Background: Cholesterol modulates inwardly rectifying potassium (Kir) channels.
Results: A two-way molecular cytosolic switch controls channel modulation by cholesterol and PI(4,5)P2.
Conclusion: Cholesterol and PI(4,5)P2 induce a common gating pathway of Kir2.1 despite their opposite impact on channel function.
Significance: These findings provide insights into structure-function relationship of ion channels and contribute to understanding of the mechanisms underlying their regulation by lipids.
Keywords: Cholesterol, Ion Channels, Lipids, Membrane Lipids, Potassium Channels, Kir Channels
Abstract
Inwardly rectifying potassium (Kir) channels play an important role in setting the resting membrane potential and modulating membrane excitability. An emerging feature of several Kir channels is that they are regulated by cholesterol. However, the mechanism by which cholesterol affects channel function is unclear. Here we show that mutations of two distant Kir2.1 cytosolic residues, Leu-222 and Asn-251, form a two-way molecular switch that controls channel modulation by cholesterol and affects critical hydrogen bonding. Notably, these two residues are linked by a residue chain that continues from Asn-251 to connect adjacent subunits. Furthermore, our data indicate that the same switch also regulates the sensitivity of the channels to phosphatidylinositol 4,5-bisphosphate, a phosphoinositide that is required for activation of Kir channels. Thus, although cholesterol and phosphatidylinositol 4,5-bisphosphate do not interact with the same region of Kir2.1, these different modulators induce a common gating pathway of the channel.
Introduction
In recent years, cholesterol has been emerging as a major regulator of ion channel function. Multiple studies have shown that an increase in membrane cholesterol regulates the function of a variety of ion channels (1, 2), including different types of K+ channels (3–8), Ca2+ channels (9–13), Na+ channels (14, 15), and Cl− channels (16, 17). The most common effect of cholesterol on ion channels is a decrease in channel activity that may include a decrease in the open probability, in the unitary conductance, and/or in the number of active channels in the membrane. However, the molecular mechanism by which cholesterol affects channel function is not clear.
Here we focus on Kir23 channels, a subfamily of constitutively active, strongly inwardly rectifying K+ channels that set the resting membrane potential and modulate membrane excitability. Kir2.1 is a component of the inward rectifier current IK1 that provides substantial repolarizing current during the terminal repolarization phase of the cardiac action potential and is the primary conductance controlling the diastolic membrane potential (18, 19). In addition, Kir2 channels are critically involved in the regulation of the excitability and contraction of smooth muscle cells (20) and in maintaining membrane potential under resting conditions in endothelial cells (21, 22). Kir2 channels were also suggested to be one of the primary flow sensors (22).
We have shown previously that the function of Kir2 channels is suppressed by the elevation of membrane cholesterol and enhanced by cholesterol depletion (8, 23, 24). Furthermore, cholesterol levels had no effect on the unitary conductance and only a little effect on the open probability of the channels (8). We thus hypothesized that an increase in membrane cholesterol induces a conformational change of the channel protein that leads to a “silent” (inactive) state of the channel, stabilizing the closed state of the channel. Silent channels are retained on the plasma membrane but cannot be detected by single channel analysis, suggesting that cholesterol affects the number of active channels rather than the open probability. Furthermore, multiple lines of evidence suggest that cholesterol regulates Kir channels by direct sterol-protein interactions. Specifically, substitution experiments of cholesterol by its optical isomer epi-cholesterol (25) resulted in a profound increase in endothelial Kir current, suggesting that specific cholesterol-protein interactions are important in the regulation of Kir2 channels. Furthermore, incorporation into liposomes of the purified bacterial analog of Kir channels, KirBac1.1, showed that cholesterol per se is sufficient to suppress channel function (26). In addition, direct evidence suggested that cholesterol binds to KirBac1.1 and that cholesterol binding is essential for its regulatory effect (27). Similarly, using purified eukaryotic Kir2.1 channels reconstituted into liposomes, it was recently demonstrated that Kir2.1 is also suppressed by cholesterol in this pure protein-lipid environment but not by its enantiomer, ent-cholesterol (28).
Recently, we have identified a series of residues that are crucial for the sensitivity of Kir2 channels to cholesterol (23, 24, 29). Unexpectedly, these residues were located in the C and N termini of the channel, suggesting a critical role for the cytosolic domain in cholesterol modulation of Kir channels. Specifically, we first showed that cholesterol sensitivity of Kir2 channels critically depends on specific residues in the pore-facing CD loop (23, 24). We then identified an additional series of residues that together with the CD loop residues form a structured belt that surrounds the cytosolic pore of the channel close to its interface with the transmembrane domain and modulate the cholesterol sensitivity of Kir channels (29). Our analysis implicated the cholesterol sensitivity belt residues in channel gating, correlating each of these residues with residues located in the apex of the G loop, which is regarded as the major cytosolic gate of the channel (30).
In this study, we used a combination of computational and experimental approaches to elucidate further the mechanism responsible for cholesterol sensitivity of Kir2 channels. Surprisingly, we identified a switch that controls cholesterol sensitivity of Kir2.1 and that is comprised of two distant cytosolic residues, Leu-222 of the cholesterol sensitivity belt and Asn-251. Whereas Leu-222 is located in the CD loop close to the interface of the C terminus with the transmembrane domain, Asn-251 is located further away from the transmembrane domain in the EF loop ∼24 Å from Leu-222. Moreover, we show that the same switch also regulates the sensitivity of the channels to PI(4,5)P2, another major regulator of channel function, which is required for activation of Kir channels (31–34).
EXPERIMENTAL PROCEDURES
Expression of Recombinant Channels in Xenopus Oocytes
Point mutations were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). cRNAs were transcribed in vitro using the mMESSAGE mMACHINE kit (Ambion, Austin, TX). Oocytes were isolated and microinjected as described previously (35). Expression of channel proteins in Xenopus oocytes was accomplished by injection of the desired amount of cRNA. Oocytes were injected with 0.5 ng of cRNA of the channel. All oocytes were maintained at 17 °C. Two-electrode voltage clamp recordings were performed 1 day following injection.
Cells and Cell Transfection
HEK293 cells were grown as described previously (23) in minimum essential medium containing GlutaMAX, 10% fetal bovine serum, 1% minimum essential medium non-essential amino acids, 50 units ml−1 penicillin, and 50 units ml−1 streptomycin in a 5% CO2 humidified atmosphere at 37 °C. All media and reagents were from Invitrogen. Point mutations of HA-Kir2.1 were generated using the QuikChange site-directed mutagenesis kit (Stratagene). HA-Kir2.1 WT or its single point mutants were transiently co-transfected with enhanced GFP (cmv-pcDNA3.1-GFP-TOPO, Invitrogen) using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's protocol. Experiments were conducted 2–3 days after transfection.
Cholesterol Enrichment of Xenopus Oocytes
Treatment of Xenopus oocytes with a mixture of cholesterol and lipids has been shown to increase the cholesterol/phospholipid molar ratio of the plasma membrane of the oocytes (36). Thus, to enrich the oocytes with cholesterol we used a 1:1:1 (w/w/w) mixture containing cholesterol, porcine brain l-α-phosphatidylethanolamine, and 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine (Avanti Polar Lipids, Birmingham, AL). The mixture was evaporated to dryness under a stream of nitrogen. The resultant pellet was suspended in a buffered solution consisting of 150 mm KCl and 10 mm Tris/HEPES at pH 7.4 and sonicated at 80 kHz in a bath sonicator (Laboratory Supplies, Hicksville, NY). Xenopus oocytes were treated with cholesterol for 1 h.
Cellular Cholesterol Enrichment
HEK293 cells transfected with Kir2.1 were enriched with cholesterol by treatment with methyl-β-cyclodextrin (MβCD) saturated with cholesterol, a well known cholesterol donor as described previously (37). 5 mm MβCD solution in DMEM without serum mixed with saturated cholesterol was sonicated and shaken overnight in a 37 °C incubator. HEK293 cells were incubated with the MβCD solution with or without cholesterol for 1 h to enhance or reduce the cellular cholesterol level. The effect of this approach on the cholesterol levels in HEK293 cells was confirmed by using an Amplex Red cholesterol assay kit (Molecular Probes) to measure the cellular cholesterol in the cells according to the manufacturer's specifications.
