Abstract
A critical event in the history of biological chemistry was the chemical identification of the first neurotransmitter receptor, the nicotinic acetylcholine receptor. Disciplines as diverse as electrophysiology, pharmacology, and biochemistry joined together in a unified and rational manner with the common goal of successfully identifying the molecular device that converts a chemical signal into an electrical one in the nervous system. The nicotinic receptor has become the founding father of a broad family of pentameric membrane receptors, paving the way for their identification, including that of the GABAA receptors.
Keywords: GABA Receptors, Ion Channels, Neurobiology, Neuroscience, Neurotransmitter Receptors, Neurotransmitters, Nicotinic Acetylcholine Receptors, Receptors
Introduction
It has been 42 years since the isolation of the nicotinic acetylcholine receptor (nAChR)2 from fish electric organ, the first ligand-gated ion channel and the first ion channel ever identified; 25 years since the first GABAA and glycine receptor subunits were cloned and sequenced and concomitantly their homology with the nAChRs recognized; and 5 years since the discovery that closely homologous ligand-gated ion channels are present in prokaryotes (1). In this minireview, I briefly retrace the main steps in the discovery of the nAChR, the titular head of this receptor superfamily.
The Concept of Receptor and the Chemical Identification of the Acetylcholine Receptor
The English physiologist John Newport Langley, working with neuromuscular preparations, proposed in 1905 that muscle tissue possesses “a substance that combines with nicotine and curare … receives the stimulus and transmits it.” He called the muscle entity the “receptive substance.” In the subsequent 50 years, the concept of pharmacological receptors inspired three main lines of research: first, the pharmacological approach aimed at characterizing the specificity of the receptor site by using novel chemical ligands (e.g. the distinction between nicotinic and muscarinic AChRs by Sir Henry Dale); second, the electrophysiological approach exemplified by Bernard Katz and John Eccles aimed at understanding the ionic responses to endogenous neurotransmitter signals; and third, the chemical tradition aimed at the chemical identification of the receptor molecule(s).
In the late 1960s, lipids, polysaccharides, proteins, and even nucleic acids were considered as potential receptors. The early independent efforts of Carlos Chagas, Eduardo de Robertis, and David Nachmansohn to identify the receptor for acetylcholine (ACh) in the electric organ of the fish Electrophorus electricus with radioactive ligands were abandoned because their tissue extracts lacked specificity (2). However, in the course of these studies, Nachmansohn recognized the extraordinarily rich content of nicotinic synapses in the electric organ (2). With Ernest Schoffeniels, he devised a method for preparing individual cells, or electroplaques, from the electric organ. This offered the opportunity to investigate, simultaneously, the electrophysiological, pharmacological, and biochemical characteristics of the response to ACh within the same biological system (2). At this time, there were also speculations that the enzyme acetylcholinesterase (AChE) and the physiological receptor site for ACh could reside on the same protein complex.
The introduction of new biochemical methods radically changed the field of receptor identification. One such method is affinity labeling, which relies on the use of compounds that are structural homologs of the neurotransmitter and also possess a highly reactive group. This combination allows for specific binding to the receptor site, and once bound, the probe covalently links to the protein. For instance, the molecule p-trimethylammonium benzenediazonium fluoroborate (TDF) carries a trimethylammonium group (as does ACh) as well as a reactive diazonium group (3). As anticipated, TDF interacted covalently with E. electricus electroplaque as an irreversible competitive antagonist, and curare protected against this covalent attachment (4). The method was subsequently improved upon with the synthesis of 4-(N-maleimido)phenyltrimethylammonium iodide, whereby the diazonium is substituted with a maleimide group (5). The latter selectively reacts with –SH groups exposed by treating the electroplaque membrane with dithiothreitol. However, at this stage, both the method of tissue preparation and the specificity of the compounds used were insufficient to allow for isolation of the receptor in its active form from the electric organ.
A second method that significantly advanced the field was the marked improvement of procedures for fractionation and purification of membrane fragments rich in AChE from E. electricus electric organs. Electron microscopic sections of these membrane fragments revealed that they formed closed vesicles (6). Inspired by the technique used with bacterial permeases (7), it became possible to measure radioactive Na+ (or K+) ion fluxes with these microsacs by using a simple filtration method (8, 106). The microsacs responded to nicotinic agonists with specificities closely resembling those recorded by electrophysiological methods employing intact electroplaques. The signal transduction by the neurotransmitter could be reproduced in a totally acellular system in the absence of energy supply and in a chemically defined environment. Thus, it became possible to study in vitro the chemistry of the physiological response to ACh and of the signal transduction mechanism involved (8, 106). The receptor molecule was evidently present in the purified membranes in a functional state. It was now possible to follow reversible binding to these purified membranes using the nicotinic agonist decamethonium as the radioactive ligand (by the method of equilibrium dialysis that Gilbert and Müller-Hill (9) used to identify the lac repressor) (Fig. 1). The detergent deoxycholate gently extracted the binding protein without denaturing it, and bound decamethonium was displaced by various nicotinic agonists and antagonists, including curare and Flaxedil in the order of their physiological effects (10). Since then, similar receptor binding assays have been used extensively to characterize the GABAA and glycine receptors (see the accompanying Classics).
FIGURE 1.
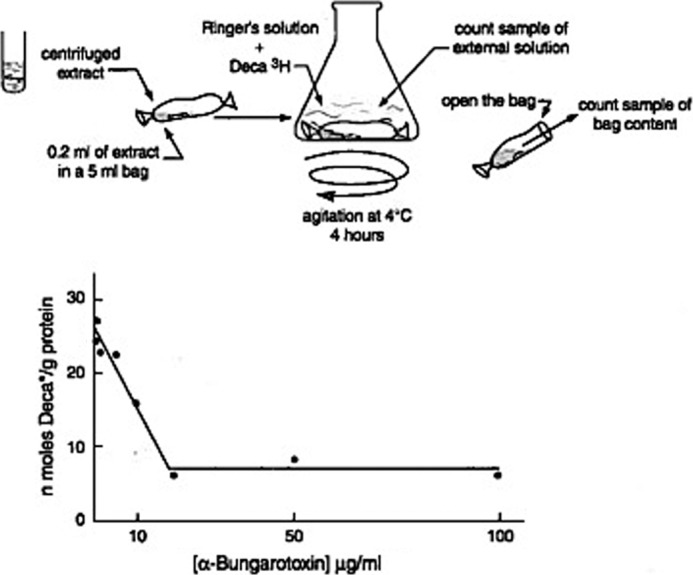
Upper, binding method by equilibrium dialysis used for the identification of the nicotinic receptor. Lower, effect of the snake toxin α-BGT on binding of the nicotinic agonist [3H]decamethonium (Deca). This figure has been reprinted from Refs. 12 (lower) and 51 (upper).
Third, Chen-Yuan Lee, a Taiwanese pharmacologist, had found that a snake venom toxin, α-bungarotoxin (α-BGT), specifically blocks in vivo neuromuscular transmission in high vertebrates at the postsynaptic level without interacting with AChE (11). Aware of Claude Bernard's lesson to use toxic compounds as chemical lancets, I asked Lee, who unexpectedly visited me at Institut Pasteur, for a sample of the toxin. A few days later, I received it and immediately tried it in the three systems just mentioned. The result was remarkable (12): α-BGT blocked the electroplaque's electrical response in vivo and the microsac's ion flux response to nicotinic agonists in vitro; α-BGT also blocked the binding of radioactive decamethonium to the detergent extract (Fig. 1). This extract contained a protein, sensitive to Pronase digestion, that bound nicotinic agonists and the snake venom toxin in a mutually exclusive manner. This nicotinic receptor (nAChR) molecule was shown to be a high molecular weight hydrophobic protein that could be physically separated from AChE (12).
