Abstract
There is growing evidence that deficits in neuronal plasticity underlie the cognitive problems seen in fetal alcohol spectrum disorders (FASD). However, the mechanisms behind these deficits are not clear. Here we test the effects of early alcohol exposure on ocular dominance plasticity (ODP) in mice and the reversibility of these effects by phosphodiesterase (PDE) inhibitors. Mouse pups were exposed to 5 g/kg of 25% ethanol i.p. on postnatal days (P) 5, 7 and 9. This type of alcohol exposure mimics binge drinking during the third trimester equivalent of human gestation. To assess ocular dominance plasticity animals were monocularly deprived at P21 for 10 days, and tested using optical imaging of intrinsic signals. During the period of monocular deprivation animals were treated with vinpocetine (20mg/kg; PDE1 inhibitor), rolipram (1.25 mg/Kg; PDE4 inhibitor), vardenafil (3 mg/Kg; PDE5 inhibitor) or vehicle solution. Monocular deprivation resulted in the expected shift in ocular dominance of the binocular zone in saline controls but not in the ethanol group. While vinpocetine successfully restored ODP in the ethanol group, rolipram and vardenafil did not. However, when rolipram and vardenafil were given simultaneously ODP was restored. PDE4 and PDE5 are specific to cAMP and cGMP respectively, while PDE1 acts on both of these nucleotides. Our findings suggest that the combined activation of the cAMP and cGMP cascades may be a good approach to improve neuronal plasticity in FASD models.
Keywords: FASD, FAS, ocular dominance, Vinpocetine, PDE
Introduction
Fetal alcohol spectrum disorder (FASD) is an umbrella term for a variety of conditions affecting the children of women who drink alcohol during pregnancy. The effects of early alcohol exposure are wide ranging and can vary from subtle behavioral changes to severe mental retardation (Guerri, 1998; Riley et al., 2004; Rasmussen et al., 2006). Recent epidemiological studies have shown that FASD is the leading cause of mental retardation in the western world (May et al., 2009).
There is growing evidence that deficits in neuronal plasticity underlie many of the neurological problems observed in FASD (Rema and Ebner, 1999; Savage et al., 2002; Medina et al., 2005; Thomas et al., 2007; Medina and Ramoa, 2005; Medina and Krahe, 2008; Vaglenova et al., 2008). Ocular dominance plasticity (ODP), which is seen in the primary visual cortex of mammals, is a commonly used model of sensory-induced cortical neuronal plasticity. This type of plasticity has been used to study the mechanisms that underlie neural plasticity in general; sharing common mechanisms with learning and memory such as dependence on the NMDA receptor (Bear et al., 1990) and the transcription factor cAMP/Calcium-dependent response element binding protein (CREB) (Mower et al., 2002).
The mechanisms of ODP have been well established, and comprise changes from the molecular (number of receptors, expression of plasticity-related genes), to the cellular (dendrites branching and pruning) and behavioral (loss of vision) level (Antonnini et al. 1999). With this variety of observable effects, ODP can be a powerful tool to investigate how external insults (such as alcohol exposure) can affect neuronal plasticity.
In rodents ODP plasticity is evident in the binocular zone of the primary visual cortex. In this area, neurons present different degrees of binocularity; from cells that are equally responsive to stimulation of either eye, to cells that respond almost exclusively to one eye (Métin et al., 1988). When both eyes are receiving normal input, the binocular zone is dominated by responses to the contralateral eye. However if the contralateral eye is sutured closed during the critical period of the visual system, which is roughly between postnatal days (P) 19 and 32 in rodents (Cang et al., 2005), the eye dominance of the binocular zone shifts to respond primarily to inputs from the ipsilateral, open eye (Smith et al., 2009). This shift in binocularity is seen in mice after as little as 1 day when a mechanism similar to long term depression (LTD) plays a role in a suppression of contralateral eye inputs (Frenkel and Bear, 2004). After 5 days of MD, mechanisms similar to long term potentiation (LTP) act to increase the strength of the ipsilateral eye inputs (Frenkel and Bear, 2004). These two components, depression and potentiation, of ODP are well established and have been extensively studied within the rodent visual system (see Smith et al, 2009 for review). Indeed ODP in rodents has been used as a model to study neuronal plasticity deficits in distinct developmental conditions such as Fragile X (Dolen et al., 2007), Neonatal hypoxia-ischemia (Failor et al., 2010) and Angelmann Syndrome (Yashiro et al., 2009).
Our group pioneered the use of ocular dominance plasticity to investigate the deficits of neuronal plasticity in FASD (Medina et al., 2003). We showed that exposure of ferrets to alcohol from P10 to P30, a period of development roughly equivalent to the third trimester equivalent of human gestation (and to P4 to P10 in rodents), leads to a persistent impairment of ocular dominance plasticity (Medina et al., 2005; Medina et al., 2003; Medina et al., 2006). Importantly, this disruption could be reversed by treating ferrets with the phosphodiesterase type 1 inhibitor (PDEi1) vinpocetine (Medina et al., 2006). PDE1, converts cAMP to AMP and cGMP to GMP (Keravis and Lugnier, 2010), therefore inhibition of this enzyme can lead to increase intracellular levels of the cyclic form of these nucleotides. Both cAMP and cGMP can activate cascades which will ultimately lead to phosphorylation of transcription factors that are important for the expression of plasticity related genes (Frank and Greenberg, 1994; Atkins et al., 1998; Etkin et al., 2006). Among these transcription factors are the cAMP responsive binding protein (CREB) and the serum response factor (SRF). In fact, overexpression of a constitutively form of SRF by viral mediated gene transfer also resulted in restoration of ODP in the ferret model of FASD (Paul et al., 2010).
