Abstract
Objectives
Intestinal dysmotility is one of the effects of cystic fibrosis (CF) but when and how this develops is not well understood. The goal of this study was to use the Cftr knockout mouse to determine when in development circular smooth muscle of the small intestine becomes dysfunctional.
Methods
Wild type (WT) and CF mice were used at postnatal day 5 (P5) through adult. Pieces of small intestine were used to measure contractile activity of the circular muscle. Bacterial overgrowth was measured by quantitative PCR of the bacterial 16S gene. Intestinal gene expression was determined by quantitative RT-PCR. Prostaglandin E2 (PGE2) and its metabolites were measured by enzyme immunoassay.
Results
CF circular muscle response to cholinergic stimulation was similar to WT at P5, became somewhat impaired at P7, and was severely impaired by P14. In the CF intestine, bacterial overgrowth occurred by P4 and was maintained into adulthood. Eicosanoid metabolic gene expression in the CF intestine did not differ from WT shortly after birth. The phospholipase A2 genes, Pla2g4c and Pla2g5 exhibited increased expression in CF mice at P24. Prostaglandin degradative genes, Hpgd and Ptgr1, showed lower expression in CF as compared to WT at P16 and P24, respectively. PGE2 levels were significantly greater in CF mice at most ages from P7 through adulthood.
Conclusions
The results clearly demonstrate that lack of CFTR itself does not cause smooth muscle dysfunction, as the circular muscle from P5 CF mice had normal activity and dysfunction developed between P7-P14.
Keywords: small intestinal bacterial overgrowth, prostaglandin, phospholipase A2, cyclooxygenase, hydroxyprostaglandin dehydrogenase, prostaglandin reductase 1
INTRODUCTION
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene. The best understood function of CFTR is as a cAMP-regulated anion channel (1). CFTR is expressed primarily in a variety of epithelia but can also be demonstrated at lower levels in numerous cell types including immune cells (2), neurons (3), neuroendocrine cells (4), and airway smooth muscle (5). The significance of CFTR expression and its loss in CF in these nonepithelial tissues is not well understood at this time.
Loss of CFTR function in epithelia can explain much of CF pathophysiology. In CF the loss of CFTR-dependent Cl−and fluid secretion results in a poorly hydrated surface on affected epithelia which fosters accumulation of mucus in affected organs such as the airways and intestines (6–8). CFTR also is required for HCO3−secretion and secreted mucins do not expand properly in the absence of bicarbonate (9) contributing to mucus accumulation in CF. This excessive mucus accumulation is a hallmark of CF, and the mucus provides a unique niche for abnormal bacterial colonization and growth (10), a form of microbial dysbiosis. In the CF intestine, there is an inflammatory response associated with microbial dysbiosis (11–13). Inflammation can lead to further mucus production creating a feed-forward spiral of destructive responses. An important component of inflammation that contributes to mucus secretion is arachidonic acid, produced by the action of phospholipase A2 (PLA2) enzymes, and which is then converted to a variety of biologically active eicosanoids including PGE2 (14). It has been shown that cytosolic PLA2 is increased in lungs of CF mice and that challenge with lipopolysaccharide or arachidonic acid increases Muc5AC, whereas PLA2 inhibition blocks this effect (15). At least two PLA2 genes have increased expression in the adult CF mouse small intestine [(16) and the current study], which is accompanied by excessive mucus accumulation.
While it is possible to model the myriad and pleiotropic pathophysiology of CF based solely on its expression in epithelia, not all aspects of the disease can be explained this way. An important issue is the over-exuberant airway immune response to infection in CF (17).
One proposal is that loss of CFTR function affects cellular fatty acid metabolism in a manner that favors a proinflammatory state (14). Evidence supporting an inherent proinflammatory state includes data showing lymphocytes from CF patients have increased arachidonic acid production (18), CF patients have elevated urinary excretion of eicosanoid metabolites (19), and cultured CFTR-deficient cells produce more PGE2 (20). There is also abundant data showing altered eicosanoid metabolism in tissues from CF patients (21). A more direct study showed that when fetal human CF and wild type airway tissue is transplanted into immunodeficient mice, only the CF tissue recruits neutrophils that progressively destroy the transplanted tissue (22). Because that study used fetal tissue, it was sterile and there was no issue of prior infection that could have activated an immune response. Negative data also exist, such as the study that showed no consistent differences in the effects of B.cepacia on cytokine and PGE2 production, comparing a variety of CF and wild type cells (23). Some of the most compelling recent data for nonepithelial effects of CFTR comes from the use of conditional transgenic mice in which Cftr expression can be activated or inactivated in specific cell types (24). For example, expression of Cftr specifically in the intestinal epithelium prevented intestinal obstruction but did not improve failure to thrive as compared to global Cftr knockout mouse (25). In another study using this mouse model it was shown that knockout of Cftr specifically in lymphocytes resulted in an exaggerated allergic response to Aspergillus fumigatus and that the Cftr-deficient lymphocytes showed an increase in intracellular [Ca2+] as compared to wild type lymphocytes in response to T cell receptor activation (2).
Our studies of the CF mouse small intestine have revealed a wide range of pathophysiological changes similar to human CF. These include microbial dysbiosis with small intestinal bacterial overgrowth (SIBO) (10), an innate immune response including altered prostaglandin metabolism (16), and impaired intestinal motility and circular smooth muscle dysfunction (26; 27). We have provided evidence that the majority of these changes in the CF mouse intestine are a consequence of microbial dysbiosis.
Because the gut is sterile at birth we hypothesized that smooth muscle dysfunction would already exist in the early postnatal period in CF mice if there is a direct role for CFTR in activity of these nonepithelial cells. We also investigated when SIBO occurs as well as when changes in expression of prostaglandin metabolic genes occur, both of which are known to happen in adult CF mice, to look for associations with impaired smooth muscle activity in CF.
