Abstract
c-Jun N-terminal kinase (JNK) activation promotes hepatocyte death during acetaminophen overdose, a common cause of drug-induced liver failure. While mitogen-activated protein kinase (MAPK) phosphatase (Mkp)-1 is a critical negative regulator of JNK MAPK, little is known about the role of Mkp-1 during hepatotoxicity. In this study, we evaluated the role of Mkp-1 during acute acetaminophen toxicity. Mkp-1+/+ and Mkp-1−/− mice were dosed ip with vehicle or acetaminophen at 300 mg/kg (for mechanistic studies) or 400 mg/kg (for survival studies). Tissues were collected 1–6 hr post 300 mg/kg dosing to assess glutathione levels, organ damage, and MAPK activation. Mkp-1−/− mice exhibited more rapid plasma clearance of acetaminophen than did Mkp-1+/+ mice, indicated by a quicker decline of plasma acetaminophen level. Moreover, Mkp-1−/− mice suffered more severe liver injury, indicated by higher plasma alanine transaminase activity and more extensive centrilobular apoptosis and necrosis. Hepatic JNK activity in Mkp-1−/− mice was higher than in Mkp-1+/+ mice. Finally, Mkp-1−/− mice displayed a lower overall survival rate and shorter median survival time after dosing with 400 mg/kg acetaminophen. The more severe phenotype exhibited by Mkp-1−/− mice indicates that Mkp-1 plays a protective role during acute acetaminophen overdose, potentially through regulation of JNK.
Keywords: toxicity, hepatic, mouse, acetaminophen, Mkp-1, JNK
Introduction
The analgesic acetaminophen is currently the most frequent cause of drug-induced hepatic failure in the United States, with intentional and unintended overdose contributing to 10,000 to 50,000 hospitalizations and 500 deaths annually (Nourjah et al. 2006). The mechanism mediating acetaminophen toxicity is well characterized as a consequence of saturation of metabolism (Mitchell et al. 1973a; Nelson 1990). Under prescribed dosing, the majority of acetaminophen is rapidly metabolized in the liver by phase II glucuronide and sulfate conjugation enzymes to nontoxic compounds (Mitchell et al. 1973a). Additionally, a relatively minor portion of the drug is converted by phase I cytochrome p450 enzymes to the highly reactive intermediate metabolite N-acetyl-p-benzoquinone-imine (NAPQI; Dahlin et al. 1984; Gonzalez and Kimura 2003). NAPQI has an extremely short half-life and is rapidly excreted after conjugation with glutathione under normal physiological conditions (Mitchell et al. 1973b). Acetaminophen overdose overwhelms the major metabolic pathway of acetaminophen elimination, causing both increased NAPQI formation and depletion of the reduced form of cellular glutathione (Mitchell et al. 1973b). Excess NAPQI can covalently bind to essential cellular macromolecules, causing hepatocyte death and liver injury (Jollow et al. 1974; Jollow et al. 1973; Mitchell et al. 1973b). Recently, specific downstream biochemical signaling cascades have been identified that perpetuate acetaminophen-induced hepatotoxicity (reviewed in Han et al. 2010), including the JNK family (Gunawan et al. 2006; Latchoumycandane et al., 2007; Henderson et al. 2007).
JNK proteins are members of the MAPK family that regulate numerous hepatic cellular processes and diseases, including hepatocyte apoptosis and development of steatohepatitis in mouse models (Qiao et al. 2003; Singh et al. 2009). During acetaminophen overdose, JNK is activated, likely by the upstream kinases, including apoptosis signal-regulating kinase 1 (Nakagawa et al. 2008). Activated JNK perpetuates hepatic damage by altering redox status (Nakagawa et al. 2008), modulating bcl-2 family activity (Gunawan et al. 2006; Latchoumycandane et al. 2007), and altering mitochondrial biogenesis (Hanawa et al. 2008). Inhibition of JNK reduces hepatic injury by preventing mitochondrial stress and reducing peroxynitrate formation (Saito et al. 2010). Both JNK1 and JNK2 isoforms are expressed in the liver (Bogoyevitch 2006) and blocking the activation of both isoforms simultaneously protects mice against acetaminophen-induced hepatotoxicity (Gunawan et al. 2006; Hanawa et al. 2008). Since JNK is emerging as a key mediator in acetaminophen-induced liver injury, recent studies have examined endogenous or exogenous JNK-inhibitors for potential therapeutic use (Hanawa et al. 2008; Saito et al. 2010). One such class of inhibitors is the MkP subfamily of the dual specificity protein phosphatase (DUSP) family.
In mammalian cells, MkPs are the primary phosphatases responsible for dephosphorylating MAPKs (Keyse 2000). MkPs are upregulated by many of the same extracellular stimuli that activate MAPKs, including mitogens and stress (Chi et al. 2006; Salojin et al., 2006; Zhao et al., 2006, Lau and Nathans 1985; Wang and Liu 2007). MkP-1, also referred to as DUSP1, is the prototypical member of the MkP family and it preferentially inactivates the stress-induced MAPKs JNK and p38 in most tissues (Liu et al. 1995; Raingeaud et al. 1995; Franklin and Kraft, 1997; Franklin et al., 1998; Chi et al., 2006; Hammer et al., 2006; Zhao et al. 2006). While Mkp-1 is dispensable for development, growth, and maturation (Dorfman et al. 1996), loss of Mkp-1 prolongs JNK and p38 activation and enhances host susceptibility to systemic inflammatory challenges such as endotoxemia and bacterial infections (Chi et al. 2006; Frazier et al. 2009; Hammer et al. 2006; Wang et al. 2007; Zhao et al. 2006). While MkP-1 is expressed in human and rodent hepatocytes (Tomasi et al. 2010) and inhibition ofMkP-1 prolongs JNK activation (Boutros et al. 2008), relatively little is known about the role of Mkp-1 during toxic hepatic injuries. The goal of this study was to examine the effects of Mkp-1 deficiency in a mouse model of acute acetaminophen overdose. Our data support a role for Mkp-1 in protection against acute acetaminophen-induced hepatic toxicity, likely through inhibiting JNK activity.
Materials and Methods
Experimental Animals
Mkp-1−/− mice were originally generated from embryos provided by Bristol-Myers Squibb Pharmaceutical Research Institute and the creation of these mice has been described previously (Dorfman et al. 1996). Male Mkp-1+/+ and Mkp-1−/− mice 8–16 weeks of age on a C57/129 mixed background were used for all experiments. All mice were housed at 25°C in a vivarium with humidity between 30% and 70%, with a 12-hr alternating light–dark cycle. All experiments were performed according to National Institutes of Health guidelines and the experimental protocols were approved by the Institutional Animal Care and Use Committee at the Research Institute at Nationwide Children’s Hospital.
Acetaminophen Overdose
In all experiments, Mkp-1+/+ and Mkp-1−/− mice were fasted for 16–18 hr to deplete glutathione stores prior to acetaminophen dosing, as has been performed previously (Hirayama et al. 1983). Mice then received a single ip injection of vehicle (phosphate buffered saline), 300, or 400 mg/kg acetaminophen (Sigma). These doses are within the hepatotoxic ranges reported previously for mouse strains such as C57BL/6 (Hanawa et al. 2008) and ICR (Rogers et al. 2000). The 300 mg/kg dose was selected to model a severe but nonfatal overdose, while the 400 mg/kg dose was selected as a potentially lethal dose for use in a survival study. Mice receiving vehicle or 300 mg/kg acetaminophen were euthanized by overdose of pentobarbital 1, 2, 4, or 6 hr after dosing, and blood and liver were collected for further analyses. To evaluate the effect of Mkp-1 deficiency on survival, mice received a 400 mg/kg ip injection of acetaminophen, were re-fed standard chow 6 hr post drug administration (to allow for multiday monitoring), and observed through 120 hr to calculate overall survival.