An α-toxin from Naja nigricollis, closely homologous to α-BGT, was then covalently coupled to Sepharose beads without losing its binding activity. Mixing the toxin beads with the membrane extract revealed that 75–100% of the nAChR protein bound to the toxin beads, whereas 85–100% of the AChE remained in the supernatant. The data (13) confirmed that AChE and the nAChR molecule were distinct protein entities. These studies also introduced Cuatrecasas' technique of affinity chromatography to the nAChR field. Many groups then became aware of these distinct methods (14–16). We (17, 18) and others (19) used alternative affinity columns with immobilized quaternary ammonium agonists or antagonists (Fig. 2), extending the use, by Miledi et al. (20), of radioactive 131I-labeled α-BGT (which, according to them, selectively binds to the receptor in its resting state).
FIGURE 2.
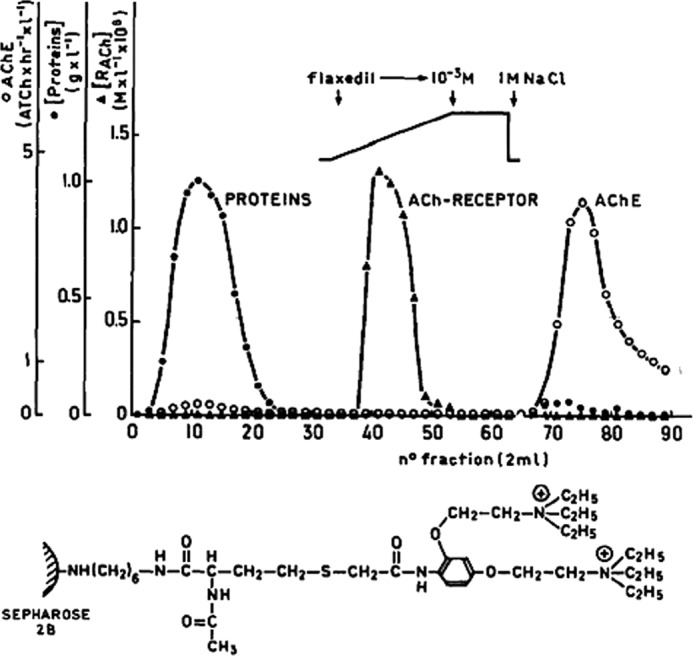
Purification of the nAChR by affinity chromatography. This figure has been reprinted from Ref. 17.
Another rather simple technological development that, retrospectively, had an important impact on nAChR research was the isolation of a novel generation of excitable microsacs exceptionally rich in nAChR (20–40% of total protein) prepared from homogenates of Torpedo marmorata electric organ (21), a finding that was readily confirmed by other groups. The nAChR-rich membranes made the structural and functional properties of the membrane-bound nAChR accessible to a variety of biochemical and biophysical methods, such as purification in large quantities (22), fluorescence spectroscopy (23), electron spin resonance (24), and x-ray diffraction (25).
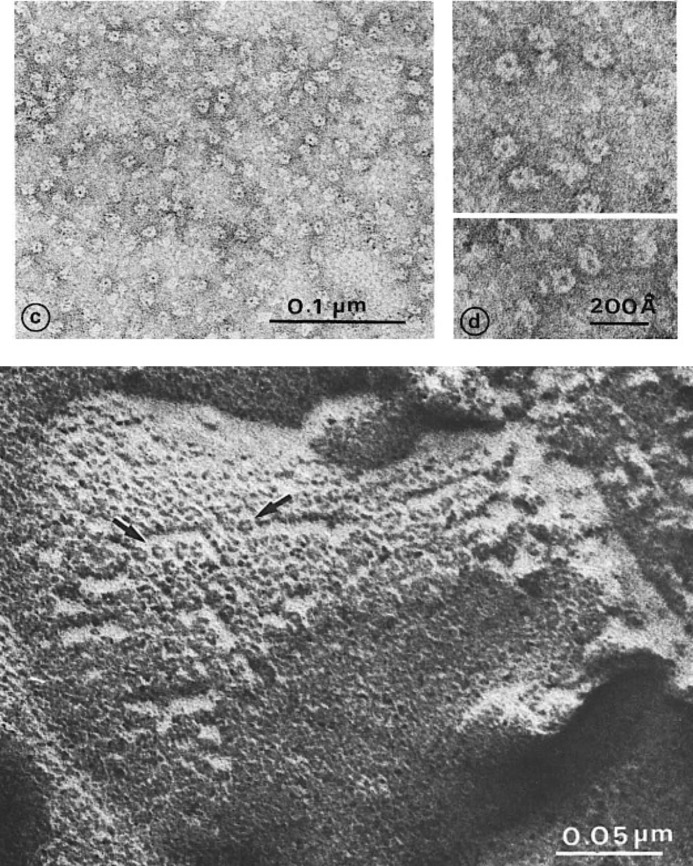
Finally, the nAChR protein purified from E. electricus and the purified nAChR-rich membranes from T. marmorata were examined by electron microscopy and revealed ring-like particles (8–9 nm in diameter) with a hydrophilic core linked to a compact bundle (Fig. 3) (26). Made up of several (five to six) subunits, they formed closely packed two-dimensional assemblies in T. marmorata postsynaptic membranes (∼8,000–12,000 μm2) (Fig. 3) (26, 27). These nAChR images were the first ever of the structure of a neurotransmitter receptor. They were subsequently described in greater details by Nigel Unwin (reviewed in Ref. 28) and others. Similar pictures later became available for the GABAA and glycine receptors (see the accompanying Classics).
FIGURE 3.
First structural observation of the purified nicotinic receptor proteins from E. electricus (upper) and from purified subsynaptic membrane fragments from T. marmorata (lower). This figure has been reprinted from Ref. 26.
The Pentameric Organization of the Nicotinic Receptor and the Complete Sequence of the Subunits
The amount of purified nAChR was sufficient to identify the subunit organization of the protein. A first study using partial cross-linking of the purified E. electricus nAChR revealed five well defined bands, suggesting a pentameric organization (29). The pentameric organization was rapidly confirmed by the teams of Karlin and Raftery, who, in addition, discovered that the nAChR molecule is composed of four distinct types of subunits with slight differences in molecular mass that assemble into a 2α1β1γ1δ1 heteropentamer (30–33).
Nothing was known about the chemistry of the subunits. However, with the recently developed new technology of high resolution microsequencing, amino acid sequences could be determined from small quantities of protein. The sequence of 20 amino acids comprising the N-terminal domain of the α-subunit of the T. marmorata receptor was established in my laboratory (34). A chemical identity card of the receptor was made available, the first ever established for a neurotransmitter receptor. It was confirmed in the Raftery laboratory with the α-subunit of Torpedo californica (35) and extended to the N-terminal sequence of the four subunits, revealing a number of sequence identities among the subunits (36). Consistent with the Monod-Wyman-Changeux (1965) model (37), the nAChR protein was an authentic oligomer, but pseudosymmetrical, with a 5-fold axis of rotation perpendicular to the plane of the postsynaptic membrane.