MATERIALS AND METHODS
Animals
Cftrtm1UNC+/− mice, originally obtained from the Jackson Laboratories (Bar Harbor ME USA), have been bred onto the C57BL/6J background until congenic. They are periodically backcrossed with wild type (WT) C57BL/6J mice and were at generation 31 of backcrossing in this study. Cftrtm1UNC+/− mice were bred to obtain Cftrtm1UNC−/− (CF) and Cftrtm1UNC+/+ (WT) mice. Mice aged from 4 days to 12 weeks and of both genders were used; no gender differences in the measured parameters were observed in this study. Cftrtm1UNC+/− mice are phenotypically normal and were used occasionally when needed as WTs; none of the parameters measured in this study were different between Cftr homozygous wild type and Cftr heterozygous mice. WT and CF mice were fed a liquid diet (Peptamen, Nestle Nutrition; Florham Park, NJ, USA) from weaning which prevents lethal intestinal obstruction in CF mice. All animal use was approved by the University of Kansas Medical Center IACUC.
Organ Bath Analysis of Intestinal Circular Smooth Muscle Activity
These measurements were made as previously described (26). Briefly, a ring of small intestine from about 1/3 the way between the gastro-duodenal and ileocecal junctions was mounted in a 20 mL bath filled with Krebs–Henseleit bicarbonate buffer at 0.5g of tension. The bath was maintained at 37°C and continuously gassed with 95% O2/5% CO2. After equilibration for 1 hr, isometric force data were recorded using an MP35 System and BSL Pro software (Biopac; Goleta, CA, USA). The cholinergic agonist carbamylcholine chloride (CCh) was added to the bath at 2-min intervals in 10-fold steps from 108 to 104 mol L−1 to stimulate contraction. Contractile activity data were analyzed using Biopac and PeakFit software (Systat; Chicago, IL, USA) and are expressed as mN per gram tissue wet weight.
Measurement of Intestinal Bacterial Load
Quantitative PCR for the bacterial 16S gene was used to estimate bacterial load, as previously described (27). Briefly, the intestinal lumen was flushed with saline containing the mucolytic agent dithiothreitol (10 mM), and DNA that was isolated from the flushed material was used as template in a real-time PCR reaction using universal 16S PCR primers and a kit from Qiagen (Valencia, CA, USA). A cloned 16S PCR product was used in serial dilutions to prepare a standard curve for quantitation.
Quantitative RT-PCR of Prostaglandin Metabolic Gene Expression
qRT-PCR was performed as previously described (16) using total RNA prepared from the entire small intestine and a kit from Qiagen. The gene-specific primers used are as in 16) (Ptgr1 was referred to as Ltb4dh in that report), and primers for Pla2g4c were forward: tcctcagtgagatgaggaaagtg; reverse: agagccttggggatccttta. Values were normalized to the mRNA for the ribosomal protein Rpl26. Data were analyzed by ∆∆Ct (threshold cycle) method with correction for differential PCR efficiencies (28). Data are expressed relative to the WT P4 average set to unity.