Biochemical Plasma Measurements
Plasma alanine transaminase (ALT) activity was determined using a Thermo Scientific assay reagent (Infinity ALT(GPT) Liquid Stable Reagent) as previously described (Bergmeyer, Scheibe, and Wahlefeld 1978; Henry et al. 1960). Briefly, this assay measures the ALT-mediated conversion of alanine to pyruvate. Pyruvate is reduced to lactate by lactate dehydrogenase with the simultaneous oxidation of NADH to NAD, which is monitored by measuring the rate of decrease in absorbance at 340 nm. IL-6 concentration in plasma was determined using an ELISA kit (BD Biosciences).
Acetaminophen levels were measured in mouse plasma by high-performance liquid chromatography (HPLC) with UV detection at 248 nm using a modification of the method previously described (Buckpitt et al. 1977; To and Wells 1985). Briefly, 20 µL of plasma was mixed with 25 µL ice-cold acetonitrile and placed on ice for 10 min. The sample was then centrifuged at 10,000 rpm for 5 min at 4°C. The supernatant was collected and the pellet was washed with 25 µL ice-cold acetonitrile and centrifuged again. The supernatants were pooled, dried under a stream of nitrogen gas, and reconstituted in 100 µL of mobile phase A. Analysis was carried out on a Shimadzu HPLC (Shimadzu Scientific Instruments, Columbia, MD) equipped with a Zorbax SB-C18 3.0 × 150 mm (5 µm) column (Agilent Technologies, Santa Clara, CA). Acetaminophen was separated using a solvent gradient at room temperature with a flow rate of 0.6 mL/min. Initial conditions were 95% mobile phase A (0.1% acetic acid in water with 2% methanol) and 5% mobile phase B (0.1% acetic acid in methanol with 2% water). After a 3-min hold, mobile phase B was increased to 20% over 12 min using a solvent curve of −8 (convex) and held at 20% for 5 min. The column was then washed with 80% mobile phase B and reequilibrated with starting conditions. Acetaminophen concentrations were calculated by experimentally derived standard curves.
Histopathology
Liver samples were fixed in formalin, routinely processed into paraffin blocks, and cut into 4-µm sections. The sections were stained with H&E for histological analysis. The evaluator scored the slides while blinded to the genotype, treatment group, and time point. Lesion scores were assigned as follows (adapted from Blazka et al. 1996): grade 0 (no evidence of histological lesions), 1 (minimal congestion and mild hepatocellular cytoplasmic degeneration immediately adjacent to the central vein), 2 (moderate congestion and degeneration with individual necrotic hepatocytes in the periportal region), 3 (marked congestion and degeneration with necrotic hepatocytes extending into the midzonal regions), and 4 (severe congestion and degeneration and necrosis that bridges between most centrilobular zones). TUNEL staining was performed following the manufacturer’s instructions using an In Situ Cell Death Detection Kit (Roche), and counterstained with hemotoxylin. Slides were scored by selecting ten 400× fields that were centered on a centrilobular vein and counting the number of TUNEL-positive nuclei in each field.
Total Hepatic Glutathione Levels
Hepatic levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured as previously described (Rogers et al. 2000). Briefly, frozen tissues were placed into buffer at a ratio of 10% (tissue weight/volume of buffer). Tissue homogenates were prepared in 0.1 M phosphate buffer (pH 7.4) containing ethylenediaminetetraacetic acid (EDTA) either supplemented with 0.01 M N-ethylmaleimide (for assessing GSSG) or without N-ethylmaleimide (for assessing GSH). Tissue contents of both GSH and GSSG were measured by the enzyme recycling method as previously described (Adams, Lauterburg, and Mitchell 1983).
Western Blot Analysis
Western blot analysis was performed as previously described (Chen et al. 2002), using liver homogenates. Rabbit polyclonal antibodies against p-JNK (Cell Signaling Technology), p-p38 (Cell Signaling Technology), JNK1 (Santa Cruz Biotechnology), and p38 (Santa Cruz Biotechnology) were used. Immunoreactivity was visualized using an enhanced chemiluminescent (ECL plus) detection kit (GE Healthcare Life Sciences). Scanned radiographic films were analyzed for band intensity using ImageQuant (GE Healthcare Life Sciences) to determine densitometry values.
Statistical Analysis
Differences in plasma IL-6, plasma ALT, plasma acetaminophen levels, hepatic GSH and GSSG concentrations, and densitometry measurements between groups were compared using a t-test. Survival differences between Mkp-1+/+ and Mkp-1−/− mice were assessed by Kaplan-Meier curve analysis with a log-rank test. Prism statistics software (GraphPad Software) was used for all analyses and a value of p < .05 was considered statistically significant.
Results
Plasma ALT, IL-6, and Acetaminophen Levels
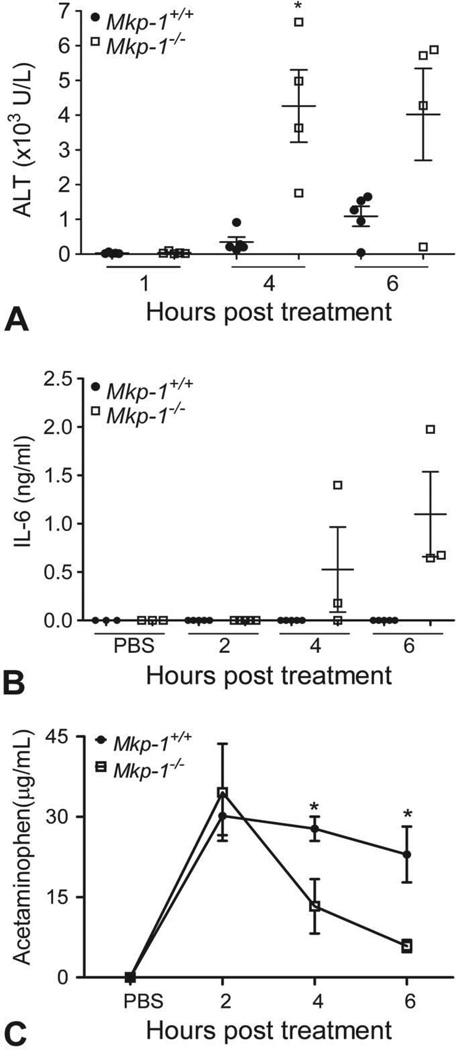
To determine whether Mkp-1−/− mice exhibit higher levels of biomarkers of liver damage and inflammation, plasma levels of ALT and the acute inflammatory cytokine IL-6 were measured in Mkp-1+/+ and Mkp-1−/− mice treated with 300 mg/kg acetaminophen. While ALT levels were comparable between the two strains at 1 hr post dosing, Mkp-1−/− mice had significantly higher ALT levels by 4 hr posttreatment, which continued to trend higher at 6 hr (Fig. 1A). Additionally, while plasma levels of IL-6 were below detectable levels in Mkp-1+/+ mice at all time points, plasma levels of IL-6 were detectable in the Mkp-1−/− at 4 hr and remained elevated through 6 hr post dosing (Fig. 1B). Finally, acetaminophen levels were measured in both strains after dosing with PBS or acetaminophen for 2, 4, or 6 h. Mkp-1−/− mice demonstrated a more rapid clearance of acetaminophen from the plasma, with significantly lower plasma levels at 4 and 6 hr post dosing (Fig. 1C).