Two-electrode Voltage Clamp Recording and Analysis in Xenopus Oocytes
Whole-cell currents were measured by conventional two-microelectrode voltage clamp with a GeneClamp 500 amplifier (Axon Instruments, Union City, CA) as reported previously (35). A high potassium solution was used to superfuse oocytes (96 mm KCl, 1 mm NaCl, 1 mm MgCl2, and 5 mm KOH/HEPES, pH 7.4). Basal currents represent the difference of inward currents obtained (at −80 mV) in the presence of 3 mm BaCl2 in high potassium solution from those in the absence of Ba2+. A minimum of two batches of oocytes was tested for each normalized recording shown. Recordings from different batches of oocytes were normalized to the mean of whole-cell basal currents obtained from control untreated oocytes. The mean of each batch of control untreated oocytes was normalized to 1. Statistics (i.e. mean and S.E.) of each construct were calculated from all of the normalized data recorded from different batches of oocytes.
Macropatch Recording and Analysis in Xenopus Oocytes
Macropatch channel activity was recorded on oocytes under the inside-out mode of standard patch clamp methods (38) as described previously (39). The bath and pipette solutions of ND96K+EGTA were composed of 96 mm KCl, 1 mm MgCl2, 5 mm EGTA, and 10 mm HEPES, pH 7.4. Currents were recorded at a holding membrane potential of −80 mV. Recordings were made using the Axon 200A patch clamp amplifier. Data were sampled at 5 kHz, filtered at 2 kHz, and stored on a PC-compatible computer. Analysis was carried out using Clampfit 9 (Axon Instruments). DiC8 PI(4,5)P2 phosphoinositides (Avanti Lipids) were dissolved in water, and aliquots of the stock solution were kept at −80 °C. All dilutions were prepared on the day of the experiment and applied to excised patches.
Single Channel Recording and Analysis in Xenopus Oocytes
Single channel currents were recorded from oocytes under a standard cell-attached configuration. The pipette solution contained 96 mm K-Mes, 10 mm HEPES, and 2 mm MgCl2 with pH 7.4 adjusted with KOH. The composition of bath solutions was 96 mm K-Mes, 10 mm HEPES, and 5 mm EGTA with pH 7.4 adjusted with KOH. Single channel conductance values were determined by the slope of current-voltage curves where current-voltage data could be well fitted to a linear line. The sampling interval was 200 μs, and the low pass filter frequency was 2 KHz.
Electrophysiology Studies in HEK Cells
Whole-cell Kir2.1 currents in HEK293T cells were recorded using the patch clamp technique. Briefly, the external solution contained 150 mm NaCl, 6 mm KCl, 1.0 mm MgCl2, 1.5 mm CaCl2, 10 mm HEPES, and 1.0 mm EGTA at pH 7.3 (pH adjusted with NaOH). The pipette solution contained 145 mm KCl, 1.0 mm MgCl2, 10 mm HEPES, 1.0 mm EGTA, and 4 mm ATP at pH 7.3 (pH adjusted with KOH). Borosilicate glass electrodes were used with resistances in the range of 2–6 megaohms when filled and connected to a patch clamp amplifier (HEKA Electronik, Lambrecht, Germany). Currents were monitored by 500-ms linear voltage ramps from −100 to +60 mV at an interpulse interval of 3 s. The time course of Kir2.1 currents was recorded at a holding potential of −60 mV. Only cells exhibiting fluorescence were used for recordings. All the recordings were carried out at room temperature (22–25 °C). A minimum of three independent experiments was carried out for each recording shown. Recordings of cholesterol-treated cells from each day were normalized to the mean of the currents obtained from control untreated cells from the same day. The mean of each group of control untreated cells was normalized to 1. Statistics (i.e. mean and S.E.) of each construct were calculated from all of the normalized data recorded from the different experiments.
Immunostaining
Cells were transfected with HA-Kir2.1 construct or mutants as described above. For immunostaining, HA-Kir2.1-transfected cells were seeded on glass coverslips, fixed with 4% para-formaldehyde, and blocked in a solution containing 1% BSA in Dulbecco's phosphate-buffered saline containing calcium and magnesium (DPBS; Cellgro) for 1 h, then incubated with primary antibodies (1:600 in DPBS solution containing 1% BSA) overnight at 4 °C, washed, incubated with secondary antibodies (1:200 dilution in DPBS solution containing 1% BSA for 2 h), and washed with DPBS. The primary antibody used for Kir2.1-HA was mouse monoclonal anti-HA antibody (HA 1.1, Covance) that recognizes HA-Kir2 constructs, and the secondary antibody used was Alexa Fluor 555 red anti-mouse (Molecular Probes). The samples were mounted and viewed using a Zeiss AxioVert 200M microscope equipped with an LD Plan-Neofluar 40× objective controlled by AxioVision Release 4.7 software.
Molecular Dynamics Simulations
The model of the channel used (KDB database ID H011)4 (41) was based on the chimera between the cytosolic domain of Kir3.1 and the transmembrane domain of KirBac1.3 (Protein Data Bank code 2QKS; resolution 2.2 Å) (42). Comparison between the crystal structures (30) of the cytosolic domains of Kir2.1 (Protein Data Bank code 1U4F; 2.41-Å resolution) and Kir3.1 (Protein Data Bank code 1U4E; 2.09-Å resolution) shows that the structural similarity between the cytosolic domains of the two eukaryotic inwardly rectifying potassium channels is high with a root mean square deviation of only 1.1Å between the backbone Cα atoms. The simulation system was constructed using the membrane insertion protocol developed within the CHARMM-GUI project (43). The simulation box contains the channel and a soluble cytosolic domain, bound K+ ions in sites S2 and S4 of the selectivity filter, and 161 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine lipid molecules solvated in an explicit 150 mm KCl aqueous solution represented with TIP3 water model (44) and optimized CHARMM-27 ion parameters (45). All computations were carried out by NAMD version 2.7b1 (46), and analysis was done with CHARMM version c36b2 with the CHARMM-27 force fields for proteins and lipids (47). The protein structure was minimized in cycles with a gradual decrease of harmonic restraints on heavy atoms. Each minimization cycle consists of 500 steps of the steepest descent and 500 steps of the adopted basis Newton-Raphson algorithms. The minimized structures were embedded next into a lipid membrane using a multistep membrane building procedure used in previous studies (43). The molecular dynamics simulation methods used here are similar to those used in previous studies of membrane systems utilizing the NPAT ensemble. Briefly, constant temperature/constant pressure algorithms were applied (with pressure at 1 atm and temperature at 303 K). Electrostatic interactions were treated with the particle mesh Ewald algorithm with a 92/92/144-Å grid for fast Fourier transform. The temperature and pressure in the system were maintained with a combination of a Langevin thermostat acting on heavy atoms of the lipid bilayer with a Langevin dumping parameter of 5 ps−1 and a Nosé-Hoover Langevin piston method. The piston oscillation period was set to 100 fs with a dumping time of 50 fs. The non-bonded interactions were smoothly switched off at 10–13.5 Å. All simulation systems were equilibrated for 1 ns each without any constraints, and the production was run for another 10 ns. Figures of models were made using the PyMOL Molecular Graphics System, Version 1.1 (Schrödinger, LLC). Analysis was carried out using the Visual Molecular Dynamics package (VMD) (48) and the data analysis and graphing software Origin (OriginLab).
Hydrogen Bonding
The cutoffs for hydrogen bonding used are as follows: the hydrogen-acceptor distance is ≤2.5 Å, and the donor hydrogen-acceptor angle is above 90° (49). The total percentage of hydrogen bonding is the percentage of structures that form any hydrogen bond (one or more) between the relevant residues. Minimal hydrogen-bonding distances were calculated as described previously (50).
RESULTS
Effect of L222I on Kir2.1: a Computational Analysis
To obtain initial insights into the molecular basis underlying the role of the cholesterol sensitivity belt residues in channel modulation, we carried out extensive molecular dynamics simulations of both the WT Kir2.1 and the L222I mutant. Fig. 1A depicts the absolute changes in the distances between the location of the backbone central carbon (Cα) of position 222 and the Cα atom of each C-terminal residue following the L222I mutation and irrespective of the direction of the change in distance, whether closer or further away from position 222. When examining the directions of the changes in the distances, it was evident that whereas several molecular surfaces in the C terminus exhibited increased distances relative to position 222 the distance between a number of other molecular surfaces and position 222 decreased (Fig. 1B). Notably, with this extensive structural perturbation spanning the majority of the C-terminal residues, intersubunit interactions were also affected by the L222I mutation, explicating our previous data demonstrating that the L222I mutant has a dominant-negative effect on the sensitivity of the WT channel to cholesterol (23).