Prostaglandin E2 Immunoassay
PGE2 was measured by immunoassay of flushed intestinal fluid, as previously described (16). The instructions supplied with the kit were followed, the first step of which is to treat the sample with an alkaline incubation that converts PGE2 and metabolites to a single compound that is then measured in the immunoassay (catalog number 514531, Cayman Chemical; Ann Arbor MI, USA).
Statistics
Data are presented as mean standard error (SE). Statistical significance was determined by ANOVA followed by Tukey's posthoc test, or by Kruskal–Wallis test where data were not normally distributed, using Systat software (San Jose, CA, USA). P<0.05 was considered significant.
RESULTS
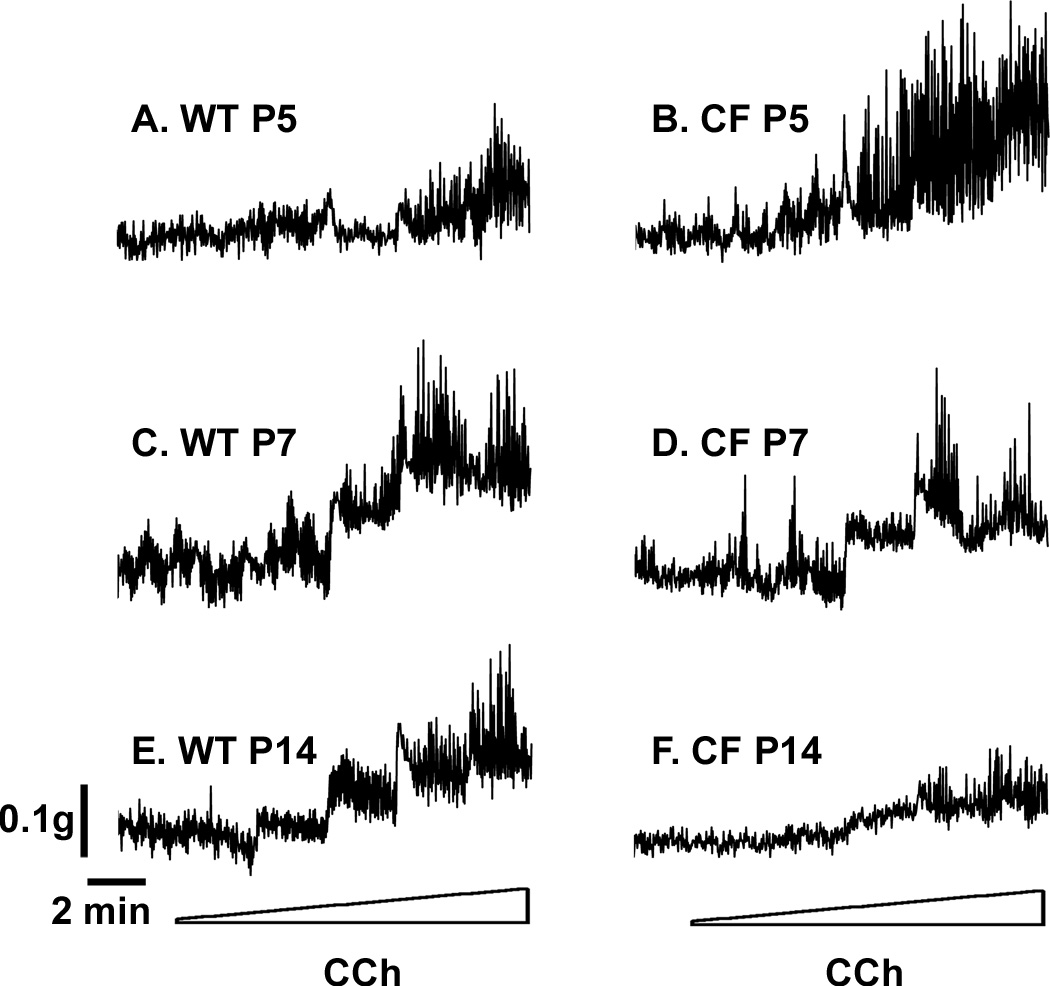
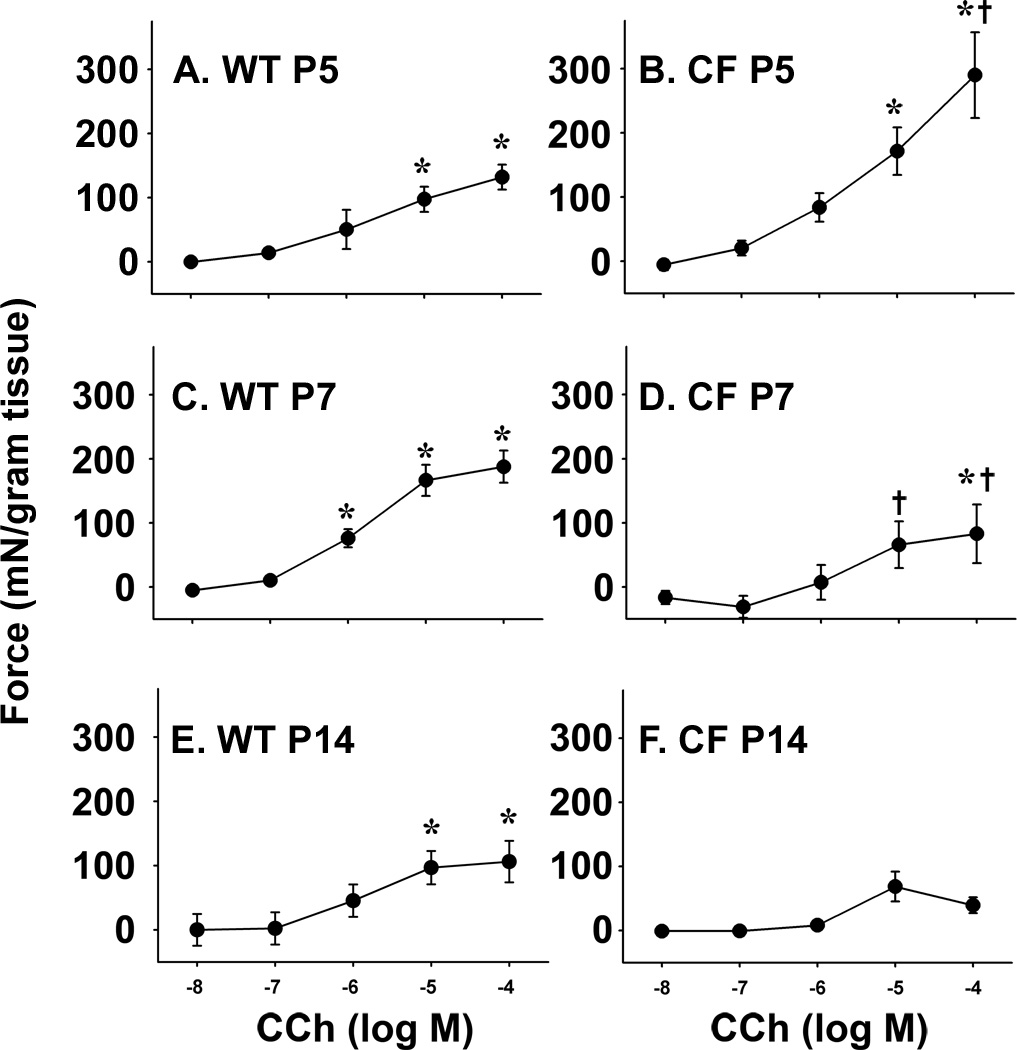
To test the hypothesis that loss of CFTR results in intestinal smooth muscle dysfunction in CF, we measured the ex vivo activity of intestinal rings from neonatal mice aged 5 days and older, comparing CF to WT mice. Tissue rings were mounted in an organ bath to measure contractile activity of the circular smooth muscle in response to cholinergic stimulation (carbachol, from 10−8 to 10−4 M). In tissue from 5 day old mice (P5), there was a response to cholinergic stimulation in both WT and CF samples, examples of which are shown in Fig.1A and B, respectively. The response to cholinergic stimulation was statistically significant compared to unstimulated at 10−5 and 10−4 M carbachol in both WT and CF P5 tissues (Fig.2A and B, respectively). At 10−4 M carbachol, the response of the CF P5 tissue was statistically greater than the P5 WT tissue (Fig.2). A robust response to stimulation was also observed at P7 and P14 in WT tissue (Fig.1C and 2C, and Fig.1E and 2E, respectively). In contrast, the response in CF P7 tissue was lessened (Fig.1D and 2D). At 10−5 and 10−4 M carbachol, the P7 CF tissue response was significantly less than that of WT tissue (Fig.2D). At P14, the CF tissue lacked a statistically significant response to all carbachol concentrations (Fig.1F and 2F).
Fig.1.
Contractile behavior of WT and CF intestinal circular smooth muscle response to cholinergic stimulation during the postnatal period. Tissue rings were mounted in an organ bath and preincubated for 1 hr. Examples of typical responses to cholinergic stimulated contractility, recorded under isometric conditions. (A) WT P5; (B) CF P5; (C) WT P7; (D) CF P7; (E) Wt P14; (F) CF P14. Tissue was stimulated with stepwise additions of carbachol from 10−8 M to 10−4 M at 2 min intervals. (See Fig.2 for complete data and statistical analysis.
