Abstract
Aging is usually accompanied by diminished immune protection upon infection or vaccination. While aging results in well-characterized changes in the T-cell compartment of long-lived, outbred, and pathogen-exposed organisms, their relevance for primary antigen responses remain unclear. Therefore, it remains unclear whether and to what extent the loss of naïve T-cells, their partial replacement by oligoclonal memory populations, and the consequent constriction of T-cell receptor (TCR) repertoire, limit the antigen responses in aging primates.
We show here that aging rhesus monkeys (Macaca mulatta) exhibit poor CD8 T-cell and B cell responses in the blood and poor CD8 responses in the lungs upon vaccination with the modified vaccinia strain Ankara (MVA). The function of antigen presenting cells appeared to be maintained in aging monkeys, suggesting that the poor response was likely intrinsic to lymphocytes. We found that the loss of naïve CD4 and CD8 T-cells, and the appearance of persisting T-cell clonal expansions (TCE) predicted poor CD8 responses in individual monkeys. There was strong correlation between early CD8 responses in the transitory CD28+ CD62L− CD8+ T-cell compartment, and the peak antibody titers upon boost in individual animals, as well as a correlation of both parameters of immune response to the frequency of naïve CD8+ T-cells in old, but not in adult monkeys. Therefore, our results argue that T-cell repertoire constriction and naïve cell loss have prognostic value for global immune function in aging primates.
INTRODUCTION
Protective immunity declines with age, exposing the elderly to an increased risk from severe infections. The underlying causes of immunosenescence are multiple and not completely understood, yet the T-cell compartment exhibits consistent and pronounced age related changes (1). These include thymic involution and decreased production of naïve T-cells, diminished responsiveness to TCR signaling that culminates in blunted T-cell proliferation, reduced IL-2 production and effector T-cell differentiation (2), lowering or inversion of CD4: CD8 T-cell ratios (3), reduction in naïve and an increase in memory cells (4), telomere shortening (5), and T-cell receptor (TCR) repertoire constriction (6, 7). It was shown that the occurrence of T-cell clonal expansions (TCE) in mice (8) and humans (9) is an age-dependent phenomenon, associated with dramatic losses of TCR repertoire (10). Consistent with all of the above changes, there is some evidence that in mice impaired CD8 response to virus infections (11, 12) may partially correlate to age-related repertoire losses (10, 13).
We and others have shown previously that old primates harbor diminished naïve T-cell pools (14, 15), and that the percentage of naïve cells correlates inversely to the occurrence of TCE in rhesus monkeys (16). Associations between age-related TCR repertoire constriction and diminished immune function were experimentally demonstrated in rodents (13, 17). However, experiments in short-lived inbred animals, maintained in specific pathogen free conditions throughout their lifetime, often cannot predict the relevance of these homeostatic changes for the T-cell function in elderly humans.
To address these issues, we investigated CD8 T-cell and antibody responses to primary vaccination with modified vaccinia Ankara (MVA) in long-lived, outbred, and pathogen-exposed rhesus monkeys (Macaca mulatta, RM in the text). We observed age-related losses in primary responses to vaccine, despite largely unaltered dendritic cell (DC) function. Old monkeys exhibited a loss of T-cell repertoire diversity as well as an overall loss of naïve cells, which both correlated to poor CD8 response to antigen. Initial CD8 responses, in turn, correlated to maximum antibody responses to MVA. Therefore, our data argue that loss of naïve T-cell populations and repertoire predicts poor responses to vaccination, and might be a major contributor to immune senescence in aging primates.
MATERIAL AND METHODS
Animals, virus
Colony-bred male and female Rhesus macaques of Indian origin were maintained according to the federal, state and local guidelines. All experiments were approved by the Animal Care and Use Committee at the Oregon National Primate Research Center (ONPRC). Animals with tumors, amyloidosis, or signs of clinical disease were excluded from the study. Cohorts tested for Ab responses consisted of a large group of 21 adult (age 6–10 years, 13 male, 9 female) and 28 old (age 18–27, 12 male, 16 female) RM. For detailed analysis of CD8 response kinetic, a subset of 11 younger adult (˜=8.5 y, range 7–10, 6 males, 5 females) and 9 old (˜=23.2 y, range 20–27, 4 males, 5 females) was randomly selected. Pooled data from three independent experiments are shown throughout. MVA(18) was used for immunization, whereas vaccinia virus (VACV) strain WR was used for restimulation of CD8 T-cells. Both viruses were grown on chicken Embryo fibroblasts (Charles River) and purified by sonication and ultracentrifugation on a 36% Sucrose cushion.
Immunization, FITC painting and sampling
Animals were immunized by two subcutaneous injections of 5×107 PFU of MVA each into the left and right pectoral area respectively. Animals were anesthesized by Ketamine injection for bleeding, Bronchoalveolar lavage (BAL) collection and biopsies at indicated time points. BAL cells were harvested by a 40 ml saline wash of one lung side. 2 skin biopsies were sampled from painted and unpainted sites respectively by punch biopsy. Draining lymph nodes were surgically resected and analyzed by flow cytometry (FCM) as described below.
ELISA
Costar EIA/RIA plates (Corning Incorporated, Corning, New York) were coated with VACV-WR lysates as a source of antigen. Duplicates of serially diluted plasma were incubated on the plates for 1.5 hours, followed by three washes. The plates were then incubated for 1 hour with goat anti-monkey IgG(γ)-HRP (Nordic Immunological Laboratories, Tilburg, The Netherlands). The plates were again washed three times before o-Phenylenediamine (Sigma, St. Louis, Missouri) in citrate buffer was added. The reaction was stopped with 1M HCl. The 490nM absorbance was assessed in a Molecular Device optical reader using the Soft Max program. The titers were calculated from the linear phase, as 0.1 intersecting points, essentially as described elsewhere(19).