In contrast to the results obtaining with PD1 inhibition, the PDE4 inhibitor rolipram, which acts specifically on the cAMP cascade, failed to restore OD plasticity in the ferret model of FASD even when tested with different doses (0.5mg/kg, 1.25mg/kg and 2.5 mg/kg). Because of the distinct results obtained with rolipram (acting specifically on cAMP) and vinpocetine (acting on both cAMP and cGMP) it is possible that the positive effects of PDE1 inhibitors are due to activation of the cGMP cascade. Alternatively, it is also conceivable that the restoration of ODP plasticity by vinpocetine is due to a synergistic effect between cGMP and cAMP cascades.
While ferrets can be very useful as animal models, the lower cost of the mouse facilitates using larger samples enabling us to test multiple experimental groups and testing dose-response curves. In addition, the use of mice also allows for the study of transgenic animals in order to better tease out the molecular mechanisms behind the damaging effects of early ethanol exposure. Therefore, extending our previous results to mice may be extremely useful.
Our first goal would be to test whether ocular dominance plasticity in mice is disrupted after early alcohol exposure and whether this disruption can be reversed by the PDEi1, vinpocetine. Because PDEi1 acts on both cyclic nucleotides, it is not know whether the positive effects of vinpocetine treatment are due cAMP or cGMP cascades. Therefore, our second goal is to test the efficacy of a PDEi4 (specific for cAMP) and a PDEi5 (specific to cGMP) in restoring ODP in mice.
Methods
Animals
All procedures described in this paper were approved by the Institutional Animal Care and Use Committee.
Visibly pregnant C57/BL6 female mice were obtained from a commercial supplier (Harlan), and singularly housed in the university animal facility, with a 12 hour light/dark cycle at 22° C. Animals were fed standard chow diet ad libitum. Pregnant dams were checked twice daily until pups were born. The day of birth was considered P0. Typically, 2/3 of animals within a litter were injected with alcohol (total n = 29) and the remaining with control saline (total n = 16). A total of 16 litters were used. These animals were then randomly sorted into the experimental groups saline (n = 5), saline + MD (n = 5), ethanol (n = 3), ethanol + MD (n = 2), Ethanol + MD + Vehicle (n=4), ethanol + MD + vinpocetine (n = 5), saline + MD + vinpocetine (n = 6), ethanol + MD + rolipram (n = 6), ethanol + MD + vardenafil (n = 5), and ethanol + MD + rolipram + vardenafil (n = 4). Males and females were randomly distributed between groups. Results from Ethanol + MD and Ethanol + MD + Vehicle were similar and these groups were pooled together (n=6, Figures 4 and 5).
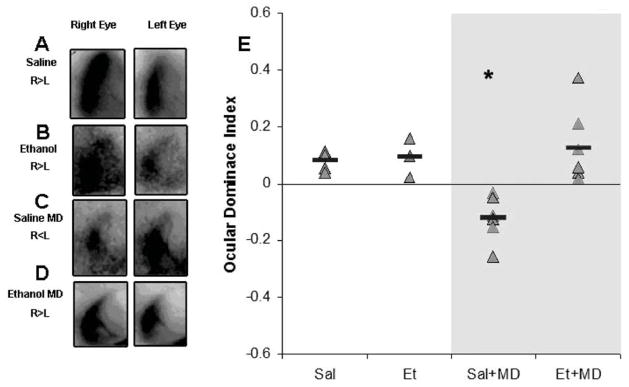
Figure 4. Effect of early alcohol exposure on ODP.
A–D) Optical imaging of intrinsic signals from the left visual cortex of representative cases of each group. Darker maps indicated stronger signals after visual stimulation. Note that in non-deprived animals (A and B) stimulation of the right eye leads to stronger signals than left eye stimulation, which is consistent with the contralateral bias seen in the primary visual cortex of rodents. After 10 days of monocular deprivation (MD) an ocular dominance shift is seen; note the stronger signal after left eye stimulation (C). This shift was not seen in ethanol exposed animals (D) which indicates impairment in ocular dominance plasticity. (E) Quantification of optical imaging findings. Ocular dominance indexes were calculated (see methods); positive and negative values represent whether the cortex is more responsive to the contralateral (right) or ipsilateral (left) eye respectively. Red bars represent average ODI values for each group (* p < 0.0001) Sal=Saline treated; Et= Alcohol-treated; MD= Monocular deprivation.
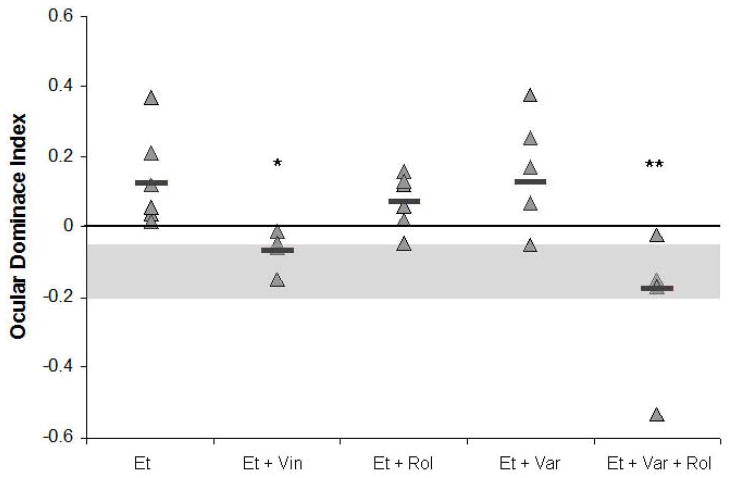
Figure 5. Effect of different types of PDE inhibitors on ODP.
Vinpocetine (Vin) restores ODP induced by MD in alcohol-treated animals (* p = 0.05). This rescue is not achieved by either rolipram (Rol) or vardenafil (Var). However, when vardenafil and rolipram are given together ODP is restored (** p = 0.001). Grey area show range of values obtained from saline-treated animals after a monocular deprivation. Et = alcohol.