Fig.2.
WT and CF intestinal circular smooth muscle response to cholinergic stimulation during the postnatal period. Tissue rings were mounted in an organ bath and preincubated for 1 hr. Examples of responses to cholinergic stimulated contractility, recorded under isometric conditions. (A) WT P5; (B) CF P5; (C) WT P7; (D) CF P7; (E) Wt P14; (F) CF P14. Tissue was stimulated with stepwise additions of carbachol from 10−8 M to 10−4 M at 2-min intervals. Data are presented as force per gram tissue wet weight (mN/g) above unstimulated baseline. (7–12 litters of mice were used per age; n=68 WT P5, 35 WT P7, 41 WT P14, 12 CF P5, 11 CF P7, 11 CF P14) (*) p<0.05 comparing to unstimulated of same genotype; (+) p<0.05 comparing CF to WT of the same age and same CCh concentration.
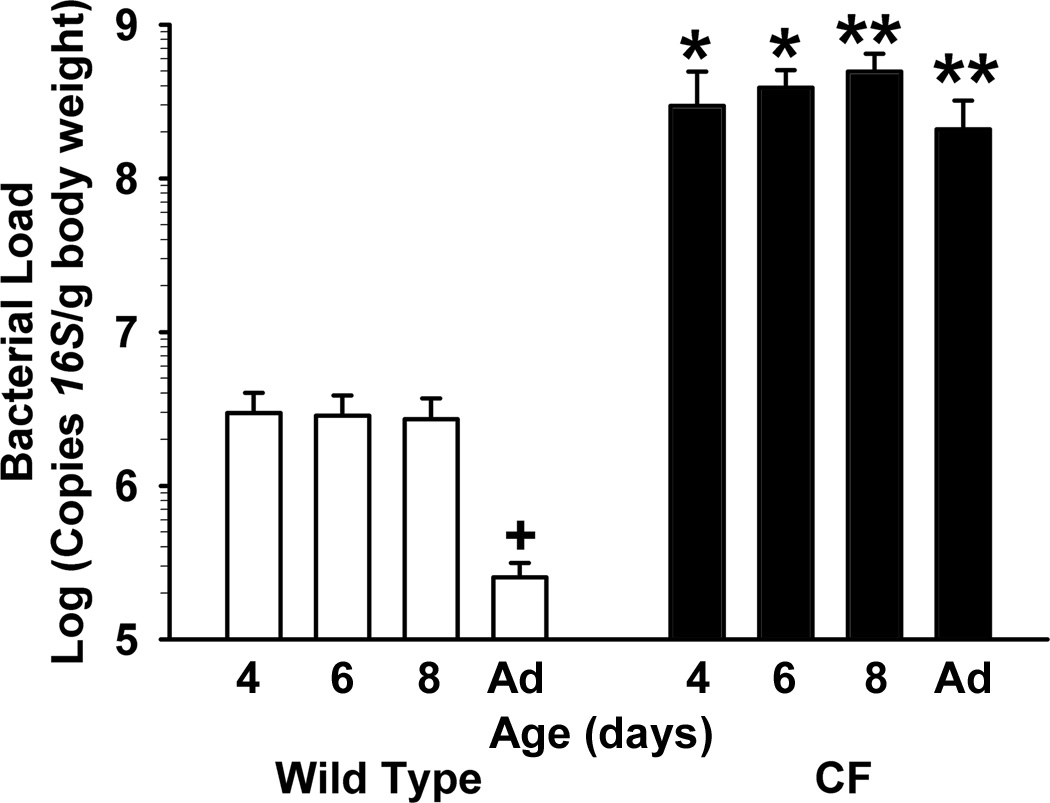
Normal intestinal smooth activity is required for proper gut motility, which has an important role in maintaining low bacterial load in the small intestine. Therefore, we next investigated when small intestinal bacterial overgrowth (SIBO) occurs in CF mice. Amplification by real-time PCR of the bacterial 16S gene in material flushed from the small intestinal lumen was used to estimate bacterial load, as previously described (27). In CF mice as young as 4 days of age, the bacterial load was >100× that of WT mice of the same age (Fig.3). SIBO in CF intestine was maintained through adulthood. Interestingly, there was a 10× decrease in bacterial load in adult WT mice as compared to WT neonates (Fig.3, p<0.05).
Fig.3.
Bacterial load in the intestines of WT and CF during the postnatal period. The contents of the small intestine were flushed with a mucolytic agent, and the flushed material was processed to measure copies of the bacterial 16S rRNA gene by quantitative PCR with universal primers (Materials and Methods). (n=4–7 samples per age and genotype) (*) p<0.05 CF vs WT value of same age; (**) p<0.01 CF vs P4 WT value of same age; (+) p<0.05 vs P4 WT value.
