Abstract
Strychnine-sensitive glycine receptors (GlyRs) mediate synaptic inhibition in the spinal cord, brainstem, and other regions of the mammalian central nervous system. In this minireview, we summarize our current view of the structure, ligand-binding sites, and chloride channel of these receptors and discuss recently emerging functions of distinct GlyR isoforms. GlyRs not only regulate the excitability of motor and afferent sensory neurons, including pain fibers, but also are involved in the processing of visual and auditory signals. Hence, GlyRs constitute promising targets for the development of therapeutically useful compounds.
Keywords: Chloride Channels, Glycine Receptors, Homology Modeling, Neurotransmitter Receptors, Synapses, GluCl, Glycine Neurotransmission, Hyperekplexia, Inhibitory Synapse, Ligand-gated Ion Channel
Introduction
The normal functioning of the CNS depends on the balanced interplay of both excitatory and inhibitory neurons. Glutamate is the principal excitatory and GABA and glycine are the major inhibitory neurotransmitters in the adult mammalian CNS. Glycine serves, in addition, as a co-agonist of glutamate at the NMDA subtype of excitatory glutamate receptors.
Glycinergic synapses mediate fast inhibitory neurotransmission mainly in the spinal cord, brainstem, and caudal brain and control a variety of motor and sensory functions, including vision and audition (1). Glycine exerts its inhibitory effects via specific glycine receptors (GlyRs)2 that are highly enriched in the postsynaptic membrane. Binding of glycine leads to the opening of the GlyR integral anion channel, and the resulting influx of Cl− ions hyperpolarizes the postsynaptic cell, thereby inhibiting neuronal firing. The alkaloid strychnine antagonizes glycine binding with high affinity and has proven to be a unique tool in radioligand binding studies (2) and affinity purification (3) of GlyRs. Since these original studies, three decades of GlyR research have generated a wealth of genetic, functional, and structural data, which are summarized here.
Composition of GlyR Proteins
GlyRs are group I ligand-gated ion channels (LGICs) that belong to the Cys loop receptor family, which, in addition, includes nicotinic acetylcholine (nAChR), serotonin type 3 (5-HT3), and the closely related GABAA (GABAAR) receptors (4, 5). Affinity-purified GlyR preparations contain three polypeptide species (3): the GlyRα (48 kDa) and GlyRβ (58 kDa) subunits and a tightly bound cytosolic scaffolding protein (93 kDa) later named gephyrin (6). Cross-linking experiments showed that purified GlyRs are heteropentameric proteins, similar to the nAChRs in Torpedo electric organ and skeletal muscle (7). Their subunit stoichiometry was originally thought to be 3α:2β (7, 8), but more recently, this was revised to 2α:3β (9, 10).
Molecular cloning identified four vertebrate genes (called Glra1–4) encoding GlyRα subunits (α1–α4) and a single gene (Glrb) encoding the GlyRβ subunit (11–15). All GlyRα subunits display high sequence identity (>80%) (supplemental Fig. S1) and, upon heterologous expression, form functional homomeric glycine-gated channels with properties closely resembling those of GlyRs in vivo (4, 5). By photoaffinity labeling with [3H]strychnine, the GlyRα subunits were shown to possess critical determinants of ligand binding (16). GlyRβ displays significant sequence differences compared with the α subunits (<50% identity) (supplemental Fig. S1) and does not generate functional receptors when expressed alone (11) but is retained in the endoplasmic reticulum (17). However, the GlyRβ subunit is more than simply a structural subunit because it contributes to agonist binding (9) and has an essential role in the intracellular trafficking and synaptic clustering of postsynaptic GlyRs (18, 19). Its extended cytoplasmic loop region binds to the postsynaptic scaffolding protein gephyrin with high affinity (18, 20) and interacts with Vps35 and neurobeachin, proteins implicated in intracellular membrane protein transport (21).
The overall topology of GlyR subunits is shared with that of other group I LGIC proteins (22). All of these polypeptides contain a large N-terminal extracellular domain (ECD), four transmembrane segments (TM1–TM4), a long intracellular loop connecting TM3 and TM4, and a short extracellular C terminus. Sequence homologies are particularly high within the transmembrane segments and a conserved disulfide-bonded motif of 15 amino acids (“Cys loop”) located in the ECD. Although the number of GlyR subunit genes (n = 5) appears to be modest compared with those found in the mammalian nAChR (n = 17) and GABAAR (n = 20) families, alternative splicing of exons encoding segments of the extracellular N-terminal or intracellular loop regions further extends GlyR subunit heterogeneity (4, 5, 23). In the case of GlyRα2, splice variants in the ECD have been shown to differ in agonist efficacies (24), and intracellular loop variants of GlyRα3 have been found to differ in receptor desensitization (25) and subcellular targeting (26). Furthermore, post-transcriptional editing of a minor fraction of the GlyRα3 mRNA at a single nucleotide position has been reported to result in a gain-of-function receptor (27). This editing reaction leads to the substitution of proline 185 with leucine in the ECD and increases the apparent agonist affinity of α3-GlyRs.
Structure of the GlyR and Its Extracellular Binding Sites
An atomic resolution structure of a GlyR is not available yet despite efforts to obtain suitable amounts of purified protein for structural studies (28). Therefore, structural information has been inferred from other Cys loop receptors or bacterial homologs of the group I LGIC family. Electron microscopy data collected from the muscle-type nAChR from Torpedo (29) and the coordinates of the acetylcholine-binding protein AChBP, a soluble homopentameric protein that is structurally homologous to the ECD of group I LGICs (30), have been widely used to model the ECD of many different receptors, including the GlyR (9, 31, 32). Recently, the first crystal structure of an anion-conducting Cys loop receptor, the glutamate-gated channel (GluCl) from Caenorhabditis elegans, was solved at atomic resolution (33). GluCl is a close homolog (up to 43% sequence identity) (supplemental Fig. S1) of GlyR subunits and also contains their hallmark feature, the conserved disulfide bridge in loop C. Therefore, GluCl-based models of GlyRs, as shown in Fig. 1, are considered highly reliable templates for ligand binding studies and the rational design of novel selective drugs.
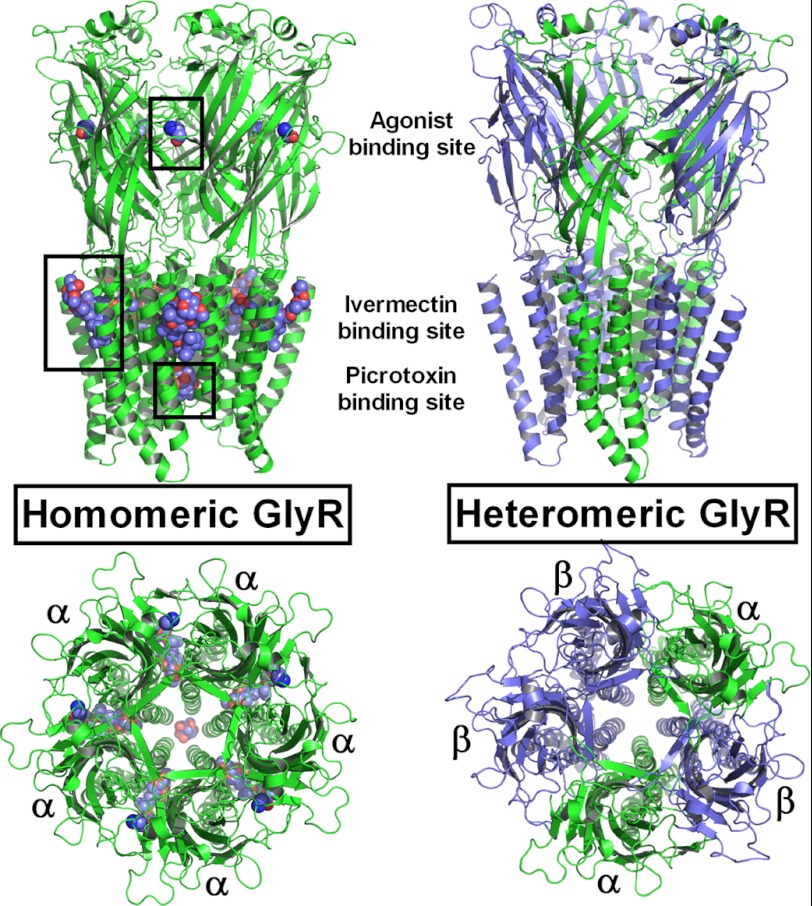
FIGURE 1.