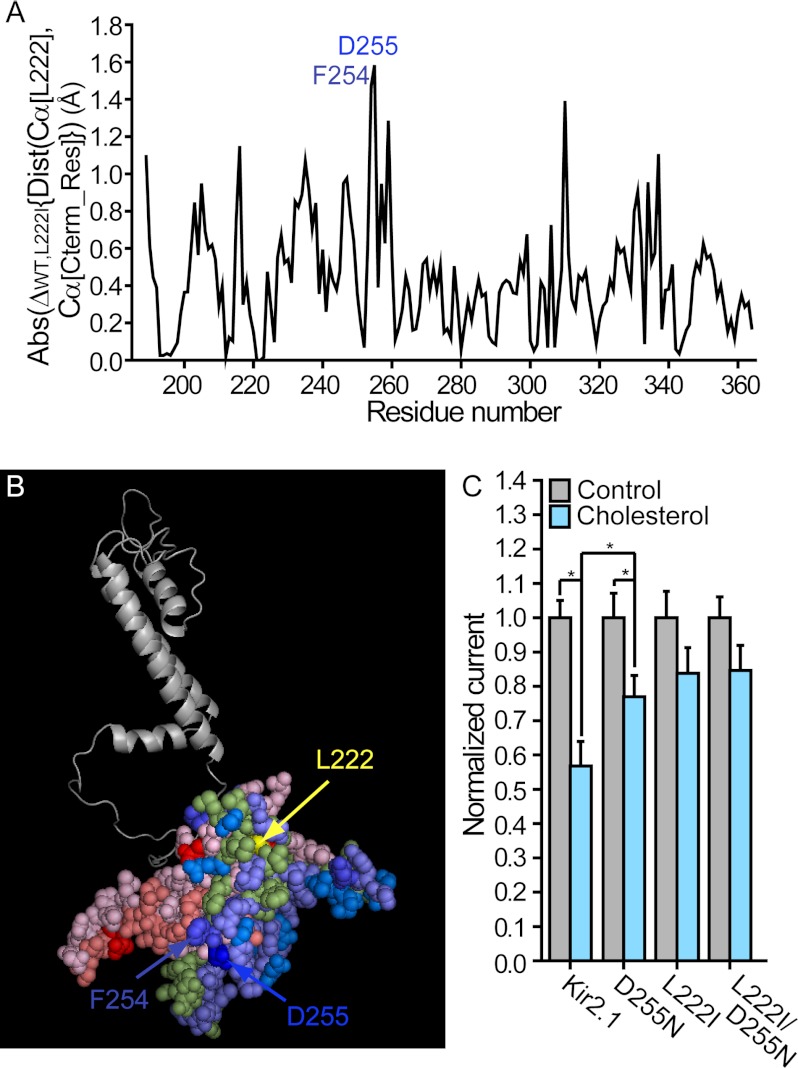
FIGURE 1.
A, absolute (Abs) changes in the average distances (Dist) between the location of the backbone central carbon (Cα) of position 222 and the Cα atom of each C-terminal residue (Cterm_Res) following the L222I mutation in Kir2.1. B, changes in the average distances between the location of the Cα atom of position 222 and the Cα atom of each C-terminal residue following the L222I mutation plotted on a model of one subunit of Kir2.1. The blue, red, and green shades represent an increase, a decrease, and a very limited or no change in these distances, respectively. The darker the shade, the larger the absolute change. Specifically, among the blue shades, blue represents 1.5 Å ≤ Δ, TV-blue (blue slate) represents 1.0 Å ≤ Δ < 1.5 Å, marine represents 0.6 Å ≤ Δ < 1.0 Å, and slate represents 0.2 Å ≤ Δ < 0.6 Å. Among the red shades, red represents Δ ≤ −1.0 Å, deep salmon represents −1.0 Å < Δ ≤ −0.6 Å, and light pink represents −0.6 Å < Δ ≤ −0.2 Å. The green shade represents −0.2 Å < Δ < 0.2 Å. C, whole-cell basal currents recorded in Xenopus oocytes at −80 mV showing the effect of cholesterol enrichment on Kir2.1 and the mutants D255N, L222I, and L222I/D255N (n = 9–35). Significant differences are indicated by asterisks (*, p ≤ 0.05). Error bars represent S.E.
Thus, in view of the large number of changes in intrasubunit Cα-Cα distances between the C-terminal residues and position 222, we focused on the residue that exhibited the maximal change. As evident in Fig. 1A, the largest change in distance relative to position 222 due to the L222I mutation was observed for position 255 followed by position 254. Surprisingly, Asp-255 is located away from the cholesterol sensitivity belt and from the transmembrane domain in the center of the EF loop facing the cytoplasmic pore of the channel (Fig. 1B). Our first question, therefore, was whether Asp-255 was also involved in cholesterol sensitivity of the channel. We thus examined the effect of mutating the aspartate at position 255 to an asparagine. As can be seen in Fig. 1C, the D255N mutation decreased the sensitivity of Kir2.1 to cholesterol, indicating that this region is also involved in conferring cholesterol sensitivity to this channel. We also evaluated whether the D255N mutation altered the effect of the L222I mutation. However, the D255N mutation did not alter the effect of the L222I mutant (Fig. 1C).
Reversal of the Cholesterol Sensitivity of L222I: a Comparison between Kir2.1 and Kir2.2 in Proximity to Asp-255
Kir2.2 has an isoleucine in the position corresponding to position 222 of Kir2.1 but surprisingly exhibits similar sensitivity to cholesterol. We thus hypothesized that the combination/interaction of position 222 with other residues that are distinct between Kir2.1 and Kir2.2 is responsible for the cholesterol sensitivity of Kir2.2 with an isoleucine at a location equivalent to Leu-222 of Kir2.1. We therefore examined whether the sensitivity of the L222I mutant could be restored by mutations of residues proximal to positions 254 and 255, and which differ between Kir2.1 and Kir2.2.
Comparing the sequences of Kir2.1 and Kir2.2 in the vicinity of positions 254 and 255, three differences can be identified between the two channels in positions 251, 256, and 258 (see Fig. 2A). As can be seen in Fig. 2B, the N251D mutation fully restored the sensitivity of the L222I mutant to cholesterol as is apparent from the similar cholesterol sensitivities of the L222I/N251D double mutant and the WT channel. In contrast, mutations of the other two residues, S256K and I258L, did not restore cholesterol sensitivity to L222I mutant as evidenced by the L222I/S256K/I258L triple mutant being cholesterol-insensitive similar to L222I (Fig. 2B).
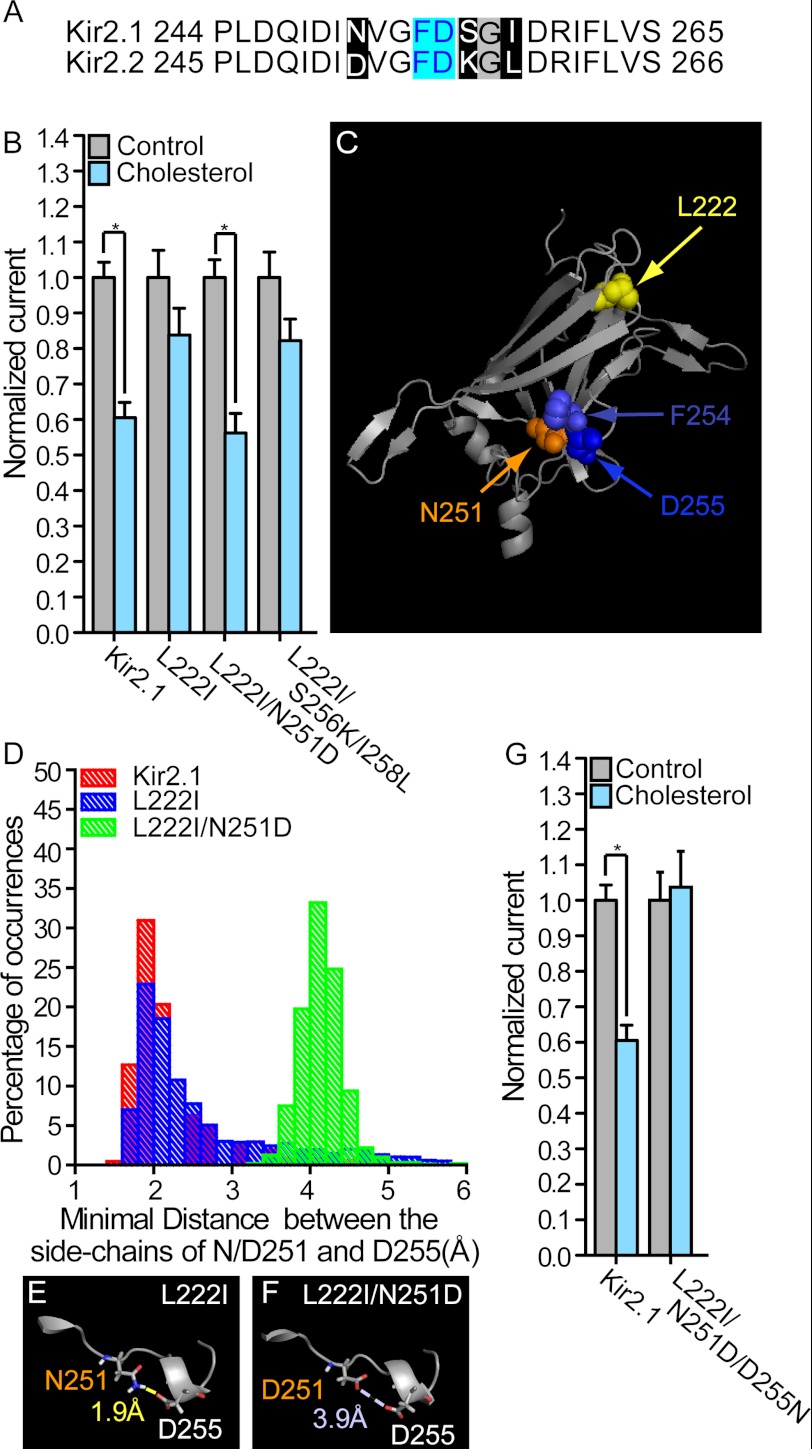
FIGURE 2.
