criteria (BIC). The autoregressive covariance structure best fit the comet and the 8-oxo-dG data for the daily treatment effect in which period was the repeated measure. The compound symmetry structure best fit the 8-oxodG data for the daily treatment experiment. The acute challenge treatment experiment had two repeated factors, period and hour, and thus required a Kronecker product covariance structure to model covariance. For both the comet assay and the urinary 8-oxodG measurements, the data were best fit by a crossed unstructured × autoregressive model.
In the statistical model for the analysis of the daily treatment effect, the following were treated as fixed effects in the model: baseline (fasting sample from day 1), baseline by treatment, sex, period, sequence, treatment, GSTM1 genotype, GSTT1 genotype, and XPD genotype. Study participant was treated as a random effect. For analysis of the acute challenge treatment, hour and hour by treatment were added to the model. Prior to performing these analyses, the data were tested for carryover effects by the inclusion of the treatment by period interaction term. This interaction was nonsignificant in all cases and therefore was excluded from the model. Determination of significant terms was by backward elimination of nonsignificant terms (P< 0.05). Because period and sequence were integral to the structure of the experimental design, these terms were retained in all models even though they were nonsignificant in every case. Model effects are reported as least squares means (LS means). Because LS means are a linear combination of effects in a model, and the models for the 10-day treatment intake and the acute treatment challenge were different (e.g., there was no hour effect to be estimated during the 10-day treatment intake), LS means computed for day 11 hour 0 were statistically similar but not identical for the 10-day intake and acute treatment challenge models. Least squares means of logit-transformed percent tail DNA data were inverse transformed to their original (%) scale. Standard errors on the original scale of the LS means from the comet data were calculated using the Delta method with the R software package [44, 45].
Results
Effect of daily treatment intervention on DNA strand breaks
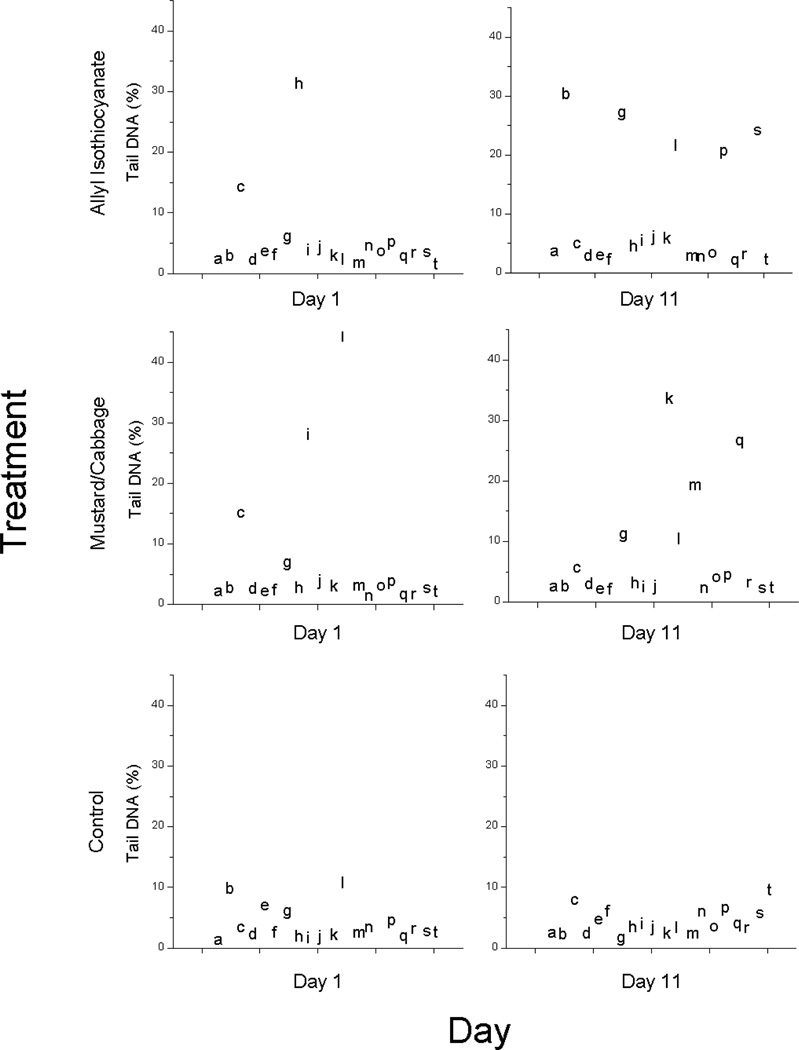
The percent of DNA in comet tails of PBMCs isolated from participants after 10 days on the different dietary interventions did not differ, either in the entire group of participants (n=46) or in the sub-population (n=20) selected for comet measurements in the acute treatment challenge (Table 2). Participants with the GSTM1 genotype had higher % tail DNA compared to those with the GSTM1 null genotype (6.7 ± 0.5% and 4.1 ± 0.6%, respectively). During the treatment period in which participants consumed the basal diet, % tail DNA was generally below 10% (Fig. 1). At baseline, before the controlled dietary interventions commenced (day 1 hour 0), the majority of participants had % tail DNA less than 10% although that of some participants exceeded this. By day 11, there were more values above 10% for the AITC and M/C treatments compared to the basal diet, but the corresponding increases in the LS means did not rise to the level of statistical significance.
Table 2.
Percent tail DNA in peripheral blood mononuclear (PBMCs) measured by the comet assay in response to 10-day intake of basal diet, basal diet with allylisothiocyanate, or basal diet with mustard and cabbage1,2,3, 4.
| Treatment | % tail DNA in PBMCs from total study group (n=46) |
% tail DNA in PBMCs in sub- population (n=20) |
|---|---|---|
| Control | 4.8 ± 0.6 | 3.9 ± 0.7 |
| Allylisothiocyanate | 5.7 ± 0.7 | 6.2 ± 1.1 |
| Mustard/Cabbage | 5.3 ± 0.6 | 4.9 ± 0.9 |
Values represent LS mean ± SEM
The sub-population (n=20) of the total participant group (n=46) subsequently was measured for response to acute treatment challenge.
No treatment effects were detected for either the total study group (n=46) or the sub-population (n=20).
Because LS means are a linear combination of effects in a model, and the models for the 10-day treatment intake and the acute treatment challenge were different (e.g., there was no hour effect to be estimated during the 10-day treatment intake), LS means computed for day 11 hour 0 were similar but not identical for the 10-day intake (LS mean % DNA in tail ± SEM: 3.86 ± 0.72 for control, 6.23 ± 1.09 for AITC, 4.85 ± 0.86 for C/M) and acute treatment challenge models (LS mean % DNA in tail ± SEM: 3.90 ± 0.84 for control, 6.17 ± 1.30 for AITC, 4.90 ± 1.04 for M/C).