Knowledge of the initial sequence data opened the nAChR field to recombinant DNA technologies. The teams of Shosaku Numa (38–40), Stephen Heinemann (41, 42), and Eric Barnard (43), as well as Anne Devillers-Thiéry, and Jérôme Giraudat (44, 45) in my laboratory, struggled to clone the complementary DNAs of the different subunits from electric organ and muscle and to establish their complete sequence. Experiments by Eric Barnard and Ricardo Miledi had demonstrated that messenger RNA extracted from the electric organ of Torpedo injected into Xenopus oocytes led to the synthesis and incorporation of functional AChRs into the membrane of the oocyte (46). Injection of the four mRNAs transcribed from the cloned cDNAs yielded functional nAChRs (47), confirming earlier biochemical experiments (48, 49) demonstrating that assembly of the four types of subunits suffices to recover a fully operational nAChR.
Examination of the complete cDNA sequences revealed several common structural domains along the sequences of the subunits that led to the first model of transmembrane organization of nAChR subunits (39, 40, 42, 45). It was proposed that the long hydrophilic N-terminal segment, four hydrophobic stretches, and a short hydrophilic segment were organized into an extracellular (synaptic) domain, four transmembrane α-helices, and an intracellular (cytoplasmic) domain. In 1986 and after, closely homologous sequences and the organization of the subunits, including a Cys loop, were found in neuronal nicotinic ACh receptors, including α7- and α4β2-nAChRs (Ref. 50; reviewed in Ref. 51), GABAA, glycine, 5-HT3, and glutamate-gated chloride channel (GluCl), thus creating the superfamily of pentameric receptors that is the subject of the accompanying Classics. The recent discovery of cationic orthologs in prokaryotes (52, 53) has extended the superfamily, plunging its evolutionary origins back 3 billion years (1).
Identification of the ACh-binding Sites
The actual tridimensional topology of the AChR protein and of the various sites it carries still could not be directly inferred from recombinant DNA technologies. Identification of the amino acids composing the ACh-binding site and the ion channel relied upon different technologies. The previously mentioned method of affinity labeling proved to be useful at this stage. A first result was obtained by Karlin's group using 4-(N-maleimido)phenyltrimethylammonium iodide (5), which labels the sulfhydryl groups of the ACh-binding site (see above). This led to the identification of a pair of adjacent cysteines (positions 192 and 193) located in the N-terminal domain of the α-subunit (54). Despite these results, the pharmacological specificity of the ACh-binding site remained unknown.
Our group demonstrated that the snake 3H-labeled α-toxin itself, without additional modification, could be used as a photolabel. UV irradiation of the 3H-labeled α-toxin-Torpedo receptor complex resulted in the incorporation of covalently bound radioactivity not only into the α-subunit but also into the γ- and δ-subunits (55). From this observation, it was concluded that the ACh-binding sites were located at the interface between subunits (55) and were therefore non-equivalent. This was confirmed in subsequent functional studies.
The use of p-N,N-dimethylammonium benzenediazonium difluoroborate (DDF), an affinity probe similar to TDF (3, 4), provided additional important information (56). The dimethylammonium group of DDF created a resonant molecule that could be photoactivated by energy transfer from the protein. Indeed, eight amino acids were found labeled by DDF, six of them with an aromatic side chain, and all of them located in the long hydrophilic N-terminal domain of the α-subunit. These amino acids were distributed into three main loops, forming a sort of electronegative aromatic pocket in which the quaternary ammonium group of ACh was lodged (56–58), thus pointing to an analogy with the AChE-binding site, where π bonding is exhibited as well. These three loops, located on the α-subunit side of the binding site and referred to as the “principal component,” were named A, B, and C (58), a nomenclature that has been adopted by the receptor community. In agreement with the snake 3H-labeled α-toxin photolabeling data, the affinity probe DDF labeled the γ- and δ-subunits in addition to the α-subunit (51, 56–59). The various groups working on the receptor, including those of Arthur Karlin, Jonathan Cohen, and ourselves, further documented this notion and identified additional loops D, E, and F on the non-α-subunit side of the interface (Refs. 51 and 59 and references therein). These loops form a “complementary” component of the ACh-binding site on the γ- and δ-subunits. These biochemical data were supported by site-directed mutagenesis studies of the labeled amino acids identified in these studies (Refs. 51 and 59 and references therein).
Confirmation of the binding site organization has come from the crystal structure of a soluble snail protein that binds ACh, the ACh-binding protein, a close homolog of the nAChR extracellular domain (60) and of the full-length eukaryotic GluCl receptor (61) and the prokaryotic Erwinia chrysanthemi receptor (ELIC) bound with GABA (62) and ACh (as an antagonist) (Ref. 63; reviewed in Ref. 1).
Identification of the Ion Channel
By the early 1980s, no biochemical structure of any ion channel was known. The question was how to chemically identify the amino acids that line the pore through which ions flow. The quest (1974–1999) proved to be long and difficult (see Refs. 51 and 64). Pharmacological agents, such as local anesthetics, known for decades to block ion currents elicited by nicotinic agonists in an indirect noncompetitive manner, proved to be essential tools for chemical labeling the channel. The first experiments, performed with both E. electricus and T. marmorata receptor-rich membranes, demonstrated in vitro that, at pharmacologically active concentrations, the local anesthetics did not directly displace nicotinic ligands from the ACh-binding site but reversibly bound to a different allosteric site (65, 66). One of these compounds, chlorpromazine, displayed, in addition, the remarkable property of covalently linking to the receptor protein by simple UV irradiation. In receptor-rich membranes from T. marmorata, chlorpromazine labeled the four types of subunits of the nAChR (67), and precise quantitative measurements demonstrated that it bound to just one high affinity site per 2α1β1γ1δ1 oligomer (68). The kinetics of access of chlorpromazine to this site increased by 100-fold when rapidly mixed with ACh under conditions expected to generate functional ion channels (69, 70). We proposed that chlorpromazine binds to a site located within the ion channel along the pseudosymmetry axis that becomes accessible to chlorpromazine when the ion channel opens. The conditions under which the channel could be specifically labeled were thus established.
It took more than a year to demonstrate that chlorpromazine labels serine 262, within the second transmembrane segment (TM2) of the δ-subunit (71), a finding that was rapidly confirmed by another group using the same protocol but with a different probe (72). Further identification of the chlorpromazine-labeled amino acids on the other subunits not only showed that the serines form a ring (73) but also revealed the adduct of other amino acids (leucines and threonines) located at a distance of three to four amino acids on both sides of the ring of serines (74). It was concluded that (a) the TM2 segments contribute to the channel walls, (b) these segments are folded into an α-helix, (c) the chlorpromazine-binding site is located at a near-equatorial position in the channel's pseudosymmetry axis; and (d) a positive reciprocal allosteric interaction exists between ACh and the chlorpromazine-binding sites.