In vitro antigenic restimulation
Blood lymphocytes were enriched as published(16). BAL lymphocytes were centrifuged at 600xg and their concentration was adjusted. 106 cells were cultivated in 200 ml of RPMI, supplemented with 10% FBS, β-mercaptoethanol and penicillin/streptomycin. Antigen-stimulated cells were incubated for 15h with vaccinia virus WR strain, at a multiplicity of infection of 1, upon which brefeldin A was added for 2 additional hours. Intracellular IFNγ and TNFα responses were measured by FCM. Control cells, cultivated in parallel in the absence of virus, showed high TNFα background signal (Fig. 1D); thus, we focused on IFNγ responses. Ag-specific responses were defined by subtracting the background IFNγ responses in uninfected controls from IFNγ values observed in vaccinia-stimulated samples.
Figure 1. Age-related decline in RM primary CD8 response to vaccination.
8 experimental cohorts of Old (n=28) and adult (n=21) RM were vaccinated with MVA as described in methods. MVA-specific antibody responses were calculated and displayed as fold increase of ELISA titers over baseline (panelA). Cohorts of young and old animals were compared at indicated time points post prime (Upper panel) or boost (lower panel) by repeated measures ANOVA, and assessed for significance (n.s.-p>0.05; *-p<0.05). Histograms indicate group means, error bars show Standard errors of the mean (SEM). (B) Kinetic of IFNγ CD8+ cell responses in blood on days 7, 14, 28 and 42. Cells were in vitro stimulated with VACV WR at MOI=1 for 15h, followed by additional 2h incubation with BrefeldinA. Upon surface staining with αCD4 and αCD8, cells were fixed, permeablized and stained for cytokine expression. Frequency of IFNγ+ cells in uninfected controls were subtracted from vaccinia-infected samples to eliminate background IFNγ expression. Results are shown as means±SEM from three pooled experiments. p-value from repeated measures ANOVA is indicated. (C) Kinetic of IFNγ responding CD8+ cells in BAL on days 14, 28 and 42 of adult and old monkeys. Note that age-related differences were more pronounced in BAL than in blood (see panel B). Pooled means±SEM from three independent experiments and p-value from repeated measures ANOVA are shown. (D) Three representative contour plots of IFNγ (y axes) and TNFα (x axes) expression in control CD8 T-cells (upper panels – CTRL) or vaccinia stimulated cells (lower panels – VACV)
Antibody staining and flow cytometry
Surface staining was performed as shown earlier (16), with following modifications: in baseline phenotype stainings we used anti(α)CD4-APCCy7 (clone Oct-4, Biolegend) antibodies, instead of αCD4 - clone L200; we added αCD45RA-PECy5.5 (clone MEM-56, Invitrogen), and αCCR7-PECy7 (clone 3D12, BD) to the phenotypization antibody panel. For intracellular cytokine staining, cells were first surface stained with αCD4Percp-Cy5.5, αCD8β-ECD, αCD95-APC, αCD62L-PE (clone SK11, BD, San Jose, CA) and αCD28-biotin antibodies, followed by incubation with streptavidin-Qdot525. Cells were subsequently fixed for 5’ with 100µl of IC Fixation buffer (eBioscience, San Diego, CA), followed by 5’ permeabilization with 100 µl of Permeabilization Buffer (eBioscience) and 30 min incubation with αIFNγ-FITC (clone 4S.B3, BD) and αTNFα-PECy7 (clone MAb11, BD). Cells were washed and results acquired using a custom, 3-laser LSR-II cytometer (BD). Cytometric results were analyzed by FlowJo 8.2 software (Treestar, Ashland, OR). To define the % of naïve CD8 cells, we multiplied the % of CD8 cells in the CD45RA+CD11a− gate by the % of CD31+CD95− in the CD45RA+CD11a− subset, and divided their product by 100. Frequency of IFNγ responding cells were defined by adding values from IFNγ+TNFα− and IFNγ+TNFα+ quadrants. Responses in CD62L−CD28+ or CD62L−CD28− subsets were defined by progressive gating.
IL-12 release from stimulated DC
DC stimulation and IL-12 detection were performed essentially as described (20). In brief, DC were enriched by a 5-day incubation of CD14+ blood monocytes with1000U/ml GMCSF and 100U/ml IL-4, followed by a 2 day stimulation with 1µg/ml of CD40-L. IL-12 in supernatants was quantified by ELISA in a SpectraMax Plus reader (Molecular Devices, Sunnyvale, CA).
MHC 1b- restricted antigen processing and presentation
Primate DC were generated as above. Human DCs were isolated by flask adherence and then incubated with IL-4/GM-CSFfor 5 days(21). To test the ability of DC to process and present antigen to CD8+ T cells, DC were used as APC in limiting quantities and responding T cells in excess in an IFN-γ ELISPOT assay(22). We used readily available human MHC Ib-restricted CD8+ T cell clones recognizing an antigen contained in Mycobacterium tuberculosis (Mtb) cell wall, because the MHC Ib molecule is conserved sufficiently between Rhesus monkeys and humans such that Rhesus DC can present to the human CD8+ T cells (Lewinsohn, D.A. et al., unpublished observations). Specifically, DCs (10,000 cells/well) were incubated with Mtb cell wall (30ug/ml) for 1 hour. DC and then T cell clones (10,000 cells/well) were added and supplemented with with IL-2 (0.5ng/ml) and tested in the IFN-γ ELISPOT assay.
