Abstract
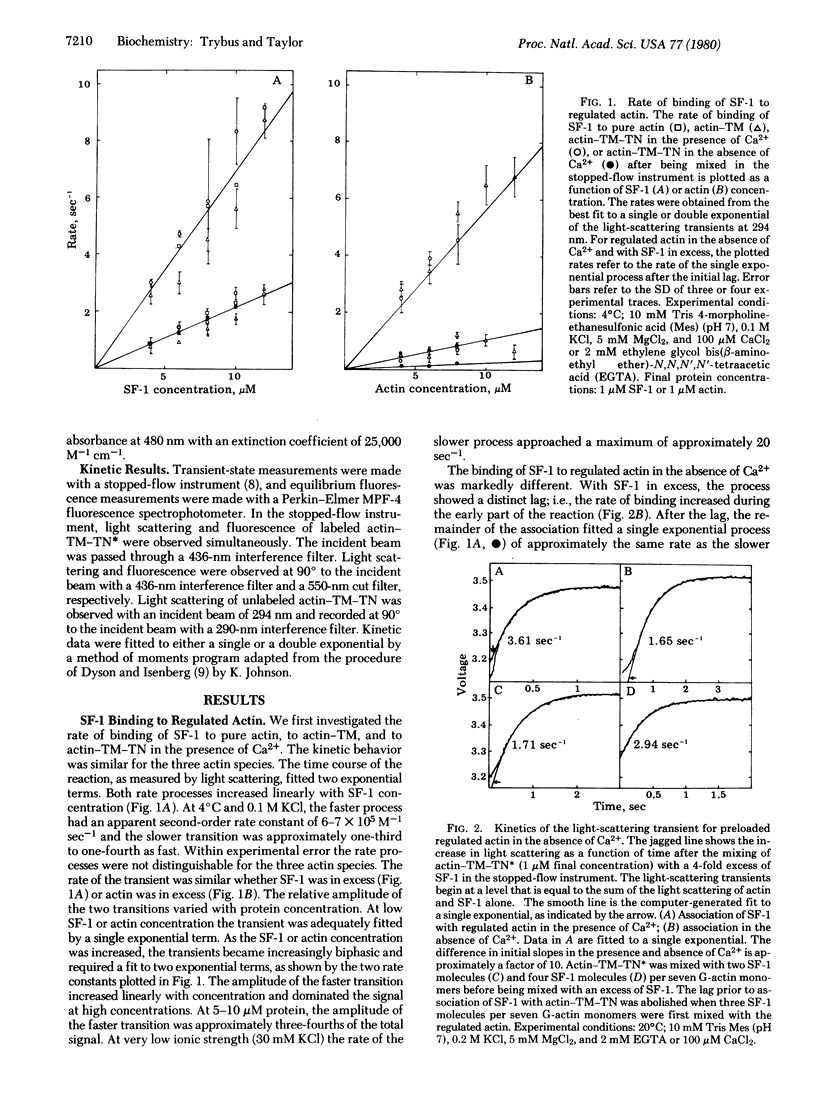
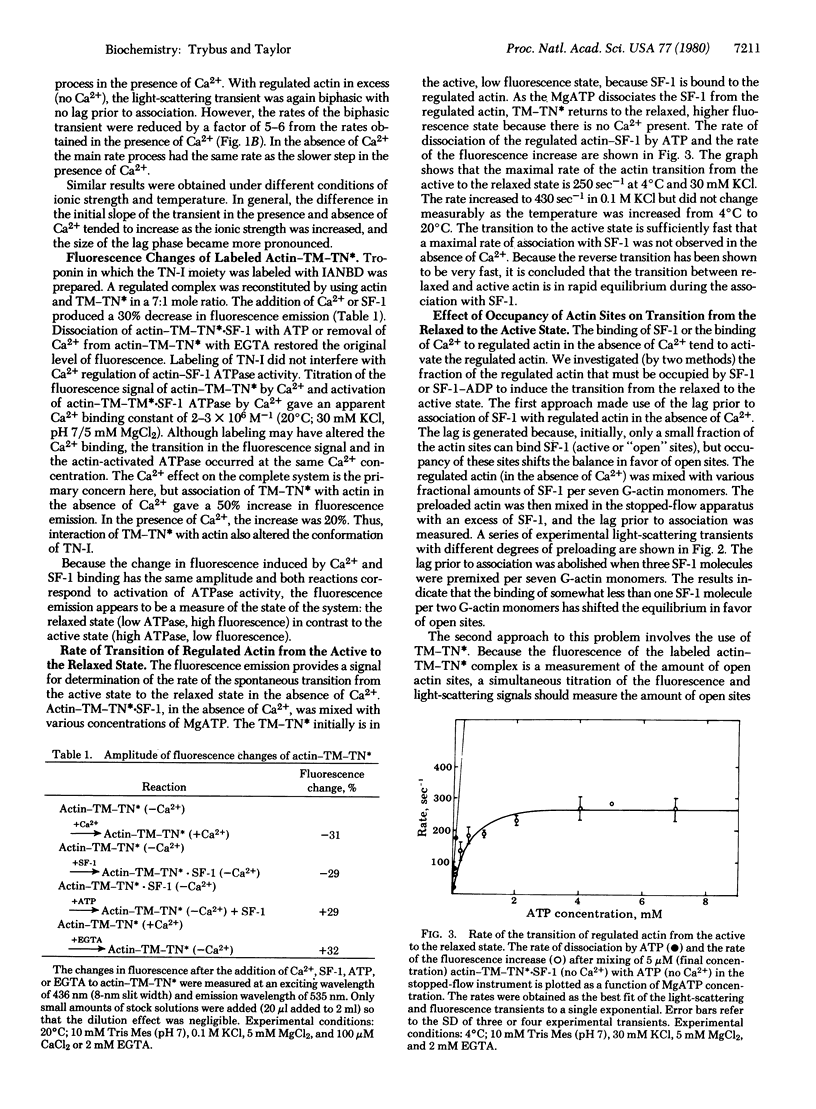
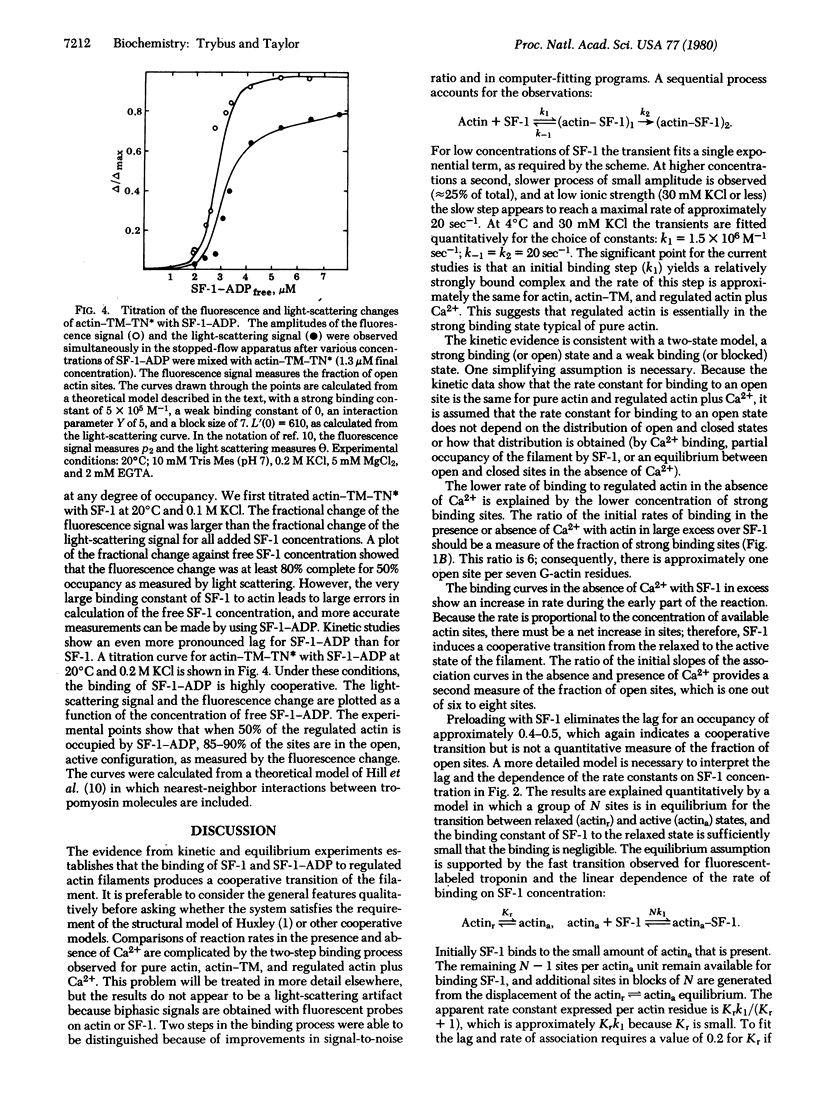
The transient-state kinetics of binding of myosin subfragment 1 (SF-1) to regulated actin in the presence and absence of Ca2+ were investigated. The binding of SF-1 to pure actin, to actin-tropomyosin (actin-TM), or to actin-tropomyosin-troponin (actin-TM-TN) in the presence of Ca2+ was kinetically the same. In each case, the light-scattering transients were biphasic, suggesting a two-step binding of SF-1 to actin. Binding of SF-1 to regulated actin in the absence of Ca2+ was different from binding in its presence and also varied depending on whether SF-1 or regulated actin was in excess. The kinetic results in the absence of CA2+ are explained by a cooperative binding model, in which the initial binding of SF-1 molecules to open (active) actin sites increases the number of open sites. TN-I labeled with the fluorophore 4-(N-iodoacetoxyethyl-N-methyl)-7-nitrobenz-2-oxa-1,3 diazole (TN*) was used to probe the state of the actin-TM-TN complex. Binding of SF-1 or CA2+ to regulated actin (in the absence of