In parallel site-directed mutagenesis experiments in which single channel recordings were carried out after reconstitution in Xenopus oocytes, a region located in the δ-subunit that comprises the putative TM2 segment and the adjacent bend portion between TM2 and TM3 was shown to be responsible for a conductance difference between Torpedo and bovine channels (75). Subsequent analysis (76) identified rings of negatively charged glutamine residues that were classified as external, intermediate, and cytoplasmic and that beautifully framed the amino acid clusters labeled by chlorpromazine, thus confirming their proposed location within the ion path (68–70). The teams of Henry Lester and Norman Davidson reached a similar conclusion (77).
Further studies identified amino acids that contribute to the ionic selectivity of the channel (78–80). A group of three residues was found to drive the conversion of the cationic selectivity of the ion channel into one of anionic selectivity (79, 80). For the first time, an excitatory receptor could be transformed into an inhibitory one. This finding, as well as the converse operation (from anionic to cationic), was reproduced with other receptors: GABAA, glycine, GluCl, and 5-HT3 (Ref. 51; see the accompanying Classics). A functional chimera was successfully constructed that joined the synaptic domain of α7-nAChR and the transmembrane domain of the 5-HT3 receptor (81). Even combinations of prokaryotic and eukaryotic receptor domains were found functional (82). This demonstrates unambiguously a conservation of tertiary organization between members of the receptor superfamily. Finally, the high resolution x-ray data from prokaryotic ELIC and GLIC (from Gloeobacter violaceus) (83–85) are consistent with the biochemical data and EM structure (reviewed in Refs. 28 and 51) of the nAChR ion channel (1). They demonstrate further that the channel domain is topographically distinct from the neurotransmitter-binding domain and that the interaction between the neurotransmitter and the ion transport mechanism is an allosteric interaction (1, 37, 64, 86).
Allosteric Transitions of the Nicotinic Receptor: The Quaternary Twist Mechanism
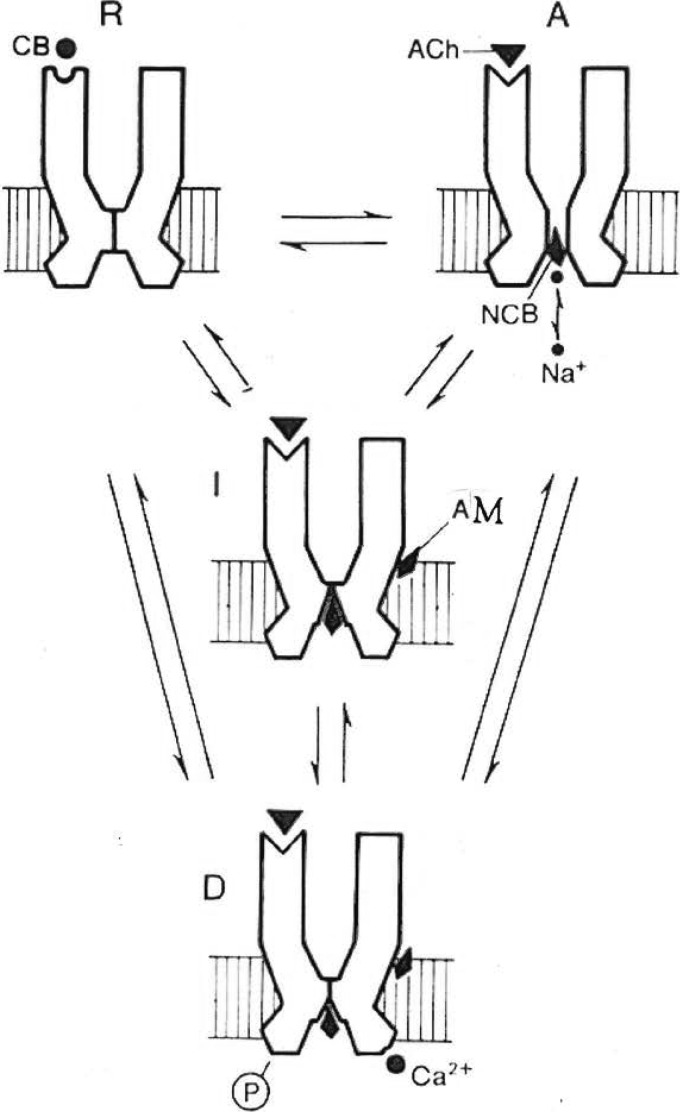
Direct evidence for the conformational changes that mediate this interaction was still unavailable. Early rapid mixing experiments using snake 3H-labeled α-toxin as a probe and receptor-rich membranes from T. marmorata revealed changes in conformation that took seconds to reach a high affinity state, possibly desensitized, from a low affinity resting state (87). Consistent findings were subsequently reported using muscle cells (88) and Torpedo membranes (89, 90, 107, 108). A refined kinetic analysis of the binding interaction of the fluorescent nicotinic agonist dansyl-C6-choline with receptor-rich membranes (91, 92, 109) and correlation with the in vitro measurement of ion transport through the ion channel (93) resulted in the demonstration of allosteric transitions between several conformational states: a resting closed channel state (R) stabilized by snake α-toxin and nicotinic antagonists; an active transient open channel state with low affinity for ACh and nicotinic agonists (A), and at least one desensitized and slowly accessible refractory state (D) with high affinity for both agonists and antagonists (in addition to a fast desensitized state (I)) (Fig. 4).
FIGURE 4.
Minimal four-state model for the allosteric transitions of the nicotinic receptor. CB, competitive (orthosteric) blocker; NCB, noncompetitive (channel) blocker; AM, allosteric modulator; P, phosphorylation site. This figure has been reprinted from Ref. 64.
Moreover, under resting conditions, a sizeable fraction (∼20%) of the receptor was found to be present in the high affinity desensitized state (91), and spontaneous channel openings of the muscle nAChR were recorded in the absence of ACh (94). This ruled out the induced fit mechanism to the benefit of the conformational selection (Monod-Wyman-Changeux) scheme (see Ref. 86). Still, the situation appeared more complex than for regulatory enzymes. There exists not only one but a cascade of discrete transitions between open and closed conformational states (Fig. 4) (see Refs. 1, 51, and 64).
Up until recently, little new information became available to help explain the structural transitions of the nAChR, except for in situ electron microscopy studies of the Torpedo receptor (95). In silico modeling from the available structural data brought novel insight into the conformational transitions of the receptor protein (96, 97). Normal mode analysis performed on a three-dimensional model of the α7-nAChR gave a breakdown of the protein movements into discrete modes. Among the first 10 lowest frequency modes, the first mode produced a structural reorganization that caused a wide opening of the channel pore resulting from a concerted and symmetrical transition (a quaternary twist motion of the protein) with opposing rotations of the upper (extracellular) and lower (transmembrane) domains and significant tertiary reorganizations within each subunit, in particular at the domain interface. The global quaternary twist motion accounted reasonably for the available experimental data on the gating process (97). Strong evidence emerged from the comparison studies of the x-ray structures of the prokaryotic receptors GLIC (from G. violaceus), which showed an open channel conformation, and ELIC, which displayed a closed channel conformation (83–85). Comparison of the two structures indicated that at least 29% of the quaternary twist transition model accounts for channel opening (83). Future developments include the molecular dynamics of the transition on a microsecond time scale (98).
Allosteric Modulatory Sites
The signal transduction process mediated by the nAChR is regulated by at least three main categories of allosteric “modulators,” which bind to sites distinct from the neurotransmitter site and the ion channel. These modulators are thought to selectively shift the allosteric equilibrium in favor of either an active (positive modulators) or a resting/desensitized (negative modulators) conformation without competing with the neurotransmitter binding to the orthosteric sites (Refs. 64 and 86; Ref. 1 and references therein).