Figure 1.
Loss of Mkp-1 function elevates systemic markers of liver damage and inflammation and increases plasma clearance of acetaminophen. Mice were fasted, treated with vehicle or 300 mg/kg acetaminophen ip, and euthanized at the indicated time points. Plasma levels of ALT (A), IL-6 (B), and acetaminophen (C) were measured. Values represent mean ± standard error (n = 3–8 mice per group). *p < .05.
Histopathology and TUNEL Staining
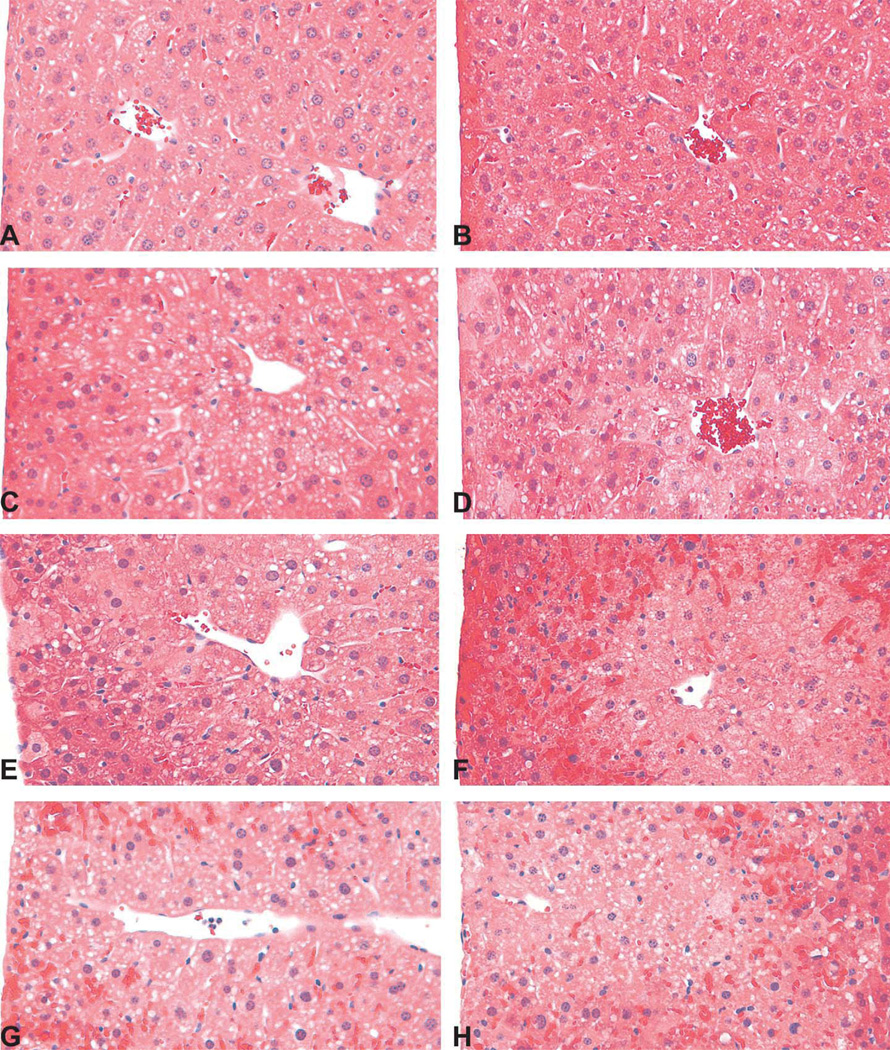
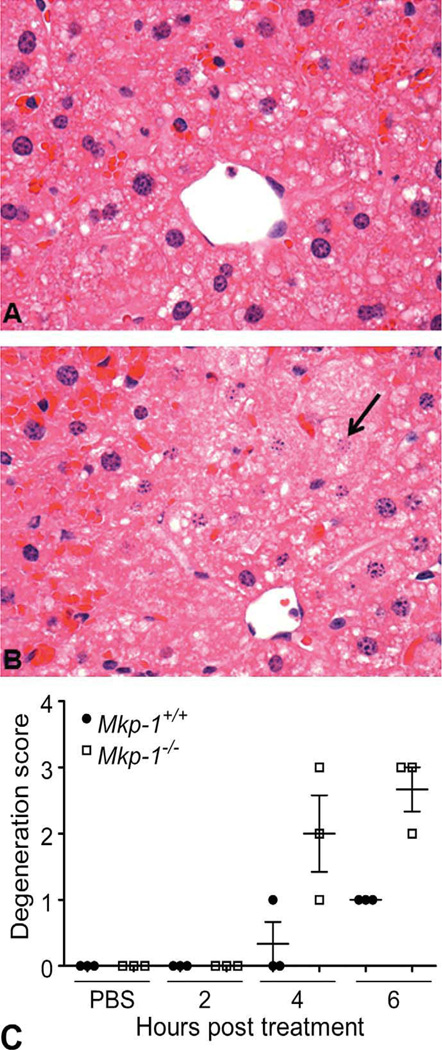
There were no appreciable histopathological lesions in Mkp-1+/+ (Fig. 2A) or Mkp-1−/− (Fig. 2B) mice treated with vehicle. Likewise, no histopathological abnormality was seen in either Mkp-1+/+ (Fig. 2C) or Mkp-1−/− (Fig. 2D) mice treated with 300 mg/kg acetaminophen for 2 hr. At 4 hr post dosing, sections from Mkp-1+/+ mice generally lacked histopathological lesions and were similar to the vehicle-treated groups (Fig. 2E). In contrast, at 4 hr post dosing, livers from Mkp-1−/− showed evidence of cytoplasmic degeneration, including marked vacuolation of centrilobular hepatocytes, centrilobular congestion, and scattered necrotic hepatocytes (Fig. 2F). By 6 hr post acetaminophen dosing, Mkp-1−/− livers displayed widespread congestion, hepatocellular vacuolation, and necrosis of centrilobular hepatocytes, with lesions extending into the midzonal regions (Figs. 2H and 3B). In contrast, lesions in Mkp-1+/+ mice at 6 hr post dosing were limited to mild degenerative changes such as hepatocellular vacuolation and mild congestion, with rare necrotic cells (Figs. 2G and 3A). When comparing lesion grades, livers from Mkp-1−/− mice had higher mean degeneration scores than livers from Mkp-1+/+ mice at both 4 and 6 hr post acetaminophen dosing (Fig. 3C).
Figure 2.
Enhanced centrilobular congestion, hepatocellular degeneration, and necrosis in acetaminophen-treated Mkp-1−/− mice. Mice were fasted and then treated with vehicle (A, B) or 300 mg/kg acetaminophen ip for 2 (C, D), 4, (E, F), or 6 (G, H) hr. Representative photomicrographs from Mkp-1+/+ mice are (A, C, E, and G); sections from Mkp-1−/− mice are (B, D, F, and H; 40 ).
Figure 3.
Enhanced centrilobular congestion, hepatocellular degeneration, necrosis, and histological grade in Mkp-1−/− mice at 6 hr post acetaminophen treatment. Mice were fasted and then treated with 300 mg/kg acetaminophen ip for 6 hr. (A) Representative photomicrograph of liver from a Mkp-1+/+ mouse showing mild degeneration and occasional necrotic hepatocytes (400×). (B) Representative photomicrograph of liver from a Mkp-1−/− mouse showing diffuse centrilobular hepatic necrosis, indicated by loss of nuclei and dissolution of nuclear membranes (arrow) (400×). (C) Group scores for hepatocellular degeneration/necrosis in centrilobular regions of liver sections. Values represent mean ± standard error from three mice.