Structures of homomeric α1- and heteromeric α1β-GlyRs modeled using GluCl as a template. Left, α1-GlyR shown with five glycine and ivermectin molecules each and a single picrotoxin molecule bound (ligands depicted as van der Waals spheres). Right, unliganded heteromeric α1β-GlyR with the different interfaces indicated.
Agonists and competitive antagonists are known to bind to the ECD at the interfaces of two adjacent subunits (4, 5, 22). The GlyR binding pocket is formed by distinct “loop regions” of the principal (+) and complementary (−) subunit surfaces (Fig. 2). Residues whose substitution affects agonist and antagonist binding have been identified in loops D, B, E, and C (4, 5, 31). In addition, the disulfide bond between α1Cys-198 and α1Cys-209 constraining loop C appears to be critical for both cell surface expression and ligand binding (34). The binding determinants of the β subunit are less well studied; two GlyRβ residues (Arg-86 and Glu-180, equivalent to α1Arg-65 and α1Glu-157, respectively) (Fig. 2) have been found to influence ligand binding to heteromeric α1β-GlyRs (9). Docking of the agonist glycine and the antagonist strychnine into homology models of the α1-α1 and α1-β interfaces resulted in complex structures that agreed well with previous mutational data (9). However, the properties of the β-β interface had remained enigmatic, as GlyRβ alone does not form functional channels. A novel mutagenesis strategy guided by homology modeling has recently allowed reproduction of all potential agonist-binding sites (α-β, β-α, and β-β) present in heteromeric GlyRs by single-subunit expression (35). Although fully functional, the resulting β-β binding site displayed the most distinct pharmacological profile toward a range of agonists and modulators tested, indicating that it might be selectively targeted to modulate the activity of synaptic GlyRs.
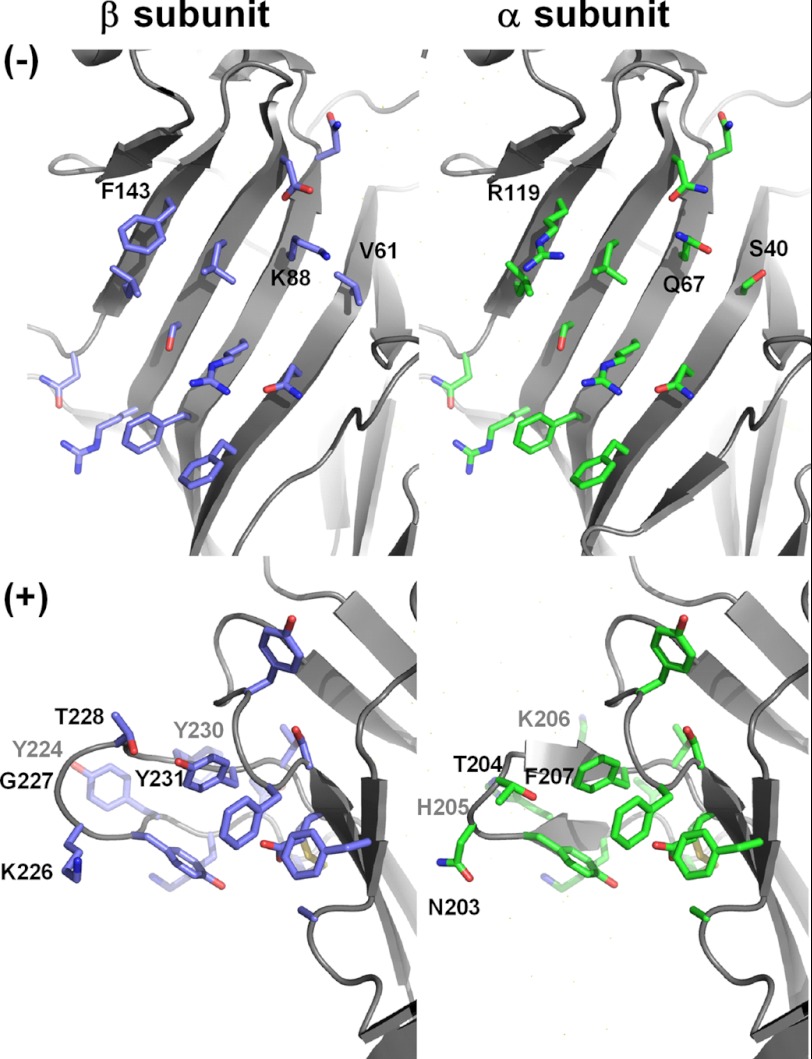
FIGURE 2.
Models of the principal (+) and complementary (−) surfaces of the GlyRα1 and GlyRβ agonist-binding regions. In heteromeric GlyRs, the + and − surfaces of the α1 and β subunit ECDs generate non-equivalent agonist-binding sites at the α-β, β-α, or β-β interface, which are all functional. Note the significant sequence divergence (up to eight substitutions) between α1 and β, in particular within loop C.
The ECD of GlyRs binds not only agonists and competitive antagonists but also compounds that allosterically modulate the agonist responses of different LGICs. Neurosteroids, general anesthetics, and ethanol potentiate GlyR currents, whereas the divalent cation Zn2+, tropeines, and endogenous cannabinoids, such as anandamide and 2-arachidonylglycerol, have both potentiating (at low concentrations) and inhibitory (at high concentrations) effects. Because of their potential therapeutic importance, the binding sites and isoform selectivity of these modulators have been intensely investigated (4, 5, 31, 36), and their in vivo roles have been studied in transgenic and knock-in mouse models (37, 38). Zn2+ and tropeines bind within or in the vicinity of the extracellular agonist-binding sites (39, 40). In contrast, hydrophobic modulators, such as neurosteroids and endocannabinoids (41) and anesthetics and ethanol (42), all appear to act by binding to the transmembrane domain (see below), although residues located in the ECD and/or the large intracellular loop may also contribute to current modulation (43, 44). Additionally, the principal excitatory transmitter glutamate has been reported to allosterically potentiate native and recombinant GlyRs; this may be important for balancing excitation and inhibition in the CNS (45).