Figure 1.
Percent tail DNA measured by the comet assay in peripheral blood mononuclear cells isolated from fasting blood samples on day 1 and day 11. Treatments were allyl isothiocyanate, mustard/cabbage, and control, and were consumed at dinner on days 1 through 10. Data points correspond to individual participants; the comet assay was used to assess the acute challenge treatment effect in these same participants (n=20).
Acute response of % tail DNA to the treatment challenge on day 11
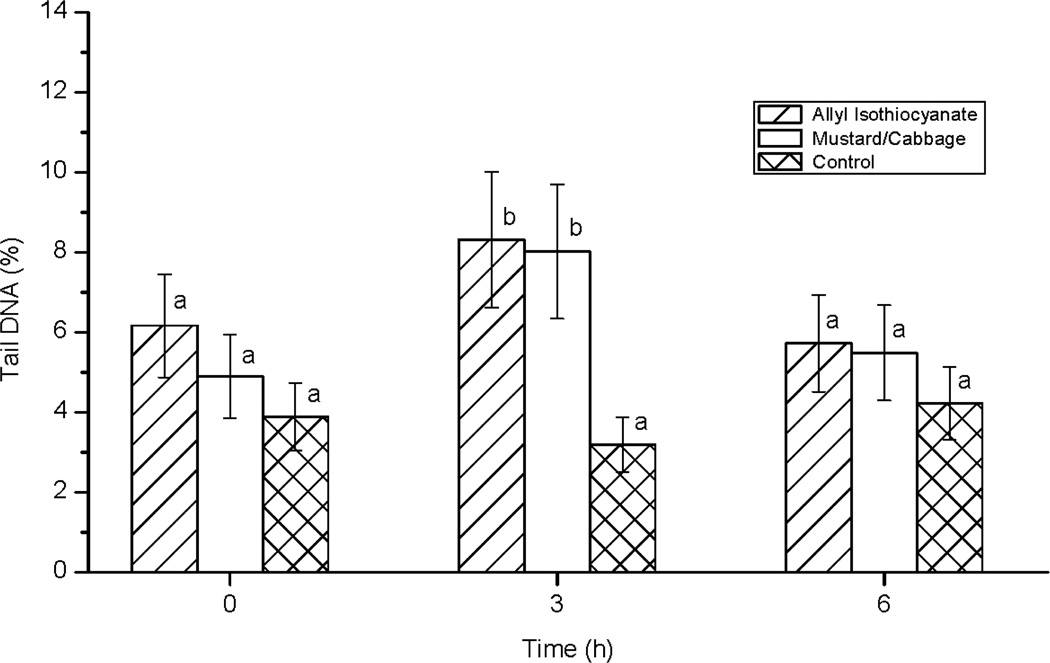
There was a statistically significant (P < 0.05) treatment by hour interaction for DNA strand breakage as reflected in % tail DNA measured in PBMCs collected immediately before the acute treatment intervention on day 11 (hour 0) and at 3 and 6 h after the intervention. At hour 0, there was no treatment effect but at 3 hours post acute intervention, the % tail DNA was higher in the AITC and M/C treatments compared to the control treatment (Fig. 2). At 6 hours after consuming the treatments, the % tail DNA values of the treatment groups were not different.
Figure 2.
Percent DNA in comet tail from peripheral mononuclear blood cells collected immediately before treatment consumption (hour 0) and at three and six hours after consumption. Analysis of variance was performed on logit-transformed data by the MIXED procedure in SAS. The results reported here are inverse transformed. Data are expressed as least square means. Error bars represent standard errors of the means. Means with different letters within an hour differ at P ≤ 0.05. Because LS means are a linear combination of effect in a model, and the models for the 10-day treatment intake and the acute treatment challenge were different (e.g., there was no hour effect to be estimated during the 10-day treatment intake), LS means computed for day 11 hour 0 were similar but not identical for the 10-day intake and acute treatment challenge models.
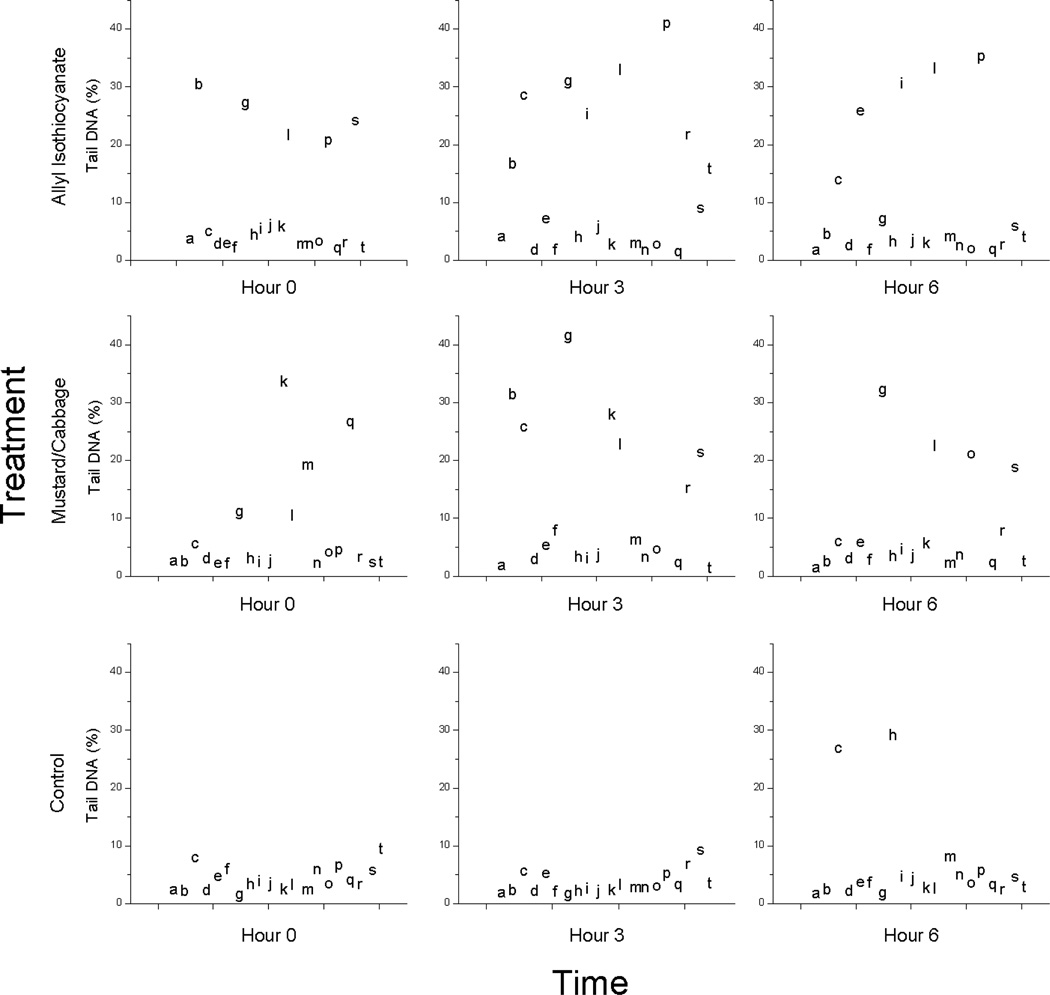
As was observed in the daily treatment portion of the experiment, the % tail DNA of PBMCs from individuals from the control group were predominantly less than 10% (Fig. 3). In contrast, the % tail DNA in PBMCs from both the AITC and the M/C groups were comparatively more variable at day 11 hours 0, 3, and 6. Although most of the values for % tail DNA were less than 10% for the AITC and the M/C groups at each hour, at hour 3, there was a sufficient number of individuals with relatively higher % tail DNA in the AITC and M/C treatments compared to the control that the LS means were significantly higher in the AITC and M/C groups compared to the control group. By hour 6, % tail DNA in the AITC and M/C groups was still more variable than that of the control group, although the treatment effect at hour 6 was nonsignificant.