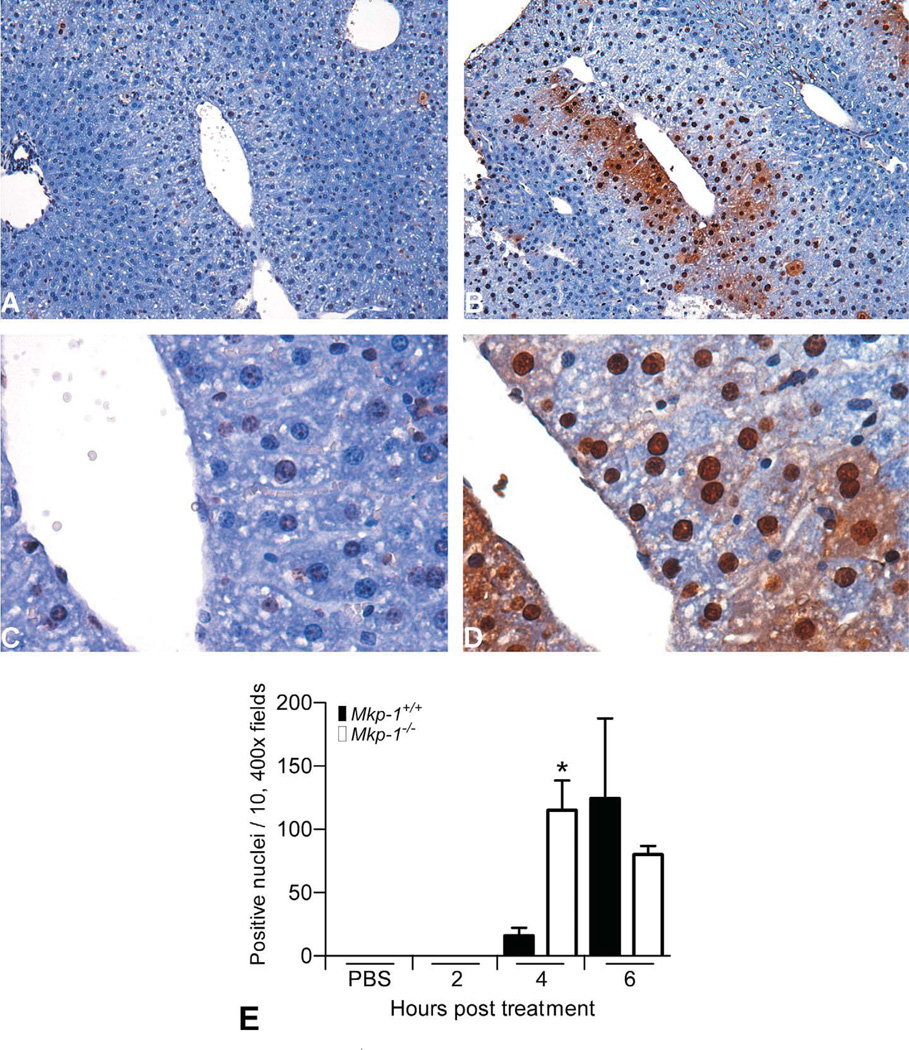
In addition to increased centrilobular necrosis, livers from Mkp-1−/− mice underwent widespread centrilobular apoptosis at earlier time points. TUNEL-positive nuclei were absent in mice treated with PBS or acetaminophen for 2 hr. By 4 hr posttreatment, TUNEL-positive nuclei remained rare in Mkp-1+/+ livers (Fig. 4A and C), while Mkp-1−/− livers had numerous TUNEL-positive nuclei surrounding central veins and foci of necrotic cell debris (Fig. 4B and C). The number of TUNEL-positive nuclei at 4 hr was significantly higher in Mkp-1−/− livers (Fig. 4E). By 6 hr, TUNEL-positive nuclei were common in Mkp-1+/+ livers, while centrilobular cells in Mkp-1−/− livers included large numbers of both TUNEL-positive and necrotic cells.
Figure 4.
Enhanced centrilobular apoptosis in Mkp-1−/− mice at 4 hr post acetaminophen treatment. Mice were fasted and then treated with vehicle or 300 mg/kg acetaminophen ip for the time points indicated. Representative photomicrographs of liver sections from Mkp-1+/+ mice (A and C) and Mkp-1−/− mice (B and D) with TUNEL staining to identify apoptotic nuclei (dark brown staining). A and B are 100× and C and D are 400×. (C) Number of positive nuclei in ten, 400× fields. Values represent mean ± standard error from three mice.
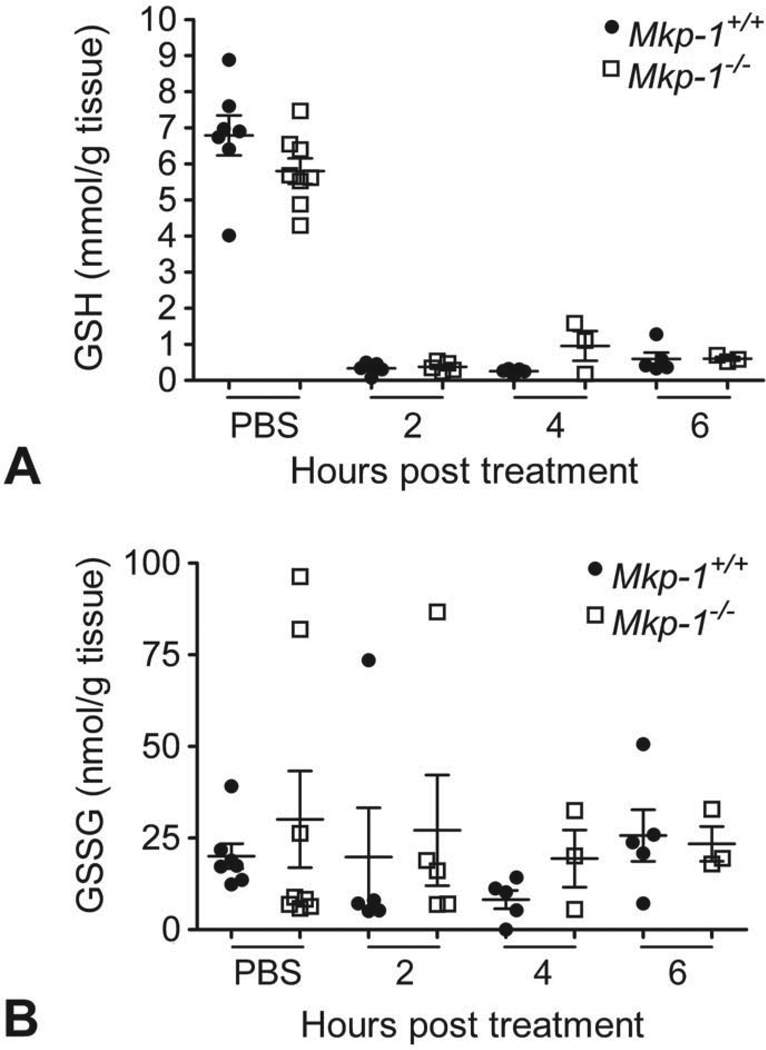
Hepatic Glutathione
While the hepatic levels of both GSH and GSSG trended slightly higher in vehicle-treated Mkp-1−/− mice compared to wild-type liver samples, acetaminophen treatment rapidly depleted hepatic GSH and GSSG in both genotypes (Fig. 5A and B). The hepatic GSH and GSSG levels did not substantially recover during the 6-hr post dosing interval in either wild-type or Mkp-1−/− mice, indicating prolonged glutathione depletion in this model.