GlyR Chloride Channel
The transmembrane domain of GlyR subunits and other group I LGICs consists of a four-α-helix bundle, in which the transmembrane segments are arranged in a clockwise order due to multiple interactions between specific hydrophobic residues that are essential for proper receptor assembly (46). TM1-TM2 and TM2-TM3 are connected by short loops, whereas TM3 is linked to TM4 by long intracellular loops that contain phosphorylation and ubiquitination sites as well as binding motifs for interacting proteins. The amphiphilic TM2 forms the ion channel; to facilitate comparisons between different LGICs, its residues are often numbered from 1′ to 19′, with position 1′ corresponding to the cytoplasmic N-terminal end and residue 19′ to the extracellular C-terminal end. Substituted cysteine accessibility experiments disclosed that Gly-2′, Thr-6′, and Arg-19′ line the pore of the α1-GlyR (47). Additional pore-lining residues were inferred by sequence comparison with other LGICs and include Thr-7′, Leu-9′, Thr-10′, Thr-13′, Ser-16′, and Gly-17′ (48). A model of the pore domain of GlyRs based on the recently determined structure of GluCl confirms that most of these residues are indeed facing the channel lumen (supplemental Fig. S2).
Agonist binding to the ECD of GlyRs triggers the opening of the anion-selective channel spanning the plasma membrane. Extensive work on different LGICs has demonstrated that the conformational changes within the five subunits induced upon agonist binding are consistent with an allosteric transition model that predicts rotational movements of the TM2 segments around about position 9′ (29). This model was recently validated by comparing the structures of two bacterial LGIC homologs crystallized in the open and closed states, respectively (49) and is likely to similarly apply to the GlyR. Residues located at the ends or outside of TM2 have been shown to participate in the gating process, such as the GlyRα1 residues Arg-271, located in the M2-M3 linker (34), and Lys-276, a pore-facing residue at the extracellular end of TM2 (50). Presumably, these and other residues that reside at the interface between the ECD and transmembrane domain are essential for coupling the conformational changes occurring in the ECD upon agonist binding to channel opening (4, 5, 51).
The GlyR channel is largely anion-selective but has been reported to also permeate Na+ and K+ ions, albeit at low efficiency (52). GlyRs display a permeability sequence of SCN− > NO3− > I− > Br− >Cl−, which is inversely correlated to the hydration energies of these ions, implying that removal of hydration water molecules facilitates ion permeation (53). A “ring of charges,” consisting of arginine residues (Arg-0′) in TM2, is thought to concentrate permeating chloride ions and to repel oppositely charged cations. Consistent with this view, the subunits of cation-conducting nAChRs have a negatively charged residue (−Glu-1′) at the equivalent position, and a respective substitution (A−1′E) was found to convert the GlyR into a cation-selective receptor (52). Residues located at the extracellular ends of the TM2 segments and in the adjacent loops (Arg-19′ and Lys-24′) provide additional rings of charge, whose substitution also reduced the unitary Cl− conductance (54). Besides these positively charged residues, a conserved proline at position 2′ appears to play an important role in determining the selectivity and functional properties of homomeric GlyR channels (55); its deletion increased the pore size of the α1-GlyR and reduced its anion/cation permeability ratio (56). The new GluCl structure shows that this proline residue is located at the narrowest point of the channel and thus directly restricts ion flow (supplemental Fig. S2).
The GlyR channel is blocked by a number of non-competitive antagonists, such as picrotoxin, ginkgolide B, and cyanotriphenyl borate (4, 5). Previous mutagenesis data had indicated that picrotoxin occludes the channel by binding to pore-exposed residues of TM2 (57), although other sites of interaction have also been proposed (58, 59). More recently, Pro-2′ and Thr-6′ of TM2 were found to constitute important determinants of picrotoxin block (60). The crystal structure obtained from the GluCl-picrotoxin complex confirmed this assignment by showing that these residues constitute the binding site for this open channel blocker (33). Furthermore, it supports the idea that the low inhibitory potency of picrotoxin observed at heteromeric compared with homomeric GlyRs (57) is due to steric hindrance by the bulky Phe-282 side chain extending from TM2 of GlyRβ (Fig. 3).
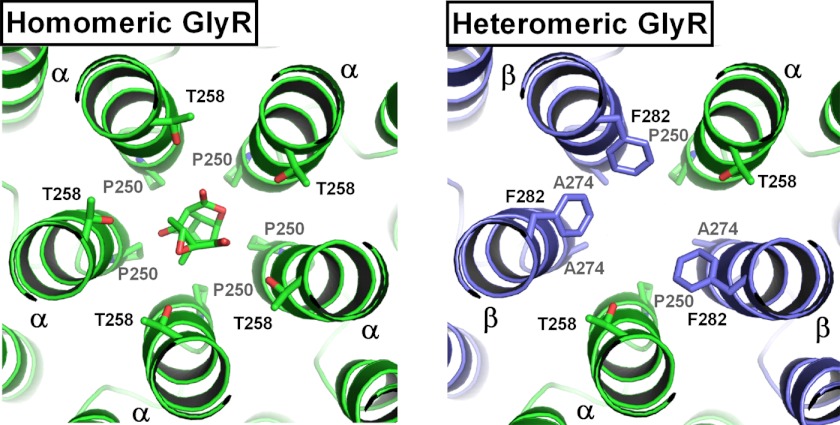
FIGURE 3.
Transmembrane domains of homo- and heteromeric GlyRs. Left, picrotoxin bound in the chloride channel of the homomeric α1-GlyR. Binding determinants include five Pro-250 and Thr-258 residues each. Right, in the α1β-GlyR, three of the Thr-258 residues are replaced by Phe-282, and three of the Pro-250 residues by Ala-274 of the β subunit. These substitutions explain the ∼50-fold lower affinity of picrotoxin for heteromeric GlyRs.
Several hydrophobic modulators of the GlyR are thought to interact with external sites of the transmembrane domain (42, 61). These include neurosteroids, general anesthetics, and alcohols, which all potentiate GlyR function (31). Early mutagenesis experiments had identified a common intrasubunit binding site for these compounds in both GlyRs and GABAARs (42), which, because of its predicted size and shape, was termed “big cavity” (62). Within this cavity, the polar residues Ser-267 and Thr-264 located in TM2 and TM3 were shown to influence the enhancing effect of volatile anesthetics and high dose ethanol in GlyRs (42). Recently, the crystal structures of complexes between a bacterial LGIC ortholog from Gloeobacter violaceus (GLIC) and two anesthetics (propofol and desflurane) have been solved (63). These structures nicely confirmed the concept of a common intrasubunit binding site in group I LGICs, which pre-exists in the GLIC apo structure in the upper part of the transmembrane domain of each monomer and recruits anesthetics via van der Waal interactions. This binding may displace crucial lipids and thereby positively or negatively influence channel gating (63). In addition to the big cavity, a second intramembrane site located at the interfaces between adjacent subunits appears to be crucial for potentiation by hydrophobic modulators. The existence of an intersubunit binding site for general anesthetics was first shown in GABAARs by photoaffinity labeling with etomidate (64). In GlyRs, this site is targeted by the antihelminthic ivermectin, which allosterically activates both GlyR and GluCl currents. The recent crystal structure of the GluCl-ivermectin complex (33) delineates ivermectin bound at the interface between TM3 from the principal (+) and TM1 from the complementary (−) subunits (Fig. 1). Furthermore, it shows that ivermectin stabilizes the open state of GluCl by inducing conformational changes in both the transmembrane domain and ECD. Although most of the ivermectin-binding residues of GluCl appear to be conserved in GlyRs, the GlyR binding site for ivermectin has been suggested to differ (65) and hence should be further challenged experimentally.