A, sequence alignment between Kir2.1 and Kir2.2 around Phe-254 and Asp-255 of Kir2.1 (10 residues before and after the two residues). Segments (251, 256–258) that included differences between Kir2.1 and Kir2.2 were grouped based on their location relative to F254 and D255 (cyan). Within these segments, residues that were different were highlighted black, and residues that were identical in the two channels were highlighted gray. B, whole-cell basal currents recorded in Xenopus oocytes at −80 mV showing the effect of cholesterol enrichment on Kir2.1 and the mutants L222I, L222I/N251D, and L222I/S256K/I258L (n = 19–43). C, ribbon model of the cytosolic domain of one subunit of Kir2.1 showing the positions of Leu-222, Asn-251, Phe-254, and Asp-255. D, hydrogen bonding between the side chains of Asn-251 and Asp-255 (Hδ21,22 of Asn-251 and Oδ1,2 of Asp-255) in Kir2.1 and its L222I mutant. Also shown is the minimal distance between the side chains of Asp-251 and Asp-255 (Oδ1,2 of Asp-251 and Oδ1,2 of Asp-255) in L222I/N251D. E, representative conformation showing hydrogen bonding between the side chains of Asn-251 and Asp-255 in the L222I mutant. F, representative conformation showing the distance between the side chains of Asp-251 and Asp-255 in the L222I/N251D mutant. G, whole-cell basal currents recorded in Xenopus oocytes at −80 mV showing the effect of cholesterol enrichment on Kir2.1 and the L222I/N251D/D255N triple mutant (n = 11–35). Significant differences in B and G are indicated by asterisks (*, p ≤ 0.05). Error bars represent S.E.
Notably, Asn-251 is adjacent to both Phe-254 and Asp-255 in the three-dimensional structure of the cytosolic domain of the WT Kir2.1 as illustrated in Fig. 2C. Moreover, analysis by molecular dynamics simulation suggested that in the WT Kir2.1 channel there was hydrogen bonding between the amide side-chain group of Asn-251 and the side-chain carboxyl of Asp-255 about 78% of the time with an average minimal distance between the possible interacting atoms (Hδ21 and Hδ22 of Asn-251 and Oδ1 and Oδ2 of Asp-255) of 2.24 ± 0.01 Å. Following the L222I mutation, analysis of the simulation suggests there is only a slight shift in the average minimal distance between Asn-251 and Asp-255 to 2.63 ± 0.02 Å and that hydrogen bonding is preserved between these residues ∼63% of the time. In contrast, the L222I/N251D mutant exhibited a striking increase in the distance between positions 251 and 255 as a result of the electrostatic repulsion introduced by the N251D mutation (see Fig. 2, E and F). This ultimately resulted in the loss of hydrogen bonding between these positions as illustrated in Fig. 2D, which depicts the distribution of the minimal distances between the side chains of positions 251 and 255. Specifically, compared with both the WT and the L222I mutant, the histogram is right-shifted to non-hydrogen-bonding distances for the L222I/N251D double mutant with an average minimal distance of 4.14 ± 0.01 Å.
Thus, based on this analysis and in view of the capability of N251D to restore the cholesterol sensitivity of L222I, we hypothesized that the loss of hydrogen bonding between positions 251 and 255 may be critical for restoration of the cholesterol sensitivity of the L222I mutation. To test this hypothesis and corroborate the role of the interaction between positions 251 and 255 in the reversal of the effect of the L222I mutation on the cholesterol sensitivity of Kir2.1, we mutated Asp-255 in the L222I/N251D double mutant to an asparagine to form the L222I/N251D/D255N triple mutant to restore the hydrogen bonding between positions 251 and 255 that exists in the L222I mutant. Specifically, compared with the L222I mutant that has an asparagine in position 251 and an aspartate in position 255, in this triple mutant, positions 251 and 255 are exchanged, and there is an aspartate in position 251 and an asparagine in position 255. In accord with our hypothesis, the L222I/N251D/D255N triple mutant was not cholesterol-sensitive (Fig. 2G), indicating that the D255N mutation countered the effect of N251D and abrogated the restoring effect of N251D on the cholesterol sensitivity of the L222I mutant.
The Effect of N251D on Kir2.1: Computational Analysis and Implications for Cholesterol Sensitivity
With the effect of the L222I/N251D mutation compared with L222I extending throughout the C terminus to alter the distances between the backbone central carbon atoms of the C-terminal residues and position 222, we next examined the effect of the N251D mutation on the WT Kir2.1 channel. As can be seen in Fig. 3, A and B, also on the background of the WT, the changes in the distances between the Cα atoms of the C-terminal residues and position 222 following the N251D mutation extended throughout the C terminus. Moreover, when comparing the effect of the N251D mutation on the WT with the effect of the L222I mutation, an interesting pattern emerged. Accordingly, the distances between position 222 and the Cα atoms of residues in several key regions for channel gating are affected similarly following both mutations (see Fig. 3A). In addition to residues in the vicinity of Asn-251, these include residues in the proximal C terminus, the G loop, and the CD loop. Based on this similarity between the structural effects of the two mutations, we hypothesized that they would also have a similar modulatory effect on the channel. We thus first tested the effect of the N251D mutation on the cholesterol sensitivity of Kir2.1 using Xenopus oocytes. As Fig. 3C shows, similarly to the L222I mutation, N251D also abrogates cholesterol sensitivity. Thus, because the double mutant L222I/N251D was cholesterol-sensitive, this indicates that the effect of each of the mutations on cholesterol sensitivity is reversed by the second mutation (Fig. 2B).
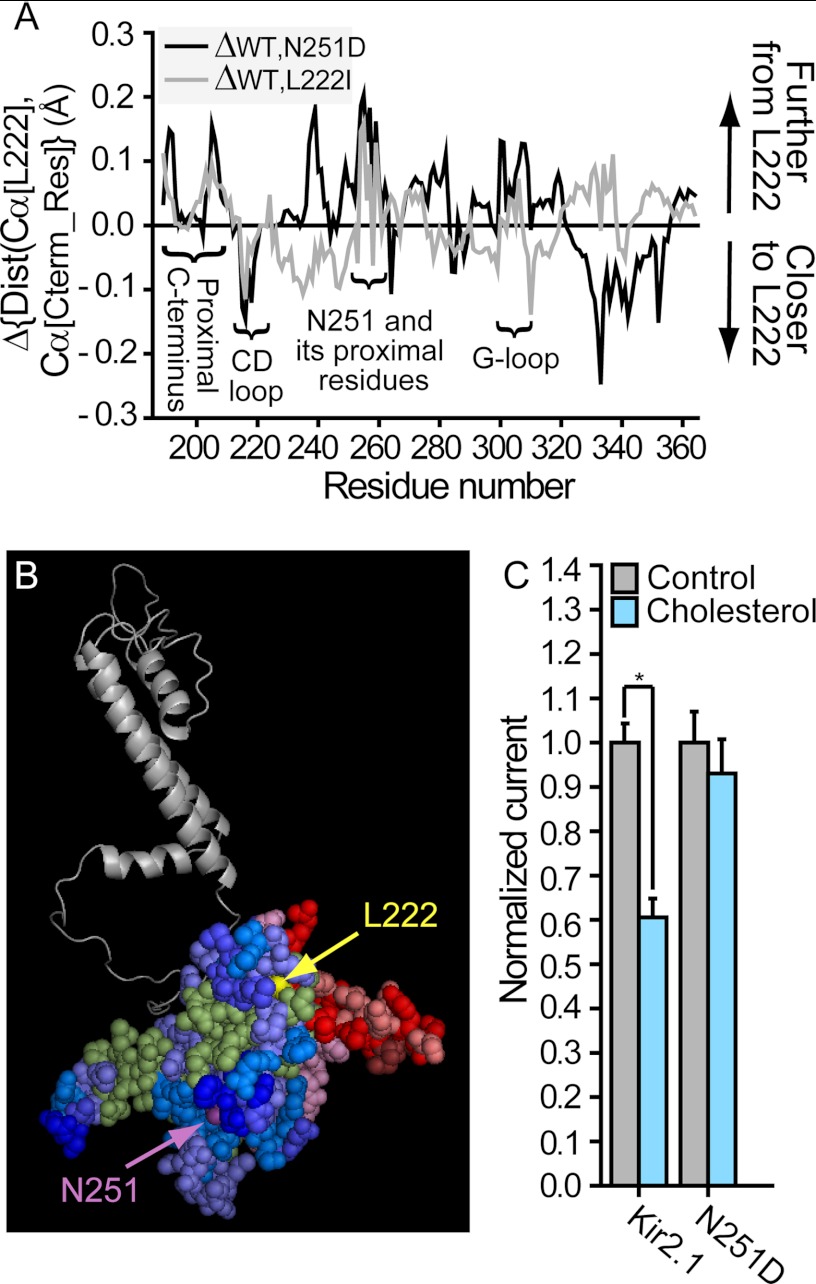
FIGURE 3.