DC migration assay
Animal hair was clipped, but not shaved and skin was painted with a solution of 100mg/ml fluorescein in a solution of 10%DMSO, 45% Acetone and 45% DBT.
A day later, skin and draining lymph node bioptic samples were collected from painted and control (unpainted contralateral) sites from each monkey. The epidermis was removed from the dermis, fixed with paraformaldehyde and stained for HLA-DR-Cy3 Streptavidin or isotype control. Langerhans cells were enumerated by fluorescent microscopy.
Cells from the inguinal (draining) and axillary (control) lymph nodes were harvested twenty-four hours after hind quarter FITC painting. Cells were surface stained using CD83-PE (Immunotech), CD3-PerCP (BDPharmingen), CD86-Allophycocyanin (BD Pharmingen) CD11c-PeCy7 (Biolegend) and CD20-Pacific Blue (Biolegend). FCM data were acquired and analyzed as above. CD3-CD20- cells that were positive for CD11c, CD86 or CD83 were defined as DC and analyzed for frequency of FITC+ signal.
CDR3 length polymorphism assay
TCR length polymorphism was assayed essentially as described previously (16). In brief, cDNA from 106 to 5×106 PBMC was subjected to 24 separate TCR Vβ-specific PCR reactions. PCR products were separated by polyacrylamide gel electrophoresis and quantified by densitometry.
Statistical analysis
All statistical analysis was calculated with SAS software, version 9.1.3, (SAS, Cary, NC) using repeated-measure ANOVA or non-parametric two-tailed statistical analysis. Single time-point comparisons of CD8 subsets from young and old monkeys were done by t distribution-based Wilcoxon-Mann-Whitney test. Kinetics of CD8 responses in young and old monkeys were compared by repeated measures ANOVA. Due to skewed data distribution, non-parametric correlations were calculated throughout unless indicated otherwise.
RESULTS
Poor response to vaccination in old monkeys
To define the immune response to vaccination in aging primates, and exclude possible influences associated with the maturation of the immune system, we compared cohorts of old (18–25 years old, average 21.8) and adult, non-juvenile RM (7–10 years old, average 8.1). Monkeys were primed, and boosted 8 weeks later, with MVA, as a representative replication-defective live vaccine that would be considered a safe candidate for vaccination of older adults. We analyzed a large group of 21 adult and 29 old rhesus monkeys, divided in eight independent cohorts, consisting of at least two adult and three old monkeys/cohort. Since antibody is responsible for long-term anti-pox immunity after vaccination, we tested antibody responses by indirect ELISA to MVA at baseline, on days 28 and 42 following priming, and days 14, 28 and 49 post-boost. Responses were quantified as logarithmic fold increases of antibody titer over baseline, and all experimental results were pooled and compared between age cohorts. Average antibody responses to MVA were weaker in old than in young monkeys at all times, and this difference became significant upon boost (Fig. 1A), strongly arguing that humoral immune response to MVA vaccination may be weaker in aging primates than in young ones.
MVA vaccination is known to induce CD8 responses, believed to play a role in clearing primary poxvirus infection. To analyze broad CD8 responses to MVA, we developed an in vitro stimulation assay with infectious vaccinia as source of antigen (see materials and methods). Preliminary results showed suboptimal in vitro re-stimulation of frozen samples (data not shown). Therefore, we performed all stimulation assays on fresh samples. We measured by flow cytometry the CD8 IFNγ response to antigenic restimulation by a 16h in vitro infection of blood and BAL cells with VACV. Due to the high technical demands of this protocol, this procedure was performed on three randomly selected cohorts consisting of 11 adult and 9 old monkeys. We compared the kinetics of CD8 responses in blood on days 7, 14, 28 and 42-post vaccination. The response peaked at day 14 in both age groups, and decreased thereafter, yet was overall significantly lower in old monkeys (Fig 1B). Therefore, CD8 T-cells from old monkeys exhibited significantly weaker IFNγ responses upon brief in vitro antigen restimulation than their adult counterparts. We next tested whether this result represented an overall reduction in antigen-specific response, or merely a redistribution of antigen-specific CD8 T-cells from the bloodstream into tissues. To that effect, we collected BAL cells on days 14, 28 and 42 post infection, and used them in antigen-stimulation assays to monitor for the development of antigen-specific CD8 T-cell response in tertiary tissues. In the adult monkeys, the BAL response peaked at day 28, rather than 14, and was even more intense than observed in the blood (Fig. 1C). By contrast, the response was undetectable in the majority of old monkeys, and the age related difference was more pronounced than in blood. Therefore, the age-related loss of CD8 function manifested itself in multiple compartments.
No drastic loss in dendritic cell function in old monkeys
Antigen processing and presentation by DC is essential to T-cell and, indirectly, most B-cell responses. Thus, age-related decline in uptake, migration, processing and/or presentation by DC would all have the potential to decrease T-cell (and, indirectly, via poor CD4 stimulation, B-cell) responses to an antigen. For example, a reduction in numbers (23, 24) and migration (25) was seen in young monkeys infected with SIV, and reduced DC numbers were reported, (26), but not confirmed (27), in aging mice. Therefore, we examined DC phenotype and function in adult and old monkeys. We quantified DC in situ, by morphometric immunohistology of bioptic skin specimens and observed no age-related difference in the number of HLA-DR+ cells (Fig 2A). Painting the skin of our monkeys with fluorescein, and enumerating the fluorescently labeled HLA-DR+ cells in the skin or CD11c+ ones in draining LN showed no aging-related decrease in the number of DCs leaving the skin, nor reaching the draining LN. Likewise, fluorescein-labelled CD80+ or CD86+ cells reaching draining LN did not show age-related differences (data not shown). Therefore, DC numbers and in vivo migration were intact in old monkeys.