Roles of GlyR Isoforms in the Mammalian CNS
The physiological functions of the different GlyR isoforms have been incompletely analyzed due to the lack of subtype-specific antagonists. Our present picture of GlyR subtypes is based primarily on immunocytochemical studies and the analysis of knock-out mice.
α1-GlyRs
GlyRs containing the α1 subunit represent the predominant adult GlyR isoform, and heteromeric α1β receptors account for the majority of synaptically localized GlyRs in the mammalian CNS. The importance of the GlyRα1 subunit is underlined by the existence of various disease mutations in the Glra1 genes of different mammalian species, including mouse, cattle, and human (5). The spasmodic mouse carries a substitution at position 52 (A52S) of Glra1 that reduces the agonist binding affinity (66, 67). The oscillator strain constitutes a natural Glra1 null mutation due to a microdeletion in exon 8 that truncates the α1 subunit after TM3 (68). Notably, GlyR function of the oscillator polypeptide can be rescued upon coexpression of a C-terminal fragment containing TM4 (69). This result and complementation experiments with different GlyR and 5-HT3 receptor fragments (46) have revealed an essential role of TM interactions in group I LGIC assembly.
Upon recombinant expression, the GlyRα1 subunit forms channels characterized by short mean open times and fast decay kinetics, as found for glycinergic spontaneous inhibitory postsynaptic currents (sIPSCs) in adult spinal cord neurons (70). In retinas from oscillator mice, glycinergic sIPSCs in A-type ganglion cells are strikingly reduced and their kinetics slowed due to Glra1 inactivation (71). Together, the available data indicate that the GlyRα1 subunit defines receptors that are crucial for fast regulation of both motor and sensory functions.
α2-GlyRs
This GlyR isoform is highly expressed at the embryonic and neonatal stages but postnatally is largely replaced by α1 subunit-containing receptors. Synaptically localized α2 staining has been detected in different adult CNS regions, including the spinal cord, brainstem, midbrain, olfactory bulb, and retina, and corresponds to heteromeric α2β receptors (72). At early developmental stages, GlyRα2 forms homo-oligomeric receptors (73), which are extrasynaptically localized and thought to mediate non-synaptic tonic transmission caused by non-vesicular glycine release and/or spillover from adjacent nerve terminals.
Despite the availability of Glra2−/− mice (74, 75), the precise physiological roles of GlyRα2 are still largely enigmatic because the knock-out animals are phenotypically normal. Electrophysiological recordings of neonatal GlyRs in spinal neurons (70) and glycinergic sIPSCs in narrow-field amacrine cells of Glra2−/− mice (74) indicate that α2-GlyRs mediate IPSCs characterized by slow decay kinetics. In Glra2−/− mice, receptive field center responses in the retinal on and off pathways are impaired (76), and hyperalgesia induced by the injection of zymosan is prolonged compared with wild-type littermates (77). Both its known sites of expression and the available physiological data suggest that, in adult mammals, synaptic GlyRα2 is involved mainly in regulating sensory pathways.
α3-GlyRs
Originally, GlyRα3 was considered a minor adult GlyR isoform with an expression pattern resembling that of GlyRα1 (14, 78). In the spinal cord, GlyRα3 staining is found at synaptic sites in laminae I and II of the dorsal horn, where it inhibits the propagation of nociceptive signals to higher brain regions and serves as molecular substrate of pain sensitization by the inflammatory mediator prostaglandin E2 (PGE2) (79). PGE2 binds to prostaglandin EP2 receptors and thereby activates protein kinase A, which phosphorylates GlyRα3 and thus down-regulates glycine currents in dorsal horn neurons. In Glra3−/− mice, this down-regulation of glycine inhibition by PGE2 is abolished, and PGE2 fails to sensitize pain responses (79). Also, the analgesic effect of cannabinoids in mouse models of chronic inflammatory and neuropathic pain is absent in Glra3−/− animals (80). Hence, GlyRα3 constitutes a promising target for the development of novel drugs for the treatment of chronic pain syndromes.
GlyRα3 immunoreactivity has also been detected in other pathways involved in sensory processing. In retina, GlyRα3 is localized at synapses that are distinct from GlyRα1-containing ones (71). By analyzing Glra3−/− mice, amacrine cells of the AII subtype have been shown to contain synaptic α3-GlyRs, which confer medium-fast kinetics on sIPSCs (74). These receptors regulate the receptive fields of ON-ganglion cells by enhancing the excitatory center response (76). Furthermore, GlyRα3 has been found at inhibitory synapses in the inner ear (81). Together, the available data indicate that like GlyRα2, α3-GlyRs have an important role in sensory information processing.
α4-GlyRs
GlyRα4 is the least understood subtype of the GlyR family; this mainly reflects the low abundance of α4 mRNA and protein in the mammalian CNS. In chicken embryos, GlyRα4 transcripts have been detected in the spinal cord, peripheral ganglia, and male genital ridge (82). In the adult rodent retina, GlyRα4-immunoreactive synapses have been found on displaced ON-cholinergic amacrine cells (71). Upon heterologous expression, α4-GlyRs form channels with pharmacological properties largely resembling those of α1 receptors (82). Notably, in humans, the GLRA4 gene is a pseudogene. Based on the analysis of sIPSCs in narrow-field amacrine cells of Glra2−/− mice, α4 receptors have been suggested to display ultra-slow decay kinetics (74).
GlyRβ Subunit
The Glrb gene encodes the rodent β subunit, which is present three times in adult pentameric α1β-GlyRs (9, 10). By in situ hybridization, high levels of GlyRβ mRNA have been detected throughout the embryonic and postnatal CNS (78). However, this widespread transcription of Glrb is not reflected in a corresponding abundance of the β protein. A recently generated monoclonal antibody revealed GlyRβ immunoreactivity exclusively at GlyR-positive synapses but not in CNS regions lacking glycinergic neurons, such as the cortex (72). This discrepancy between GlyRβ transcript and protein levels likely reflects a retention and rapid degradation in the endoplasmic reticulum of GlyRβ in the absence of α subunits. Immunostainings of adult mouse retina have confirmed a high extent (>90%) of co-localization of GlyRβ with GlyRα1–3 (72). In the case of GlyRα4, however, immunoreactive synapses that lack GlyRβ staining were found in the inner plexiform layer. Thus, a minor subpopulation of synaptic GlyRs might not be heteromeric.
The functional importance of GlyRβ is underlined by the spastic mutation in mice, which produces a phenotype identical to that seen in spasmodic animals (83). Spastic animals express reduced levels of the major adult GlyR isoform α1β due to an intronic insertion of a LINE-1 element in the Glrb gene (84, 85). Causative for the reduced expression is exon skipping resulting from a polymorphism of a splice signal amplified by the LINE-1 insertion (86).