A, changes in the distances (Dist) between the Cα atoms of the C-terminal residues (Cterm_Res) and position 222 following the N251D mutation compared with the WT Kir2.1 (black). Also shown are the changes in the distances between the Cα atoms of the C-terminal residues and position 222 following the L222I mutation compared with the WT Kir2.1 (gray). Critical cytosolic regions for channel gating are labeled. B, changes in the average distances between the location of the Cα atom of position 222 and the Cα atom of each C-terminal residue following the N251D mutation plotted on a model of one subunit of Kir2.1. The blue, red, and green shades represent an increase, a decrease, and a very limited or no change in these distances, respectively, as described in Fig. 1B. C, whole-cell basal currents recorded in Xenopus oocytes at −80 mV showing the effect of cholesterol enrichment on Kir2.1 and the N251D mutant (n = 19–43). A significant difference is indicated by an asterisk (*, p ≤ 0.05). Error bars represent S.E.
To verify further that the L222I/N251D double mutant rescues the loss of cholesterol sensitivity in both L222I and N251D mutants, all the mutants were expressed in HEK293 cells, a mammalian cell line that is devoid of endogenous Kir channels. Cholesterol sensitivity of the channels was tested by exposing the cells to MβCD saturated with cholesterol (MβCD-cholesterol), a method that is well established to enrich cells with cholesterol (37). Indeed, as expected, exposing the cells to MβCD-cholesterol resulted in about 50% increase in the level of membrane cholesterol (supplemental Fig. 1). Consistent with our previous data in other mammalian cells (8, 23, 25), an increase in membrane cholesterol resulted in about 2-fold decrease in Kir2.1 WT current, whereas the L222I mutant was cholesterol-insensitive. Here we show that similarly to our data in oocytes, both L222I and N251D were not suppressed by cholesterol, whereas the double mutant L222I/N251D had the same sensitivity as the WT (Fig. 4).
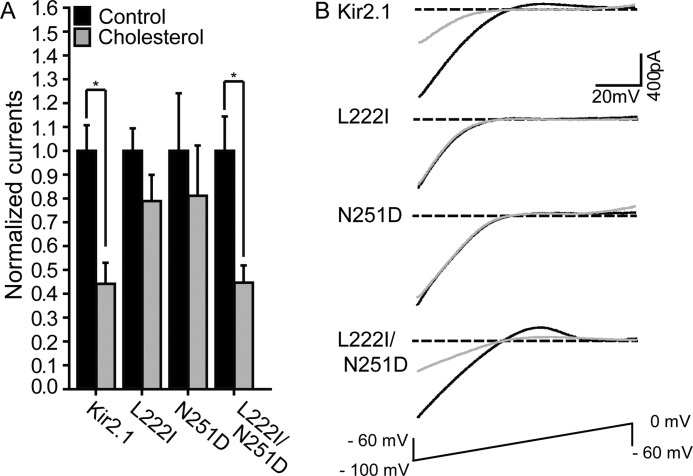
FIGURE 4.
A, mean peak currents for control and cholesterol-enriched HEK293 cell populations co-transfected with enhanced GFP and HA-Kir2.1 or its L222I, N251D, and L222I/N251D mutants (n = 10–53). Significant differences are indicated by asterisks (*, p ≤ 0.05). B, typical traces for Kir2.1 WT, L222I, N251D, and L222I/N251D in control and cholesterol-enriched cell populations. Error bars represent S.E.
Both N251D and L222I Reduce the Strength of Channel-PI(4,5)P2 Interactions Separately but Cancel Each Other's Effect Together
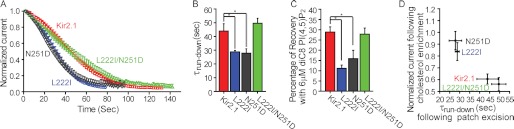
To further corroborate our hypothesis that L222I and N251D affect channel modulation in a similar manner, we next examined the effect of the N251D mutation on channel-PI(4,5)P2 interactions. In contrast to cholesterol that may stabilize the closed state of the channel, PI(4,5)P2 is required for its activation. It has been shown previously (39) that the L222I mutation reduces the strength of the interaction between the channel and PI(4,5)P2. As can be seen in Fig. 5, A and B, similarly to the effect of the L222I mutation, the currents ran down significantly faster for the N251D mutant compared with the WT Kir2.1, indicating that the N251D mutation also reduced the strength of channel-PI(4,5)P2 interactions. Moreover, in accord with the ability of N251D to restore the cholesterol sensitivity of L222I in the L222I/N251D double mutant, N251D also restored the strength of the interaction of the channel with PI(4,5)P2 of L222I to the levels exhibited by the WT channel. To confirm this observation, we applied 6 μm diC8 PI(4,5)P2 to excised patches expressing Kir2.1 and the above mutants after run-down. As can be seen in Fig. 5C, the effect of diC8 PI(4,5)P2 on the WT Kir2.1 channel was significantly higher than its effect on the L222I and N251D mutants, further corroborating that the interaction between these two mutants and PI(4,5)P2 is weaker compared with the WT channel. In contrast, the effect of 6 μm diC8 PI(4,5)P2 on the L222I/N251D double mutant was not significantly different from its effect on Kir2.1, indicating that the strength of its interaction with PI(4,5)P2 is comparable with the WT channel. Notably, there is correspondence between the effect of these single and double mutations on the sensitivity of the channel to cholesterol and on the strength of the interaction between the channel and PI(4,5)P2 (Fig. 5D).
FIGURE 5.
A, normalized current run-down at −80 mV in excised inside-out macropatches from Xenopus oocytes expressing Kir2.1 and its L222I, N251D, and L222I/N251D mutants. B, summary data based on Fig. 5A showing τrun-down for Kir2.1, L222I, N251D, and L222I/N251D. In A and B, significant differences are indicated by asterisks (*, p ≤ 0.05). C, percentage of recovery after run-down following application of 6 μm diC8 PI(4,5)P2 to Xenopus oocytes in standard excised inside-out configuration. D, correspondence between the effect of the L222I, N251D, and L222I/N251D mutations on the sensitivity of the channel to cholesterol and on the strength of the interaction between the channel and PI(4,5)P2. Error bars represent S.E.
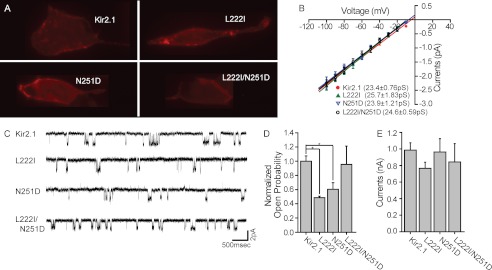
The L222I, N251D, and L222I/N251D Mutations Have No Effect on the Basic Channel Properties of Kir2.1
To characterize further the impact of L222I, N251D, and the double mutant on the basic properties of the channels, we compared the membrane expression, unitary conductance, open probability, and whole-cell currents under basal conditions. To test whether these mutations alter channel expression or their ability to traffic to the plasma membrane, the channels were tagged with an extracellular HA tag that allows selective identification of the channels that are inserted into the plasma membrane as described earlier (8, 51). All the mutants were expressed in HEK cells at comparable levels, suggesting that membrane trafficking is not affected (Fig. 6A). Furthermore, the unitary conductance of the three mutants was also the same as that of the WT Kir2.1 channel (Fig. 6, B and C). On the other hand and in agreement with the decrease in the strength of channel-PI(4,5)P2 interactions in these mutants compared with the WT, the open probability of the single mutants was significantly decreased as compared with the WT channel (Fig. 6, C and D). This observation is in agreement with our earlier data showing that cholesterol effects are not visible in single channel analysis (8). In contrast, whole-cell currents were similar for Kir2.1 WT and the three mutants under basal conditions in HEK293 cells (Fig. 6E). With the mutants decreasing both the sensitivity of the channel to cholesterol and the strength of channel-PI(4,5)P2 interactions, this lack of effect on whole-cell currents is likely to be a manifestation of these two opposite effects combined. Specifically, because cholesterol suppresses channel function, loss of cholesterol sensitivity is expected to increase channel activity. In contrast, PI(4,5)P2 is required to activate the channel, and therefore, a decrease in the strength of the interaction between the channel and PI(4,5)P2 is expected to lower Kir2.1 activity.