One group of modulators includes Ca2+, which potentiates most neuronal nAChRs (99, 100) and binds to the extracellular domain below the ACh site at residues contributed from both sides of the subunit interface (96). Another includes Zn2+.
A second important group consists of modulators, such as galantamine, that bind at “non-agonist” interfaces, which, in heteropentameric nAChRs, differ from the neurotransmitter-binding site and appear to be homologs of the benzodiazepine site on GABAA receptors (see observations made by Richard Olsen in the accompanying Classics).
Another group of allosteric modulators interacts with the transmembrane domain. The antihelminthic ivermectin was originally discovered to behave as a strong positive modulator of α7-nAChR (101). Its action was altered by mutations within the TM2 domain (101). General anesthetics (both intravenous and volatile) negatively modulate excitatory nAChRs but positively enhance inhibitory GABA receptors. Photolabeling studies with GABAA receptors (see observations made by Richard Olsen and colleagues; see in the accompanying Classics) and x-ray structures of GLIC complexes with propofol or desfurane reveal a site within the upper part of the transmembrane domain of each subunit (102) with which nicotinic allosteric modulators may also interact in neuronal nAChRs (AM in Fig. 4) (103).
Allosteric modulatory sites have also been identified in the cytoplasmic loop that links TM3 and TM4 in all eukaryotic (but not prokaryotic) pentameric receptors. In nAChRs, several phosphorylation sites (104) that control desensitization in muscle and α7-nAChR also contribute to end plate localization by agrin-induced tyrosine phosphorylation of the cytoskeletal protein 43K-rapsyn (22, 51). The cytoplasmic domain of the α4-nAChR subunit also binds a variety of scaffold proteins that interact with cytoskeletal proteins and with G protein systems that are involved in intracellular signaling pathways (105).
Conclusion
Since the isolation of the nAChR and the discovery that GABAA and glycine receptor subunits are close orthologs of the nAChR, thereby founding the superfamily of pentameric ligand-gated ion channels, the whole field of pentameric receptors for neurotransmitters has blossomed, including the discovery of homologous receptors in prokaryotes. Several of these are the target of the most commonly used drugs, such as benzodiazepines, barbiturates, curare, and general anesthetics. The recent advances in the x-ray structures of several of these receptors (1) open new avenues for the rational design of pharmacological agents acting on the brain, in parallel with the abundant studies on the G protein-coupled receptors, which were identified several years later.
Acknowledgments
I gratefully thank the Woods Hole Marine Biological Laboratory, where a significant part of the minireview was written, and Leonard Warren and Albert Grossman for carefully editing the manuscript.
This is the first article in the Thematic Minireview Series on Celebrating the Discovery of the Cysteine Loop Ligand-gated Ion Channel Superfamily.
- nAChR
- nicotinic acetylcholine receptor
- ACh
- acetylcholine
- AChE
- acetylcholinesterase
- TDF
- p-trimethylammonium benzenediazonium fluoroborate
- α-BGT
- α-bungarotoxin
- GluCl
- glutamate-gated chloride channel
- DDF
- p-N,N-dimethylammonium benzenediazonium difluoroborate
- ELIC
- E. chrysanthemi ligand-gated ion channel
- GLIC
- G. violaceus ligand-gated ion channel
- TM
- transmembrane segment.
REFERENCES
- 1. Corringer P. J., Poitevin F., Prevost M. S., Sauguet L., Delarue M., Changeux J. P. (2012) Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure 20, 941–956 [DOI] [PubMed] [Google Scholar]
- 2. Nachmansohn D. (1959) The Chemical and Molecular Basis of Nerve Activity, Academic Press, New York [Google Scholar]
- 3. Fenton J. W., 2nd, Singer S. J. (1965) Affinity labeling of antibodies to the p-azophenyltrimethylammonium hapten and a structural relationship among antibody active sites of different specificities. Biochem. Biophys. Res. Commun. 20, 315–320 [DOI] [PubMed] [Google Scholar]
- 4. Changeux J. P., Podleski T. R., Wofsy L. (1967) Affinity labeling of the acetylcholine receptor. Proc. Natl. Acad. Sci. U.S.A. 58, 2063–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karlin A., Winnik M. (1968) Reduction and specific alkylation of the receptor for acetylcholine. Proc. Natl. Acad. Sci. U.S.A. 60, 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Changeux J. P., Gautron J., Israël M., Podleski T. (1969) Separation of excitable membranes from the electric organ of Electrophorus electricus. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 269, 1788–1791 [PubMed] [Google Scholar]
- 7. Cohen G. N., Monod J. (1957) Bacterial permeases. Bacteriol. Rev. 21, 169–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasai M., Changeux J. P. (1970) Demonstration of the excitation by cholinergic agonists from fractions of purified membranes in vitro. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 270, 1400–1403 [PubMed] [Google Scholar]
- 9. Gilbert W., Müller-Hill B. (1966) Isolation of the lac repressor. Proc. Natl. Acad. Sci. U.S.A. 56, 1891–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Changeux J. P., Kasai M., Huchet M., Meunier J. C. (1970) Extraction from electric tissue of Electrophorus of a protein presenting several typical properties characteristic of the physiological receptor of acetylcholine. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 270, 2864–2867 [PubMed] [Google Scholar]
- 11. Chang C. C., Lee C. Y. (1963) Isolation of neurotoxins from the venom of Bungarus multicinctus and their modes of neuromuscular blocking action. Arch. Int. Pharmacodyn. Ther. 144, 241–257 [PubMed] [Google Scholar]
- 12. Changeux J. P., Kasai M., Lee C. Y. (1970) Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc. Natl. Acad. Sci. U.S.A. 67, 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meunier J. C., Huchet M., Boquet P., Changeux J. P. (1971) Separation of the receptor protein of acetylcholine and acetylcholinesterase. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 272, 117–120 [PubMed] [Google Scholar]
- 14. Karlsson E., Heilbronn E., Widlund L. (1972) Isolation of the nicotinic acetylcholine receptor by biospecific chromatography on insolubilized Naja naja neurotoxin. FEBS Lett. 28, 107–111 [DOI] [PubMed] [Google Scholar]
- 15. Klett R. P., Fulpius B. W., Cooper D., Smith M., Reich E., Possani L. D. (1973) The acetylcholine receptor. I. Purification and characterization of a macromolecule isolated from Electrophorus electricus. J. Biol. Chem. 248, 6841–6853 [PubMed] [Google Scholar]
- 16. Lindstrom J., Patrick J. (1974) in Synaptic Transmission and Neuronal Interaction (Bennett M. V. L., ed) pp. 191–216, Raven Press, New York [Google Scholar]
- 17. Olsen R. W., Meunier J. C., Changeux J. P. (1972) Progress in purification of the cholinergic receptor protein from Electrophorus electricus by affinity chromatography. FEBS Lett. 28, 96–100 [DOI] [PubMed] [Google Scholar]