Figure 5.
Acetaminophen reduces hepatic GSH and GSSG levels to a comparable degree in wild-type and knockout animals. Liver GSH (A) and GSSG (B) levels were determined for fasted Mkp-1+/+ and Mkp-1−/− mice dosed with vehicle or 300 mg/kg acetaminophen and sacrificed at the time points indicated. Values represent mean ± standard error (n = 3–8 mice/group).
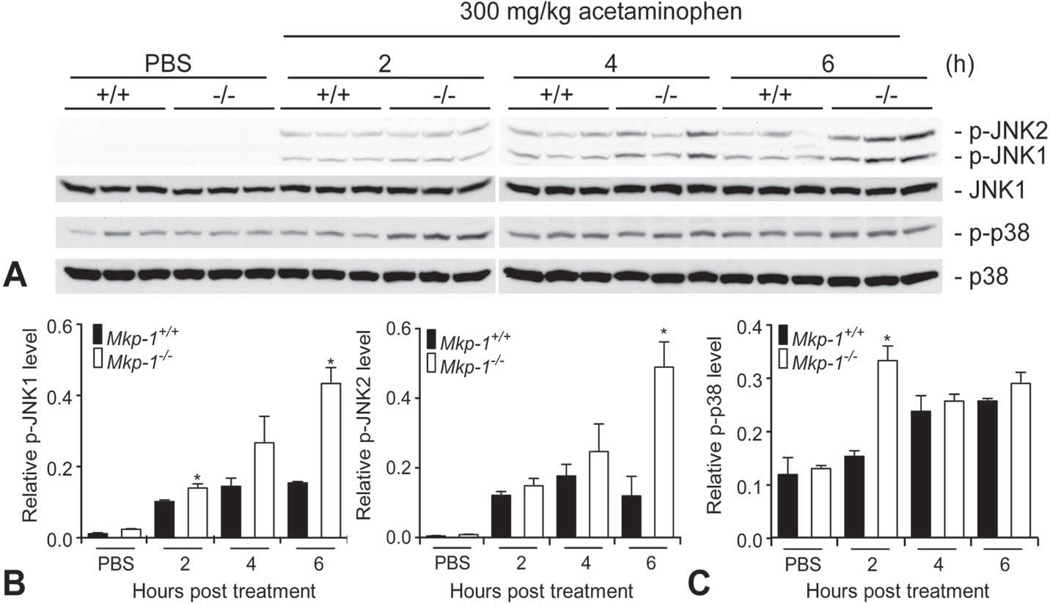
Western Blot
Since previous studies have demonstrated that JNK activation is a key event that perpetuates hepatocellular damage during acetaminophen overdose (Gunawan et al. 2006; Hanawa et al. 2008; Nakagawa et al. 2008; Saito et al. 2010), we assessed JNK activity in liver homogenates from Mkp-1+/+ and Mkp-1−/− mice treated with vehicle or 300 mg/kg acetaminophen. Phospho-JNK1 and phospho-JNK2 levels were very low in livers from vehicle-treated wild-type and Mkp-1 knockout mice. In Mkp-1+/+ mice, acetaminophen dosing resulted in JNK activation within 2 hr that plateaued by 4 hr (Fig. 6A, top panel). In contrast, JNK1 and JNK2 activity in Mkp-1−/− livers continued to increase between 2 and 6 hr. Quantitation of the levels of phosphorylated JNK indicates that at 6 hr, levels of both phospho-JNK1 and phospho-JNK2 were significantly higher in the livers of Mkp-1−/− mice than in livers of wild-type mice (Fig. 6B). In contrast to phospho-JNK, the level of another stress-induced Mkp-1 substrate, phospho-p38, was only moderately increased over the time course examined (Fig. 5A bottom panel). The only time point where p38 activity was significantly different between the two genotypes was at 2 hr (Fig. 6C), which occurred prior to the development of elevated plasma ALT levels and histopathological lesions in Mkp-1−/− mice.
Figure 6.
Loss of Mkp-1 function prolongs hepatic JNK MAPK activation. Liver homogenates were prepared from fasted Mkp-1+/+ and Mkp-1−/− mice treated with vehicle or 300 mg/kg acetaminophen and euthanized at the time points indicated. Homogenates were probed with antibodies against p-JNK, JNK1, p-p38, and p38. (A) Scans of representative Western blot radiographs. (B) Densitometry histograms of scans for p-JNK1 and p-JNK2 in part (A) normalized to JNK1. (C) Densitometry histograms of scans for p-p38 in part (A) normalized to total p38. Values represent mean ± standard error from three mice; n = 3–5 mice/group over all blots performed. *p < .05.
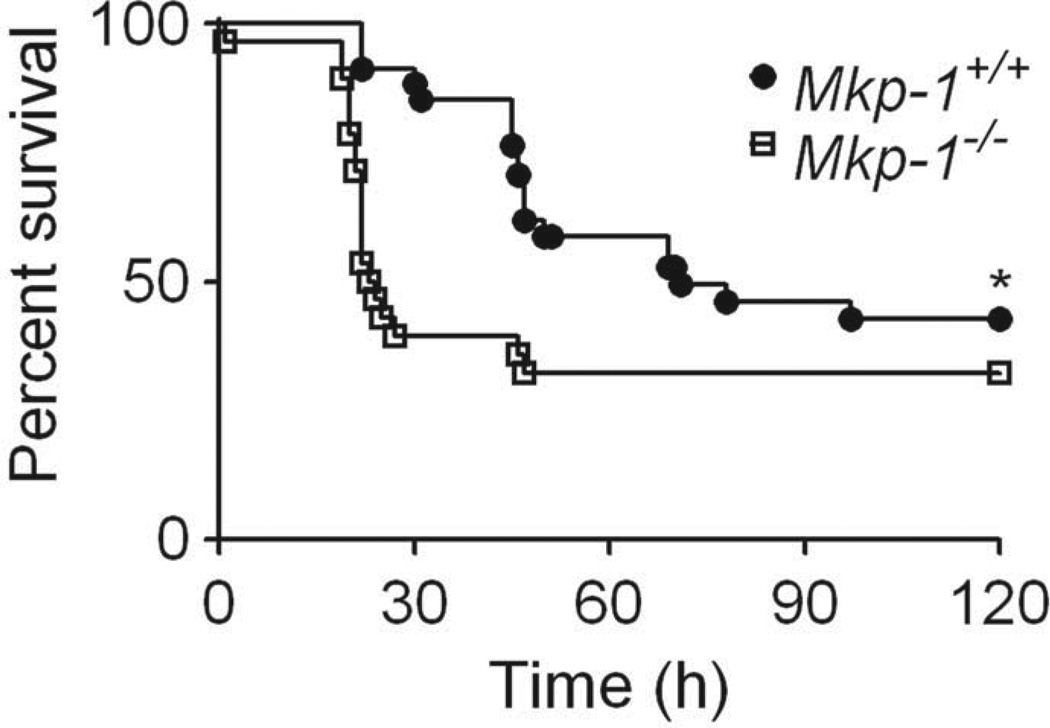
Survival
To assess survival, fasted Mkp-1+/+ (n = 34) and Mkp-1−/− (n = 28) mice were administered a single high dose of acetaminophen (400 mg/kg), re-fed 6 hr later, and monitored through 120 hr (Fig. 7). Mkp-1−/− mice displayed significantly decreased overall survival (32%) compared to Mkp-1+/+ mice (43%). Additionally, wild-type mice displayed a longer median survival time of 71 hr, compared to a median survival time of only 23 hr in Mkp-1−/− mice.