Developmental Changes in GlyR Function and Isoform Expression
In contrast to its hyperpolarizing action on adult neurons, glycine depolarizes motor neurons during embryonic development and around birth (87). Due to high intracellular chloride concentrations at these stages, GlyR channel opening leads to chloride efflux and thereby may induce neuronal firing, although inhibition also may occur as a result of the shunting conductance produced by GlyR activation. A depolarizing excitatory function of GlyRs at early developmental stages may be important for synaptogenesis because GlyR-triggered activation of voltage-gated Ca2+ channels appears to be crucial for GlyR clustering at postsynaptic sites (88). Also, it may be essential for the regulation of glutamate release by presynaptic GlyRs that have been detected electrophysiologically in large adult nerve terminals, such as the calyx of Held (89) and hippocampal mossy fiber terminals (90). Postnatally, the neuronal chloride equilibrium potential shifts to negative values due to chloride extrusion upon expression of the K+/Cl− cotransporter KCC2 (91). Thus, GlyR currents become hyperpolarizing, i.e. inhibitory.
In the spinal cord and brainstem, the developmental change in GlyR function described above is paralleled by changes in subunit composition (92). Embryonic and neonatal GlyRs are thought to be extrasynaptically localized homopentamers of α2 subunits (73), whereas adult synaptic GlyRs are heteromers containing α1 (or other α) and β subunits (7, 20). This change in subunit composition alters the biophysical properties of GlyR currents, resulting in faster decay kinetics and a smaller channel conductance (70, 93). Recombinant α2-GlyRs show slower response kinetics and larger subconductance state distributions than α1β receptors, consistent with the in vivo properties of neonatal versus adult GlyRs (94). Apparently, the postnatal change in GlyR subunit composition fine-tunes inhibitory transmission by shortening the mean channel open time and accelerating the decay of glycinergic IPSCs.
Mutations in Human GlyR Genes Cause Hyperekplexia
Mutations affecting glycinergic neurotransmission cause the hereditary neuromotor disorder hyperekplexia (HKPX; startle disease). The hallmark symptoms of this genetically heterogeneous disorder are an exaggerated startle response to auditory or tactile stimuli and, particularly in neonates, transient muscle rigidity (“stiff baby syndrome”). In 1993, positional cloning disclosed mutations in the GLRA1 gene localized on chromosome 5q33.1 (termed the HKPX1 locus) as a major cause of hyperekplexia (95). In this pioneering study, substitutions of the highly conserved residue Arg-271 within the extracellular loop connecting TM2 and TM3 were found in four families with autosomal dominant hyperekplexia. These substitutions decrease the agonist sensitivity and single-channel conductance of recombinant GlyRα1 (54, 96). Subsequently, additional dominant and recessive inheritance patterns and compound heterozygosity have been described for other patient families in which various GLRA1 missense or null mutations have been identified (4, 5, 97). Some of these mutations have been found to affect GlyR intracellular trafficking rather than agonist binding or channel gating (69, 98). Consistent with an important role of the β subunit in postsynaptic GlyR function, mutations in the GLRB gene have been associated with HKPX2 (97, 99). A third major form of hyperekplexia of presynaptic origin (HKPX3) is due to mutations in the gene encoding the neuronal glycine transporter GlyT2 (SLC6A5) (97).
Recent studies have implicated other GlyR genes, in particular GLRA3, in the pathology of other neurological disorders, such as autism, human immunodeficiency virus-associated dementia, generalized epilepsy, and amyotrophic lateral sclerosis (4, 5). Furthermore, autoantibodies against GlyRα1 have been found in patients suffering from progressive encephalomyelitis with rigidity and myoclonus (100). Together, all of these studies underline the importance of proper GlyR function for human health.
Conclusion and Perspectives
Since its purification in 1982, considerable progress has been made in elucidating the structure and pharmacology of the GlyR and in identifying physiological functions of its distinct isoforms. Furthermore, mouse models for different GlyR subunit deficiencies have become available, and the importance of GlyR mutations for the pathogenesis of human neuromotor disease is now well understood. However, many unanswered questions await further investigation, including the precise mechanism of GlyR channel gating, the individual roles of the different GlyR isoforms in various normal and diseased brain regions, and the development of isoform-specific ligands.
Supplementary Material
Acknowledgments
We thank all colleagues on whose work this minireview is based.
This is the second article in the Thematic Minireview Series on Celebrating the Discovery of the Cysteine Loop Ligand-gated Ion Channel Superfamily.

This article contains supplemental Figs. S1 and S2.
- GlyR
- glycine receptor
- LGIC
- ligand-gated ion channel
- nAChR
- nicotinic acetylcholine receptor
- GABAAR
- GABAA receptor
- ECD
- extracellular domain
- TM
- transmembrane segment
- GluCl
- glutamate-gated channel
- sIPSC
- spontaneous inhibitory postsynaptic current
- PGE2
- prostaglandin E2
- HKPX
- hyperekplexia.
REFERENCES
- 1. Legendre P. (2001) The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 58, 760–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young A. B., Snyder S. H. (1973) Strychnine binding associated with glycine receptors of the central nervous system. Proc. Natl. Acad. Sci. U.S.A. 70, 2832–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pfeiffer F., Graham D., Betz H. (1982) Purification by affinity chromatography of the glycine receptor of rat spinal cord. J. Biol. Chem. 257, 9389–9393 [PubMed] [Google Scholar]
- 4. Betz H., Laube B. (2006) Glycine receptors: recent insights into their structural organization and functional diversity. J. Neurochem. 97, 1600–1610 [DOI] [PubMed] [Google Scholar]
- 5. Lynch J. W. (2004) Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 84, 1051–1095 [DOI] [PubMed] [Google Scholar]
- 6. Prior P., Schmitt B., Grenningloh G., Pribilla I., Multhaup G., Beyreuther K., Maulet Y., Werner P., Langosch D., Kirsch J. (1992) Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron 8, 1161–1170 [DOI] [PubMed] [Google Scholar]
- 7. Langosch D., Thomas L., Betz H. (1988) Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc. Natl. Acad. Sci. U.S.A. 85, 7394–7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burzomato V., Groot-Kormelink P. J., Sivilotti L. G., Beato M. (2003) Stoichiometry of recombinant heteromeric glycine receptors revealed by a pore-lining region point mutation. Receptors Channels 9, 353–361 [DOI] [PubMed] [Google Scholar]
- 9. Grudzinska J., Schemm R., Haeger S., Nicke A., Schmalzing G., Betz H., Laube B. (2005) The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 45, 727–739 [DOI] [PubMed] [Google Scholar]