- 18. Meunier J. C., Sealock R., Olsen R., Changeux J. P. (1974) Purification and properties of the cholinergic receptor protein from Electrophorus electricus electroplax. Eur. J. Biochem. 45, 371–394 [DOI] [PubMed] [Google Scholar]
- 19. Schmidt J., Raftery M. A. (1972) Use of affinity chromatography for acetylcholine receptor purification. Biochem. Biophys. Res. Commun. 49, 572–578 [DOI] [PubMed] [Google Scholar]
- 20. Miledi R., Molinoff P., Potter L. T. (1971) Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature 229, 554–557 [DOI] [PubMed] [Google Scholar]
- 21. Cohen J. B., Weber M., Huchet M., Changeux J. P. (1972) Purification from Torpedo marmorata electric tissue of membrane fragments particularly rich in cholinergic receptor. FEBS Lett. 26, 43–47 [DOI] [PubMed] [Google Scholar]
- 22. Sobel A., Weber M., Changeux J. P. (1977) Large-scale purification of the acetylcholine receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur. J. Biochem. 80, 215–224 [DOI] [PubMed] [Google Scholar]
- 23. Cohen J. B., Changeux J. P. (1973) Interaction of a fluorescent ligand with membrane-bound cholinergic receptor from Torpedo marmorata. Biochemistry 12, 4855–4864 [DOI] [PubMed] [Google Scholar]
- 24. Brisson A. D., Scandella C. J., Bienvenüe A., Devaux P. F., Cohen J. B., Changeux J. P. (1975) Interaction of a spin-labeled long chain acylcholine with the cholinergic receptor protein in its membrane environment. Proc. Natl. Acad. Sci. U.S.A. 72, 1087–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dupont Y., Cohen J. B., Changeux J. P. (1974) X-ray diffraction study of membrane fragments rich in acetylcholine receptor protein prepared from the electric organ of Torpedo marmorata. FEBS Lett. 40, 130–133 [DOI] [PubMed] [Google Scholar]
- 26. Cartaud J., Benedetti E. L., Cohen J. B., Meunier J. C., Changeux J. P. (1973) Presence of a lattice structure in membrane fragments rich in nicotinic receptor protein from the electric organ of Torpedo marmorata. FEBS Lett. 33, 109–13 [DOI] [PubMed] [Google Scholar]
- 27. Nickel E., Potter L. T. (1973) Ultrastructure of isolated membranes of Torpedo electric tissue. Brain Res. 57, 508–517 [DOI] [PubMed] [Google Scholar]
- 28. Unwin N. (2005) Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J. Mol. Biol. 346, 967–989 [DOI] [PubMed] [Google Scholar]
- 29. Hucho F., Changeux J. P. (1973) Molecular weight and quaternary structure of the cholinergic receptor protein extracted by detergents from Electrophorus electricus electric tissue. FEBS Lett. 38, 11–15 [DOI] [PubMed] [Google Scholar]
- 30. Weill C. L., McNamee M. G., Karlin A. (1974) Affinity labeling of purified acetylcholine receptor from Torpedo californica. Biochem. Biophys. Res. Commun. 61, 997–1003 [DOI] [PubMed] [Google Scholar]
- 31. Raftery M. A., Vandlen R., Michaelson D., Bode J., Moody T., Chao Y., Reed K., Deutsch J., Duguid J. (1974) The biochemistry of an acetylcholine receptor. J. Supramol. Struct. 2, 582–592 [DOI] [PubMed] [Google Scholar]
- 32. Lindstrom J., Walter B., Einarson B. (1979) Immunochemical similarities between subunits of acetylcholine receptors from Torpedo, Electrophorus, and mammalian muscle. Biochemistry 18, 4470–4480 [DOI] [PubMed] [Google Scholar]
- 33. Saitoh T., Oswald R., Wennogle L. P., Changeux J. P. (1980) Conditions for the selective labelling of the 66,000 Dalton chain of the acetylcholine receptor by the covalent noncompetitive blocker 5-azido-[3H]trimethisoquin. FEBS Lett. 116, 30–36 [DOI] [PubMed] [Google Scholar]
- 34. Devillers-Thiéry A., Changeux J. P., Paroutaud P., Strosberg A. D. (1979) The amino-terminal sequence of the 40,000 molecular weight subunit of the acetylcholine receptor protein from Torpedo marmorata. FEBS Lett. 104, 99–105 [DOI] [PubMed] [Google Scholar]
- 35. Hunkapiller M. W., Strader C. D., Hood L., Raftery M. A. (1979) Amino-terminal amino acid sequence of the major polypeptide subunit of Torpedo californica acetylcholine receptor. Biochem. Biophys. Res. Commun. 91, 164–169 [DOI] [PubMed] [Google Scholar]
- 36. Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. (1980) Acetylcholine receptor: complex of homologous subunits. Science 208, 1454–1456 [DOI] [PubMed] [Google Scholar]
- 37. Monod J., Wyman J., Changeux J. P. (1965) On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 [DOI] [PubMed] [Google Scholar]
- 38. Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. (1982) Primary structure of α-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature 299, 793–797 [DOI] [PubMed] [Google Scholar]
- 39. Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T., Numa S. (1983) Primary structures of β- and δ-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature 301, 251–255 [DOI] [PubMed] [Google Scholar]
- 40. Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T., Numa S. (1983) Structural homology of Torpedo californica acetylcholine receptor subunits. Nature 302, 528–532 [DOI] [PubMed] [Google Scholar]
- 41. Ballivet M., Patrick J., Lee J., Heinemann S. (1982) Molecular cloning of cDNA coding for the γ-subunit of Torpedo acetylcholine receptor. Proc. Natl. Acad. Sci. U.S.A. 79, 4466–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Claudio T., Ballivet M., Patrick J., Heinemann S. (1983) Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor γ-subunit. Proc. Natl. Acad. Sci. U.S.A. 80, 1111–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sumikawa K., Houghton M., Smith J. C., Bell L., Richards B. M., Barnard E. A. (1982) The molecular cloning and characterisation of cDNA coding for the α-subunit of the acetylcholine receptor. Nucleic Acids Res. 10, 5809–5822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giraudat J., Devillers-Thiéry A., Auffray C., Rougeon F., Changeux J. P. (1982) Identification of a cDNA clone coding for the acetylcholine binding subunit of Torpedo marmorata acetylcholine receptor. EMBO J. 1, 713–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Devillers-Thiéry A., Giraudat J., Bentaboulet M., Changeux J. P. (1983) Complete mRNA coding sequence of the acetylcholine binding α-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc. Natl. Acad. Sci. U.S.A. 80, 2067–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barnard E. A., Miledi R., Sumikawa K. (1982) Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc. R. Soc. Lond. B Biol. Sci. 215, 241–246 [DOI] [PubMed] [Google Scholar]
- 47. Mishina M., Kurosaki T., Tobimatsu T., Morimoto Y., Noda M., Yamamoto T., Terao M., Lindstrom J., Takahashi T., Kuno M., Numa S. (1984) Expression of functional acetylcholine receptor from cloned cDNAs. Nature 307, 604–608 [DOI] [PubMed] [Google Scholar]
- 48. Hazelbauer G. L., Changeux J. P. (1974) Reconstitution of a chemically excitable membrane. Proc. Natl. Acad. Sci. U.S.A. 71, 1479–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Epstein M., Racker E. (1978) Reconstitution of carbamylcholine-dependent sodium ion flux and desensitization of the acetylcholine receptor from Torpedo californica. J. Biol. Chem. 253, 6660–6662 [PubMed] [Google Scholar]