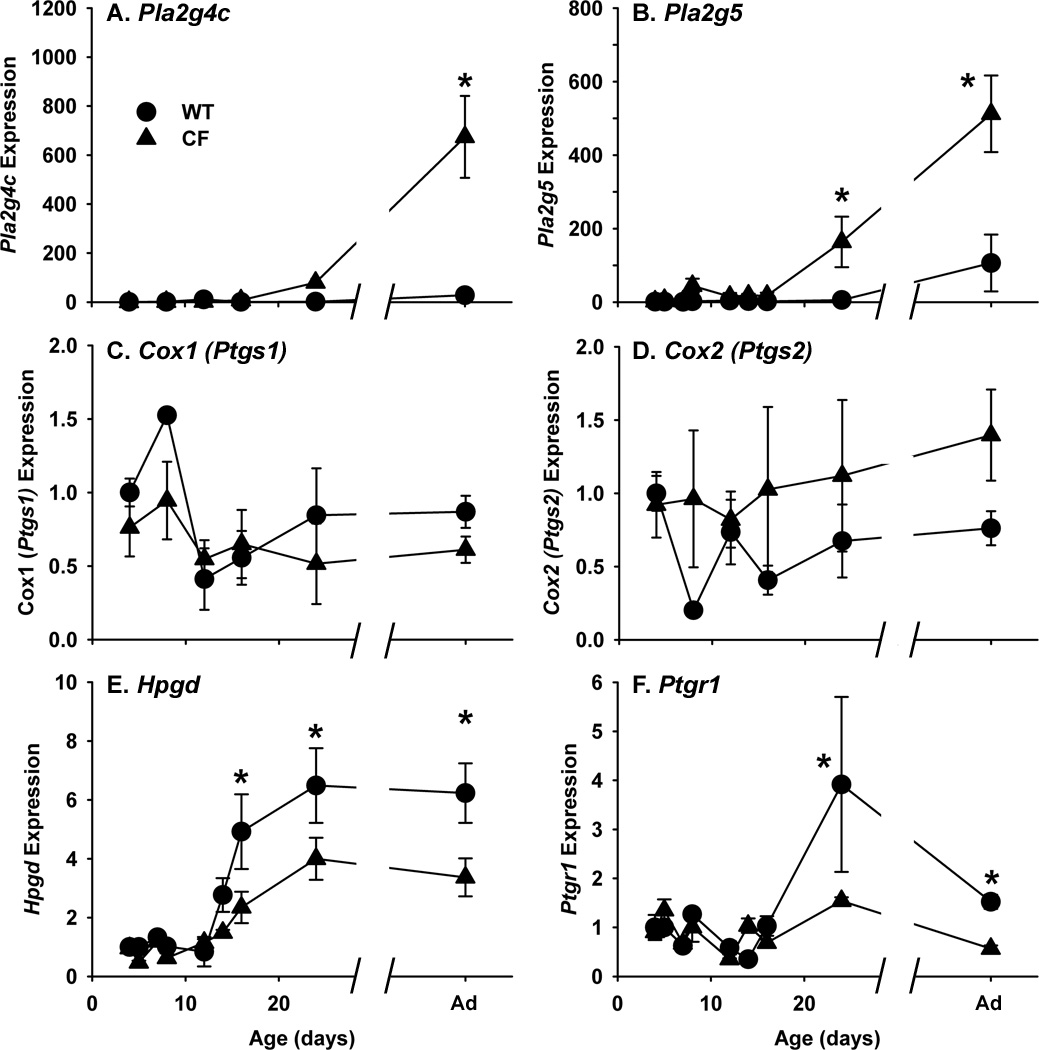
We next investigated expression of prostaglandin synthetic and degradative genes during postnatal development in CF mice as compared to WT. Two phospholipase A2 genes, Pla2g4c (a cytosolic, Ca2+-independent PLA2, also known as cPLA2() and Pla2g5 (a secreted PLA2 with antibacterial activity), which produce the eicosanoid precursor arachidonic acid from membrane phospholipids were analyzed. During postnatal development, Pla2g4c expression was fairly constant in the WT intestine (Fig.4A). In CF mice, Pla2g4c expression started to become elevated at P24, and was significantly elevated in adult CF mice as compared to WT mice (Fig.4A). Pla2g5 expression in WT mice was also fairly constant during postnatal development (Fig.4B). In CF mice, Pla2g5 expression was significantly elevated at P24 and in adult (Fig.4B) as compared to WT mice.
Fig.4.
Expression of eicosanoid metabolic genes in the intestines of WT and CF mice during the postnatal period. Total RNA was isolated from the entire small intestine and was used as a template in quantitative reverse transcription-polymerase chain reaction (qRT-PCR) reactions with gene-specific primers (Materials and Methods). The genes measured are (A) Pla2g4c (phospholipase A2, group 4c); (B) Pla2g5 (phospholipase A2, group V); (C) Cox1 (cyclooxygenase 1, also known as Ptgs1); (D) Cox2 (cyclooxygenase 2, also known as Ptgs2); (E) Hpgd (hydroxyprostaglandin dehydrogenase); and (E) Ptgr1 (prostaglandin reductase 1, also known as Ltb4dh). Data are mean standard error of measurement calculated using the ∆∆Ct method with correction for differential PCR efficiencies. Data are normalized to the ribosomal L26 protein (Rpl26) mRNA and are expressed relative to P4 WT. (n=3 P4 through P28, and 9 each for adult WT and CF samples) (*) P<0.05 vs WT P4 value.
The two synthetic enzymes that convert arachidonic acid to prostaglandins are the cyclooxygenase enzymes prostaglandin synthase 1 (Ptgs1 or Cox1) and prostaglandin synthase 2 (Ptgs2 or Cox2). In both WT and CF mice, expression of these two Cox genes was constant throughout development and neither of these genes showed any significant difference in expression at any age in the CF intestine as compared to WT (Fig.4C, D).
Prostaglandins are usually rapidly inactivated by the degradative enzymes hydroxyprostaglandin dehydrogenase (Hpgd) and prostaglandin reductase 1 (Ptgr1, previously referred to as leukotriene B4 dehydrogenase, Ltb4dh) (28). As shown in Fig.4E, Hpgd expression is low in both WT and CF small intestine until P16 when levels increase ~5 fold in the WT mouse relative to P4 WT. In contrast, Hpgd expression was only ~2.5 fold increased in the P16 CF intestine, significantly less than in WT mice (Fig.4E). Lesser Hpgd expression was maintained through adulthood in CF mice, as compared to WT. Similarly Ptgr1 expression also increased at P24, relative to P4 WT, in both WT and CF mice, but the increase was significantly less in CF as compared to WT (Fig.4F).