- 10. Yang Z., Taran E., Webb T. I., Lynch J. W. (2012) Stoichiometry and subunit arrangement of α1β glycine receptors as determined by atomic force microscopy. Biochemistry 51, 5229–5231 [DOI] [PubMed] [Google Scholar]
- 11. Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. (1990) Cloning and expression of the 58-kDa β subunit of the inhibitory glycine receptor. Neuron 4, 963–970 [DOI] [PubMed] [Google Scholar]
- 12. Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. (1987) The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature 328, 215–220 [DOI] [PubMed] [Google Scholar]
- 13. Grenningloh G., Schmieden V., Schofield P. R., Seeburg P. H., Siddique T., Mohandas T. K., Becker C. M., Betz H. (1990) α subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 9, 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuhse J., Schmieden V., Betz H. (1990) Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J. Biol. Chem. 265, 22317–22320 [PubMed] [Google Scholar]
- 15. Matzenbach B., Maulet Y., Sefton L., Courtier B., Avner P., Guénet J. L., Betz H. (1994) Structural analysis of mouse glycine receptor α subunit genes. Identification and chromosomal localization of a novel variant. J. Biol. Chem. 269, 2607–2612 [PubMed] [Google Scholar]
- 16. Graham D., Pfeiffer F., Betz H. (1983) Photoaffinity labelling of the glycine receptor of rat spinal cord. Eur. J. Biochem. 131, 519–525 [DOI] [PubMed] [Google Scholar]
- 17. Griffon N., Büttner C., Nicke A., Kuhse J., Schmalzing G., Betz H. (1999) Molecular determinants of glycine receptor subunit assembly. EMBO J. 18, 4711–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kneussel M., Betz H. (2000) Clustering of inhibitory neurotransmitter receptors at developing postsynaptic sites: the membrane activation model. Trends Neurosci. 23, 429–435 [DOI] [PubMed] [Google Scholar]
- 19. Maas C., Tagnaouti N., Loebrich S., Behrend B., Lappe-Siefke C., Kneussel M. (2006) Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. J. Cell Biol. 172, 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer G., Kirsch J., Betz H., Langosch D. (1995) Identification of a gephyrin binding motif on the glycine receptor β subunit. Neuron 15, 563–572 [DOI] [PubMed] [Google Scholar]
- 21. del Pino I., Paarmann I., Karas M., Kilimann M. W., Betz H. (2011) The trafficking proteins vacuolar protein sorting 35 and neurobeachin interact with the glycine receptor β subunit. Biochem. Biophys. Res. Commun. 412, 435–440 [DOI] [PubMed] [Google Scholar]
- 22. Corringer P. J., Le Novère N., Changeux J. P. (2000) Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 40, 431–458 [DOI] [PubMed] [Google Scholar]
- 23. Oertel J., Villmann C., Kettenmann H., Kirchhoff F., Becker C. M. (2007) A novel glycine receptor β subunit splice variant predicts an unorthodox transmembrane topology. Assembly into heteromeric receptor complexes. J. Biol. Chem. 282, 2798–2807 [DOI] [PubMed] [Google Scholar]
- 24. Miller P. S., Harvey R. J., Smart T. G. (2004) Differential agonist sensitivity of glycine receptor α2 subunit splice variants. Br. J. Pharmacol. 143, 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nikolic Z., Laube B., Weber R. G., Lichter P., Kioschis P., Poustka A., Mülhardt C., Becker C. M. (1998) The human glycine receptor subunit α3. GLRa3 gene structure, chromosomal localization, and functional characterization of alternative transcripts. J. Biol. Chem. 273, 19708–19714 [DOI] [PubMed] [Google Scholar]
- 26. Melzer N., Villmann C., Becker K., Harvey K., Harvey R. J., Vogel N., Kluck C. J., Kneussel M., Becker C. M. (2010) Multifunctional basic motif in the glycine receptor intracellular domain induces subunit-specific sorting. J. Biol. Chem. 285, 3730–3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meier J. C., Henneberger C., Melnick I., Racca C., Harvey R. J., Heinemann U., Schmieden V., Grantyn R. (2005) RNA editing produces glycine receptor α3(P185L), resulting in high agonist potency. Nat. Neurosci. 8, 736–744 [DOI] [PubMed] [Google Scholar]
- 28. Breitinger U., Breitinger H. G., Bauer F., Fahmy K., Glockenhammer D., Becker C. M. (2004) Conserved high affinity ligand binding and membrane association in the native and refolded extracellular domain of the human glycine receptor α1 subunit. J. Biol. Chem. 279, 1627–1636 [DOI] [PubMed] [Google Scholar]
- 29. Miyazawa A., Fujiyoshi Y., Unwin N. (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949–955 [DOI] [PubMed] [Google Scholar]
- 30. Brejc K., van Dijk W. J., Klaassen R. V., Schuurmans M., van Der Oost J., Smit A. B., Sixma T. K. (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276 [DOI] [PubMed] [Google Scholar]
- 31. Laube B., Maksay G., Schemm R., Betz H. (2002) Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol. Sci. 23, 519–527 [DOI] [PubMed] [Google Scholar]
- 32. Nevin S. T., Cromer B. A., Haddrill J. L., Morton C. J., Parker M. W., Lynch J. W. (2003) Insights into the structural basis for zinc inhibition of the glycine receptor. J. Biol. Chem. 278, 28985–28992 [DOI] [PubMed] [Google Scholar]
- 33. Hibbs R. E., Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys loop receptor. Nature 474, 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rajendra S., Lynch J. W., Pierce K. D., French C. R., Barry P. H., Schofield P. R. (1995) Mutation of an arginine residue in the human glycine receptor transforms β-alanine and taurine from agonists into competitive antagonists. Neuron 14, 169–175 [DOI] [PubMed] [Google Scholar]
- 35. Dutertre S., Drwal M., Laube B., Betz H. (2012) Probing the pharmacological properties of distinct subunit interfaces within heteromeric glycine receptors reveals a functional ββ agonist-binding site. J. Neurochem. 122, 38–47 [DOI] [PubMed] [Google Scholar]
- 36. Miller P. S., Topf M., Smart T. G. (2008) Mapping a molecular link between allosteric inhibition and activation of the glycine receptor. Nat. Struct. Mol. Biol. 15, 1084–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blednov Y. A., Benavidez J. M., Homanics G. E., Harris R. A. (2012) Behavioral characterization of knock-in mice with mutations M287L and Q266I in the glycine receptor α1 subunit. J. Pharmacol. Exp. Ther. 340, 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hirzel K., Müller U., Latal A. T., Hülsmann S., Grudzinska J., Seeliger M. W., Betz H., Laube B. (2006) Hyperekplexia phenotype of glycine receptor α1 subunit mutant mice identifies Zn2+ as an essential endogenous modulator of glycinergic neurotransmission. Neuron 52, 679–690 [DOI] [PubMed] [Google Scholar]
- 39. Maksay G., Laube B., Schemm R., Grudzinska J., Drwal M., Betz H. (2009) Different binding modes of tropeines mediating inhibition and potentiation of α1 glycine receptors. J. Neurochem. 109, 1725–1732 [DOI] [PubMed] [Google Scholar]
- 40. Miller P. S., Da Silva H. M., Smart T. G. (2005) Molecular basis for zinc potentiation at strychnine-sensitive glycine receptors. J. Biol. Chem. 280, 37877–37884 [DOI] [PubMed] [Google Scholar]
- 41. Xiong W., Wu X., Lovinger D. M., Zhang L. (2012) A common molecular basis for exogenous and endogenous cannabinoid potentiation of glycine receptors. J. Neurosci. 32, 5200–5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mihic S. J., Ye Q., Wick M. J., Koltchine V. V., Krasowski M. D., Finn S. E., Mascia M. P., Valenzuela C. F., Hanson K. K., Greenblatt E. P., Harris R. A., Harrison N. L. (1997) Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature 389, 385–389 [DOI] [PubMed] [Google Scholar]