- 50. Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. (1986) Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor α-subunit. Nature 319, 368–374 [DOI] [PubMed] [Google Scholar]
- 51. Changeux J. P., Edelstein S. (2005) Nicotinic Acetylcholine Receptors: From Molecular Biology to Cognition, Odile Jacob, New York [Google Scholar]
- 52. Tasneem A., Iyer L. M., Jakobsson E., Aravind L. (2004) Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys loop ion channels. Genome Biol. 6, R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bocquet N., Prado de Carvalho L., Cartaud J., Neyton J., Le Poupon C., Taly A., Grutter T., Changeux J. P., Corringer P. J. (2007) A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature 445, 116–119 [DOI] [PubMed] [Google Scholar]
- 54. Kao P. N., Dwork A. J., Kaldany R. R., Silver M. L., Wideman J., Stein S., Karlin A. (1984) Identification of the α-subunit half-cystine specifically labeled by an affinity reagent for the acetylcholine receptor binding site. J. Biol. Chem. 259, 11662–11665 [PubMed] [Google Scholar]
- 55. Oswald R. E., Changeux J. P. (1982) Cross-linking of α-bungarotoxin to the acetylcholine receptor from Torpedo marmorata by UV light irradiation. FEBS Lett. 139, 225–229 [DOI] [PubMed] [Google Scholar]
- 56. Dennis M., Giraudat J., Kotzyba-Hibert F., Goeldner M., Hirth C., Chang J. Y., Changeux J. P. (1986) A photoaffinity ligand of the acetylcholine binding site predominantly labels the region 179–207 of the α-subunit on native acetylcholine receptor from Torpedo marmorata. FEBS Lett. 207, 243–249 [Google Scholar]
- 57. Dennis M., Giraudat J., Kotzyba-Hibert F., Goeldner M., Hirth C., Chang J. Y., Lazure C., Chrétien M., Changeux J. P. (1988) Amino acids of the Torpedo marmorata acetylcholine receptor subunit labeled by a photoaffinity ligand for the acetylcholine binding site. Biochemistry 27, 2346–2357 [DOI] [PubMed] [Google Scholar]
- 58. Galzi J. L., Revah F., Black D., Goeldner M., Hirth C., Changeux J. P. (1990) Identification of a novel amino acid α-tyrosine 93 within the cholinergic ligands-binding sites of the acetylcholine receptor by photoaffinity labeling. Additional evidence for a three-loop model of the cholinergic ligands-binding sites. J. Biol. Chem. 265, 10430–10437 [PubMed] [Google Scholar]
- 59. Karlin A. (2002) Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3, 102–114 [DOI] [PubMed] [Google Scholar]
- 60. Brejc K., van Dijk W. J., Klaassen R. V., Schuurmans M., van Der Oost J., Smit A. B., Sixma T. K. (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276 [DOI] [PubMed] [Google Scholar]
- 61. Hibbs R. E., Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys loop receptor. Nature 474, 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zimmermann I., Dutzler R. (2011) Ligand activation of the prokaryotic pentameric ligand-gated ion channel ELIC. PLoS Biol. 9, e1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pan J., Chen Q., Willenbring D., Yoshida K., Tillman T., Kashlan O. B., Cohen A., Kong X. P., Xu Y., Tang P. (2012) Structure of the pentameric ligand-gated ion channel ELIC co-crystallized with its competitive antagonist acetylcholine. Nat. Commun. 3, 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Changeux J. P. (1990) in Fidia Research Foundation Neuroscience Award Lectures (Changeux J. P., Llinas R. R., Purves D., Bloom F. F., eds) Vol. 4, pp. 17–168, Raven Press, New York [Google Scholar]
- 65. Weber M., Changeux J. P. (1974) Binding of Naja nigricollis [3H]α-toxin to membrane fragments from Electrophorus and Torpedo electric organs. III. Effect of local anaesthetics on the binding of the tritiated α-neurotoxin. Mol. Pharmacol. 10, 35–40 [PubMed] [Google Scholar]
- 66. Cohen J. B., Weber M., Changeux J. P. (1974) Effects of local anesthetics and calcium on the interaction of cholinergic ligands with the nicotinic receptor protein from Torpedo marmorata. Mol. Pharmacol. 10, 904–932 [PubMed] [Google Scholar]
- 67. Oswald R., Changeux J. P. (1981) Ultraviolet light-induced labeling by noncompetitive blockers of the acetylcholine receptor from Torpedo marmorata. Proc. Natl. Acad. Sci. U.S.A. 78, 3925–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Heidmann T., Oswald R., Changeux J. P. (1982) The high affinity binding site for chlorpromazine is present only as a single copy per cholinergic receptor molecule and is shared by four polypeptide chains. C. R. Seances Acad. Sci. III 295, 345–349 [PubMed] [Google Scholar]
- 69. Heidmann T., Changeux J. P. (1984) Time-resolved photolabeling by the noncompetitive blocker chlorpromazine of the acetylcholine receptor in its transiently open and closed ion channel conformations. Proc. Natl. Acad. Sci. U.S.A. 81, 1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Heidmann T., Changeux J. P. (1986) Characterization of the transient agonist-triggered state of the acetylcholine receptor rapidly labeled by the noncompetitive blocker [3H]chlorpromazine: additional evidence for the open channel conformation. Biochemistry 25, 6109–6113 [DOI] [PubMed] [Google Scholar]
- 71. Giraudat J., Dennis M., Heidmann T., Chang J. Y., Changeux J. P. (1986) Structure of the high affinity site for noncompetitive blockers of the acetylcholine receptor: serine 262 of the δ-subunit is labeled by [3H]chlorpromazine. Proc. Natl. Acad. Sci. U.S.A. 83, 2719–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Oberthür W., Muhn P., Baumann H., Lottspeich F., Wittmann-Liebold B., Hucho F. (1986) The reaction site of a noncompetitive antagonist in the δ-subunit of the nicotinic acetylcholine receptor. EMBO J. 5, 1815–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hucho F., Oberthür W., Lottspeich F. (1986) The ion channel of the nicotinic acetylcholine receptor is formed by the homologous helices M II of the receptor subunits. FEBS Lett. 205, 137–142 [DOI] [PubMed] [Google Scholar]
- 74. Giraudat J., Dennis M., Heidmann T., Haumont P. Y., Lederer F., Changeux J. P. (1987) Structure of the high affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H] chlorpromazine labels homologous residues in the β- and δ-chains. Biochemistry 26, 2410–2418 [DOI] [PubMed] [Google Scholar]
- 75. Imoto K., Methfessel C., Sakmann B., Mishina M., Mori Y., Konno T., Fukuda K., Kurasaki M., Bujo H., Fujita Y., Numa S. (1986) Location of a δ-subunit region determining ion transport through the acetylcholine receptor channel. Nature 324, 670–674 [DOI] [PubMed] [Google Scholar]
- 76. Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. (1988) Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature 335, 645–648 [DOI] [PubMed] [Google Scholar]
- 77. Leonard R. J., Labarca C. G., Charnet P., Davidson N., Lester H. A. (1988) Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science 242, 1578–1581 [DOI] [PubMed] [Google Scholar]
- 78. Villarroel A., Herlitze S., Koenen M., Sakmann B. (1991) Location of a threonine residue in the α-subunit M2 transmembrane segment that determines the ion flow through the acetylcholine receptor channel. Proc. Biol. Sci. 243, 69–74 [DOI] [PubMed] [Google Scholar]