Figure 3.
Percent tail DNA measured by the comet assay using peripheral blood mononuclear cells isolated from blood samples collected on day 11 immediately before treatment consumption and at 3 and 6 hours after consumption. Data points correspond to individual participants. Treatments were allyl isothiocyanate, mustard/cabbage, and control.
Effect of daily treatment intervention on urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG) concentration
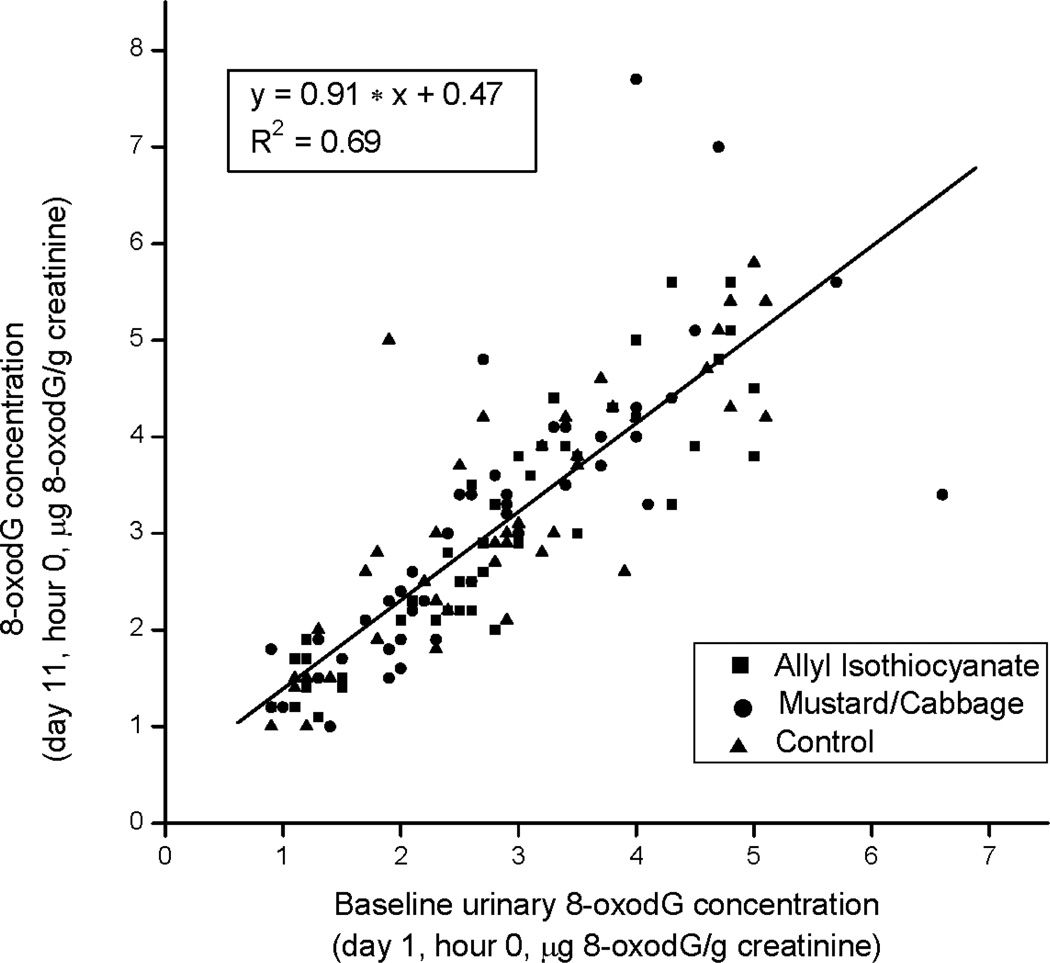
There was no significant treatment effect for urinary 8-oxodG in response to 10-day treatment intake (LS means ± SEMs of 2.95 ± 0.09 for control, 2.88 ± 0.09 for AITC, and 3.06 ± 0.09 for mustard/cabbage). Baseline (day 1 hour 0) 8-oxodG was the only significant effect in the repeated measures mixed models analysis of variance (ANOVA; P < 0.0001). The significant predictive contribution of the baseline 8-oxodG values to the day 11 hour 0 values is evident in the scatter plot and linear regression of Fig. 4 (R2 = 0.69). As determined by the ANOVA and characterized by the random dispersion of the 3 symbols in the plot corresponding to the 3 treatments, the dietary interventions did not significantly influence 8-oxodG over the 11-day test period.
Figure 4.
Urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG) concentration (µg 8-oxodG/g creatinine) at day 11, hour 0,vs. baseline urinary 8-oxodG (day 1,hour 0) Data points represent individual participants and corresponding treatments.
Acute response of urinary 8-oxodG to the treatment challenge on day 11
There was no treatment or treatment by hour effect on urinary 8-oxodG (Table 3). Baseline was an important covariate (P < 0.0001). The sex of the study participants was a significant term in the ANOVA model (P < 0.05) with men having higher levels of 8-oxodG compared to women (3.44 ± 0.14 compared to 2.98 ± 0.09 µg 8-oxodG/g creatinine, respectively, LS mean ± SEM).
Table 3.
Urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG) concentration on day 11 in response to an acute challenge of mustard/cabbage orallyl isothiocyanate1,2.
| 8-oxodG (µg 8-oxodG /g creatinine) | |||
|---|---|---|---|
| Hour | Control | Treatment Mustard/Cabbage |
Allylisothiocyanate |
| 0 | 3.11 ± 0.11 | 3.21 ± 0.11 | 3.04 ± 0.11 |
| 3 | 3.43 ± 0.12 | 3.22 ± 0.12 | 3.24 ± 0.12 |
| 6 | 3.25 ± 0.12 | 3.23 ± 0.12 | 3.18 ± 0.12 |
Values represent LS mean ± SEM for µg 8-oxodG/g creatinine.
Because LS means are a linear combination of effects in a model, and the models for the 10-day treatment intake and the acute treatment challenge were different (e.g., there was no hour effect to be estimated during the 10-day treatment intake), LS means computed for day 11 hour 0 were similar but not identical for the 10-day intake (LS means ± SEMs of 2.95 ± 0.09 for control, 3.06 ± 0.09 for mustard/cabbage, and 2.88 ± 0.09 for AITC) and acute treatment challenge models (LS means ± SEMs of 3.11 ± 0.11 for control, 3.21 ± 0.11 for mustard/cabbage, and 3.04 ± 0.11 for AITC).
Change in AllylIsothiocyanate Concentrations in Cabbage and Mustard
During the course of the study, the AITC concentration in the cabbage decreased by 48% (0.34 uM ± 0.02 to 0.18 ± 0.01 uM/150-g dose, mean ± SEM) and the AITC concentration in the mustard decreased by 89% (114.39 ± 2.01 to 12.20 ± 0.63 uM/30-g dose, mean ± SEM). The composition of the food-grade AITC was characterized by GC-MS analysis where the chromatogram had a single peak and its mass spectrum was the same as others reported for AITC [46, 47].
Discussion
This study was designed to test the potential effects of pure AITC and food products containing AITC on biomarkers of cancer risk. Cabbage was chosen as one of the food products because sinigrin content of cabbage can reach approximately 0.7 µmol per gram wet weight [34]. Thus, 100 g pureed cabbage would be expected to provide up to 70 µmol AITC. However, sinigrin content of cabbage varies with season, cultivar, and growing location [34, 35]. We pureed the cabbage to maximize the conversion of sinigrin to AITC. However, upon analysis of the AITC in the test foods, we found that the test cabbage contained unexpectedly small amounts of AITC. Therefore, we rebalanced the proportions of cabbage and mustard with an emphasis on mustard. The result was that mustard was the primary provider of AITC in the food treatment arm. Although cabbage provided little AITC, the food arm of the study was intended to model real-world consumption of AITC where bioactive components in foods such as cabbage have the potential to modulate effects produced by pure AITC.
Previous studies of the effect of allylisothiocyanateon DNA integrity have produced mixed results, some suggesting that AITC protects DNA, and others suggesting that AITC is genotoxic. Brassica extracts or components have provided protection to DNA exposed to chemical carcinogens or H2O2in vitro. When human lymphocytes were preincubated with Brussels sprout extract or sinigrin, the lymphocytes were afforded protection against H2O2 exposure as assessed by SCGE [48]. Pretreatment of a human colon cell line with sulforaphane or indole [3,2-b] carbazole (glucosinolate metabolites) provided protection against DNA strand breaks caused by benzy(a)pyrene (BaP) [49]. Murinehepatoma cells were protected against H2O2 induced DNA strand breaks by incubation with Brussels sprout extract as well as various glucosinolates [50]. Phenethylisothiocyanate and indole-3-carbinol, both glucosinolate metabolites, protected HepG2 cells from DNA damage induced by chemical carcinogens (NDBA and NPIP), while AITC at the same concentrations did not [51]. AITC at 1 µM did not cause measurable DNA damage and attenuated damage caused by two chemical carcinogens (NPYR and NDMA), while AITC at 50 µM caused significant DNA strand breakage to HepG2 cells [52]. In another study, extracts of Brussels sprouts reduced formation of 8-oxodG in calf thymus DNA in vitro [53]. In each of these studies, the cells were challenged with H2O2 or a chemical carcinogen, and the Brassica extract or component provided protection of DNA integrity.
In contrast to DNA protection observed in many studies, other studies have suggested that isothiocyanates can be genotoxic. In one of the earliest studies, crude juices of Brassica vegetables, including Brussels sprouts, white cabbage, cauliflower, green cabbage, kohlrabi, broccoli, turnip, and black radish, caused point mutations in Salmonella, repairable DNA damage in E.coli, and chromosomal abnormalities in mammalian cells [54]. The vast majority of genotoxicity was derived from the isothiocyanate fraction. Follow-up studies tested specific isothiocyanates to find that genotoxicity, as assessed through assays such as the differential DNA repair assay with E.coli, the micronucleus assay with human HepG2 cells, and SCGE with hepatocytes and gastrointestinal cells, was facilitated by benzyl isothiocyanate [55], allylisothiocyanate [56], phenethylisothiocyanate [56], and methyl isothiocyanate [57]. Benzyl and phenethylisothiocyanateboth increased DNA strand breakage in Caco-2 cells [58]. In a study of two human cell lines, T lymphoblastoid Jurkat and human umbilical vein endothelial cells, sulforaphane induced DNA breakage in both cell lines [59].
Generally, protective effects of isothiocyanates or Brassica extracts are observed when cells are challenged with H2O2 or chemical carcinogens, and DNA damage is observed when cells are incubated with isothiocyanates or Brassica extracts alone. However, AITC acted synergistically with B(a)P to induce strand breaks in HepG2 cells, doubling the strand breakage observed with B(a)P alone [60], even though Brussels sprout extract was protective in the same study. This observation was confirmed in another study of HepG2 cells in which the combination of AITC plus B(a)P caused more DNA damage than B(a)P itself, while mustard extract, which contains AITC, afforded protection against B(a)P induced DNA damage [61].
Despite the increase in DNA strand breaks observed in PBMCs when volunteers consumed AITC or mustard and cabbage, DNA repair as assessed by 8-oxodG was not increased. This result may have occurred if the rate of repair of single strand breaks differed from the base excision repair process that produces 8-oxodG. Indeed, the removal of single strand breaks and 8-hydroxyguanine (8-OH-gua) in HeLa cells and lymphocytes after incubation with H2O2 have different kinetics. In this system, single strand breaks were repaired within an hour whereas for formamidopyrimidineglycosylase-sensitive lesions (FPG acts on 8-OH-gua), removal was not observed over 4 hours [41].