Fig. 2. No age-related differences in number and migration efficacy of DC.
(A) Skin bioptic samples were stained with HLA-DR antibodies to define DC, and DC per visual field were counted in duplicate, with 10 fields/replicate. Median values and interquartile ranges for adult (n=11) and old (n=9) monkeys are shown. (B) Dendritic cells were enriched from blood CD14+ cells by a 5-day cultivation in GM-CSF and IL-4 media, and stimulated with 1µg/ml CD40L for 48h. IL-12 release in supernatants was measured by ELISA and normalized to cell numbers. Median values and interquartile ranges for adult (n=13) and old (n=22) monkeys are shown. (C) DC obtained as in panel B were used to process and present MHC1b- restricted peptides to the indicated CD8 cell clones. Functional CD8 responses to DC presentation were measured by ELISPOT, and normalized to reflect fold increases over background. Geometric means and 95% confidence intervals are shown for old (n=36) and adult (n=18) cohorts.
We next addressed the possibility that DC from old primates provide inferior costimulatory response. For instance, infant monkeys exhibit a DC defect in IL-12 responses to in vitro stimulation, resulting in strongly decreased response to MVA vaccination despite relatively intact T-cell function (D.A. Lewinsohn et al., in preparation). However, in our cohorts of adult and old monkeys we observed no differences in IL-12 DC response to moderate CD40-ligand stimulation (Fig. 2B), or to strong stimulation with a combination (20) of TNFα, Prostaglandin E2, IL-1β and IL-6 (not shown).
Finally, the functional ability of DC to present class I antigen was directly measured by their ability to present mycobacterial antigens in a class Ib-restricted manner to human CD8+ T-cell clones where sufficient conservation between MHC1b alleles allows recognition of antigen expressing Rhesus monkey DC by these human T cells without the need for Mamu matching (Lewinsohn, D.A. et al, unpublished results). There was no difference in response to adult and old DC as measured by ELISPOT (Fig. 2C). In conclusion, within the constraints of our experimental models, we failed to uncover age-related differences in DC numbers, phenotype, migration or Ag-presentation function between adult and old rhesus monkeys, suggesting that other elements of the aging immune system, including lymphocytes themselves, likely cause suboptimal antigen-specific responses.
Loss of naïve CD8 cells and repertoire diversity in old monkeys
We have previously shown that age-dependent loss of naïve T-cells in aging monkeys correlates to increases in naïve cell proliferation and turnover (16). Since naïve lymphocytes, which carry broadly diverse TCR repertoires, are required for the recognition of novel antigens, it was conceivable that the poor CD8 response to MVA in old monkeys reflected the age-related loss of naïve CD8 cell numbers and TCR repertoire diversity.
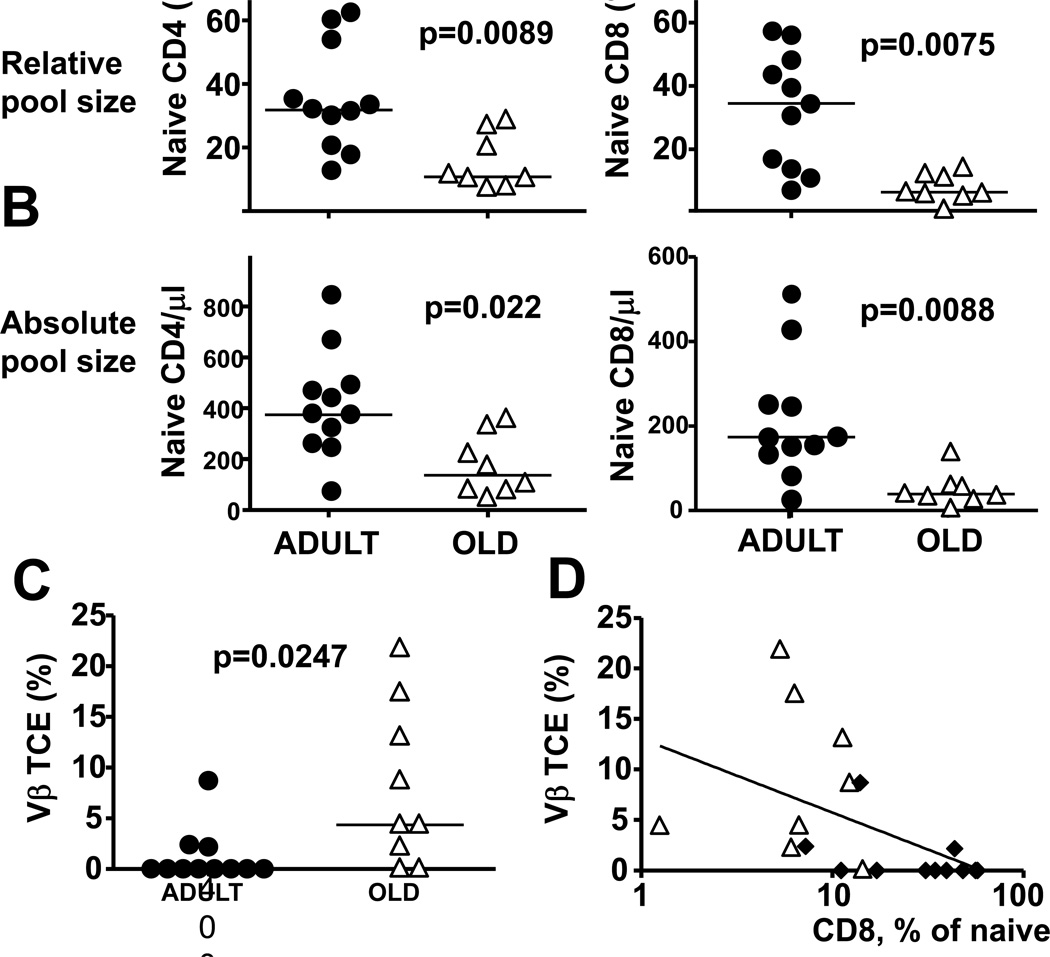
Three weeks before immunization, we defined the percentage of naïve CD4 and CD8 T-cells in blood by flow cytometry (FCM). Naive CD4 T-cells were defined as CD45RA+CD11a− CD28+CD95−CD31+CCR7−, and naïve CD8 as CD45RA+CD11a− CD31+CD95−, which allowed stringent calculation of naïve T-cell fractions. Significantly lower frequency of naïve CD4 (p=0.0089) and CD8 T-cells (p=0.0075) was observed in old monkeys (Fig 3A). This relative loss was a reflection of the loss of naïve cells in absolute terms, counted as the number of naïve CD4 and CD8 T-cells per volume unit of blood, because the aged cohort showed significantly lower absolute counts of naïve CD4 (p=0.022), and CD8 cells (p=0.0088) (Fig. 3B).
Fig. 3. Age-related differences in CD8 naïve pool size and stable TCE occurrence correlate inversely.