Figure 7.
Mkp-1 promotes survival during high dose acetaminophen toxicity. Fasted Mkp-1+/+ and Mkp-1−/− mice were treated with 400 mg/kg acetaminophen, re-fed 6 hr later, and survival monitored through 120 hr. n = 34 Mkp-1+/+; 28 Mkp-1−/−. *p < .05.
Discussion
This current study evaluated the role of Mkp-1 in mice exposed to a single hepatotoxic or lethal dose of acetaminophen. We found that Mkp-1−/− mice displayed biochemical and histological evidence of more severe hepatocellular injury and systemic inflammation compared to wild-type animals. The enhanced hepatocellular damage in Mkp-1−/− mice correlated with prolonged hepatic JNK activation. Finally, Mkp-1−/− mice had a lower overall survival rate and a shorter mean survival time compared to wild-type mice.
Elevations in ALT are characteristic of acute acetaminophen overdose (Larson et al. 2005; Ostapowicz et al. 2002), with mean ALT values in human patients generally > 4,000 IU/L, which is markedly higher than typical ALT levels in other drug or viral-induced causes of hepatic failure. The high plasma ALT levels in Mkp-1−/− mice (Fig. 1A) closely parallel the levels previously reported in mouse models (Blazka et al. 1996; Rogers et al. 2000). In addition to markers of direct hepatocellular damage, acetaminophen-induced cell injury often leads to activation of MAPKs and subsequent release of pro-inflammatory cytokines, such as IL-6 (Connolly et al. 2011). IL-6 (Fig. 1B) is a proinflammatory cytokine known to be upregulated in Mkp-1−/− mice in response to other injurious stimuli, such as lipopolysaccharide (LPS) (Chi et al. 2006; Salojin et al. 2006; Zhao et al. 2006) and Staphylococcus aureus (Wang et al. 2007). In response to hepatic toxins such as acetaminophen, IL-6 levels can be rapidly released by resident hepatic cells, including Kupffer and dendritic cells (Connolly et al. 2011; Lacour et al. 2005). IL-6 can also serve as a prognostic biomarker for decreased survival in human acute liver failure (Berry et al. 2010) and is a potential downstream mediator of acetaminophen-induced hepatic injury in mice (Bourdi et al. 2007). While we did not directly examine other pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α or interleukin (IL)-1β, it is likely that those would also be elevated in acetaminophen-treated Mkp-1−/− mice. Upregulation of TNF-α and IL-1β often parallels higher IL-6 release in Mkp-1−/− mice treated with bacterial ligands (Chi et al. 2006; Salojin et al. 2006; Zhao et al. 2006).
The finding that Mkp−/− mice more rapidly cleared plasma levels of acetaminophen in this model was a novel finding (Fig. 1C). Previous studies have demonstrated interactions and even modulation of cytochrome P450 activity by JNK (Liu et al. 2002a; Liu et al. 2002b;, Yasunami et al., 2004, Tang, Pettersson, and Norlin 2008; Wang et al. 2010). Although we did not directly investigate the mechanism that led to the increased plasma clearance at 4 and 6 hr post dosing, we postulate that the elevated JNK levels due to Mkp-1 deficiency in the Mkp-1−/− mice might speed up the metabolism in general, and could enhance cytochrome P450 activity and thus increase the metabolism of acetaminophen to reactive metabolites. Indeed, it has been shown that, compared to wild-type mice, Mkp-1−/− mice have higher JNK-mediated metabolic activity rate in certain pathways (Wu et al. 2006). The increased production of cytochrome P450 metabolites could cause a more rapid depletion of glutathione and more rapid alkylation of other protein thiols, thus enhancing toxicity.
The centrilobular congestion and necrosis seen in the livers of both genotypes (but more severe in Mkp-1−/− animals; Figs. 2 and 3) is the pattern commonly reported both in animal models (Blazka et al. 1996; Placke et al. 1987) and in human cases of acetaminophen toxicity (Ramachandran andKakar 2009; Walker, Racz, andMcElligott 1983, Mitchell et al., 1973b, Ostapowicz et al., 2002). Localized tissue hypoxia is one of the contributing factors leading to necrosis and initial loss of centrilobular hepatocytes. Hypoxia is known to enhance activation of JNK in the liver (McCloskey et al. 2004) andwas recently shown to upregulate Mkp-1 expression in a model of ischemia and reperfusion (Boutros et al. 2008), suggesting thatMkP-1may play a role in the hepatic response to the stress of decreased oxygen tension. Apoptotic cell death is now recognized as a component of hepatic acetaminophen toxicity, and the results of the TUNEL-staining indicate a more rapid onset of centrilobular apoptosis in Mkp-1 −/− mice (Fig 4). MAPK play a key role in mediating the apoptotic response, and it is probable that prolonged activation of MAPK such as JNK may lead to the more rapid onset of apoptosis in the Mkp-1−/− mice.
There were no significant differences in GSH or GSSG levels in wild-type and knockout mice during acetaminophen overdose (Fig. 5), indicating that the enhanced hepatotoxicity and decreased survival in Mkp-1−/− animals was not due to altered depletion of glutathione. As was seen in our model, acetaminophen treatment can rapidly exhaust cytoplasmic and mitochondrial GSH stores (Hirayama et al. 1983) concurrent with GSSG depletion. This depletion of both GSH and GSSG likely occurs secondary to the formation of thioether metabolites of NAPQI bound to GSH, thereby preventing the normal shunting of GSH to GSSG (Adams, Lauterburg, and Mitchell 1983; Nelson 1990).
JNK1 and JNK2 activation were prolonged in Mkp-1 deficient livers in our model (Fig. 6A, B), correlating with enhanced hepatic injury in Mkp-1−/− mice. Activation of hepatic JNK is recognized as a key event in the progression and exacerbation of acetaminophen toxicity, and inhibition of both JNK1 and JNK2 has been shown to protect mice against acetaminophen-induced hepatotoxicity (Gunawan et al. 2006; Hanawa et al. 2008). Our results are consistent with other models in which Mkp-1−/− mice display prolonged JNK and p38 activation during systemic diseases such as endotoxemia and bacterial infection (Chi et al. 2006;Hammer et al. 2006; Zhao et al., 2006; Frazier et al., 2009; Wang et al. 2007). While p38 activation has not been identified as contributing to hepatic damage in acetaminophen overdose (Nakagawa et al. 2008) and p38 activity was only mildly elevated at 2 hr in Mkp-1 deficient mice (Fig. 6C), we cannot rule out a contribution of p38 toward the enhanced hepatic injury in acetaminophen-treated Mkp-1−/− mice.