Pharmacological Treatment
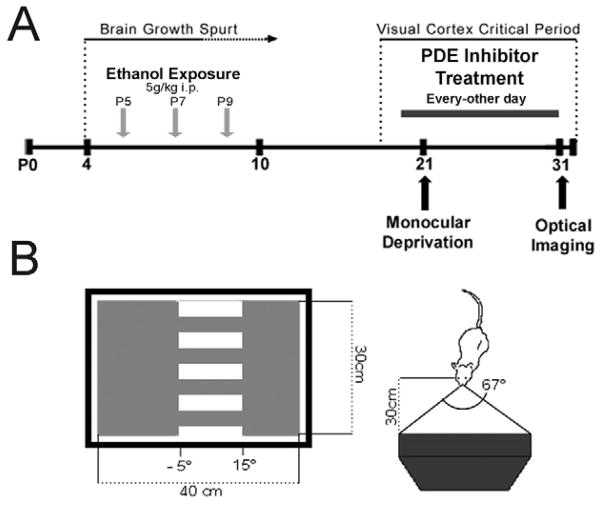
Pups received a single abdominal intraperitoneal (i.p.) injection of 5g/kg of alcohol (25% ethanol in normal saline, i.p.) or saline as a control on days P5, 7 and 9. Animals then were allowed an alcohol free period until P20. Beginning at P21 (one day prior to monocular deprivation), animals were treated with 20 mg/kg i.p. of vinpocetine (Sigma, St. Louis, MO), 1.25 mg/kg i.p. of rolipram (Sigma, St. Louis, MO), 3 mg/kg oral of vardenafil (Bayer pharmaceuticals) or volume matched vehicle as a control every other day for 10 days, until optical imaging of intrinsic signals was performed (Figure 1A). Dosage of vinpocetine was chosen based on our lab’s previous studies in ferrets (Medina et al., 2006), while dosages of rolipram (Barad et al., 1998; Rutten et al., 2007) and vardenafil (Prickaerts et al., 2002) were chosen based on previous studies which demonstrated behavioral effects.
Figure 1. Experimental paradigm.
(A) Mouse pups receive a single injection of 5g/kg of ethanol on postnatal days 5, 7 and 9. At P21 animals are monocularly deprived and treated with PDE inhibitors or vehicle every-other-day until P31 when an optical imaging of intrinsic signals is performed. B) Visual stimulus; mice are placed 30cm from a high refresh rate monitor displaying a drifting grating.
Monocular Deprivation
At P21, 12 days after last alcohol injection, pups were anesthetized using vaporized isoflurane (Baxter, Deerfield, IL). Anesthesia was characterized as absence of the paw withdrawal reflex. Once the animal was anesthetized small portions of the upper and lower right eyelid were trimmed. The eyelids were then sutured together using 4 interrupted sutures of 7.0 Prolene (Ethicon, Inc, Cornelia, GA). The sutured eye was then covered with tissue-glue (CP Medical, Portland, OR. The animal remained monocularly deprived for 10 days, until optical imaging of intrinsic signal experiments were performed.
Optical Imaging of Intrinsic Signals
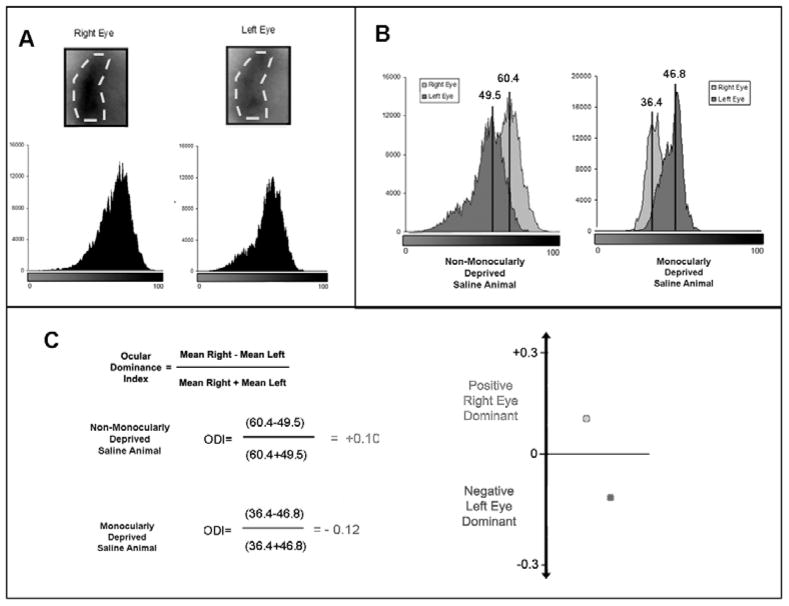
Optical imaging of intrinsic signals was performed using Imager 3001 VSD+ (Optical Imaging System, Germantown, NY) as previously described, with some modifications (Medina, 2005; Medina, 2006; Antonini et al., 1999). Briefly, mice were anesthetized using 10mg of chlorprothixene (Sigma) and 15g/kg of urethane (Sigma). The scalp was then cut open, and the animal’s head attached to a metal plate with a window that allows for the exposure of the skull. The skull was covered with agar (2.5% in normal saline) and a cover slip. The MD eye was then cut open to allow for deprived eye stimulation. An image of the cortical vascular pattern was obtained through the skull by illumination with a green filter (550 nm); this type of image was possible due to the transparency of the mouse skull at this age. Images of intrinsic signals were then obtained by using a red filter (700 nm). Recordings were made using VDAQ (Optical Imaging Inc.) software. Visual stimulation was then performed using a horizontal banding of downward drifting grey and black bars on a 21 inch monitor (Sony Trinitron; Sony) using a SGT+ graphics board and STIM software (Optical Imaging Inc.). Gratings were presented only in the center 12cm of the monitor, which was placed 30cm from the nose of the animal (See Figure 1). A single trial consisted of this stimulus and a blank screen presented to each eye for 9s in sequence, with data acquisition during the last 8s. Eye shutters (Optical Imaging Inc.) controlled by the acquisition computer were used to stimulate each eye individually. A total of 20 trials were performed for each eye, and the summed images were used to obtain a monocular response map by subtracting responses to a blank screen from responses to visual stimulation of each eye. To obtain a quantitative estimate of intensity, differential maps were mixed with the floating point files clipped at ±2SD from the median. All pixels with a value higher than 133 (256 being white) were excluded as noise. An Ocular Dominance Index (ODI) was calculated for each animal using the average pixel intensity for each eye. Pixel intensity for both the contra- and ipsi- lateral eye signals were calculated by selecting a region of interest based on the summed signal from bilateral eye stimulation. Using this region of interest mean pixel intensity for individual eye input was calculated using the histogram function in Image J (version 1.40g, National Institutes of Health, USA). The following equation was used to calculate ODI, where C and I represent average pixel intensities after stimulation of the contralateral and ipsilateral eye, respectively;
Positive ODI values would indicate a contralateral eye bias (unshifted), and negative values would indicate an ipsilateral eye bias (shifted)(See Figure 2).