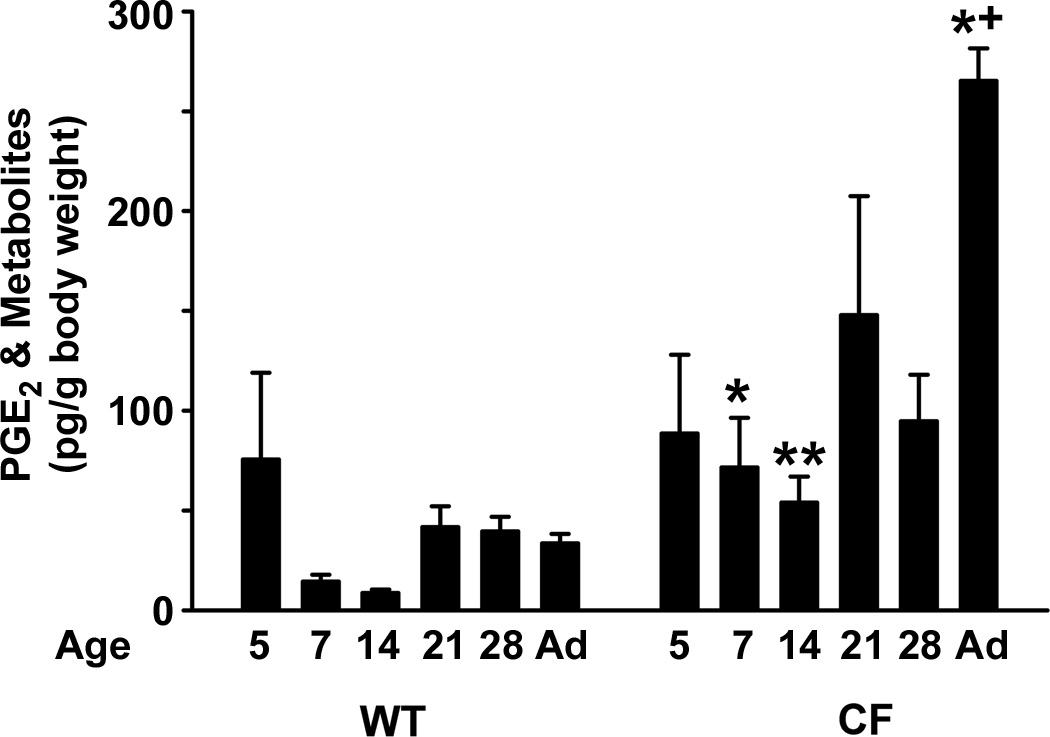
Finally, we measured levels of PGE2 during postnatal development of WT and CF mice. Since PGE2 is very unstable in vivo, a commercial immunoassay kit that employs an alkaline incubation step to convert PGE2 and its metabolites to a common compound 13,14-dihydro-15-keto-PGA2) was used (16). At P5 in both WT and CF intestines, PGE2 levels were similar (Fig.5). Thereafter, PGE2 levels tended to decrease in the WT intestine (statistically significant at P14 but not other ages). In contrast, in the CF intestine PGE2 levels remained elevated throughout development as compared to WT, becoming most increased in adult CF mice (Fig.5).
Fig.5.
PGE2 levels in the intestines of WT and CF mice during the postnatal period. The intestinal lumen was lavaged and the lavage fluid was processed for enzyme immunoassay of PGE2 and its metabolites (Materials and Methods). Data are mean standard error of measurement (n=5–6 samples per age and genotype). (*) p<0.05 comparing CF to WT of the same age; (+) p<0.05 comparing P5 of the same genotype.
DISCUSSION
In this study we sought to answer the question of whether intestinal smooth muscle dysfunction in CF mice is present at early postnatal ages or develops later. If dysfunction is present at early ages it would be consistent with a direct effect of loss of CFTR in the smooth muscle cells; and if it develops later it would argue that environmental effects, secondary to loss of CFTR, are responsible. To test this we examined the behavior of enteric circular smooth muscle as a function of postnatal age in CF and WT mice. We also investigated bacterial load in the small intestine (SIBO), and expression of prostaglandin metabolic genes and levels of PGE2 which we have proposed contribute to the smooth muscle dysfunction in CF. We found that smooth muscle dysfunction develops postnatally, and, therefore, cannot be directly caused by loss of CFTR in smooth muscle cells.
Comparing CF to WT mice during the postnatal period, the earliest difference observed was that SIBO occurred by P4 in CF mice, with >100× higher levels as compared to WT mice. The high level of bacteria in the small intestine of CF mice remained fairly constant through adulthood. Interestingly, the bacterial load in WT mice showed a significant 10× decrease between P8 and adulthood. The decrease likely occurs at weaning when more antimicrobials such as Ang4 are expressed by Paneth cells and other epithelial cells (29; 30). These data indicate that the CF small intestine provides a more hospitable niche for bacteria, and that this niche is present shortly after birth and is maintained through adulthood in CF. The likely causative factor in CF that creates this new niche is the accumulation of mucus in the intestinal lumen (31), which becomes heavily colonized by bacteria (10).
During the postnatal period, we observed that the response to cholinergic stimulation of CF enteric circular smooth muscle was strong at P5, became lesser at P7, and was almost totally lacking at P14. Activity of WT smooth muscle was fairly consistent between P5 and P14. Thus, in CF mice, smooth muscle activity is normal in the early postnatal period even though they lack Cftr expression. Because it is technically impractical to measure small intestine transit rates in suckling mice, we used an ex vivo approach to measure smooth muscle activity as a proxy for intestinal transit. Of course, normal ex vivo muscle activity does not prove that in vivo motility is normal, and it remains a formal possibility that motility and small intestinal transit are not normal in the early postnatal period in CF mice. Nevertheless, these data showing normal muscle activity in young CF mice are consistent with the interpretation that smooth muscle activity is independent of CFTR. Previous studies showed that altering the gut lumen environment using oral osmotic laxative in CF mice could improve small intestinal transit and smooth muscle activity (26; 32), which is consistent with the interpretation that there is not a direct role for CFTR in smooth muscle function. Also, CF patients that are well-nourished, indicating healthy small intestinal function, had normal fasting migrating motor complex activity (33). These data are consistent with the idea that impaired motility is secondary to loss of CFTR, probably due to altered luminal environmental conditions that variably occur in CF.