As in our model, acetaminophen overdose can be fatal in humans, with clinical patients dying from liver failure–related sequelae (Larson et al. 2005; Ostapowicz et al. 2002). Unfortunately, once high doses of acetaminophen are ingested, the efficacy of therapies such as N-acetyl cysteine decreases rapidly over time (Rumack et al. 1981), leaving a narrow interval for effective intervention. Our results demonstrate a markedly shortened median survival time in Mkp-1 deficient mice (23 hr vs. 71 hr in wild-type mice, Fig. 7). While we did not provide interventional care in this model, we can speculate that a therapy such as N-acetyl cysteine might have reduced mortality in the wild-type mice, but not in Mkp-1−/− mice, due to the short interval to death in the Mkp-1 deficient strain. Additionally, since JNK inhibitors have been shown to protect wild-type mice from acetaminophen overdose (Gunawan et al. 2006; Hanawa et al. 2008), we speculate that a JNK-specific inhibitor, when available, would also provide protection to Mkp-1 knockout mice. It is possible that the magnitude of protection from acetaminophen overdose may be different in knockout and wild-type animals, as there may be other factors (e.g., differences in cytochrome p450 induction) that are also influencing the severe phenotype in Mkp-1 knockout mice.
Since Mkp-1 deficiency correlates with a more severe phenotype in our model, we can speculate that induction of Mkp-1 may provide protection against an acute hepatotoxic insult. While we did not directly test Mkp-1 induction as a therapeutic intervention, several treatments can upregulate Mkp-1 expression in hepatocytes, including glucagon (Schliess, Kurz, and Haussinger 2000), S-adenosylmethionine (Tomasi et al. 2010), and high dose dexamethasone (Scheving et al. 2007). While these agents have a multitude of effects in vivo, MkP-1 induction may be one component of their clinically protective effects in related diseases. Additionally, there is precedence for a protective effect of systemic induction of Mkp-1, as glutamine has been shown to protect wild-type mice against endotoxemia (Ko et al. 2009) and correlate with reduced signs of acute respiratory distress syndrome (Singleton et al. 2005) in rodent models.
While these results demonstrate that Mkp-1 deficiency is detrimental in acute acetaminophen toxicity, Mkp-1 may not be uniformly hepatoprotective in all diseases. Indeed, Mkp-1 appears to promote the development of hepatic steatosis in a mouse model. Mice lacking both the leptin receptor (db/db) and Mkp-1 were resistant to peroxisome proliferator-activated receptor-γ-mediated hepatic steatosis compared to db/db mice (Flach et al. 2011), and Mkp-1−/− mice are generally resistant to other lipid-mediated diseases such as obesity (Wu et al. 2006).
In our mouse model, Mkp-1 deficiency enhanced hepatotoxicity during acute acetaminophen overdose, correlating with more rapid plasma clearance of acetaminophen, prolonged hepatic JNK activation and decreased total survival compared to wild-type animals. These results suggest that Mkp-1 activation is a novel potential therapeutic target for acute acetaminophen toxicity. Several available compounds are already known to upregulate MkP-1 expression in hepatocytes (Schliess, Kurz, and Haussinger 2000; Tomasi et al. 2010; Scheving et al. 2007). While future studies will be required to verify the therapeutic potential of Mkp-1 induction within the liver, data from this and related studies provide promising indications that MKP-1 may be a novel endogenous hepatoprotective factor during acute hepatotoxicity.
Acknowledgments
The authors thank Katherine Heyob for technical assistance with performing the glutathione assays and Molly Augustine for performing the HPLC analysis of plasma acetaminophen.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Allergy and Infectious Disease at the National Institutes of Health [grant numbers R01 AI057798 and AI068956 to Y.L.], the National Center for Complementary & Alternative Medicine at the National Institutes of Health [R01 AT006880 to L.K.R], and the National Center for Research Resources at the National Institutes of Health [T32 RR007073 for stipend support of L.M.W and K01 RR032139 to L.M.W., which is currently supported by the Office of Research Infrastructure Programs as /OD K01 OD010985].
Abbreviations
- ALT
alanine transaminase
- DUSP
dual specificity protein phosphatase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HPLC
high-performance liquid chromatography
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated proteins kinases
- Mkp-1
mitogen-activated protein kinase phosphatase-1
- NAPQI
N-acetyl-p-benzoquinone imine
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Adams JD, Jr, Lauterburg BH, Mitchell JR. Plasma glutathione and glutathione disulfide in the rat: Regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983;227:749–754. [PubMed] [Google Scholar]
- Bergmeyer HU, Scheibe P, Wahlefeld AW. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clin Chem. 1978;24:58–73. [PubMed] [Google Scholar]
- Berry PA, Antoniades CG, Hussain MJ, McPhail MJ, Bernal W, Vergani D, Wendon JA. Admission levels and early changes in serum interleukin-10 are predictive of poor outcome in acute liver failure and decompensated cirrhosis. Liver Int. 2010;30:733–740. doi: 10.1111/j.1478-3231.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- Blazka ME, Elwell MR, Holladay SD, Wilson RE, Luster MI. Histopathology of acetaminophen-induced liver changes: Role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol Pathol. 1996;24:181–189. doi: 10.1177/019262339602400206. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. Bioessays. 2006;28:923–934. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- Bourdi M, Eiras DP, Holt MP, Webster MR, Reilly TP, Welch KD, Pohl LR. Role of IL-6 in an IL-10 and IL-4 double knockout mouse model uniquely susceptible to acetaminophen-induced liver injury. Chem Res Toxicol. 2007;20:208–216. doi: 10.1021/tx060228l. [DOI] [PubMed] [Google Scholar]
- Boutros T, Nantel A, Emadali A, Tzimas G, Conzen S, Chevet E, Metrakos PP. The MAP kinase phosphatase-1 MKP-1/DUSP1 is a regulator of human liver response to transplantation. Am J Transplant. 2008;8:2558–2568. doi: 10.1111/j.1600-6143.2008.02420.x. [DOI] [PubMed] [Google Scholar]
- Buckpitt AR, Rollins DE, Nelson SD, Franklin RB, Mitchell JR. Quantitative determination of the glutathione, cysteine, and N-acetyl cysteine conjugates of acetaminophen by high-pressure liquid chromatography. Anal Biochem. 1977;83:168–177. doi: 10.1016/0003-2697(77)90522-x. [DOI] [PubMed] [Google Scholar]
- Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–9. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly MK, Ayo D, Malhotra A, Hackman M, Bedrosian AS, Ibrahim J, Cieza-Rubio NE, Nguyen AH, Henning JR, Dorvil-Castro M, Pachter HL, Miller G. Dendritic cell depletion exacerbates acetaminophen hepatotoxicity. Hepatology. 2011;54:959–968. doi: 10.1002/hep.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman K, Carrasco D, Gruda M, Ryan C, Lira SA, Bravo R. Disruption of the erp/mkp-1 gene does not affect mouse development: Normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 1996;13:925–931. [PubMed] [Google Scholar]