- 43. Perkins D. I., Trudell J. R., Crawford D. K., Alkana R. L., Davies D. L. (2010) Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol. Ther. 127, 53–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yévenes G. E., Zeilhofer H. U. (2011) Molecular sites for the positive allosteric modulation of glycine receptors by endocannabinoids. PLoS ONE 6, e23886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu J., Wu D. C., Wang Y. T. (2010) Allosteric potentiation of glycine receptor chloride currents by glutamate. Nat. Neurosci. 13, 1225–1232 [DOI] [PubMed] [Google Scholar]
- 46. Haeger S., Kuzmin D., Detro-Dassen S., Lang N., Kilb M., Tsetlin V., Betz H., Laube B., Schmalzing G. (2010) An intramembrane aromatic network determines pentameric assembly of Cys loop receptors. Nat. Struct. Mol. Biol. 17, 90–98 [DOI] [PubMed] [Google Scholar]
- 47. Lynch J. W., Han N. L., Haddrill J., Pierce K. D., Schofield P. R. (2001) The surface accessibility of the glycine receptor M2-M3 loop is increased in the channel open state. J. Neurosci. 21, 2589–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu M., Akabas M. H. (1996) Identification of channel-lining residues in the M2 membrane-spanning segment of the GABAA receptor α1 subunit. J. Gen. Physiol. 107, 195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bocquet N., Nury H., Baaden M., Le Poupon C., Changeux J. P., Delarue M., Corringer P. J. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114 [DOI] [PubMed] [Google Scholar]
- 50. Lewis T. M., Sivilotti L. G., Colquhoun D., Gardiner R. M., Schoepfer R., Rees M. (1998) Properties of human glycine receptors containing the hyperekplexia mutation α1(K276E), expressed in Xenopus oocytes. J. Physiol. 507, 25–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pless S. A., Leung A. W., Galpin J. D., Ahern C. A. (2011) Contributions of conserved residues at the gating interface of glycine receptors. J. Biol. Chem. 286, 35129–35136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Keramidas A., Moorhouse A. J., Pierce K. D., Schofield P. R., Barry P. H. (2002) Cation-selective mutations in the M2 domain of the inhibitory glycine receptor channel reveal determinants of ion-charge selectivity. J. Gen. Physiol. 119, 393–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bormann J., Hamill O. P., Sakmann B. (1987) Mechanism of anion permeation through channels gated by glycine and γ-aminobutyric acid in mouse cultured spinal neurons. J. Physiol. 385, 243–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Langosch D., Laube B., Rundström N., Schmieden V., Bormann J., Betz H. (1994) Decreased agonist affinity and chloride conductance of mutant glycine receptors associated with human hereditary hyperekplexia. EMBO J. 13, 4223–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gunthorpe M. J., Lummis S. C. (2001) Conversion of the ion selectivity of the 5-HT3A receptor from cationic to anionic reveals a conserved feature of the ligand-gated ion channel superfamily. J. Biol. Chem. 276, 10977–10983 [PubMed] [Google Scholar]
- 56. Lee D. J., Keramidas A., Moorhouse A. J., Schofield P. R., Barry P. H. (2003) The contribution of proline 250 (P-2′) to pore diameter and ion selectivity in the human glycine receptor channel. Neurosci. Lett. 351, 196–200 [DOI] [PubMed] [Google Scholar]
- 57. Pribilla I., Takagi T., Langosch D., Bormann J., Betz H. (1992) The atypical M2 segment of the β subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 11, 4305–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dibas M. I., Gonzales E. B., Das P., Bell-Horner C. L., Dillon G. H. (2002) Identification of a novel residue within the second transmembrane domain that confers use-facilitated block by picrotoxin in glycine α1 receptors. J. Biol. Chem. 277, 9112–9117 [DOI] [PubMed] [Google Scholar]
- 59. Lynch J. W., Rajendra S., Barry P. H., Schofield P. R. (1995) Mutations affecting the glycine receptor agonist transduction mechanism convert the competitive antagonist picrotoxin into an allosteric potentiator. J. Biol. Chem. 270, 13799–13806 [DOI] [PubMed] [Google Scholar]
- 60. Yang Z., Cromer B. A., Harvey R. J., Parker M. W., Lynch J. W. (2007) A proposed structural basis for picrotoxinin and picrotin binding in the glycine receptor pore. J. Neurochem. 103, 580–589 [DOI] [PubMed] [Google Scholar]
- 61. Lu H., Xu T. L. (2002) The general anesthetic pentobarbital slows desensitization and deactivation of the glycine receptor in the rat spinal dorsal horn neurons. J. Biol. Chem. 277, 41369–41378 [DOI] [PubMed] [Google Scholar]
- 62. Jenkins A., Greenblatt E. P., Faulkner H. J., Bertaccini E., Light A., Lin A., Andreasen A., Viner A., Trudell J. R., Harrison N. L. (2001) Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J. Neurosci. 21, RC136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nury H., Van Renterghem C., Weng Y., Tran A., Baaden M., Dufresne V., Changeux J. P., Sonner J. M., Delarue M., Corringer P. J. (2011) X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469, 428–431 [DOI] [PubMed] [Google Scholar]
- 64. Li G. D., Chiara D. C., Sawyer G. W., Husain S. S., Olsen R. W., Cohen J. B. (2006) Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J. Neurosci. 26, 11599–11605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lynagh T., Webb T. I., Dixon C. L., Cromer B. A., Lynch J. W. (2011) Molecular determinants of ivermectin sensitivity at the glycine receptor chloride channel. J. Biol. Chem. 286, 43913–43924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ryan S. G., Buckwalter M. S., Lynch J. W., Handford C. A., Segura L., Shiang R., Wasmuth J. J., Camper S. A., Schofield P., O'Connell P. (1994) A missense mutation in the gene encoding the α1 subunit of the inhibitory glycine receptor in the spasmodic mouse. Nat. Genet. 7, 131–135 [DOI] [PubMed] [Google Scholar]
- 67. Saul B., Schmieden V., Kling C., Mülhardt C., Gass P., Kuhse J., Becker C. M. (1994) Point mutation of glycine receptor α1 subunit in the spasmodic mouse affects agonist responses. FEBS Lett. 350, 71–76 [DOI] [PubMed] [Google Scholar]
- 68. Buckwalter M. S., Cook S. A., Davisson M. T., White W. F., Camper S. A. (1994) A frameshift mutation in the mouse α1 glycine receptor gene (Glra1) results in progressive neurological symptoms and juvenile death. Hum. Mol. Genet. 3, 2025–2030 [DOI] [PubMed] [Google Scholar]
- 69. Villmann C., Oertel J., Ma-Högemeier Z. L., Hollmann M., Sprengel R., Becker K., Breitinger H. G., Becker C. M. (2009) Functional complementation of Glra1spd-ot, a glycine receptor subunit mutant, by independently expressed C-terminal domains. J. Neurosci. 29, 2440–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Singer J. H., Talley E. M., Bayliss D. A., Berger A. J. (1998) Development of glycinergic synaptic transmission to rat brainstem motoneurons. J. Neurophysiol. 80, 2608–2620 [DOI] [PubMed] [Google Scholar]