In previous work, we noted an association between intestinal dysmotility and SIBO in CF mice (27; 32). It is known that impaired intestinal motility can lead to development of SIBO (34). Since SIBO was already present by P4 in CF mice, when muscle activity is normal, these data indicate that a loss of smooth muscle activity is not the major factor in development of SIBO in the CF mouse. These results suggest that normal smooth muscle activity is not sufficient to clear the intestine of the accumulated mucus in CF which provides the niche for bacterial overgrowth.
We also measured expression of prostaglandin metabolic genes in the intestines of CF and WT mice postnatally. In the CF intestine, there was elevated expression of the two phospholipase A2 genes measured, Pla2g4c (an intracellular, Ca2+-independent PLA2) and Pla2g5 (a secreted PLA2 with antibacterial properties). Phospholipase A2 enzymes produce arachidonic acid, the prostaglandin precursor, from membrane phospholipids. The increase in expression of these PLA2 genes in the CF intestine as compared to WT occurred after weaning. There were no significant differences at any age in expression of the two major enzymes that convert arachidonic acid to prostaglandins, Cox1 and Cox2, comparing CF and WT mice. There was less expression in CF vs WT intestine of the two major prostaglandin degradative genes, Hpgd and Ptgr1, and these changes occurred at P16 and P24, respectively.
Intestinal PGE2 levels in CF mice were significantly greater at most ages as compared to age matched WT. In WT mice there was a tendency for greater PGE2 at P5 but the difference compared to older ages was not significant except vs P14 WT. The transient elevation in PGE2 in the WT intestine may suggest an initial response to bacterial colonization of the gut after birth, followed by tolerance at older postnatal ages. If this is correct, it suggests that the CF intestine fails to become tolerant to its bacteria, perhaps because of the greater numbers and different composition of the bacterial population (10) as compared to the WT bacteria.
We had previously shown that elevated PGE2 was at least partially responsible for the impaired cholinergic response of the CF enteric circular smooth muscle in adult mice, as incubation of the tissue with the COX inhibitor indomethacin could significantly restore the response of the CF muscle to carbachol (26). In the postnatal period, elevated PGE2 in the CF intestine cannot totally account for the impaired muscle activity as compared to WT. Both WT and CF intestine had elevated PGE2 levels at P5, as compared to older WT mice, but the cholinergic response of the muscle was substantial in both at P5. At P7 the CF muscle activity was significantly less than in WT, and PGE2 was now greater than that of WT, but the same as at P5 in CF. Therefore, there are other factors involved in circular smooth muscle dysfunction in addition to altered prostaglandin metabolism in the CF intestine.
Although there were differences in PGE2 levels during postnatal development in CF as compared to WT mice, the differences did not correspond well to expression levels of prostaglandin metabolic genes. This indicates that non-genetic factors are also changed in the CF intestine. A reasonable possibility is altered levels of allosteric modulators of COX activity such as fatty acids (35) which are known to be altered in CF (14; 36).
Cystic fibrosis affects many aspects of gastrointestinal tract activity and function, resulting in poor nutrition. The mechanisms by which loss of CFTR affects the gut are not completely understood and it is unclear whether the primary factor is the altered environment caused by loss of CFTR in the intestinal epithelium, or if there are cell autonomous effects caused by loss of CFTR in cell types other than the epithelium. In this study we demonstrate that alterations in the CF intestine occur during the postnatal period, with normal smooth muscle activity early on which is later impaired. Changes in prostaglandin metabolic gene expression also occur post-weaning in the CF intestine, after smooth muscle function becomes impaired. The data on altered prostaglandin metabolic gene expression and PGE2 levels do not fully explain impaired smooth muscle activity in the CF, indicating there are still to be discovered factors at play in the CF intestine. Our data do not exclude the possibility that loss of CFTR has direct effects on cells important for normal intestinal function but it is clear that the enteric smooth muscle can function normally in the absence of CFTR.
Acknowledgments
Supported by the Cystic Fibrosis Foundation, a pilot project as part of P20 RR024214 COBRE on Molecular Regulation of Cell Development and Differentiation from the National Center for Research Resources, and NIH grant R21AI083479.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Seidler U, Singh A, Chen M, et al. Knockout mouse models for intestinal electrolyte transporters and regulatory PDZ adaptors: new insights into cystic fibrosis, secretory diarrhoea and fructose-induced hypertension. Exp Physiol. 2009;94:175–179. doi: 10.1113/expphysiol.2008.043018. [DOI] [PubMed] [Google Scholar]
- 2.Mueller C, Braag SA, Keeler A, et al. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol. 2011;44:922–929. doi: 10.1165/rcmb.2010-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y, Su M, McNutt MA, et al. Expression and distribution of cystic fibrosis transmembrane conductance regulator in neurons of the human brain. J Histochem Cytochem. 2009;57:1113–1120. doi: 10.1369/jhc.2009.953455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan J, Luk C, Kent G, et al. Pulmonary neuroendocrine cells, airway innervation, and smooth muscle are altered in Cftr null mice. Am J Respir Cell Mol Biol. 2006;35:320–326. doi: 10.1165/rcmb.2005-0468OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michoud MC, Robert R, Hassan M, et al. Role of the CFTR Channel in Human Airway Smooth Muscle. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2006-0444OC. [DOI] [PubMed] [Google Scholar]