- Flach RJ, Qin H, Zhang L, Bennett AM. Loss of mitogen-activated protein kinase phosphatase-1 protects from hepatic steatosis by repression of cell death-inducing DNA fragmentation factor A (DFFA)-like effector C (CIDEC)/fat-specific protein 27. J Biol Chem. 2011;286:22195–22202. doi: 10.1074/jbc.M110.210237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci U S A. 1998;95:3014–3019. doi: 10.1073/pnas.95.6.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier WJ, Wang X, Wancket LM, Li XA, Meng X, Nelin LD, Cato AC, Liu Y. Increased inflammation, impaired bacterial clearance, and metabolic disruption after gram-negative sepsis in Mkp-1-deficient mice. J Immunol. 2009;183:7411–7419. doi: 10.4049/jimmunol.0804343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FJ, Kimura S. Study of P450 function using gene knockout and transgenic mice. Arch Biochem Biophys. 2003;409:153–158. doi: 10.1016/s0003-9861(02)00364-8. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Shinohara M, Ybanez MD, Saberi B, Kaplowitz N. Signal transduction pathways involved in drug-induced liver injury. Handb Exp Pharmacol. 2010:267–310. doi: 10.1007/978-3-642-00663-0_10. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. Critical role of c-jun (NH2) terminal kinase in paracetamol- induced acute liver failure. Gut. 2007;56:982–990. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RJ, Chiamori N, Golub OJ, Berkman S. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, and lactic acid dehydrogenase. Am J Clin Pathol. 1960;34:381–398. doi: 10.1093/ajcp/34.4_ts.381. [DOI] [PubMed] [Google Scholar]
- Hirayama C, Murawaki Y, Yamada S, Aoto Y, Ikeda F. The target portion of acetaminophen induced hepatotoxicity in rats: modification by thiol compounds. Res Commun Chem Pathol Pharmacol. 1983;42:431–448. [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- Jollow DJ, Thorgeirsson SS, Potter WZ, Hashimoto M, Mitchell JR. Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and nontoxic doses of acetaminophen. Pharmacology. 1974;12:251–271. doi: 10.1159/000136547. [DOI] [PubMed] [Google Scholar]
- Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Ko HM, Oh SH, Bang HS, Kang NI, Cho BH, Im SY, Lee HK. Glutamine protects mice from lethal endotoxic shock via a rapid induction of MAPK phosphatase-1. J Immunol. 2009;182:7957–7962. doi: 10.4049/jimmunol.0900043. [DOI] [PubMed] [Google Scholar]
- Lacour S, Gautier JC, Pallardy M, Roberts R. Cytokines as potential biomarkers of liver toxicity. Cancer Biomark. 2005;1:29–39. doi: 10.3233/cbm-2005-1105. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–421. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- Lau LF, Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985;4:3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Jones BE, Bradham C, Czaja MJ. Increased cytochrome P-450 2E1 expression sensitizes hepatocytes to c-Jun-mediated cell death from TNF-a. Am J Physiol Gastrointest Liver Physiol. 2002a;282:G257–G266. doi: 10.1152/ajpgi.00304.2001. [DOI] [PubMed] [Google Scholar]
- Liu H, Lo CR, Czaja MJ. NF-κB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology. 2002b;35:772–778. doi: 10.1053/jhep.2002.32534. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- McCloskey CA, Kameneva MV, Uryash A, Gallo DJ, Billiar TR. Tissue hypoxia activates JNK in the liver during hemorrhagic shock. Shock. 2004;22:380–386. doi: 10.1097/01.shk.0000140660.78744.bf. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of drug metabolism. J Pharmacol Exp Ther. 1973a;187:185–194. [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973b;187:211–217. [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15:398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- Placke ME, Ginsberg GL, Wyand DS, Cohen SD. Ultrastructural changes during acute acetaminophen-induced hepatotoxicity in the mouse: A time and dose study. Toxicol Pathol. 1987;15:431–438. doi: 10.1177/019262338701500407. [DOI] [PubMed] [Google Scholar]
- Qiao L, Han SI, Fang Y, Park JS, Gupta S, Gilfor D, Amorino G, Valerie K, Sealy L, Engelhardt JF, Grant S, Hylemon PB, Dent P. Bile acid regulation of C/EBPbeta, CREB, and c-Jun function, via the extracellular signal-regulated kinase and c-Jun NH2-terminal kinase pathways, modulates the apoptotic response of hepatocytes. Mol Cell Biol. 2003;23:3052–3066. doi: 10.1128/MCB.23.9.3052-3066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009;62:481–492. doi: 10.1136/jcp.2008.058248. [DOI] [PubMed] [Google Scholar]
- Rogers LK, Valentine CJ, Szczpyka M, Smith CV. Effects of hepatotoxic doses of acetaminophen and furosemide on tissue concentrations of CoASH and CoASSG in vivo. Chem Res Toxicol. 2000;13:873–882. doi: 10.1021/tx0000926. [DOI] [PubMed] [Google Scholar]
- Rumack BH, Peterson RC, Koch GG, Amara IA. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981;141:380–385. doi: 10.1001/archinte.141.3.380. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- Scheving LA, Buchanan R, Krause MA, Zhang X, Stevenson MC, Russell WE. Dexamethasone modulates ErbB tyrosine kinase expression and signaling through multiple and redundant mechanisms in cultured rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G552–G559. doi: 10.1152/ajpgi.00140.2007. [DOI] [PubMed] [Google Scholar]
- Schliess F, Kurz AK, Haussinger D. Glucagon-induced expression of the MAP kinase phosphatase MKP-1 in rat hepatocytes. Gastroenterology. 2000;118:929–936. doi: 10.1016/s0016-5085(00)70179-x. [DOI] [PubMed] [Google Scholar]
- Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton KD, Beckey VE, Wischmeyer PE. Glutamine prevents activation of NF-kappaB and stress kinase pathways, attenuates inflammatory cytokine release, and prevents acute respiratory distress syndrome (ARDS) following sepsis. Shock. 2005;24:583–589. doi: 10.1097/01.shk.0000185795.96964.71. [DOI] [PubMed] [Google Scholar]
- Tang W, Pettersson H, Norlin M. Involvement of the PI3K/Akt pathway in estrogen-mediated regulation of human CYP7B1: Identification of CYP7B1 as a novel target for PI3K/Akt and MAPK signalling. J Steroid Biochem Mol Biol. 2008;112:63–73. doi: 10.1016/j.jsbmb.2008.08.004. [DOI] [PubMed] [Google Scholar]
- To EC, Wells PG. Repetitive microvolumetric sampling and analysis of acetaminophen and its toxicologically relevant metabolites in murine plasma and urine using high performance liquid chromatography. J Anal Toxicol. 1985;9:217–221. doi: 10.1093/jat/9.5.217. [DOI] [PubMed] [Google Scholar]
- Tomasi ML, Ramani K, Lopitz-Otsoa F, Rodriguez MS, Li TW, Ko K, Yang H, Bardag-Gorce F, Iglesias-Ara A, Feo F, Pascale MR, Mato JM, Lu SC. S-adenosylmethionine regulates dualspecificity mitogen-activated protein kinase phosphatase expression in mouse and human hepatocytes. Hepatology. 2010;51:2152–2161. doi: 10.1002/hep.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RM, Racz WJ, McElligott TF. Scanning electron microscopic examination of acetaminophen-induced hepatotoxicity and congestion in mice. Am J Pathol. 1983;113:321–330. [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu Y. Regulation of innate immune response by MAP kinase phosphatase-1. Cell Signal. 2007;19:1372–1382. doi: 10.1016/j.cellsig.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Meng X, Kuhlman JR, Nelin LD, Nicol KK, English BK, Liu Y. Knockout of Mkp-1 enhances the host inflammatory responses to gram-positive bacteria. J Immunol. 2007;178:5312–5320. doi: 10.4049/jimmunol.178.8.5312. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bell JC, Keeney DS, Strobel HW. Gene regulation of CYP4F11 in human keratinocyte HaCaT cells. Drug Metab Dispos. 2010;38:100–107. doi: 10.1124/dmd.109.029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Roth RJ, Anderson EJ, Hong EG, Lee MK, Choi CS, Neufer PD, Shulman GI, Kim JK, Bennett AM. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 2006;4:61–73. doi: 10.1016/j.cmet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Yasunami Y, Hara H, Iwamura T, Kataoka T, Adachi T. C-jun N-terminal kinase modulates 1,25-dihydroxyvitamin D3-induced cytochrome P450 3A4 gene expression. Drug Metab. Dispos. 2004;32:685–688. doi: 10.1124/dmd.32.7.685. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]