Figure 2. Analysis of optical imaging of intrinsic signals.
To analyze the images acquired using OI, first a region of interest (ROI) is created around the binocular zone for each animal. This area is the same for both right and left eye responses (A). Using this ROI, a histogram of the intensity of each pixel is created for each eye. These responses can then be over laid and the mean pixel intensity for each histogram can be calculated (B). These values are then used to calculate an ocular dominance index for the animal. If this value is greater than one there is a bias towards the right eye, if this value is negative (as for an MD animal) there is a bias towards the left eye (C).
Blood Alcohol Concentration
Blood was collected from a separate subset of C57/BL6 pups at P7 via cardiac puncture. Blood was collected at several time points after ethanol injection (0.5h, 1h, 2h, 3h, 4h and 6h), then centrifuged at 4,000 rpm for 5 minutes and serum was collected. BAC was assessed using a commercial kit (333-A diagnostics kit; Sigma, St. Louis, MO).
Statistics
Statistical analysis was performed using SPSS software. For analysis of pup weight a repeated measures ANOVA was performed with a post-hoc t-test. The effects of ethanol and MD on CBI values were analyzed using an ANOVA followed by post-hoc t-tests. CBIs from PDEi treatments were compared using an ANOVA followed by a Dunnet’s post-hoc (< control). Data is presented at Mean ± Standard Error of the mean (SEM), and the alpha level was set at 0.05.
Results
Blood alcohol concentrations and survival rate
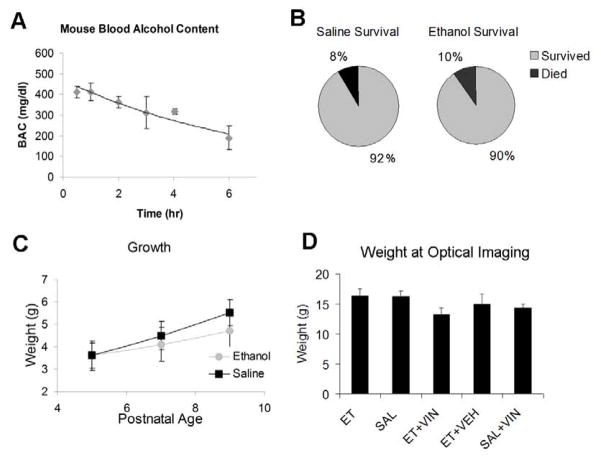
Peak of blood alcohol concentration (BAC) was 411 mg/dl (± 43) at 1 hour after i.p. injection. BAC then remained elevated over 300mg/dl for 4 hours (Figure 3A). This BAC is consistent with previous studies on maximal neuronal death after postnatal ethanol exposure in mice, and is equivalent to binge drinking in humans (Ikonomidou et al., 2000). In humans 400 mg/Kg of BAC causes loss of conscience and could lead to a comatose state. However, in chronic alcoholism this value can be reached without loss of conscience and patients can be oriented regarding self, place and time with BACs as high as 500 mg/dl (Minion et al. 1999). The majority of animals survived the ethanol and saline injections with survival rates of 90% (n=64) and 92% (n=33). No animals died during vinpocetine, rolipram, vardenafil or vehicle treatment.
Figure 3. General Effects of Alcohol Treatment.
A) Blood alcohol content was measured at various time points post injection in P7 mice. Mice exhibited a sustained BAC above 300 mg/dl for at least 4 hours. B) Saline and ethanol exposed mice exhibited similar mortality rates during the exposure paradigm. C) Mouse pups treated with ethanol gained less weight during the course of exposure than their saline treated littermates (* p < 0.001). D) At the time of optical imaging, there was no difference in weight between ethanol and saline treated littermates. Et=ethanol, Sal=saline, Veh= vehicle, Vin=vinpocetine
Pup growth
Both treatment groups gained weight during the treatment period, with a repeated measures ANOVA showing a significant within subjects effect for day of treatment (F= 169.055, df = 2, p < 0.0001), with saline and ethanol treated animals weighing 5.52g (±0.58) and 4.73g (± 0.78) on the last day of treatment, versus 3.68g (± 0.56) and 3.41g (±0.58) on the first day of treatment. Yet, ethanol exposed animals gained significantly less weight over the period of treatment than their saline exposed littermates. This effect is seen in the between subjects measure in the repeated measures ANOVA (F= 6417.74, df = 1, p < 0.0001, Figure 3C). On the last day of treatment, on P9, ethanol treated animals weighed significantly less than their saline treated littermates (post-hoc t-test, t = −5.26, df=98, p < 0.0001). At the time of optical imaging there were no difference in weight between ethanol (16.3g ± 1.1) and saline (16.2g ± 0.9) treated groups (t-test, t = 0.47, df=14, p=0.35) (Figure 3D).