Ca2+) decreased the fluorescence of actin-TM-TN* by 30%, suggesting that binding of SF-1 or CA2+ induces a similar change in state. The change in fluorescence of TN* was also used to measure the rate of the transition from the active to the relaxed state in the absence of CA2+, which was 430 sec-1 at 4 degrees C in 0.1 M KCl. The lag prior to association of SF-1 with regulated actin (in the absence of Ca2+) was abolished when three SF-1 molecules were prebound per seven G-actin monomers. Similarly, a titration of actin-TM-TN* (in the absence of Ca2+) with SF-1 or SF-1-ADP showed that most actin sites are open, as measured by the fluorescence change, when the occupancy of actin-TM-TN* by SF-1-ADP or SF-1 is approximately 50%. The evidence shows that partial occupancy of a block of G-actin sites (possibly seven) by SF-1 or SF-1-ADP stabilizes the open (active) conformation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dyson R. D., Isenberg I. Analysis of exponential curves by a method of moments, with special attention to sedimentation equilibrium and fluorescence decay. Biochemistry. 1971 Aug 17;10(17):3233–3241. doi: 10.1021/bi00793a012. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock S. E. Regulation of muscle contraction. Effect of calcium on the affinity of troponin for actin and tropomyosin. Biochemistry. 1973 Jun 19;12(13):2509–2513. doi: 10.1021/bi00737a022. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Taylor E. W. Intermediate states of subfragment 1 and actosubfragment 1 ATPase: reevaluation of the mechanism. Biochemistry. 1978 Aug 22;17(17):3432–3442. doi: 10.1021/bi00610a002. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Lattman E. E., Cummins P., Lee K. Y., Cohen C. Crystal structure and molecular interactions of tropomyosin. Nature. 1979 Mar 29;278(5703):413–417. doi: 10.1038/278413a0. [DOI] [PubMed] [Google Scholar]
- Seymour J., O'Brien E. J. The position of tropomyosin in muscle thin filaments. Nature. 1980 Feb 14;283(5748):680–682. doi: 10.1038/283680a0. [DOI] [PubMed] [Google Scholar]
- Taylor R. S., Weeds A. G. The magnesium-ion-dependent adenosine triphosphatase of bovine cardiac Myosin and its subfragment-1. Biochem J. 1976 Nov;159(2):301–315. doi: 10.1042/bj1590301. [DOI] [PMC free article] [PubMed] [Google Scholar]