(A, B). Three weeks before vaccination, blood lymphocytes were analyzed by FCM for the frequency and absolute count of naïve CD4 and CD8 T-cells Naive CD4 T-cells (left panels), and naïve CD8 T-cells (right panels) defined by restrictive progressive gating (via CD4 or CD8 , then through the CD28hiCD95lo gate) were quantified in individual monkeys in terms of their frequency with CD4 or CD8 pools (A), or in terms of their number per ml of blood (B). Symbols indicate the naïve T-cell percentage in individual old or adult monkeys, horizontal lines show means. p-values reflect Wilcoxon-Mann-Whitney test results. (C) cDNA from blood lymphocytes was analyzed yearly by PCR for TCR length polymorphism in each of the 24 V regions of the β TCR chain for 4 consecutive years, and Vβ families exhibiting consistently a single PCR band for at least the last two time points were defined as TCE+. Symbols indicate percentages of TCE+ Vβ families in individual monkeys, horizontal lines show means. (D) Naïve cell frequencies (x-axis) were correlated to the percentage of TCE+ Vβ families (y-axis) in individual adult (black diamonds) or old (white triangles) monkeys. A semilogarithmic correlation index for combined groups is indicated.
To asses the loss of TCR repertoire diversity in the aging monkey cohort, we defined the CDR3 length polymorphism of their TCR Vβ chains by a set of 24 PCR reactions specific for individual Vβ families (28). Vβ families exhibiting a single PCR peak were defined as likely containing a T-cell clonal expansion (TCE). TCE indicate severe repertoire constriction of the affected Vβ family (8), and their frequency correlates inversely to the frequency of naïve CD8 cells in RM (16). In mice, TCE wax and wane over time, with only some of them becoming stable (29), and can be classified into CD49dhi,CD122lo cells, which are unstable upon adoptive transfer and could be responding to antigen, and CD49dlo, CD122hi, which are antigen independent, stable upon adoptive transfer and arise preferentially in old rodents (30). Thus, we analyzed the TCE in our monkey cohorts over four consecutive yearly time points prior to immunization, and Vβ families showing a TCE pattern in at least two most recent time points were defined as Vβ families with a stable TCE. Old animals exhibited a significant (p=0.0247) increase in the frequency of stable TCE (Fig 3C), arguing for an age-related decrease in TCR repertoire. Moreover, naïve CD8 T-cell frequency correlated inversely (Spearman r =−0.7003, p=0.0008) to the frequency of stable TCEs (Fig 3D), and a similar correlation (Spearman r =−0.7253, p=0.0004) was observed between stable TCE and naïve CD4 frequency (data not shown), arguing for a link between the size of the naïve cell populations and repertoire diversity. In conclusion, aging monkeys showed an increase in stable TCE, and a decrease in naïve T-cell frequency and absolute counts, arguing for an aging-related loss of TCR repertoire.
Stable TCE and loss of naïve CD8 is linked to poor immune response
It was conceivable that the loss of naïve T- cells and of repertoire diversity (Fig. 3) may be responsible for the failure of CD8 cells to respond to vaccination with a novel antigen (Fig. 1). In that case, there should be direct correlation between these phenomena. The frequency of naïve blood cells correlated to peak CD8 responses (Fig. 4A). Spearm adult values were pooled, but also on adult values in isolation (Spearman r =0.6909, p=0.01an’s correlation test (r=0.8636) revealed a significant (p<0.0001) correlation when old and86). Since the majority of old monkeys displayed almost no CD8 response in lung tissue (Fig. 1C), a correlation between naïve cell counts and tissue CD8 responses could not be established in this age group (data not shown). Therefore, naïve cell frequency predicted peak CD8 responses to MVA in adult but not in old monkeys. To examine whether stable TCE affected CD8 responses, we compared CD8 responses in monkeys with at least one stable TCE-positive population (TCE+) to those without clonally expanded Vβ populations (TCE−). TCE+ animals showed significantly (p=0.0019) lower antigen responses than TCE− animals in BAL on day 28 (Fig. 4B). When the two age groups were considered separately, we could observe similar trends: The only two old animals showing any response in lung tissue were the two TCE− ones, whereas the three adult animals that exhibited TCE were among the four lowest-ranked antigen responders in their age cohort (Fig. 4B).