Ocular Dominance Plasticity in Ethanol Exposed Animals
To quantify the results obtained with optical imaging of intrinsic signals an ODI was created (see methods). An ODI of −1 indicates the animal’s binocular zone responds only to simulation of the ipsilateral eye or an ODI of +1 indicates responses to only the contralateral eye, respectively. An ODI of 0 would indicate the binocular zone responds equally to simulation of the ipsilateral eye and the contralateral eye. The shift from positive towards negative values in ODI indicates ODP. As expected non-MD ethanol and saline treated animals demonstrated the typical contralateral bias observed in rodents (ethanol: n = 3, +0.09 ± 0.03; saline: n = 5, +0.08 ± 0.01). An ANOVA showed that there was a significant effect of exposure (saline, ethanol; F= 9.6, df=1, p= 0.007), a trend in the effect of MD (MD, no MD; F= 4.14, df=1, p=0.06) and significant interaction between exposure and MD (F= 7.773, df = 1, p=0.013). The significant effect of exposure indicates that there are significant differences between saline and alcohol treated animals. While, the trend in the effect of MD was most likely due to the lack of OD shift in the ethanol treated animals, and is best shown in the interaction between treatment and MD groups, indicating that MD is necessary to show the effect of ethanol exposure. Post-hoc t-tests showed that while MD lead to significantly lower ODI in saline treated animals (n = 6, ODI = −0.12 ± 0.03, t=5.59, df=7, p < 0.0001), ethanol animals with no MD showed no ODI difference to their MD counterparts (n = 7, ODI = +0.07 ± 0.02, t= −0.39, df=8, p=0.7) (Figure 4).
Rescue of Ocular Dominance Plasticity
After showing that ocular dominance plasticity is impaired in ethanol treated animals, we examined whether treating animals with PDE inhibitors during the period of MD would restore these deficits. Using the ethanol + MD animals as control, an ANOVA showed an effect of treatment, indicating differences between vehicle and PDE inhibition treatment (F=6.47, df=4, p=0.001). To test what treatments effectively reduced ODI values we used the Dunnett test (< control). Negative ODI values would indicate an OD shift. Animals that received vinpocetine displayed an OD shift (n = 5, −0.06 ± .02), represented by a reduction in ODI when compared to controls (Dunnet’s t = 0.039). In contrast, ethanol exposed animals treated with rolipram showed values not significantly different than controls, indicating persisting contralateral eye dominance (n = 6, +0.07 ± 0.03, Dunnet’s t= 0.547). Similarly vardenafil treatment was also unable to restore ODP (n = 5, +0.13 ± 0.07, Dunnet’s t= 0.940). Yet, when animals were treated with a combination of vardenafil and rolipram, a significant reduction in ODIs was observed (n = 4, −0.17 ± 0.10, Dunnet’s t= 0.001). This result was similar to what was observed with vinpocetine, indicating a requirement of both cAMP and cGMP cascades to restore OD plasticity (see Figure 5). Some saline MD animals were also given vinpocetine treatment during the period of MD, to test for a possible further increase in plasticity, yet they displayed a normal shift in ODI (t-test; t=2.228, df=10, p=0.646; data not shown).
Response Intensity
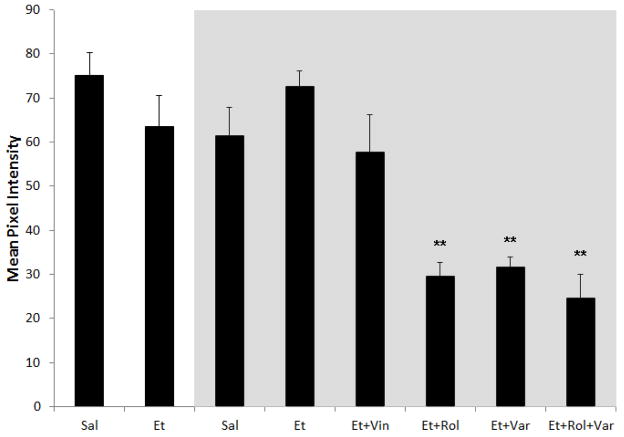
To test whether our findings could be explained by reduced visual responsiveness caused by alcohol exposure we compared the strength of the signal displayed in the magnitude maps generated by the optical imaging of intrinsic signals. To accomplish that, we compared the mean pixel intensity of maps generated after stimulation of the dominant eye. A univariate ANOVA show neither an effect of exposure (alcohol or saline; f = 0.051, p = 0.823) nor of MD (with or without MD; f = 0.139, p = 0.711). However, it showed an effect of treatment (Vehicle, Vinpocetine, Rolipram, Vardenafil, Vardenafil+Rolipram; f = 2,476.3, p < 0.0001). Post-hoc Bonferroni tests showed animals that received rolipram, vardenafil or the combination of these drugs presented a significant reduction (p < 0.0001 for all groups) of signal when compared to animals treated with vehicle (See Figure 6).
Figure 6. Average Mean Pixel Intensity.
Response magnitude was calculated as the mean pixel intensity for the strongest responding eye for each group. Groups in the grey box represent MD animals. There was no different between saline (Sal) and alcohol (Et) exposed animals with and without MD. When ethanol exposed MD animals treated with PDEis were compared to animals that did not receive PDEi treatment, they were shown to have a significantly lower response magnitude. ** p < 0.001. Vin = vinpocetine, Rol = rolipram, Var = vardenafil
Discussion
Here we have shown that mice exposed to alcohol early in development present impairment in OD plasticity when compared to saline treated controls. We also demonstrated that this impairment in ODP is reversed by a treatment with a PDE1i (vinpocetine). Although this rescue was not seen with a PDE4i (rolipram) or a PDE5i (vardenafil) alone, it was seen with a combination of these treatments. This finding suggests a requirement of the activation of both cAMP and cGMP cascades in order to rescue ethanol induced ODP deficits. But why both of these cascades would be necessary for this effect?