Since DNA damage was elevated 3 hours after consumption of AITC or cabbage and mustard, yet was no longer different from control samples at 6 hours after treatment, it is possible that in addition to DNA repair, the treatments induced apoptosis either generally or in certain cell types of PBMCs. However, it is unlikely that the comet assay could detect the nucleosome-sized fragments characteristic of apoptosis [62, 63]. DNA damage is a known trigger for apoptosis. Double strand breaks are detected by ataxia telangiectasia mutated (ATM) [64] and single stranded DNA is detected by ataxia telangiectasia and Rad3 related protein (ATR) [65], which subsequently signal a cascade of events causing cell cycle arrest [66]. P53 is ultimately activated, which signals transcription of several pro-apoptotic genes, including FAS and BAX [67]. This cascade links DNA damage, such as that observed in this study, to apoptosis, a critical mechanism for inhibiting cancer.
Volunteers in this study were assessed for XPD genotype. XPD codes for an enzyme involved in nucleotide excision repair. Studies have suggested that polymorphisms at XPD codons 751 and/or 312 are associated with increased risk of cancers of the lung [68], prostate [69, 70], bladder [71, 72], skin [73], and other various sites as second primary cancers after diagnosis of nonmelanoma skin cancer [74]. In the present study, XPD genotype did not influence the outcome of DNA damage assessed by SCGE or DNA repair as assessed by 8-oxo-dG, though it should be noted that 8-oxo-dG is not a specific product of the protein coded from XPD. These results are consistent with a recent study that reported that endogenous DNA damage and (+)-anti-benzo[a]-pyrene-7,8-dihydrodiol-9,10-epoxide-induced DNA repair capacity in PBMCs from a healthy population did not correlate with XPD751 genotypes [75].
Volunteers in this study were also evaluated for GSTM1 and GSTT1 genotype, since the glutathione S-transferase isoforms coded from these genes metabolize isothiocyanates. It has been reasoned that deletions in these genes could result in lower rates of metabolism of isothiocyanates, thus increasing exposure to circulating isothiocyanates, and causing differences in physiologic response. In support of this, GSTM1 null individuals consuming broccoli had higher plasma sulforaphane metabolite concentrations than GSTM1 positive individuals [27], though generally the studies of the influence of GST genotype on rates of ITC metabolism have had mixed results [28, 76, 77]. In addition, some studies have suggested that GSTM1 positive individuals gain greater benefit from Brassica vegetable intake than GSTM1 null individuals with respect to cancer risk [78–80]. The outcome variables measured in the present study were not associated with GSTM1 or GSTT1 genotype.
The effects of AITC on cancerous cells are different from those on non-cancerous cells. AITC inhibits proliferation of a variety of neoplastic cell cultures, while having much less impact on viability of nonneoplastic cells. For example, proliferation of two prostate cancer cell lines (LNCaP and PC-3) was significantly reduced to 36–38% upon exposure to AITC, while the effect of AITC on nonneoplastic prostate cells was much less (reducing viability to 83%) [81]. Similarly, Zhang [82] reported that the IC50 for neoplastic human bladder epithelial cells was 10 times higher than that for nonneoplastic bladder epithelial cells. AITC is significantly more toxic to cancer cells than to nonneoplastic cells.
The change in AITC concentration in the mustard and cabbage was unexpected. Unopened jars of mustard were stored at 15°C protected from light, opened jars of mustard were stored at 4°C protected from light, and pureed cabbage was stored at −20°C is containers chosen to minimize head space. Despite the apparent decrease in AITC during storage of the food products, these changes had no measurable impact on the comet and 8-oxodG results as indicated by the nonsignificance of the sequence and treatment × period interaction terms in the statistical models. Allylisothiocyanate is known to conjugate with proteins and to decompose to other sulfur compounds in aqueous solutions, particularly at high temperatures [83, 84]. It is possible that one or more of these metabolites contributed to the observed treatment effects in the comet assay. Alternatively, there may have been a saturation effect in which DNA strand breakage was induced by AITC at doses above a threshold, but DNA strand breakage was not more pronounced at the higher concentrations of AITC present at the beginning of the study.
In conclusion, the data presented here confirm the complicated relationship between Brassica vegetables and cancer risk. Like other studies in the past, these data demonstrated that components of Brassica vegetables are capable of causing DNA strand breaks. However, unexpectedly there was no concomitant increase in DNA repair observed, though only one of many types of DNA repair was assessed in this study. It is possible that other types of DNA repair were induced in response to the AITC challenge, and it is possible that vulnerable cells underwent apoptosis in response to the DNA damage, either of which might be considered beneficial. These results add important information to the body of knowledge about Brassica vegetables and processes related to cancer in vivo, and also highlight the complexity involved as well as avenues for follow-up research.
Acknowledgments
This work was supported by the U.S. Department of Agriculture and the National Cancer Institute Division for Cancer Prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
J.A.M., J.A.N., B.A.C., and C.S.C designed the study. J.A.N., C.S.C., B.A.C., and G.A.A. conducted the study. G.A.A. performed the comet assays. C.S.C. performed the GC-MS analyses. B.T.V., M.H.K., and C.S.C. completed the statistical analyses. J.A.N. and C.S.C wrote the paper, and other authors reviewed the manuscript.