Fig. 4. Age-related difference in TCE and naïve CD8 frequency predicts antigen specific responses.
(A) Frequency of naïve blood CD8 lymphocytes prior to MVA immunization (x-axis) was correlated to peak (day28) CD8 response in BAL (y-axis) in individual adult (●) or old monkeys (△).(B) Peak tissue CD8 responses from monkeys with at least one Vβ family with a clonal expansion (TCE+) were compared to those without any TCE (TCE−). Adult (●) and old (△) monkeys belonging to either TCE group are indicated by respective symbols, horizontal lines indicate means.
Loss of naïve T-cells predicts immediate CD8 responses to MVA in old monkeys
Our results indicated that the loss of TCR repertoire and of naïve T-cells might compromise CD8 responses in old primates. Since such a mechanism would affect immediate CD8 responses, we explored the initial and early responses. Naïve cells are CD28+, whereas activated effector T-cells are characterized by the CD62L−CCR7− phenotype (31), and are predominantly CD28− in rhesus monkeys (15). We showed previously that polyclonal in vitro stimulation of CD8 cells result in a progressive loss of CD28 from cell surface upon subsequent rounds of cell division (14), but the kinetics of this loss upon antigen stimulation in vivo had not been studied in old primates. IFNγ responses to antigen restimulation were significantly stronger in the CD28+ than in the CD28− subset of CD62L− CD8 T-cells on day 7 post immunization (Fig. 5A), but not at later time points (not shown). Moreover, the kinetic of CD28 expression on IFNγ+ CD8 cells showed that MVA specific cells had a significant subset of CD62L−CD28+ on day 7, and changed towards the CD62L−CD28− phenotype by day 14, which was maintained thereafter, in both adult (Fig. 5B) and old monkeys (not shown). Antigen responses to restimulation in the transitional CD28+CD62L− subset of activated T-cells were significantly reduced in old monkeys compared to adult counterparts at day 7 post vaccination (Fig 5C), arguing strongly that old animals show poor immediate CD8 responses to a neo-antigen. We correlated the frequency of naïve T-cells prior to immunization to the immediate CD8 response. Spearman’s correlation between naïve CD8 cell frequency and immediate antigen responses (CD28+ cells, blood, d7) was significant (r=0.55, p=0.015) when adult and old animals were pooled , but also in animals within the old cohort (r=0.74, p=0.037). This correlation was highly significant (r=0.82, p=0.001) in monkeys that had less than 20% of naïve cells (Fig. 5D) but not in those with large naïve populations (not shown). Monkeys with low naïve cell frequencies included all old animals, and 4 out of 11 of the adult ones, which was in line with the significant loss of naïve cells in aging (Fig. 3A and 3B). To define if the absolute count of naïve CD8 cells defined the frequency of immediate CD8 responses to MVA vaccination, we correlated the absolute count of CD8 cells in old monkeys, as shown in Figure 3 panel B to the frequency of IFNγ responders within the CD28+CD62L− pool (Fig 5E). These parameters showed a remarkably tight correlation both by non-parametric (Spearman r=0.93, p=0.0009) or parametric statistical analysis (Pearson r=0.98, p<0.0001), arguing that the naïve cell count in blood of old primates defines the initial CD8 response to vaccination. On the other hand, the absolute count of naïve CD4 cells correlated less tightly (Spearman r=0.80, p=0.047) with CD8 response (not shown). Therefore, the aging-related loss of naïve T-cells, especially CD8 T-cells, predicted the poor initial CD8 response to MVA in old primates.
Fig. 5. Early CD8 responses to MVA are attenuated in old monkeys.
(A) The frequency of IFNγ responders in CD28+ and CD28− subsets of individual adult monkeys at day 7 post vaccination was pairwise compared by Wilcoxon signed rank test and p value is shown. Similar results (p=0.0039) were observed in old animals (not shown). (B) In vitro VACV stimulation was followed by FCM as in figure 1B. The frequency of CD28+ and CD28− cells in CD62L− IFNγ responders was calculated for each individual adult monkey and time point (see supportive figure 3 for gating). Means and SEM for each time point are shown. (C) The frequency of IFNγ responders in CD28+ subsets of adult or old animals was compared by Mann-Whitney test and p value is shown. Symbols indicate values relative to individual monkeys, horizontal bars indicate means. (D) Frequency of naïve CD8 lymphocytes prior to MVA immunization (x-axis) was correlated to IFNγ responses in CD62L−CD28+ cells at day 7 following infection (y-axis) in animals with less than 20% of naïve CD8 cells prior to vaccination. Symbols show values in individual adult (●) or old monkeys (△), the line shows the linear regression curve. (E) Naïve CD8 counts per ml blood prior to MVA immunization (x axis) was correlated to IFNγ responses in CD62L−CD28+ cells at day 7 following infection (y-axis) in animals old animals. Symbols show values in individual animals, the line shows the linear regression curve. (F) Immediate CD28+CD62L− CD8+ T-cell IFNγ responses from Figure 2, panel C (x-axis) were correlated to antibody titers at day 14 post boost (y-axis). Values for individual adult (●) or old (△) animals, and the linear regression curve for combined datasets are shown.