Acting as second messengers in several processes, cAMP and cGMP are particularly important for neuronal plasticity. The cAMP-PKA and the cGMP-PKG pathways have been extensively studied and their importance for neuronal plasticity well documented (Michel et al., 2011; Nugent et al., 2009; Pilz and Broderick, 2005). Interestingly, these pathways can have overlapping functions. For instance, both PKA and PKG can act in parallel to increase CREB phosphorylation and the expression of BDNF and c-Fos (Pilz and Broderick, 2005; Lonze and Ginty, 2002; Frank and Greenberg, 1994). In addition, both PKA and the cGMP-dependent enzyme gKII can phosphorylate the GluR1 subunit of the AMPA receptor at serine 845 (Serulle et al., 2007). This phosphorylation contributes to the incorporation of AMPA receptors to the postsynaptic membrane and is crucial for synaptic plasticity (Malinow and Malenka, 2002).
In the present study, while a PDE1 inhibitor successfully restored neuronal plasticity, the use of PDE5 or PDE4 inhibitors did not. It is conceivable that simultaneous activation of the cAMP-PKA and cGMP-PKG cascades leads to a summation of their effects. This summation could occur in the same cell type, as both PDE5 and PDE4 are found in central nervous system neurons (Omori et al., 2007; Menniti et al., 2006). The restoration of neuronal plasticity observed here may not be accomplished even by using higher doses of PDE5 or PDE4 inhibitors individually due to negative feedback mechanisms (Zhang et al., 2005; Borlikova and Endo, 2009). In fact, it was demonstrated that while increased cAMP and cGMP can facilitate neuronal plasticity, high levels of either one can be ineffective or even disruptive (Barad et al., 1998; Zhang and O’Donnell, 2000). Another possibility to explain our findings is that simultaneous activation of cAMP-PKA and cGMP-PKG lead to the expression of plasticity-related molecules that, while specific for each cascade, might act in synergy.
One possibility for the lack of a restorative effect of Rolipram and vardenafil treatments is that these drugs lead to a dramatic reduction in visual responsiveness. This reduction could explain the weak magnitude maps showed by this groups (Figure 4). However, we consider this possibility unlikely because: First, animals that received the combination of rolipram and vardenafil also presented a reduction in signal in the magnitude maps, however this animals displayed restored ODP. Second, the decreases in responses observed here could be an artifact, as optical imaging of intrinsic signals relies on hemodynamic changes to detect cortical activation and PDE inhibitors can act as vasodilators (Patyar et al., 2011; Goirand et al., 2001; Kloner, 2004). It is possible that this vasodilation increases the noise in our optical imaging recordings, decreasing the signal intensity.
The negative findings observed with rolipram and vardenafil could also be attributed to the doses tested. However, the doses used here (1.25 and 3 mg/Kg respectively) were high when compared with previous studies that tested the effects of PDEis on neuronal plasticity. For instance, Rolipram in doses as low as 0.03 mg/Kg was shown to improve object recognition in rats (Rutten et al., 2006) and object retrieval in monkeys (Rutten et al., 2008). In higher doses (0.5 mg/Kg), rolipram has been shown to improve performance in the morris water maze and in the passive avoidance test (Cheng et al., 2010). Studies using vardenafil, observed that doses as low as 1 mg/Kg successfully improved object recognition (Prickaerts et al., 2002; Rutten et al., 2009).
The potential of Vinpocetine to improve neuronal plasticity, have been demonstrated using multiple paradigms. Vinpocetine has been shown to facilitate LTP (Molnar and Gaal, 1992; Molnar et al., 1994), enhance the structural dynamics of dendritical spines (Lendvai et al., 2003), improve memory retrieval (DeNoble, 1987), and enhance performance on cognitive tests in humans (Hindmarch et al., 1991). Vinpocetine was tested in several animal models of FASD. It has been shown to restore neuronal plasticity in the visual cortex of ferrets (Medina et al., 2006), improve performance in the water maze in rats (Filgueiras et al., 2010), and reduce hyperactivity in mice (Nunes et al., 2011). These findings, allied with our current results suggest that PDE1 inhibition may be useful in improving neuronal plasticity in FASD.
Here we also successfully translated our ferret model of FASD to the mouse. These findings suggest that the effects of alcohol exposure on neuronal plasticity and the restorative effect of vinpocetine are not species specific. Moreover, this translation will give us more options in the investigation of the neuronal plasticity deficits caused by early alcohol exposure. The lower cost of the mouse in comparison to ferrets facilitates using larger samples enabling us to test multiple experimental groups and test dose-responses curves. For instance, we could test in the future what is the minimal alcohol dose that can lead ODP deficits and what the minimal dose of vinpocetine necessary for a restorative effect. In addition to dosage and timing studies, the use of mice also allows for the study of transgenic animals in order to better tease out the molecular mechanisms behind the damaging effects of early ethanol exposure.
Highlights.
Early alcohol exposure impairs visual cortex plasticity in mice.
Inhibition of PDE type 1, but not types 4 or 5 alone, reverses this impairment.
Concurrent treatment with inhibitors of types 4 and 5 does rescue plasticity.
Restoration of ocular dominance plasticity in FASD requires both cAMP and cGMP cascades.