References
- 1.Seow A, Yuan J-M, Sun C-L, Van Den Berg D, Lee H-P, Yu MC. Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese Health Study. Carcinogenesis. 2002;23:2055–2061. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- 2.Kvale G, Bjelke E, Gart JJ. Dietary habits and lung cancer risk. Int J Cancer. 1983;31:397–405. doi: 10.1002/ijc.2910310402. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin JK, Gridley G, Block G, Winn DM, Preston-Martin S, Schoenberg JB, Greenberg RS, Stemhagen A, Austin DF, Ershow AG, et al. Dietary factors in oral and pharyngeal cancer. J Natl Cancer Inst. 1988;80:1237–1243. doi: 10.1093/jnci/80.15.1237. [DOI] [PubMed] [Google Scholar]
- 4.Gridley G, McLaughlin JK, Block G, Blot WJ, Winn DM, Greenberg RS, Schoenberg JB, Preston-Martin S, Austin DF, Fraumeni JF., Jr Diet and oral and pharyngeal cancer among blacks. Nutr Cancer. 1990;14:219–225. doi: 10.1080/01635589009514096. [DOI] [PubMed] [Google Scholar]
- 5.Benito E, Obrador A, Stiggelbout A, Bosch FX, Mulet M, Munoz N, Kaldor J. A population-based case-control study of colorectal cancer in Majorca. I. Dietary factors. Int J Cancer. 1990;45:69–76. doi: 10.1002/ijc.2910450114. [DOI] [PubMed] [Google Scholar]
- 6.Miller AB, Howe GR, Jain M, Craib KJ, Harrison L. Food items and food groups as risk factors in a case-control study of diet and colo-rectal cancer. Int J Cancer. 1983;32:155–161. doi: 10.1002/ijc.2910320204. [DOI] [PubMed] [Google Scholar]
- 7.Bueno de Mesquita HB, Maisonneuve P, Runia S, Moerman CJ. Intake of foods and nutrients and cancer of the exocrine pancreas: a population-based case-control study in The Netherlands. Int J Cancer. 1991;48:540–549. doi: 10.1002/ijc.2910480411. [DOI] [PubMed] [Google Scholar]
- 8.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- 9.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- 10.Powolny AA, Bommareddy A, Hahm ER, Normolle DP, Beumer JH, Nelson JB, Singh SV. Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J Natl Cancer Inst. 2011;103:571–584. doi: 10.1093/jnci/djr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiao JW, Wu H, Ramaswamy G, Conaway CC, Chung FL, Wang L, Liu D. Ingestion of an isothiocyanate metabolite from cruciferous vegetables inhibits growth of human prostate cancer cell xenografts by apoptosis and cell cycle arrest. Carcinogenesis. 2004;25:1403–1408. doi: 10.1093/carcin/bgh136. [DOI] [PubMed] [Google Scholar]
- 12.Warin R, Xiao D, Arlotti JA, Bommareddy A, Singh SV. Inhibition of human breast cancer xenograft growth by cruciferous vegetable constituent benzyl isothiocyanate. Mol Carcinog. 2010;49:500–507. doi: 10.1002/mc.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya A, Tang L, Li Y, Geng F, Paonessa JD, Chen SC, Wong MK, Zhang Y. Inhibition of bladder cancer development by allyl isothiocyanate. Carcinogenesis. 2010;31:281–286. doi: 10.1093/carcin/bgp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munday R, Munday CM. Selective induction of phase II enzymes in the urinary bladder of rats by allyl isothiocyanate, a compound derived from Brassica vegetables. Nutr Cancer. 2002;44:52–59. doi: 10.1207/S15327914NC441_7. [DOI] [PubMed] [Google Scholar]
- 15.Angeloni C, Leoncini E, Malaguti M, Angelini S, Hrelia P, Hrelia S. Modulation of phase II enzymes by sulforaphane: implications for its cardioprotective potential. J Agric Food Chem. 2009;57:5615–5622. doi: 10.1021/jf900549c. [DOI] [PubMed] [Google Scholar]
- 16.Xiao D, Zeng Y, Choi S, Lew KL, Nelson JB, Singh SV. Caspase-dependent apoptosis induction by phenethyl isothiocyanate, a cruciferous vegetable-derived cancer chemopreventive agent, is mediated by Bak and Bax. Clin Cancer Res. 2005;11:2670–2679. doi: 10.1158/1078-0432.CCR-04-1545. [DOI] [PubMed] [Google Scholar]
- 17.Choi S, Singh SV. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer Res. 2005;65:2035–2043. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- 18.Prochaska HJ, Santamaria AB, Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci U S A. 1992;89:2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 20.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 21.Munday R, Munday CM. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. J Agric Food Chem. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 22.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornalley PJ. Isothiocyanates: mechanism of cancer chemopreventive action. Anticancer Drugs. 2002;13:331–338. doi: 10.1097/00001813-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Kolm RH, Danielson UH, Zhang Y, Talalay P, Mannervik B. Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. Biochem J. 1995;311(Pt 2):453–459. doi: 10.1042/bj3110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Kolm RH, Mannervik B, Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun. 1995;206:748–755. doi: 10.1006/bbrc.1995.1106. [DOI] [PubMed] [Google Scholar]
- 26.Lin HJ, Probst-Hensch NM, Louie AD, Kau IH, Witte JS, Ingles SA, Frankl HD, Lee ER, Haile RW. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 1998;7:647–652. [PubMed] [Google Scholar]
- 27.Gasper AV, Al-janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am. J. Clin. Nutr. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 28.Steck SE, Gammon MD, Hebert JR, Wall DE, Zeisel SH. GSTM1, GSTT1, GSTP1, and GSTA1 polymorphisms and urinary isothiocyanate metabolites following broccoli consumption in humans. J Nutr. 2007;137:904–909. doi: 10.1093/jn/137.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brauer H, Libby T, Mitchell B, Li L, Chen C, Randolph T, Yasui Y, Lampe J, Lampe P. Cruciferous vegetable supplementation in a controlled diet study alters the serum peptidome in a GSTM1-genotype dependent manner. Nutrition Journal. 2011;10:11. doi: 10.1186/1475-2891-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Yu MC, Lee HP. Dietary isothiocyanates, glutathione S-transferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2001;10:1063–1067. [PubMed] [Google Scholar]