Our results provided an opportunity to test whether the early CD8 responses also correlate with the ability to mount humoral responses in aging. To that effect, we examined the intensity of the immediate antigen response in the CD28+ subset of CD8 cells and correlated it to antibody responses in individual monkeys. Most interestingly, the CD28+CD62L− CD8 response correlated to the peak antibody-specific responses, both in younger adults and old animals (Fig. 5F).
In conclusion, our results argued that the age related losses of naïve T-cells and TCR repertoire have consequences for the immune function and that frequencies of naïve T-cell populations in blood have potential value as predictors of immune responses to vaccination.
DISCUSSION
In this study, we longitudinally followed immune responses in adult and old monkeys before and during prime-boost vaccination with MVA. We have shown here that CD8 T-cell and antibody responses to vaccination with MVA are significantly diminished in old primates. Our data examining DC and CD8 phenotypes, function and diversity all suggest that this defect may not lie with DC and that it may be cell-autonomous for CD8 responses, based upon lack of observable defects in DC function and strong correlation between diminished CD8 responses and the loss of naïve CD8 T-cell numbers and TCR repertoire diversity.
In our experiments, one striking finding was that humoral and CD8 responses strongly correlated in individual monkeys. This could mean that the two arms of the immune response aged in a coordinated, synchronous fashion, which would be somewhat surprising given the general heterogeneity of the aging process. Alternatively, it was possible that another cell/process, lying upstream of these effector responses, could be undergoing age-related decline in function or structure in these animals. One candidate would be DC, and our experiments all but excluded their age-related loss of function in the experiments described above. On the other hand, Schwaiger et al. have described a CD25+ CD8+ T-helper-like cell in aging humans, whose presence strongly correlated with successful outcome of Ab-response to influenza vaccination (32), and we cannot exclude at the present that a similar cell may exist in monkeys. Finally, another candidate for a common link could be the CD4 T-cell, known to play a role in both CD8+ and B-cell responses. While our attempts to directly measure Ag-specific responses of these cells have failed, and the resolution of this issue will have to await further experimentation, our results argue that the loss of naïve cells was seen not only in CD8, but also in CD4 cell pool, which could explain reduced Ab responses. Moreover, our analysis of TCE was not subset-specific and therefore showed reduced repertoire diversity that would be expected to affect CD4 cells as well. Additional experiments will be necessary to define more precisely the loss of reperoire diversity in CD4 subsets.
Lack of any measurable defects in DC function is in concert with some (27) but not other (26) studies published in mice. Our experiments failed to reveal discernible age-related differences in antigen uptake, emigration from skin, immigration into DLN, IL-12 production and antigen presentation. If confirmed, these results would raise a question on whether and to what extent short(er) lifespan of DC compared to lymphocytes may render them relatively more resistant to the manifestations of aging. Regardless, it would be naïve to expect that any given cell type would show no effects of aging – it is more important to understand whether these effects have functional consequences, and our results suggest that DC aging may have fewer functional consequences than T-cell aging.
What defect(s), then, matter the most in CD8+ (and CD4+) T-cell aging? Three distinct defects (or clusters of defects) which are important for the response to new microbial challenge have been described in greater or lesser detail: (i) cell-autonomous defects in T-cell signaling (starting with synapse formation and culminating with transcriptional events leading to effector T-cell differentiation), which were studied chiefly in CD4 T-cells (33, 34) (ii) numerical reduction in naïve CD4 and CD8 T-cells; and (iii) loss of T-cell receptor repertoire diversity amongst total (13) and more importantly naïve (17) CD8 T-cells. It is highly likely that these three factors potentiate each other, although at the present we still lack quantitative parameters and qualitative studies that would tease their relative contributions apart. Our results do not address (i), but they speak in favor of the importance of (ii) and (iii) in functional responses of old monkeys to vaccination.
In conclusion, our data argue that old primates have poor responses to novel MHC-I restricted antigens, and that the age-related loss of CD8 function is intrinsic to the shrinking T-cell repertoire in old age, consistent with previous results in aging rodents (13). Since TCR repertoire and CD8 responses correlated to the size of the naïve cell pool, we propose that phenotyping of blood lymphocytes may yield prognostic value for the evaluation of likely responses to vaccination with live replication-defective vaccines, where low frequency of naïve CD8 T-cells might predict poor cellular responses to immunization in elderly or middle aged vaccinees.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sarah Foster for technical assistance and Drs. L. Picker and M. Slifka for useful discussion.
Non-standard abbreviations
- BAL
bronchoalveolar lavage
- CM
central memory
- DC
Dendritic cell
- EM
effector memory
- FCM
flow cytometry
- MVA
modified vaccinia strain Ankara
- N
naïve
- RM
rhesus macaque
- TCE
T-cell clonal expansion
- VACV
Vaccinia Virus
Footnotes
This research was supported by the USPHS awards AG21384 and AG23664 from the National Institute on Aging to J.N-Z., the DFG research fellowship Ci-129/1-1 to L.C-S. and the Core NPRC award RR 0163 (NCRR) to ONPRC. The authors have no competing financial interests.