FIGURE 6.
A, images of HEK293 cells transfected with HA-Kir2.1 and its L222I, N251D, and L222I/N251D mutants viewed using a Zeiss AxioVert 200M microscope. B, current-voltage curves obtained from single channel recordings of Kir2.1 and its L222I, N251D, and L222I/N251D mutants. The conductance of each construct, which was calculated from the slope of the linear fit of the currents recorded between −100 and −10 mV, is displayed in the figure. C, representative traces of single channel recordings obtained from Kir2.1 and its L222I, N251D, and L222I/N251D mutants recorded at −80 mV under a cell-attached mode. D, open probability of the channel and its L222I, N251D, and L222I/N251D mutants obtained from single channel recordings. Significant differences are indicated by asterisks (*, p ≤ 0.05). E, mean peak normalized currents for HEK293 cell populations co-transfected with enhanced GFP and HA-Kir2.1 or its L222I, N251D, and L222I/N251D mutants (n = 18–68). Error bars represent S.E.
N251D Reverses the Effect of L222I Along a Reversal Residue Chain, Restoring Critical Hydrogen Bonding between the βD and βE Strands
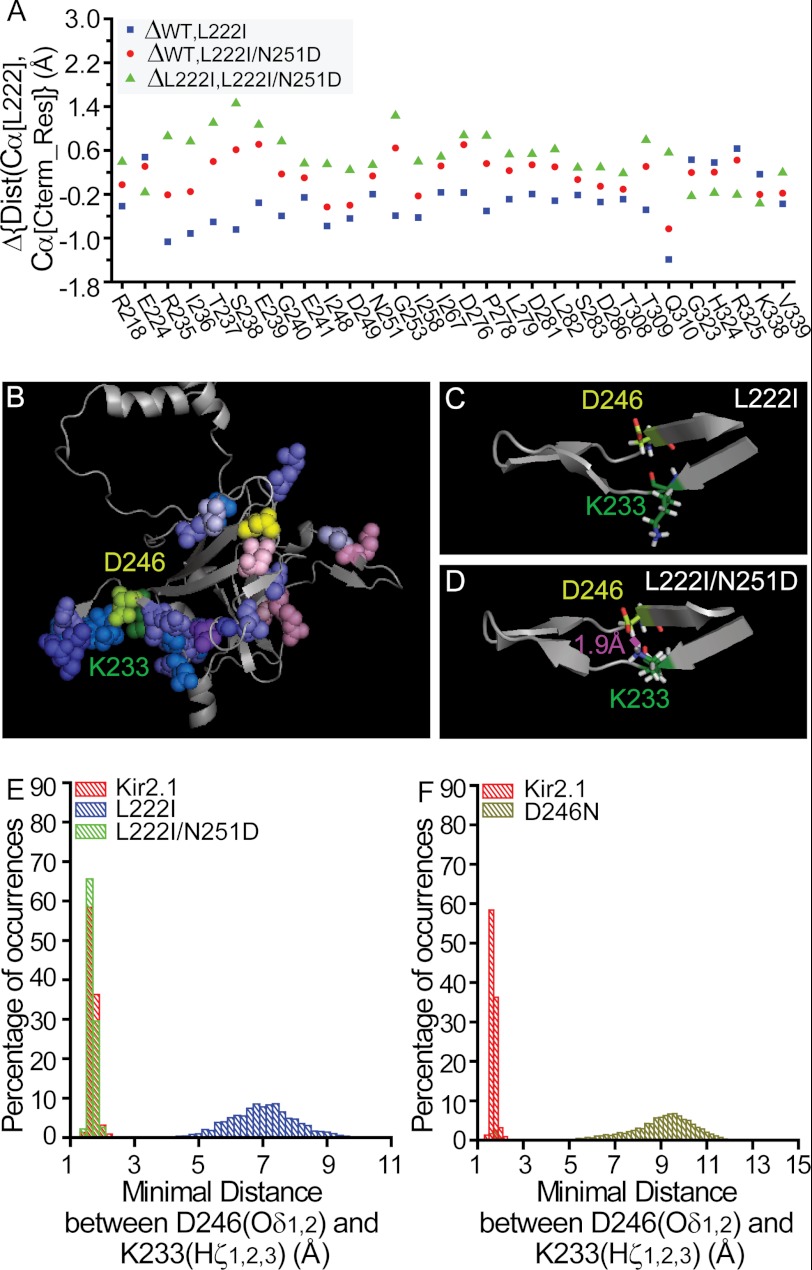
With the N251D mutation restoring cholesterol sensitivity of the channel and reversing the effect of L222I, we next compared its effect on the L222I mutant throughout the C terminus to identify structural features that are reversed by the N251D mutation. Comparison of the molecular dynamics simulations of the L222I mutant and the L222I/N251D double mutant shows that compared with the L222I mutant the effect of the N251D mutation also spans extensive regions of the C terminus. As evident in supplemental Fig. 2A, the N251D mutation results in changes in distances between the Cα atom of position 222 and the Cα atoms of large surfaces throughout the C terminus of the channel. To identify the changes in distances that are reversed by the N251D mutation, we compared the changes in distances in the L222I mutant with respect to the WT with the changes in distances in the L222I/N251D double mutant with respect to the L222I mutant. For the majority of the residues in the C-terminal crystal structure of the channel, the changes in distances are in the same direction relative to position 222 or are affected by only one of the mutations. For the 30 residues in Fig. 7A, however, the direction of the changes in the distances relative to position 222 is reversed. Specifically, for 25 of these residues, the L222I mutation leads to a decrease in the distance between the Cα atoms of position 222 and the Cα atoms of one of these residues (Fig. 7A, blue squares) with respect to the WT (e.g. Gln-310), whereas the L222I/N251D mutation results in an increase in this distance when compared with the L222I mutation (Fig. 7A, green triangles). Conversely, for the remaining five residues (e.g. Arg-325), the L222I mutation leads to an increase in this distance compared with the WT, whereas the L222I/N251D mutation results in a decrease in the distance compared with the L222I mutation.
FIGURE 7.
A, residues for which the direction of the changes in the distances (Dist) relative to position 222 following the L222I mutation is reversed in the L222I/N251D. The effect of the L222I mutation on the distances between the Cα atoms of position 222 and the Cα atoms of these residues with respect to the WT is depicted as blue squares, whereas the effect of the L222I/N251D mutation compared with the L222I mutation is depicted by the green triangles. The effect of the L222I/N251D mutation compared with the WT channel is also shown as red circles. Cterm_Res, C-terminal residues. B, changes in the average distances between the location of the Cα atom of position 222 and the Cα atoms of the 30 C-terminal residues included in Fig. 7A in the L222I/N251D mutant compared with L222I plotted on a model of one subunit of Kir2.1. The blue and red shades represent an increase or a decrease in these distances similar to the scale in Fig. 1B. L222I is shown in yellow, and Asp-251 is shown in purple-blue. Also shown are Lys-233 (forest green) and Asp-246 (lime). C, representative conformation depicting the opposite directions of the side chains of Lys-233 and Asp-246 in the L222I mutant. D, representative conformation showing hydrogen bonding between the side chains of Lys-233 and Asp-246 in the L222I/N251D mutant. E, hydrogen bonding between the side chains of Lys-233 and Asp-246 (Hζ1,2,3 of Lys-233 and Oδ1,2 of Asp-246) in Kir2.1 and its L222I/N251D mutant. Also shown is the minimal distance between the side chains of Lys-233 and Asp-246 in the L222I mutant. F, hydrogen bonding between the side chains of Lys-233 and Asp-246 (Hζ1,2,3 of Lys-233 and Oδ1,2 of Asp-246) in Kir2.1. Also shown is the minimal distance between the possible interacting atoms in the side chains of positions 233 and 246 (Hζ1,2,3 of Lys-233 and Oδ1 of Asn-246) in the D246N mutant.