- 31.Dyba M, Wang A, Noone AM, Goerlitz D, Shields P, Zheng YL, Rivlin R, Chung FL. Metabolism of isothiocyanates in individuals with positive and null GSTT1 and M1 genotypes after drinking watercress juice. Clin Nutr. 2010;29:813–818. doi: 10.1016/j.clnu.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H, Lin J, Grossman HB, Hernandez LM, Dinney CP, Wu X. Dietary isothiocyanates, GSTM1, GSTT1, NAT2 polymorphisms and bladder cancer risk. Int J Cancer. 2007;120:2208–2213. doi: 10.1002/ijc.22549. [DOI] [PubMed] [Google Scholar]
- 33.Cornelis MC, El-Sohemy A, Campos H. GSTT1 genotype modifies the association between cruciferous vegetable intake and the risk of myocardial infarction. Am J Clin Nutr. 2007;86:752–758. doi: 10.1093/ajcn/86.3.752. [DOI] [PubMed] [Google Scholar]
- 34.Charron CS, Saxton AM, Sams CE. Relationship of climate and genotype to seasonal variation in the glucosinolate-myrosinase system. I. Glucosinolate content in ten cultivars of Brassica oleracea grown in fall and spring seasons. Jornal of the Science of Food and Agriculture. 2005;85:671–681. [Google Scholar]
- 35.Kusznierewicz B, Bartoszek A, Wolska L, Drzewiecki J, Gorinstein S, Namiesnik J. Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. LWT Food Science and Technology. 2008;41:1–9. [Google Scholar]
- 36.Kennedy ET, Bowman SA, Powell R. Dietary-fat intake in the US population. J Am Coll Nutr. 1999;18:207–212. doi: 10.1080/07315724.1999.10718853. [DOI] [PubMed] [Google Scholar]
- 37.Serdula MK, Gillespie C, Kettel-Khan L, Farris R, Seymour J, Denny C. Trends in fruit and vegetable consumption among adults in the United States: Behavioral risk factor surveillance system, 1994–2000. Am J Public Health. 2004;94:1014–1018. doi: 10.2105/ajph.94.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rouzaud G, Young SA, Duncan AJ. Hydrolysis of Glucosinolates to Isothiocyanates after Ingestion of Raw or Microwaved Cabbage by Human Volunteers. Cancer Epidemiol Biomarkers Prev. 2004;13:125–131. doi: 10.1158/1055-9965.epi-085-3. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Aldini G, Johnson EJ, Rasmussen H, Kraemer K, Woolf H, Musaeus N, Krinsky NI, Russell RM, Yeum K-J. Modification of lymphocyte DNA damage by carotenoid supplementation in postmenopausal women. Am J Clin Nutr. 2006;83:163–169. doi: 10.1093/ajcn/83.1.163. [DOI] [PubMed] [Google Scholar]
- 40.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 41.Collins AR. The comet assay for DNA damage and repair - Principles, applications, and limitations. Mol. Biotechnol. 2004;26:249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 42.Bogdanov MB, Beal MF, McCabe DR, Griffin RM, Matson WR. A carbon column-based liquid chromatography electrochemical approach to routine 8-hydroxy-2'-deoxyguanosine measurements in urine and other biologic matrices: a one-year evaluation of methods. Free Radic Biol Med. 1999;27:647–666. doi: 10.1016/s0891-5849(99)00113-6. [DOI] [PubMed] [Google Scholar]
- 43.Evans MD, Olinski R, Loft S, Cooke MS, Rossner P, Jr, Sram R, Henriksen T, Poulsen HE, Weimann A, Barbieri A, Sabatini L, Violante F, Kino S, Ochi T, Sakai K, Takeuchi M, Kasai H, Meerman JHN, Gackowski D, Rozalski R, Siomek A, Halliwell B, Jenner AM, Wang H, Cerda C, Saez G, Haghdoost S, Svoboda P, Hu C-W, Chao M-R, Peng K-Y, Shih W-C, Wu K-Y, Orhan H, Istanbullu NS, Mistry V, Farmer PB, Sandhu J, Singh R, Cortez C, Su Y, Santella RM, Lambert P, Smith R. Toward consensus in the analysis of urinary 8-oxo-7,8-dihydro-2â€2- deoxyguanosine as a noninvasive biomarker of oxidative stress. FASEB Journal. 2010;24:1249–1260. doi: 10.1096/fj.09-147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oehlert GW. A note on the delta method. American Statistician. 1992;46:27–29. [Google Scholar]
- 45.A language and environment for statistical computing. R Development Core Team, R Foundation for Statistical Computing. 2011 Available from: http://www.R-project.org/ [Google Scholar]
- 46.Kjaer A, Ohashi M, Wilson J, Djerassi C. Mass spectra of isothiocyanates. Acta Chem Scand. 1963;17:2143–2154. [Google Scholar]
- 47. [accessed January 31, 2012]; http://webbook.nist.gov/cgi/cbook.cgi?Units=SI&cTG=on&cIR=on&cTC=on&cMS=on&cTP=on&cES=on&cTR=on&cPI=on&cDI=on&ID=C57067#Mass-Spec.
- 48.Zhu C-Y, Loft S. Effects of Brussels sprouts extracts on hydrogen peroxide-induced DNA strand breaks in human lymphocytes. Food Chem. Toxicol. 2001;39:1191–1197. doi: 10.1016/s0278-6915(01)00061-8. [DOI] [PubMed] [Google Scholar]
- 49.Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 50.Zhu CY, Loft S. Effect of chemopreventive compounds from Brassica vegetables on NAD(P)H:quinone reductase and induction of DNA strand breaks in murine hepa1c1c7 cells. Food Chem Toxicol. 2003;41:455–462. doi: 10.1016/s0278-6915(02)00278-8. [DOI] [PubMed] [Google Scholar]
- 51.García A, Haza AI, Arranz N, Rafter J, Morales P. Protective effects of isothiocyanates alone or in combination with vitamin C towards N-nitrosodibutylamine or N-nitrosopiperidine-induced oxidative DNA damage in the single-cell gel electrophoresis (SCGE)/HepG2 assay. J. Appl. Toxicol. 2008;28:196–204. doi: 10.1002/jat.1270. [DOI] [PubMed] [Google Scholar]
- 52.Arranz N, Haza AI, Garcia A, Moller L, Rafter J, Morales P. Protective effects of isothiocyanates towards N-nitrosamine-induced DNA damage in the single-cell gel electrophoresis (SCGE)/HepG2 assay. J Appl Toxicol. 2006;26:466–473. doi: 10.1002/jat.1163. [DOI] [PubMed] [Google Scholar]
- 53.Zhu C, Poulsen HE, Loft S. Inhibition of oxidative DNA damage in vitro by extracts of Brussels sprouts. Free Radical Research. 2000;33:187–196. doi: 10.1080/10715760000300741. [DOI] [PubMed] [Google Scholar]
- 54.Kassie F, Parzefall W, Musk S, Johnson I, Lamprecht G, Sontag G, Knasmuller S. Genotoxic effects of crude juices from Brassica vegetables and juices and extracts from phytopharmaceutical preparations and spices of cruciferous plants origin in bacterial and mammalian cells. Chem. -Biol. Interact. 1996;102:1–16. doi: 10.1016/0009-2797(96)03728-3. [DOI] [PubMed] [Google Scholar]
- 55.Kassie F, Pool-Zobel B, Parzefall W, Knasmuller S. Genotoxic effects of benzyl isothiocyanate, a natural chemopreventive agent. Mutagenesis. 1999;14:595–603. doi: 10.1093/mutage/14.6.595. [DOI] [PubMed] [Google Scholar]