REFERENCES
- 1.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 2.Gillis S, Kozak R, Durante M, Weksler ME. Immunological studies of aging. Decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest. 1981;67:937–942. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998;102:187–198. doi: 10.1016/s0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 4.Pilarski LM, Yacyshyn BR, Jensen GS, Pruski E, Pabst HF. Beta 1 integrin (CD29) expression on human postnatal T cell subsets defined by selective CD45 isoform expression. J Immunol. 1991;147:830–837. [PubMed] [Google Scholar]
- 5.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28−CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 6.Mosley RL, Koker MM, Miller RA. Idiosyncratic alterations of TCR size distributions affecting both CD4 and CD8 T cell subsets in aging mice. Cell Immunol. 1998;189:10–18. doi: 10.1006/cimm.1998.1369. [DOI] [PubMed] [Google Scholar]
- 7.Hall MA, Reid JL, Lanchbury JS. The distribution of human TCR junctional region lengths shifts with age in both CD4 and CD8 T cells. Int Immunol. 1998;10:1407–1419. doi: 10.1093/intimm/10.10.1407. [DOI] [PubMed] [Google Scholar]
- 8.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8− bearing cells in old mice. J Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 9.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to "benign monoclonal gammapathy". J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Po JL, Gardner EM, Anaraki F, Katsikis PD, Murasko DM. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech Ageing Dev. 2002;123:1167–1181. doi: 10.1016/s0047-6374(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 12.Kapasi ZF, Murali-Krishna K, McRae ML, Ahmed R. Defective generation but normal maintenance of memory T cells in old mice. Eur J Immunol. 2002;32:1567–1573. doi: 10.1002/1521-4141(200206)32:6<1567::AID-IMMU1567>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jankovic V, Messaoudi I, Nikolich-Zugich J. Phenotypic and functional T-cell aging in rhesus macaques (Macaca mulatta): differential behavior of CD4 and CD8 subsets. Blood. 2003;102:3244–3251. doi: 10.1182/blood-2003-03-0927. [DOI] [PubMed] [Google Scholar]
- 15.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, Picker LJ, Mori M, Nikolich-Zugich J. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci U S A. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J Immunol. 2009;182:784–792. doi: 10.4049/jimmunol.182.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch VM, Fuerst TR, Sutter G, Carroll MW, Yang LC, Goldstein S, Piatak M, Jr, Elkins WR, Alvord WG, Montefiori DC, Moss B, Lifson JD. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 20.Mehlhop E, Villamide LA, Frank I, Gettie A, Santisteban C, Messmer D, Ignatius R, Lifson JD, Pope M. Enhanced in vitro stimulation of rhesus macaque dendritic cells for activation of SIV-specific T cell responses. J Immunol Methods. 2002;260:219–234. doi: 10.1016/s0022-1759(01)00544-0. [DOI] [PubMed] [Google Scholar]
- 21.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewinsohn DA, Heinzel AS, Gardner JM, Zhu L, Alderson MR, Lewinsohn DM. Mycobacterium tuberculosis-specific CD8+ T cells preferentially recognize heavily infected cells. 2003;168:1346–1352. doi: 10.1164/rccm.200306-837OC. [DOI] [PubMed] [Google Scholar]
- 23.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 24.Reeves RK, Fultz PN. Disparate effects of acute and chronic infection with SIVmac239 or SHIV-89.6P on macaque plasmacytoid dendritic cells. Virology. 2007;365:356–368. doi: 10.1016/j.virol.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barratt-Boyes SM, Zimmer MI, Harshyne L. Changes in dendritic cell migration and activation during SIV infection suggest a role in initial viral spread and eventual immunosuppression. J Med Primatol. 2002;31:186–193. doi: 10.1034/j.1600-0684.2002.t01-1-02005.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi KL, Sauder DN. Epidermal Langerhans cell density and contact sensitivity in young and aged BALB/c mice. Mech Ageing Dev. 1987;39:69–79. doi: 10.1016/0047-6374(87)90087-x. [DOI] [PubMed] [Google Scholar]
- 27.Linton PJ, Li SP, Zhang Y, Bautista B, Huynh Q, Trinh T. Intrinsic versus environmental influences on T-cell responses in aging. Immunol Rev. 2005;205:207–219. doi: 10.1111/j.0105-2896.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen ZW, Kou ZC, Shen L, Reimann KA, Letvin NL. Conserved T-cell receptor repertoire in simian immunodeficiency virus-infected rhesus monkeys. J Immunol. 1993;151:2177–2187. [PubMed] [Google Scholar]
- 29.LeMaoult J, Messaoudi I, Manavalan JS, Potvin H, Nikolich-Zugich D, Dyall R, Szabo P, Weksler ME, Nikolich-Zugich J. Age-related dysregulation in CD8 T cell homeostasis: kinetics of a diversity loss. J Immunol. 2000;165:2367–2373. doi: 10.4049/jimmunol.165.5.2367. [DOI] [PubMed] [Google Scholar]
- 30.Clambey ET, White J, Kappler JW, Marrack P. Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc Natl Acad Sci U S A. 2008;105:12997–13002. doi: 10.1073/pnas.0805465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 32.Schwaiger S, Wolf AM, Robatscher P, Jenewein B, Grubeck-Loebenstein B. IL-4-producing CD8+ T cells with a CD62L++(bright) phenotype accumulate in a subgroup of older adults and are associated with the maintenance of intact humoral immunity in old age. J Immunol. 2003;170:613–619. doi: 10.4049/jimmunol.170.1.613. [DOI] [PubMed] [Google Scholar]
- 33.Garcia GG, Sadighi Akha AA, Miller RA. Age-related defects in moesin/ezrin cytoskeletal signals in mouse CD4 T cells. J Immunol. 2007;179:6403–6409. doi: 10.4049/jimmunol.179.10.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.