Acknowledgments
This work was supported by NIH (NIAAA) grant AA13023 to AEM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonini A, Fagiolini M, Stryker MP. Anatomical correlates of functional plasticity in mouse visual cortex. J Neurosci. 1999;19:4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Blokland A, Schreiber R, Prickaerts J. Improving memory: A role for phosphodiesterases. Curr Pharm Des. 2006;12:2511–2523. doi: 10.2174/138161206777698855. [DOI] [PubMed] [Google Scholar]
- Borlikova G, Endo S. Inducible cAMP early repressor (ICER) and brain functions. Mol Neurobiol. 2009;40:73–86. doi: 10.1007/s12035-009-8072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Kalatsky VA, Löwel S, Stryker MP. Optical imaging of the intrinsic signal as a measure of cortical plasticity in the mouse. Visual Neuroscience. 2005;22:685–91. doi: 10.1017/S0952523805225178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J, Tarnawski AS. Serum response factor: Discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharmacol. 2002;53:147–157. [PubMed] [Google Scholar]
- Cheng YF, Wang C, Lin HB, Li YF, Huang Y, Xu JP, Zhang HT. Inhibition of phosphodiesterase-4 reverses memory deficits produced by Abeta25–35 or Abeta1–40 peptide in rats. Psychopharmacology (Berl) 2010;212:181–191. doi: 10.1007/s00213-010-1943-3. [DOI] [PubMed] [Google Scholar]
- DeNoble VJ. Vinpocetine enhances retrieval of a step-through passive avoidance response in rats. Pharmacology, Biochemistry and Behavior. 1987;26:183–6. doi: 10.1016/0091-3057(87)90552-1. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Alarcon JM, Weisberg SP, Touzani K, Huang YY, Nordheim A, Kandel ER. A role in learning for SRF: Deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron. 2006;50:127–143. doi: 10.1016/j.neuron.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Failor S, Nguyen V, Darcy DP, Cang J, Wendland MF, Stryker MP, McQuillen PS. Neonatal cerebral hypoxia-ischemia impairs plasticity in rat visual cortex. J Neurosci. 2010;30:81–92. doi: 10.1523/JNEUROSCI.5656-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filgueiras CC, Krahe TE, Medina AE. Phosphodiesterase type 1 inhibition improves learning in rats exposed to alcohol during the third trimester equivalent of human gestation. Neurosci Lett. 2010;473:202–207. doi: 10.1016/j.neulet.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Greenberg ME. CREB: A mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Frenkel M, Bear M. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–23. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Goirand F, Bardou M, Dumas J, Rochette L, Dumas M. Effects of phosphodiesterase inhibitors on hypoxic pulmonary vasoconstriction. Influence of K (+) channels and nitric oxide. Eur J Pharmacol. 2001 Apr 6;417(1–2):141–8. doi: 10.1016/s0014-2999(01)00900-1. [DOI] [PubMed] [Google Scholar]
- Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:304–312. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Fuchs HH, Erzigkeit H. Efficacy and tolerance of vinpocetine in ambulant patients suffering from mild to moderate organic psychosyndromes. Int Clin Psychopharmacol. 1991;6:31–43. doi: 10.1097/00004850-199100610-00005. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Hörster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–60. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterases (PDE) and peptide motifs. Curr Pharm Des. 2010;16:1114–1125. doi: 10.2174/138161210790963760. [DOI] [PubMed] [Google Scholar]
- Kloner RA. Novel phosphodiesterase type 5 inhibitors, assessing hemodynamic effects and safety parameters. Clin Cardiol. 2004;27(4 Suppl 1):I20–5. doi: 10.1002/clc.4960271306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll B, Nordheim A. Functional versatility of transcription factors in the nervous system: The SRF paradigm. Trends Neurosci. 2009;32:432–442. doi: 10.1016/j.tins.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Zelles T, Rozsa B, Vizi ES. A vinca alkaloid enhances morphological dynamics of dendritic spines of neocortical layer 2/3 pyramidal cells. Brain Res Bull. 2003;59:257–260. doi: 10.1016/s0361-9230(02)00873-0. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Bukley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent school studies. Developmental Disabilities Research Reviews. 2009;15:176–92. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE. Neocortical plasticity deficits in fetal alcohol spectrum disorders: Lessons from barrel and visual cortex. J Neurosci Res. 2008;86:256–263. doi: 10.1002/jnr.21447. [DOI] [PubMed] [Google Scholar]
- Medina AE, Ramoa AS. Early alcohol exposure impairs ocular dominance plasticity throughout the critical period. Brain Res Dev Brain Res. 2005;157:107–111. doi: 10.1016/j.devbrainres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Restoration of neuronal plasticity by a phosphodiesterase type 1 inhibitor in a model of fetal alcohol exposure. The Journal of Neuroscience. 2006;26:1057–60. doi: 10.1523/JNEUROSCI.4177-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Early alcohol exposure induces persistent alteration of cortical columnar organization and reduced orientation selectivity in the visual cortex. Journal of Neurophysiology. 2005;93:1317–25. doi: 10.1152/jn.00714.2004. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Coppola DM, Ramoa AS. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. The Journal of Neuroscience. 2003;23:10002–7. doi: 10.1523/JNEUROSCI.23-31-10002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti FS, Faraci WS, Schmidt CJ. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov. 2006;5(8):660–70. doi: 10.1038/nrd2058. [DOI] [PubMed] [Google Scholar]
- Métin C, Godement P, Imbert M. The primary visual cortex in the mouse: Receptive field properties and functional organization. Experimental Brain Research. 1988;69:594–612. doi: 10.1007/BF00247312. [DOI] [PubMed] [Google Scholar]