- 56.Kassie F, Knasmuller S. Genotoxic effects of allyl isothiocyanate (AITC) and phenethyl isothiocyanate (PEITC) Chem. -Biol. Interact. 2000;127:163–180. doi: 10.1016/s0009-2797(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 57.Kassie F, Laky B, Nobis E, Kundi M, Knasmuller S. Genotoxic effects of methyl isothiocyanate. Mutat Res. 2001;490:1–9. doi: 10.1016/s1383-5718(00)00140-6. [DOI] [PubMed] [Google Scholar]
- 58.Visanji JM, Duthie SJ, Pirie L, Thompson DG, Padfield PJ. Dietary isothiocyanates inhibit Caco-2 cell proliferation and induce G2/M phase cell cycle arrest, DNA damage, and G2/M checkpoint activation. J Nutr. 2004;134:3121–3126. doi: 10.1093/jn/134.11.3121. [DOI] [PubMed] [Google Scholar]
- 59.Sestili P, Paolillo M, Lenzi M, Colombo E, Vallorani L, Casadei L, Martinelli C, Fimognari C. Sulforaphane induces DNA single strand breaks in cultured human cells. Mutat Res. 2010;689:65–73. doi: 10.1016/j.mrfmmm.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Laky B, Knasmuller S, Gminski R, Mersch-Sundermann V, Scharf G, Verkerk R, Freywald C, Uhl M, Kassie F. Protective effects of Brussels sprouts towards B[a]P-induced DNA damage: A model study with the single-cell gel electrophoresis (SCGE)/Hep G2 assay. Food Chem. Toxicol. 2002;40:1077–1083. doi: 10.1016/s0278-6915(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 61.Uhl M, Laky B, Lhoste E, Kassie F, Kundi M, Knasmüller S. Effects of mustard sprouts and allylisothiocyanate on benzo(a)pyrene-induced DNA damage in human-derived cells: A model study with the single cell gel electrophoresis/Hep G2 assay. Teratogenesis, Carcinogenesis, and Mutagenesis. 2003;23:273–282. doi: 10.1002/tcm.10051. [DOI] [PubMed] [Google Scholar]
- 62.Collins AR, Oscoz AA, Brunborg G, Gaivao I, Giovannelli L, Kruszewski M, Smith CC, Stetina R. The comet assay: topical issues. Mutagenesis. 2008;23:143–151. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- 63.Morley N, Rapp A, Dittmar H, Salter L, Gould D, Greulich KO, Curnow A. UVA-induced apoptosis studied by the new apo/necro-Comet-assay which distinguishes viable, apoptotic and necrotic cells. Mutagenesis. 2006;21:105–114. doi: 10.1093/mutage/gel004. [DOI] [PubMed] [Google Scholar]
- 64.Imreh G, Norberg HV, Imreh S, Zhivotovsky B. Chromosomal breaks during mitotic catastrophe trigger {gamma}H2AX-ATM-p53-mediated apoptosis. J Cell Sci. 2011;124:2951–2963. doi: 10.1242/jcs.081612. [DOI] [PubMed] [Google Scholar]
- 65.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Croker BA, O'Donnell JA, Nowell CJ, Metcalf D, Dewson G, Campbell KJ, Rogers KL, Hu Y, Smyth GK, Zhang JG, White M, Lackovic K, Cengia LH, O'Reilly LA, Bouillet P, Cory S, Strasser A, Roberts AW. Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci U S A. 2011;108:13135–13140. doi: 10.1073/pnas.1110358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu Z, Wei Q, Wang X, Shen H. DNA repair gene XPD polymorphism and lung cancer risk: a meta-analysis. Lung Cancer. 2004;46:1–10. doi: 10.1016/j.lungcan.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Rybicki BA, Conti DV, Moreira A, Cicek M, Casey G, Witte JS. DNA repair gene XRCC1 and XPD polymorphisms and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:23–29. doi: 10.1158/1055-9965.epi-03-0053. [DOI] [PubMed] [Google Scholar]
- 70.Bau DT, Wu HC, Chiu CF, Lin CC, Hsu CM, Wang CL, Wang RF, Tsai FJ. Association of XPD polymorphisms with prostate cancer in Taiwanese patients. Anticancer Res. 2007;27:2893–2896. [PubMed] [Google Scholar]
- 71.Chang CH, Wang RF, Tsai RY, Wu HC, Wang CH, Tsai CW, Chang CL, Tsou YA, Liu CS, Bau DT. Significant association of XPD codon 312 single nucleotide polymorphism with bladder cancer susceptibility in Taiwan. Anticancer Res. 2009;29:3903–3907. [PubMed] [Google Scholar]
- 72.Wang M, Gu D, Zhang Z, Zhou J. XPD polymorphisms, cigarette smoking, and bladder cancer risk: a meta-analysis. J Toxicol Environ Health A. 2009;72:698–705. doi: 10.1080/15287390902841029. [DOI] [PubMed] [Google Scholar]
- 73.Baccarelli A, Calista D, Minghetti P, Marinelli B, Albetti B, Tseng T, Hedayati M, Grossman L, Landi G, Struewing JP, Landi MT. XPD gene polymorphism and host characteristics in the association with cutaneous malignant melanoma risk. Br J Cancer. 2004;90:497–502. doi: 10.1038/sj.bjc.6601385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brewster AM, Alberg AJ, Strickland PT, Hoffman SC, Helzlsouer K. XPD polymorphism and risk of subsequent cancer in individuals with nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1271–1275. [PubMed] [Google Scholar]
- 75.Slyskova J, Naccarati A, Polakova V, Pardini B, Vodickova L, Stetina R, Schmuczerova J, Smerhovsky Z, Lipska L, Vodicka P. DNA damage and nucleotide excision repair capacity in healthy individuals. Environ Mol Mutagen. 2011;52:511–517. doi: 10.1002/em.20650. [DOI] [PubMed] [Google Scholar]
- 76.Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7:775–781. [PubMed] [Google Scholar]
- 77.Fowke JH, Shu X-O, Dai Q, Shintani A, Conaway CC, Chung F-L, Cai Q, Gao Y-T, Zheng W. Urinary isothiocyanate excretion Brassica consumption, and gene polymorphisms among women living in Shanghai, China. Cancer Epidemiol. Biomarkers Prev. 2003;12:1536–1539. [PubMed] [Google Scholar]
- 78.Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, Zhang YS, Marshall JR, Ambrosone CB. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr. Cancer. 2004;50:206–213. doi: 10.1207/s15327914nc5002_11. [DOI] [PubMed] [Google Scholar]
- 79.Spitz MR, Duphorne CM, Detry MA, Pillow PC, Amos CI, Lei L, de Andrade M, Gu X, Hong WK, Wu X. Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:1017–1020. [PubMed] [Google Scholar]
- 80.Wang LI, Giovannucci EL, Hunter D, Neuberg D, Su L, Christiani DC. Dietary intake of Cruciferous vegetables, Glutathione S-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes Control. 2004;15:977–985. doi: 10.1007/s10552-004-1093-1. [DOI] [PubMed] [Google Scholar]
- 81.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Molecular Nutrition and Food Research. 2010;54:127–135. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawakishi S, Kaneko T. Interaction of proteins with allyl isothiocyanate. J. Agric. Food Chem. 1987;35:85–88. [Google Scholar]
- 84.Pechacek R, Velisek J, Hrabcova H. Decomposition products of allyl isothiocyanate in aqueous solutions. J. Agric. Food Chem. 1997;45:4584–4588. [Google Scholar]