Notably, these 30 residues form a chain that links the residues in the vicinity of Leu-222 to the region proximal to position 251 and from there continues to surround the cytosolic domain of the channel (see Fig. 7B). Thus, with the N251D mutation reversing the modulatory effect of the L222I mutation, this chain of residues may underlie the functional link between these two positions and may play a critical role in the modulation of channel function. Although this residue chain seems to be discontinuous, it is joined at the interface between the subunits (see supplemental Fig. 2B) or by residues in which the interactions between their side chains are restored as demonstrated for the hydrogen bonding between Lys-233 and Asp-246 (see Fig. 7, B–D). As Fig. 7E shows, the molecular dynamics simulations suggest that the hydrogen bonding between these residues is lost following the L222I mutation (Fig. 7C) and restored following the N251D mutation (Fig. 7D). Specifically, in the simulations of both the WT Kir2.1 channel and the L222I/N251D double mutant, the hydrogen bonding between Lys-233 and Asp-246 is persistent 100% of the time with an average minimal distance between the possible interacting atoms (Hζ1, Hζ2, and Hζ3 of Lys-233 and Oδ1 and Oδ2 of Asp-246) of 1.687 ± 0.003 and 1.668 ± 0.002 Å, respectively. In contrast in the simulation of the L222I mutant, no hydrogen bonding is observed between the side chains of Lys-233 and Asp-246, and the average minimal distance is 6.99 ± 0.02 Å.
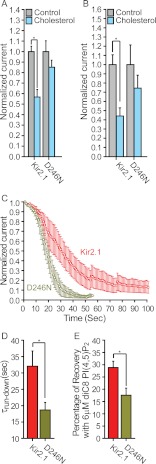
Thus, following the striking effect of the mutations of positions 222 and 251 on the hydrogen bonding between the side chains of Lys-233 and Asp-246, we hypothesized that the interaction between Lys-233 and Asp-246 would be critical for channel modulation. We thus mutated Asp-246 to break the hydrogen bond between the side chains of these two residues while keeping the channel functional. As can be seen in Fig. 7F, molecular dynamics simulations suggest that the D246N mutation would break the hydrogen bonding between the side chains of positions 233 and 246. Specifically, the average minimal distance between the side chains of positions 233 and 246 is 8.69 ± 0.03 Å for the D246N mutant. We thus tested the effect of this mutation on the sensitivity of Kir2.1 to cholesterol. As can be seen in Fig. 8, A and B, the D246N mutation abrogated the cholesterol sensitivity of the channel both in Xenopus oocytes and in HEK cells. Thus, because position 233 is located on the βD strand of the channel, whereas position 246 is located on the βE strand, this result suggests a critical role for this hydrogen bonding between the βD and the βE strands in channel modulation. We further corroborated this hypothesis by testing the effect of this mutation on channel run-down in response to neomycin, which is commonly used to sequester PI(4,5)P2 (52). As can be seen in Fig. 8, C and D, the run-down kinetics of D246N was faster than the run-down of the WT, indicating that the interaction between the D246N mutant and PI(4,5)P2 is weaker compared with the WT channel. We further confirmed this result by applying 6 μm diC8 PI(4,5)P2 to excised patches expressing Kir2.1 and the D246N mutant after run-down. As can be seen in Fig. 8E, the effect of diC8 PI(4,5)P2 on D246N was significantly lower than its effect on the WT Kir2.1 channel. Furthermore, in agreement with the decrease in the strength of channel-PI(4,5)P2 interactions, the open probability of this single mutant was significantly decreased as compared with the WT channel (supplemental Fig. 3).
FIGURE 8.
A, whole-cell basal currents recorded in Xenopus oocytes at −80 mV showing the effect of cholesterol enrichment on Kir2.1 and the D246N mutant (n = 10–18). B, mean peak currents for control and cholesterol-enriched HEK293 cell populations co-transfected with enhanced GFP and HA-Kir2.1 or its D246N mutant (n = 10–53). Significant differences are indicated by asterisks (*, p ≤ 0.05). C, normalized current run-down at −80 mV in excised inside-out macropatches from Xenopus oocytes expressing Kir2.1 and its D246N mutant. D, summary data based on Fig. 6D showing τrun-down for Kir2.1 and D246N. E, percentage of recovery after run-down following application of 6 μm diC8 PI(4,5)P2 to Xenopus oocytes in standard excised inside-out configuration. Error bars represent S.E.
DISCUSSION
Although numerous ion channels are regulated by the level of membrane cholesterol, very little is known about the structure-function relationship of their cholesterol sensitivity. Our earlier studies have shown that cholesterol sensitivity of Kir2 channels critically depends on specific residues in their cytosolic domains (23, 24, 29). Within the cytosolic domain of Kir2.1, we have identified a group of residues that form a beltlike structure around the cytosolic pore of the channel in proximity to the transmembrane domain and whose mutation to the corresponding residues in Kir2.3 results in abrogation of the cholesterol sensitivity of the channel (29). Surprisingly, however, each of the mutations of the five residues of the cholesterol sensitivity belt that abrogate the cholesterol sensitivity of Kir2.1 (H53Q, E191Q, V194L, L222I, and C311A) also converts these residues to the corresponding residues in Kir2.2. This was completely unexpected because the cholesterol sensitivities of Kir2.1 and Kir2.2 channels are very similar. In this study, we used this phenomenon to gain further insights into the cholesterol sensitivity of Kir2 channels.
To achieve this goal, we used an interactive computational-experimental approach going back and forth between molecular dynamics simulations, mutagenesis, and electrophysiology. We have shown previously that the interactive approach is instrumental in studies of the local effects of residues on channel gating and function (50, 53, 54) when the environment is implicit or partially represented in the simulations. In this study, utilization and analysis of full-membrane simulations of a large number of Kir2.1 mutants enabled us to extend this approach to guide the experiments and uncover long range effects.
Specifically, we focused on the mutation of leucine at position 222 in Kir2.1 channels to its isomer, isoleucine, which abrogates the cholesterol sensitivity of Kir2.1 channels (23, 24). Conversely, a reverse mutation in Kir2.3, which is less sensitive to cholesterol than Kir2.1 (8, 55), resulted in an increase in cholesterol sensitivity of Kir2.3 (23), underscoring the importance of this position in cholesterol sensitivity of Kir2 channels. These residues are also known to play a key role in Kir-PI(4,5)P2 interactions (38, 56). Furthermore, our analysis of a database of crystallographic structures of the cytosolic domains of eukaryotic Kir channels implicated this position in Kir channel gating, correlating it with the position equivalent to Glu-303 in Kir2.1 (29). Notably, Glu-303 is located at the apex of the G loop, the major cytosolic gate of the channel (30). The relationship between this position and the channel gating machinery was further corroborated most recently following a comparison of the crystal structures of Kir2.2 and its I223L mutant, showing that the I223L mutation induces structural changes in the G loop (57).
Here we analyzed by all-atom molecular dynamics simulation the effect of the L222I mutation on the entire crystallized cytosolic domain of Kir2.1. These simulations led to several insights into the structure-function relationship of Kir2. First, the effect of the L222I mutation was surprisingly extended to the majority of the residues of the cytosolic domain of Kir2.1. Second, our analysis linked the L222I mutation with several key regions of the cytosolic domain in addition to the G loop (Fig. 3A and supplemental Fig. 4A). Unexpectedly, our study identified a region distant from Leu-222 and the CD loop, the EF loop, that was predicted to be affected most by this mutation. To our knowledge, this loop has not been linked previously to channel modulation. Further corroboration of this result was obtained following a comparison of the crystal structures of Kir2.2 (58) and its I223L mutant (57). Although the resolution of these structures exceeds 3 Å, analysis of the differences between these structures highlights the same regions of the cytosolic domain, including the EF loop (supplemental Fig. 4B).
Within the EF loop, our functional studies linked the effect of the L222I mutation with the N251D mutation (Fig. 9A). These two mutations act as a part of a two-way switch, a device that can be controlled from two different locations, so that each location can be controlled independently but with the result depending on the relative position of the two. Specifically, each of these two mutations separately, L222I and N251D, abrogates the cholesterol sensitivity of the channel and reduces the strength of the interaction between the channel and PI(4,5)P2. Together, however, they cancel each other's effect on channel modulation.
FIGURE 9.