- 79. Galzi J. L., Devillers-Thiéry A., Hussy N., Bertrand S., Changeux J. P., Bertrand D. (1992) Mutations in the ion channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature 359, 500–505 [DOI] [PubMed] [Google Scholar]
- 80. Corringer P. J., Bertrand S., Galzi J. L., Devillers-Thiéry A., Changeux J. P., Bertrand D. (1999) Mutational analysis of the charge selectivity filter of the α7 nicotinic acetylcholine receptor. Neuron 22, 831–843 [DOI] [PubMed] [Google Scholar]
- 81. Eiselé J. L., Bertrand S., Galzi J. L., Devillers-Thiéry A., Changeux J. P., Bertrand D. (1993) Chimeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature 366, 479–483 [DOI] [PubMed] [Google Scholar]
- 82. Duret G., Van Renterghem C., Weng Y., Prevost M., Moraga-Cid G., Huon C., Sonner J. M., Corringer P. J. (2011) Functional prokaryotic-eukaryotic chimera from the pentameric ligand-gated ion channel family. Proc. Natl. Acad. Sci. U.S.A. 108, 12143–12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bocquet N., Nury H., Baaden M., Le Poupon C., Changeux J. P., Delarue M., Corringer P. J. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114 [DOI] [PubMed] [Google Scholar]
- 84. Hilf R. J., Dutzler R. (2008) X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379 [DOI] [PubMed] [Google Scholar]
- 85. Hilf R. J., Dutzler R. (2009) Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118 [DOI] [PubMed] [Google Scholar]
- 86. Changeux J. P. (2012) Allostery and the Monod-Wyman-Changeux model after 50 years. Annu. Rev. Biophys. 41, 103–133 [DOI] [PubMed] [Google Scholar]
- 87. Weber M., David-Pfeuty T., Changeux J. P. (1975) Regulation of binding properties of the nicotinic receptor protein by cholinergic ligands in membrane fragments from Torpedo marmorata. Proc. Natl. Acad. Sci. U.S.A. 72, 3443–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Colquhoun D., Range H. P. (1976) Effects of inhibitors of the binding of iodinated α-bungarotoxin to acetylcholine receptors in rat muscle. Mol. Pharmacol. 12, 519–535 [PubMed] [Google Scholar]
- 89. Grünhagen H. H., Changeux J. P. (1976) Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. IV. Quinacrine: a fluorescent probe for the conformational transitions of the cholinergic receptor protein in its membrane bound state. J. Mol. Biol. 106, 497–516 [DOI] [PubMed] [Google Scholar]
- 90. Weiland G., Georgia B., Lappi S., Chignell C. F., Taylor P. (1977) Kinetics of agonist-mediated transitions in state of the cholinergic receptor. J. Biol. Chem. 252, 7648–7656 [PubMed] [Google Scholar]
- 91. Heidmann T., Changeux J. P. (1979) Fast kinetic studies on the interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata. Eur. J. Biochem. 94, 255–279 [DOI] [PubMed] [Google Scholar]
- 92. Heidmann T., Changeux J. P. (1980) Interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata in the millisecond time range: resolution of an “intermediate” conformational transition and evidence for positive cooperative effects. Biochem. Biophys. Res. Comm. 97, 889–896 [DOI] [PubMed] [Google Scholar]
- 93. Heidmann T., Bernhardt J., Neumann E., Changeux J. P. (1983) Rapid kinetics of agonist binding and permeability response analyzed in parallel on acetylcholine receptor-rich membranes from Torpedo marmorata. Biochemistry 22, 5452–5459 [DOI] [PubMed] [Google Scholar]
- 94. Jackson M. B. (1984) Spontaneous openings of the acetylcholine receptor channel. Proc. Natl. Acad. Sci. U.S.A. 81, 3901–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Miyazawa A., Fujiyoshi Y., Unwin N. (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949–955 [DOI] [PubMed] [Google Scholar]
- 96. Le Novère N., Grutter T., Changeux J. P. (2002) Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proc. Natl. Acad. Sci. U.S.A. 99, 3210–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Taly A., Delarue M., Grutter T., Nilges M., Le Novère N., Corringer P. J., Changeux J. P. (2005) Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophys. J. 88, 3954–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nury H., Poitevin F., Van Renterghem C., Changeux J. P., Corringer P. J., Delarue M., Baaden M. (2010) One-microsecond molecular dynamics simulation of channel gating in a nicotinic receptor homologue. Proc. Natl. Acad. Sci. U.S.A. 107, 6275–6280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mulle C., Léna C., Changeux J. P. (1992) Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron 8, 937–945 [DOI] [PubMed] [Google Scholar]
- 100. Vernino S., Amador M., Luetje C. W., Patrick J., Dani J. A. (1992) Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron 8, 127–134 [DOI] [PubMed] [Google Scholar]
- 101. Krause R. M., Buisson B., Bertrand S., Corringer P. J., Galzi J. L., Changeux J. P., Bertrand D. (1998) Ivermectin: a positive allosteric effector of the α7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 53, 283–294 [DOI] [PubMed] [Google Scholar]
- 102. Nury H., Van Renterghem C., Weng Y., Tran A., Baaden M., Dufresne V., Changeux J. P., Sonner J. M., Delarue M., Corringer P. J. (2011) X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469, 428–431 [DOI] [PubMed] [Google Scholar]
- 103. Gill J. K., Dhankher P., Sheppard T. D., Sher E., Millar N. S. (2012) A series of α7 nicotinic acetylcholine receptor allosteric modulators with close chemical similarity but diverse pharmacological properties. Mol. Pharmacol. 81, 710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Teichberg V. I., Sobel A., Changeux J. P. (1977) In vitro phosphorylation of the acetylcholine receptor. Nature 267, 540–542 [DOI] [PubMed] [Google Scholar]
- 105. Kabbani N., Woll M. P., Levenson R., Lindstrom J. M., Changeux J. P. (2007) Intracellular complexes of the β2-subunit of the nicotinic acetylcholine receptor in brain identified by proteomics. Proc. Natl. Acad. Sci. U.S.A. 104, 20570–20575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kasai M., Changeux J. P. (1971) In vitro excitation of purified membrane fragments by cholinergic agonists. J. Memb. Biol. 6, 1–88 [DOI] [PubMed] [Google Scholar]
- 107. Grünhagen H. H., Changeux J. P. (1976) Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. V. Qualitative correlation between pharmacological effects and equilibration processes of the cholinergic receptor protein as revealed by the structural probe quinacrine. J. Mol. Biol. 106, 517–535 [DOI] [PubMed] [Google Scholar]
- 108. Grünhagen H. H., Iwatsubo M., Changeux J. P. (1977) Fast kinetic studies on the interaction of cholinergic agonists with the membrane-bound acetylcholine receptor from Torpedo marmorata as revealed by quinacrine fluorescence. Eur. J. Biochem. 80, 225–242 [DOI] [PubMed] [Google Scholar]
- 109. Heidmann T., Changeux J. P. (1979) Fast kinetic studies on the allosteric interactions between acetylcholine receptor and local anesthetic binding sites. Eur. J. Biochem. 94, 281–296 [DOI] [PubMed] [Google Scholar]