- 6.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 7.Sbarbati A, Bertini M, Catassi C, et al. Ultrastructural lesions in the small bowel of patients with cystic fibrosis. Pediatr Res. 1998;43:234–239. doi: 10.1203/00006450-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Lojda Z, Jodl J. [Jejunal mucosa in children with mucoviscidosis] Cesk Patol. 1975;11:135–139. [PubMed] [Google Scholar]
- 9.Garcia MAS, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norkina O, Burnett TG, De Lisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun. 2004;72:6040–6049. doi: 10.1128/IAI.72.10.6040-6049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruzzese E, Raia V, Gaudiello G, et al. Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment Pharmacol Ther. 2004;20:813–819. doi: 10.1111/j.1365-2036.2004.02174.x. [DOI] [PubMed] [Google Scholar]
- 12.Raia V, Maiuri L, De Ritis G, et al. Evidence of chronic inflammation in morphologically normal small intestine of cystic fibrosis patients. Pediatr Res. 2000;47:344–350. doi: 10.1203/00006450-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Werlin SL, uri-Silbiger I, Kerem E, et al. Evidence of Intestinal Inflammation in Patients With Cystic Fibrosis. J Pediatr Gastroenterol Nutr. 2010;51:304–308. doi: 10.1097/MPG.0b013e3181d1b013. [DOI] [PubMed] [Google Scholar]
- 14.Strandvik B. Fatty acid metabolism in cystic fibrosis. Prostaglandins Leukot Essent Fatty Acids. 2010;83:121–129. doi: 10.1016/j.plefa.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Dif F, Wu YZ, Burgel PR, et al. Critical role of cytosolic phospholipase a2{alpha} in bronchial mucus hyper-secretion in CFTR-deficient mice. Eur Respir J. 2010;36:1120–1130. doi: 10.1183/09031936.00183409. [DOI] [PubMed] [Google Scholar]
- 16.De Lisle RC, Meldi L, Flynn M, et al. Altered eicosanoid metabolism in the cystic fibrosis mouse small intestine. J Pediatr Gastroenterol Nutr. 2008;47:406–416. doi: 10.1097/MPG.0b013e31817e0f2c. [DOI] [PubMed] [Google Scholar]
- 17.Chmiel JF, Konstan MW. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin Chest Med. 2007;28:331–346. doi: 10.1016/j.ccm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Carlstedt-Duke J, Bronnegard M, Strandvik B. Pathological regulation of arachidonic acid release in cystic fibrosis: the putative basic defect. Proc Natl Acad Sci U S A. 1986;83:9202–9206. doi: 10.1073/pnas.83.23.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strandvik B, Svensson E, Seyberth HW. Prostanoid biosynthesis in patients with cystic fibrosis. Prostaglandins Leukot Essent Fatty Acids. 1996;55:419–425. doi: 10.1016/s0952-3278(96)90125-8. [DOI] [PubMed] [Google Scholar]
- 20.Medjane S, Raymond B, Wu Y, et al. Impact of CFTR DeltaF508 mutation on prostaglandin E2 production and type IIA phospholipase A2 expression by pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L816–L824. doi: 10.1152/ajplung.00466.2004. [DOI] [PubMed] [Google Scholar]
- 21.Freedman SD, Blanco PG, Zaman MM, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 22.Tirouvanziam R, Khazaal I, Peault B. Primary inflammation in human cystic fibrosis small airways. Am J Physiol Lung Cell Mol Physiol. 2002;283:L445–L451. doi: 10.1152/ajplung.00419.2001. [DOI] [PubMed] [Google Scholar]
- 23.Fink J, Steer JH, Joyce DA, et al. Pro-inflammatory effects of Burkholderia cepacia on cystic fibrosis respiratory epithelium. FEMS Immunol Med Microbiol. 2003;38:273–282. doi: 10.1016/S0928-8244(03)00169-X. [DOI] [PubMed] [Google Scholar]
- 24.Hodges CA, Cotton CU, Palmert MR, et al. Generation of a conditional null allele for Cftr in mice. Genesis. 2008 doi: 10.1002/dvg.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodges CA, Grady BR, Mishra K, et al. Cystic Fibrosis growth retardation is not correlated with loss of Cftr in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2011;301:G528–G536. doi: 10.1152/ajpgi.00052.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Lisle RC, Sewell R, Meldi L. Enteric circular muscle dysfunction in the cystic fibrosis mouse small intestine. Neurogastroenterol Motil. 2010;22:341-e87. doi: 10.1111/j.1365-2982.2009.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Lisle RC. Altered Transit and Bacterial Overgrowth in the Cystic Fibrosis Mouse Small Intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G104–G111. doi: 10.1152/ajpgi.00548.2006. [DOI] [PubMed] [Google Scholar]
- 28.Tai HH, Ensor CM, Tong M, et al. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:483–493. doi: 10.1016/s0090-6980(02)00050-3. [DOI] [PubMed] [Google Scholar]
- 29.Ganz T. Angiogenin: an antimicrobial ribonuclease. Nat Immunol. 2003;4:213–214. doi: 10.1038/ni0303-213. [DOI] [PubMed] [Google Scholar]
- 30.Hooper LV, Stappenbeck TS, Hong CV, et al. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 31.Malmberg EK, Noaksson KA, Phillipson M, et al. Increased levels of mucins in the cystic fibrosis mouse small intestine and modulator effects of the Muc1 mucin expression. Am J Physiol Gastrointest Liver Physiol. 2006;291:G203–G210. doi: 10.1152/ajpgi.00491.2005. [DOI] [PubMed] [Google Scholar]
- 32.De Lisle RC, Roach E, Jansson K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G577–G584. doi: 10.1152/ajpgi.00195.2007. [DOI] [PubMed] [Google Scholar]
- 33.Hallberg K, Abrahamsson H, Dalenback J, et al. Gastric secretion in cystic fibrosis in relation to the migrating motor complex. Scand J Gastroenterol. 2001;36:121–127. doi: 10.1080/003655201750065852. [DOI] [PubMed] [Google Scholar]
- 34.Scott LD, Cahall DL. Influence of the interdigestive myoelectric complex on enteric flora in the rat. Gastroenterology. 1982;82:737–745. [PubMed] [Google Scholar]
- 35.Yuan C, Sidhu RS, Kuklev DV, et al. Cyclooxygenase Allosterism, Fatty Acid-mediated Cross-talk between Monomers of Cyclooxygenase Homodimers. J Biol Chem. 2009;284:10046–10055. doi: 10.1074/jbc.M808634200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson C, Al-Turkmani MR, Savaille JE, et al. Cell culture models demonstrate that CFTR dysfunction leads to defective fatty acid composition and metabolism. J Lipid Res. 2008;49:1692–1700. doi: 10.1194/jlr.M700388-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]