Abstract
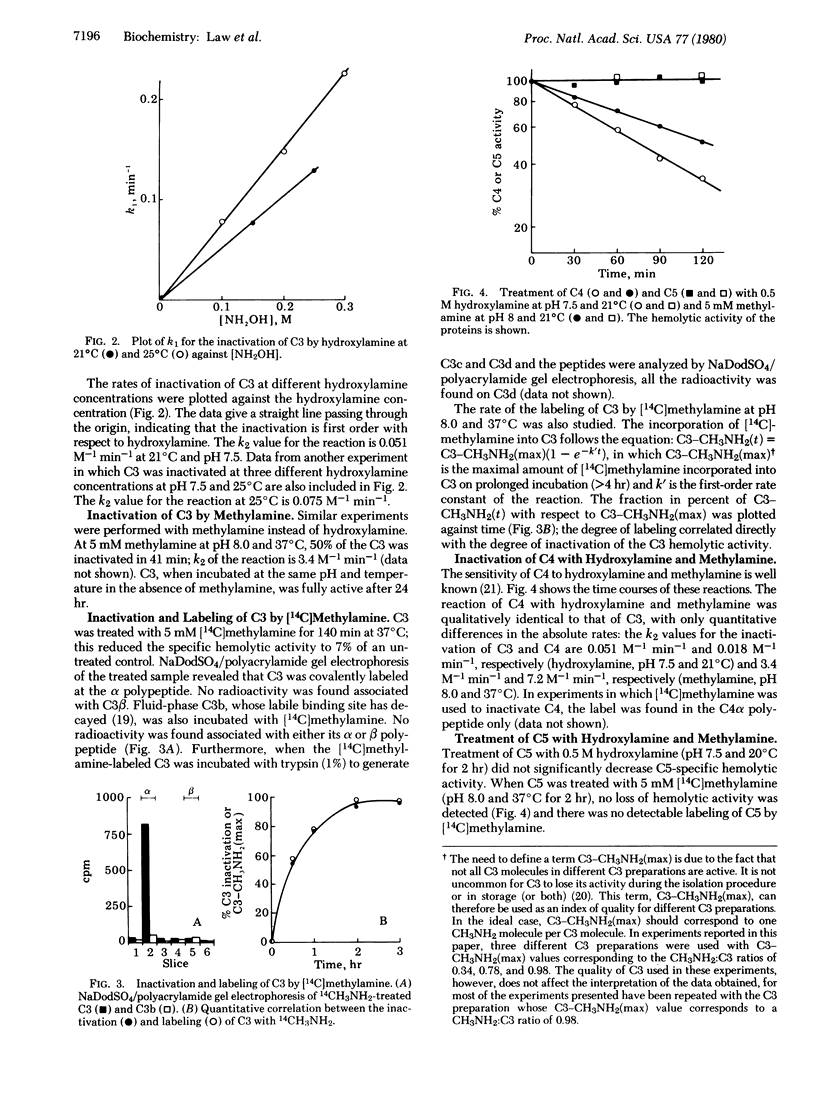
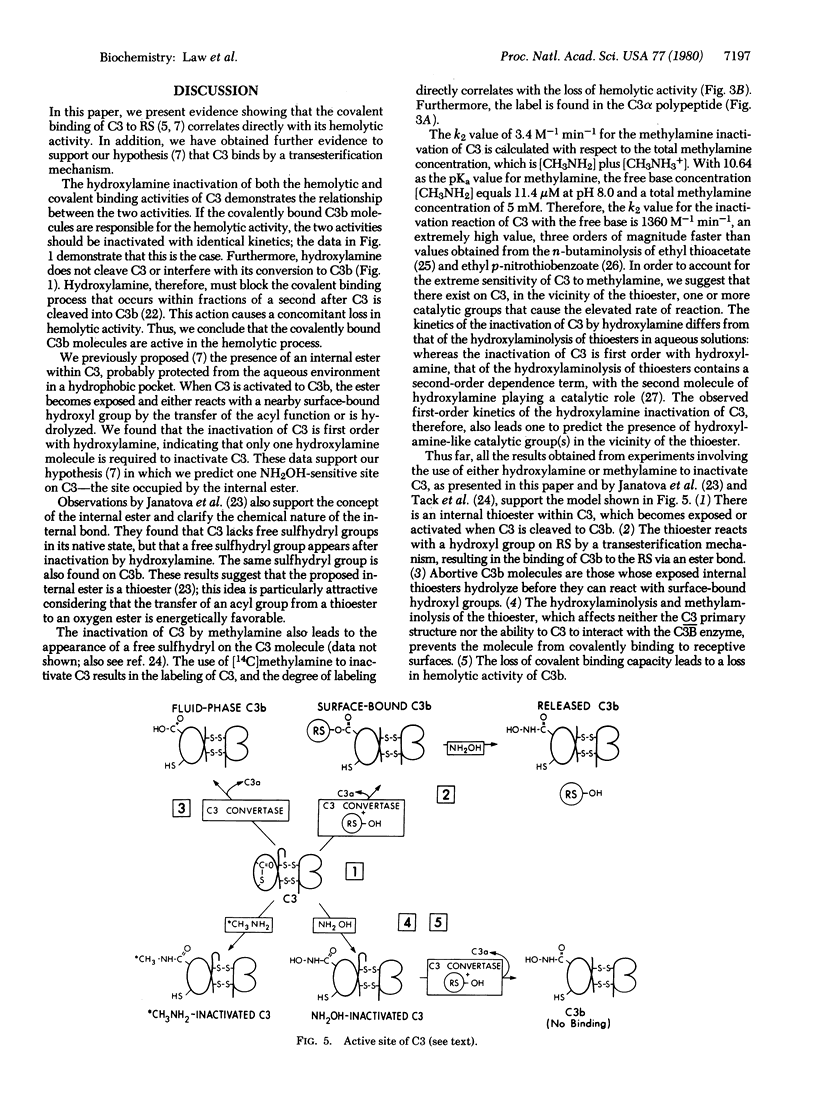
We report the inactivation of the third component of complement (C3) by hydroxylamine. C3 hemolytic and covalent binding activities decline with identical kinetics, demonstrating a direct correlation between the two activities. We conclude that covalent, surface-bound C3b is hemolytically active. The inactivation of C3 is first order with respect to hydroxylamine. We also studied C3 inactivation with [14C]methylamine. The inactivation corresponds quantitatively with the labeling of C3 in the C3d domain. The data obtained support the following hypothesis: there is an internal thioester within C3 which becomes highly reactive on activation to C3b, and C3b binds to receptive surfaces by transfer of the acyl function of the thioester to a hydroxyl group on the receptive surface. This proposed model for the reaction of C3 with receptive surfaces also applies to C4, which binds to membrane surfaces covalently and is able to be inactivated by hydroxylamine and methylamine. C5, on the other hand, is not inactivated by treatment with the amines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokisch V. A., Dierich M. P., Mūller-Eberhard H. J. Third component of complement (C3): structural properties in relation to functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):1989–1993. doi: 10.1073/pnas.72.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin C., Morris S., Tack B., Prahl J. Purification and structural analysis of the fourth component of human complement. Biochemistry. 1977 May 3;16(9):2008–2015. doi: 10.1021/bi00628a039. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade V., Nicholson A., Lee G. D., Mayer M. M. The reaction of zymosan with the properdin system: isolation of purified factor D from guinea pig serum and study of its reaction characteristics. J Immunol. 1974 May;112(5):1845–1854. [PubMed] [Google Scholar]
- DALMASSO A. P., MUELLER-EBERHARD H. J. INTERACTION OF AUTOLOGOUS COMPLEMENT WITH RED CELLS IN THE ABSENCE OF ANTIBODY. Proc Soc Exp Biol Med. 1964 Dec;117:643–650. doi: 10.3181/00379727-117-29658. [DOI] [PubMed] [Google Scholar]