- Michel M, Green CL, Eskin A, Lyons LC. PKG-mediated MAPK signaling is necessary for long-term operant memory in aplysia. Learn Mem. 2011;18:108–117. doi: 10.1101/lm.2063611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minion GE, Slovis CM, Boutiette L. Severe alcohol intoxication: a study of 204 consecutive patients. J Toxicol Clin Toxicol. 1989;27 (6):375–384. doi: 10.3109/15563658909000358. [DOI] [PubMed] [Google Scholar]
- Molnar P, Gaal L. Effect of different subtypes of cognition enhancers on long-term potentiation in the rat dentate gyrus in vivo. Eur J Pharmacol. 1992;215:17–22. doi: 10.1016/0014-2999(92)90602-z. [DOI] [PubMed] [Google Scholar]
- Molnar P, Gaal L, Horvath C. The impairment of long-term potentiation in rats with medial septal lesion and its restoration by cognition enhancers. Neurobiology (Bp) 1994;2:255–266. [PubMed] [Google Scholar]
- Mower AF, Liao DS, Nestler EJ, Neve RL, Ramoa AS. cAMP/Ca2 response element-binding protein function is essential for ocular dominance plasticity. The Journal of Neuroscience. 2002;22:2237–45. doi: 10.1523/JNEUROSCI.22-06-02237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Niehaus JL, Kauer JA. PKG and PKA signaling in LTP at GABAergic synapses. Neuropsychopharmacology. 2009;34:1829–1842. doi: 10.1038/npp.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes F, Ferreira-Rosa K, Pereira MD, Kubrusly RC, Manhaes AC, Abreu-Villaca Y, Filgueiras CC. Acute administration of vinpocetine, a phosphodiesterase type 1 inhibitor, ameliorates hyperactivity in a mice model of fetal alcohol spectrum disorder. Drug Alcohol Depend. 2011:81–7. doi: 10.1016/j.drugalcdep.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;16;100(3):309–27. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- Patyar S, Prakash A, Modi M, Medhi B. Role of vinpocetine in cerebrovascular diseases. Pharmacol Rep. 2011;63(3):618–28. doi: 10.1016/s1734-1140(11)70574-6. [DOI] [PubMed] [Google Scholar]
- Paul AP, Pohl-Guimaraes F, Krahe TE, Filgueiras CC, Lantz CL, Colello RJ, Wang W, Medina AE. Overexpression of serum response factor restores ocular dominance plasticity in a model of fetal alcohol spectrum disorders. J Neurosci. 2010;30:2513–2520. doi: 10.1523/JNEUROSCI.5840-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz RB, Broderick KE. Role of cyclic GMP in gene regulation. Front Biosci. 2005;10:1239–1268. doi: 10.2741/1616. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, van Staveren WC, Sik A, Markerink-van Ittersum M, Niewohner U, van der Staay FJ, Blokland A, de Vente J. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience. 2002;113:351–361. doi: 10.1016/s0306-4522(02)00199-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Horne K, Witol A. Neurobehavioral functioning in children with fetal alcohol spectrum disorder. Child Neuropsychol. 2006;12:453–468. doi: 10.1080/09297040600646854. [DOI] [PubMed] [Google Scholar]
- Rema V, Ebner FF. Effect of enriched environment rearing on impairments in cortical excitability and plasticity after prenatal alcohol exposure. The Journal of Neuroscience. 1999;19:10993–1006. doi: 10.1523/JNEUROSCI.19-24-10993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, McGee CL, Sowell ER. Teratogenic effects of alcohol: A decade of brain imaging. American Journal of Medical Genetics. 2004;127C:35–41. doi: 10.1002/ajmg.c.30014. [DOI] [PubMed] [Google Scholar]
- Rutten K, Prickaerts J, Blokland A. Rolipram reverses scopolamine-induced and time-dependent memory deficits in object recognition by different mechanisms of action. Neurobiol Learn Mem. 2006;85:132–138. doi: 10.1016/j.nlm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Rutten K, Prickaerts J, Hendrix M, van der Staay FJ, Sik A, Blokland A. Time-dependent involvement of cAMP and cGMP in consolidation of object memory: studies using selective phosphodiesterase type 2, 4 and 5 inhibitors. Eur J Pharmacol. 2007;8;558(1–3):107–12. doi: 10.1016/j.ejphar.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Rutten K, Basile JL, Prickaerts J, Blokland A, Vivian JA. Selective PDE inhibitors rolipram and sildenafil improve object retrieval performance in adult cynomolgus macaques. Psychopharmacology (Berl) 2008;196:643–648. doi: 10.1007/s00213-007-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, Van Donkelaar EL, Ferrington L, Blokland A, Bollen E, Steinbusch HW, Kelly PA, Prickaerts JH. Phosphodiesterase inhibitors enhance object memory independent of cerebral blood flow and glucose utilization in rats. Neuropsychopharmacology. 2009;34:1914–1925. doi: 10.1038/npp.2009.24. [DOI] [PubMed] [Google Scholar]
- Savage DD, Becher M, de la Torre AJ, Sutherland RJ. Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcohol Clin Exp Res. 2002;26:1752–1758. doi: 10.1097/01.ALC.0000038265.52107.20. [DOI] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Heynen A, Bear M. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philosophical Transactions - Royal Society. Biological Sciences. 2009;364:357–67. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behavioral Neuroscience. 2007;121:120–30. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Pandiella N, Wijayawardhane N, Vaithianathan T, Birru S, Breese C, Suppiramaniam V, Randal C. Aniracetam reversed learning and memory deficits following prenatal ethanol exposure by modulating functions of synaptic AMPA receptors. Neuropsychopharmacology. 2008;33:1071–1083. doi: 10.1038/sj.npp.1301496. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, Weinberg RJ, Ehlers MD, Philpot BD. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci. 2009;12:777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT, O’Donnell JM. Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology (Berl) 2000;150:311–316. doi: 10.1007/s002130000414. [DOI] [PubMed] [Google Scholar]
- Zhang KY, Ibrahim PN, Gillette S, Bollag G. Phosphodiesterase-4 as a potential drug target. Expert Opin Ther Targets. 2005;9:1283–1305. doi: 10.1517/14728222.9.6.1283. [DOI] [PubMed] [Google Scholar]