- 71. Wässle H., Heinze L., Ivanova E., Majumdar S., Weiss J., Harvey R. J., Haverkamp S. (2009) Glycinergic transmission in the mammalian retina. Front. Mol. Neurosci. 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weltzien F., Puller C., O'Sullivan G. A., Paarmann I., Betz H. (2012) Distribution of the glycine receptor β subunit in the mouse CNS as revealed by a novel monoclonal antibody. J. Comp. Neurol. 520, 3962–3981 [DOI] [PubMed] [Google Scholar]
- 73. Hoch W., Betz H., Becker C. M. (1989) Primary cultures of mouse spinal cord express the neonatal isoform of the inhibitory glycine receptor. Neuron 3, 339–348 [DOI] [PubMed] [Google Scholar]
- 74. Weiss J., O'Sullivan G. A., Heinze L., Chen H. X., Betz H., Wässle H. (2008) Glycinergic input of small-field amacrine cells in the retinas of wild-type and glycine receptor-deficient mice. Mol. Cell. Neurosci. 37, 40–55 [DOI] [PubMed] [Google Scholar]
- 75. Young-Pearse T. L., Ivic L., Kriegstein A. R., Cepko C. L. (2006) Characterization of mice with targeted deletion of glycine receptor α2. Mol. Cell. Biol. 26, 5728–5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nobles R. D., Zhang C., Müller U., Betz H., McCall M. A. (2012) Selective glycine receptor α2 subunit control of crossover inhibition between the on and off retinal pathways. J. Neurosci. 32, 3321–3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kallenborn-Gerhardt W., Lu R., Lorenz J., Gao W., Weiland J., Del Turco D., Deller T., Laube B., Betz H., Geisslinger G., Schmidtko A. (2012) Prolonged zymosan-induced inflammatory pain hypersensitivity in mice lacking glycine receptor α2. Behav. Brain Res. 226, 106–111 [DOI] [PubMed] [Google Scholar]
- 78. Malosio M. L., Marquèze-Pouey B., Kuhse J., Betz H. (1991) Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 10, 2401–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Harvey R. J., Depner U. B., Wässle H., Ahmadi S., Heindl C., Reinold H., Smart T. G., Harvey K., Schütz B., Abo-Salem O. M., Zimmer A., Poisbeau P., Welzl H., Wolfer D. P., Betz H., Zeilhofer H. U., Müller U. (2004) GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887 [DOI] [PubMed] [Google Scholar]
- 80. Xiong W., Cui T., Cheng K., Yang F., Chen S. R., Willenbring D., Guan Y., Pan H. L., Ren K., Xu Y., Zhang L. (2012) Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J. Exp. Med. 209, 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dlugaiczyk J., Singer W., Schick B., Iro H., Becker K., Becker C. M., Zimmermann U., Rohbock K., Knipper M. (2008) Expression of glycine receptors and gephyrin in the rat cochlea. Histochem. Cell Biol. 129, 513–523 [DOI] [PubMed] [Google Scholar]
- 82. Harvey R. J., Schmieden V., Von Holst A., Laube B., Rohrer H., Betz H. (2000) Glycine receptors containing the α4 subunit in the embryonic sympathetic nervous system, spinal cord and male genital ridge. Eur. J. Neurosci. 12, 994–1001 [DOI] [PubMed] [Google Scholar]
- 83. White W. F., Heller A. H. (1982) Glycine receptor alteration in the mutant mouse spastic. Nature 298, 655–657 [DOI] [PubMed] [Google Scholar]
- 84. Kingsmore S. F., Giros B., Suh D., Bieniarz M., Caron M. G., Seldin M. F. (1994) Glycine receptor β subunit gene mutation in spastic mouse associated with LINE-1 element insertion. Nat. Genet. 7, 136–141 [DOI] [PubMed] [Google Scholar]
- 85. Mülhardt C., Fischer M., Gass P., Simon-Chazottes D., Guénet J. L., Kuhse J., Betz H., Becker C. M. (1994) The spastic mouse: aberrant splicing of glycine receptor β subunit mRNA caused by intronic insertion of L1 element. Neuron 13, 1003–1015 [DOI] [PubMed] [Google Scholar]
- 86. Becker K., Braune M., Benderska N., Buratti E., Baralle F., Villmann C., Stamm S., Eulenburg V., Becker C. M. (2012) A retroelement modifies pre-mRNA splicing. The murine Glrbspa allele is a splicing signal polymorphism amplified by long interspersed nuclear element insertion. J. Biol. Chem. 287, 31185–31194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Reichling D. B., Kyrozis A., Wang J., MacDermott A. B. (1994) Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J. Physiol. 476, 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kirsch J., Betz H. (1998) Glycine receptor activation is required for receptor clustering in spinal neurons. Nature 392, 717–720 [DOI] [PubMed] [Google Scholar]
- 89. Turecek R., Trussell L. O. (2002) Reciprocal developmental regulation of presynaptic ionotropic receptors. Proc. Natl. Acad. Sci. U.S.A. 99, 13884–13889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kubota H., Alle H., Betz H., Geiger J. R. (2010) Presynaptic glycine receptors on hippocampal mossy fibers. Biochem. Biophys. Res. Commun. 393, 587–591 [DOI] [PubMed] [Google Scholar]
- 91. Rivera C., Voipio J., Payne J. A., Ruusuvuori E., Lahtinen H., Lamsa K., Pirvola U., Saarma M., Kaila K. (1999) The K+/Cl− cotransporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255 [DOI] [PubMed] [Google Scholar]
- 92. Becker C. M., Hoch W., Betz H. (1988) Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 7, 3717–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Takahashi T., Momiyama A., Hirai K., Hishinuma F., Akagi H. (1992) Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron 9, 1155–1161 [DOI] [PubMed] [Google Scholar]
- 94. Bormann J., Rundström N., Betz H., Langosch D. (1993) Residues within transmembrane segment M2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. EMBO J. 12, 3729–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shiang R., Ryan S. G., Zhu Y. Z., Hahn A. F., O'Connell P., Wasmuth J. J. (1993) Mutations in the α1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder hyperekplexia. Nat. Genet. 5, 351–358 [DOI] [PubMed] [Google Scholar]
- 96. Rajendra S., Lynch J. W., Pierce K. D., French C. R., Barry P. H., Schofield P. R. (1994) Startle disease mutations reduce the agonist sensitivity of the human inhibitory glycine receptor. J. Biol. Chem. 269, 18739–18742 [PubMed] [Google Scholar]
- 97. Davies J. S., Chung S. K., Thomas R. H., Robinson A., Hammond C. L., Mullins J. G., Carta E., Pearce B. R., Harvey K., Harvey R. J., Rees M. I. (2010) The glycinergic system in human startle disease: a genetic screening approach. Front. Mol. Neurosci. 3, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chung S. K., Vanbellinghen J. F., Mullins J. G., Robinson A., Hantke J., Hammond C. L., Gilbert D. F., Freilinger M., Ryan M., Kruer M. C., Masri A., Gurses C., Ferrie C., Harvey K., Shiang R., Christodoulou J., Andermann F., Andermann E., Thomas R. H., Harvey R. J., Lynch J. W., Rees M. I. (2010) Pathophysiological mechanisms of dominant and recessive GLRA1 mutations in hyperekplexia. J. Neurosci. 30, 9612–9620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Al-Owain M., Colak D., Al-Bakheet A., Al-Hashmi N., Shuaib T., Al-Hemidan A., Aldhalaan H., Rahbeeni Z., Al-Sayed M., Al-Younes B., Ozand P. T., Kaya N. (2012) Novel mutation in GLRB in a large family with hereditary hyperekplexia. Clin. Genet. 81, 479–484 [DOI] [PubMed] [Google Scholar]
- 100. Hutchinson M., Waters P., McHugh J., Gorman G., O'Riordan S., Connolly S., Hager H., Yu P., Becker C. M., Vincent A. (2008) Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology 71, 1291–1292 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.