- David G. S. Solid state lactoperoxidase: a highly stable enzyme for simple, gentle iodination of proteins. Biochem Biophys Res Commun. 1972 Jul 25;48(2):464–471. doi: 10.1016/s0006-291x(72)80074-3. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Lysis of erythrocytes by complement in the absence of antibody. J Exp Med. 1970 Nov;132(5):898–915. doi: 10.1084/jem.132.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janatova J., Lorenz P. E., Schechter A. N., Prahl J. W., Tack B. F. Third component of human complement: appearance of a sulfhydryl group following chemical or enzymatic inactivation. Biochemistry. 1980 Sep 16;19(19):4471–4478. [PubMed] [Google Scholar]
- Janatova J., Tack B. F., Prahl J. W. Third component of human complement: structural requirements for its function. Biochemistry. 1980 Sep 16;19(19):4479–4485. doi: 10.1021/bi00560a015. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Fearon D. T., Levine R. P. Action of the C3b-inactivator on the cell-bound C3b. J Immunol. 1979 Mar;122(3):759–765. [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Holcombe F. H., Levine R. P. Interaction between the labile binding sites of the fourth (C4) and fifth (C5) human complement proteins and erythrocyte cell membranes. J Immunol. 1980 Aug;125(2):634–639. [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Müllerèberhard H. J., Dalmasso A. P., Calcott M. A. The reaction mechanism of beta-1C-globulin (C'3) in immune hemolysis. J Exp Med. 1966 Jan 1;123(1):33–54. doi: 10.1084/jem.123.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Mandle R. J., Jr, McConnell-Mapes J. A. Human C3 and C5: subunit structure and modifications by trypsin and C42-C423. J Immunol. 1975 Feb;114(2 Pt 2):815–822. [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980 Oct 1;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. Activation of the complement system by antibody-antigen complexes: the classical pathway. Adv Protein Chem. 1979;33:1–71. doi: 10.1016/s0065-3233(08)60458-1. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Characterization of alkylamine-sensitive site in alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4313–4316. doi: 10.1073/pnas.76.9.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. F., Morris S. C., Prahl J. W. Third component of human complement: structural analysis of the polypeptide chains of C3 and C3b. Biochemistry. 1979 Apr 17;18(8):1497–1503. doi: 10.1021/bi00575a017. [DOI] [PubMed] [Google Scholar]