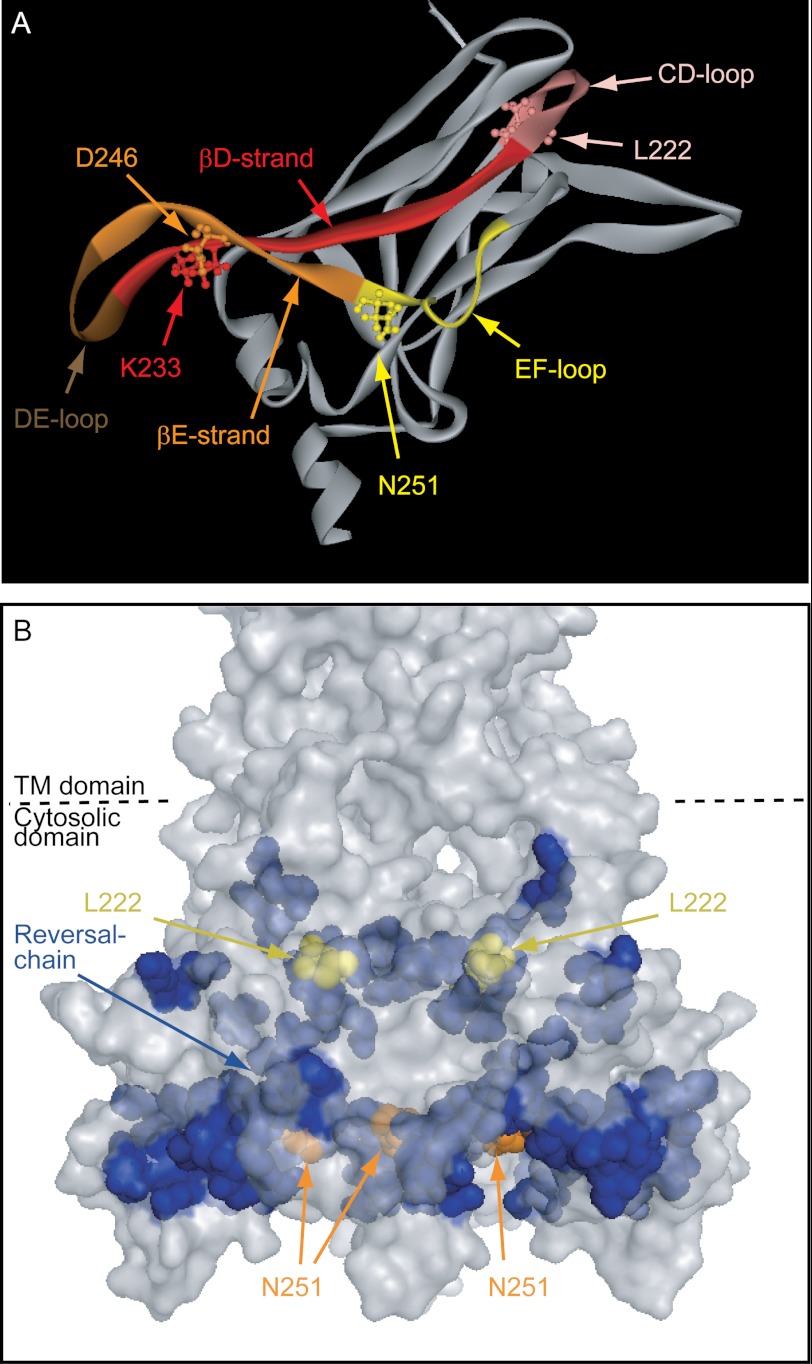
A, a ribbon model of the cytosolic domain of one subunit of Kir2.1 showing the critical residues identified in the study along with labeling of the regions in which they are located. B, surface presentation of the cytosolic domain of Kir2.1 showing the reversal residue chain (blue), L222I (yellow), and Asn-251 (orange). TM, transmembrane.
This result may be the major reason underlying the differences in cholesterol sensitivity and the strength of interaction with PI(4,5)P2 of the four WT Kir2 channels, Kir2.1, Kir2.2, Kir2.3, and Kir2.4 (see supplemental Table 1). Specifically, each of these channels possesses a different combination of residues in the positions equivalent to positions 222/251 of Kir2.1: Kir2.1, Leu/Asn; Kir2.2, Ile/Asp; Kir2.3, Ile/Asn; Kir2.4, Leu/Asp. As shown previously (8, 56), Kir2.1 and Kir2.2 exhibit similar cholesterol sensitivity and strength of interaction with PI(4,5)P2, whereas Kir2.3 and Kir2.4 exhibit decreased sensitivity to cholesterol and a decreased strength of channel-PI(4,5)P2 interaction although to a different degree. Our data show that if one of these two residues is mutated in Kir2.1, creating 222/251 combinations that are similar either to Kir2.3 (L222I mutant) or to Kir2.4 (N251I mutant), it abrogates the sensitivity of the channels to cholesterol and weakens channel-PI(4,5)P2 interactions, but if both residues are mutated together, yielding a combination that is similar to Kir2.2 channels (L222I/N251D double mutant), both cholesterol sensitivity and the strength of channel-PI(4,5)P2 interactions are fully restored. In summary, the discovery of the reciprocal relationship between Leu-222 and Asn-251 seems to be the key in elucidating a major source for the differential sensitivities of Kir2 channels to cholesterol and PI(4,5)P2.
We have shown earlier (23), however, that cholesterol sensitivity of Kir2 channels was not affected by sequestering PI(4,5)P2, suggesting that the effect of cholesterol on channel function is independent of PI(4,5)P2. Furthermore, there is only a partial overlap between the residues that are involved in the sensitivities of Kir2.1 channels to cholesterol and to PI(4,5)P2 (23, 29). Specifically, whereas several mutations that suppress the sensitivity of Kir2.1 to cholesterol are also known to affect channel-PI(4,5)P2 interactions (23, 29), cholesterol sensitivity of the channels was not affected by several positively charged residues (lysines and an arginine) (23) that have been suggested to play a key role in the electrostatic interactions between Kir2.1 and PI(4,5)P2 (32–34). Most recently, it has been shown that three of the equivalent positively charged residues in Kir2.2 interact with PI(4,5)P2 in a crystal structure of the complex of Kir2.2 with the short chain derivative of PI(4,5)P2 (57). This suggests that among the cytosolic residues that affect channel-PI(4,5)P2 interactions residues critical for PI(4,5)P2 binding per se are not pertinent for the sensitivity of the channel to cholesterol. In contrast, residues that affect channel-PI(4,5)P2 interactions by affecting the gating mechanism may also play a critical role in the sensitivity of the channel to cholesterol.
In particular, although the L222I and N251D mutations alter the strength of interaction between the channel and PI(4,5)P2, these mutations are most likely not a part of the PI(4,5)P2 binding site for the following reasons. First, functional data suggested that the binding site of PI(4,5)P2 is located at the interface between the transmembrane and cytosolic domains (32, 34, 39, 59, 60), and second, in the structure of Kir2.2 co-crystallized with the short chain derivative of PI(4,5)P2 (57), the positions equivalent to Leu-222 and Asn-251 of Kir2.1 were not included in the binding site of PI(4,5)P2. It is also unlikely that these residues would be a part of a cholesterol binding site in view of our recent docking analysis (29). On the other hand, as noted above, there is evidence that at least one of these positions (Leu-222) has a structural effect on the Kir2 gating machinery, namely the G loop (29, 57). Furthermore, our data show that L222I and N251D affect the open probability of the channel. This suggests that these mutations affect channel gating. However, further studies are required to fully discriminate between the possibilities that these mutations may indirectly affect the binding of PI(4,5)P2 and/or cholesterol or the downstream effects on channel gating.
As noted above, our data indicate that there is correspondence between the effect of combinations of single and double mutations of L222I and N251D on the sensitivity of Kir2.1 to cholesterol and on its strength of interaction with PI(4,5)P2. This observation provides further insight into the mechanism of Kir2 sensitivities to the two lipid modulators. Specifically, although cholesterol and PI(4,5)P2 do not share the same binding site, this suggests that the mechanisms underlying the effect of both gating molecules on channel function converge to a common pathway through specific regions of the cytosolic domain of the channel even though they have opposite impacts on channel activity. Thus, it is likely that conformational changes induced by cholesterol or PI(4,5)P2 to stabilize the closed or open conformations, respectively, share the transition trajectory between the two states.
Interestingly, the single mutants do not have a significant effect on whole-cell basal currents. The most likely reason for this observation is that cholesterol and PI(4,5)P2 effects may cancel each other. Specifically, because cholesterol suppresses channel function, the loss of cholesterol sensitivity is expected to increase channel activity. In contrast, PI(4,5)P2 is required to activate the channel, and therefore, a decrease in the strength of the interaction between the channel and PI(4,5)P2 is expected to lower Kir2.1 activity. Thus, depending on the contribution of each of these opposite effects to channel function, the overall effect of the mutants on Kir2.1 whole-cell currents may be canceled out. In contrast, whereas the single mutants do not affect the conductance of the channel, they exhibit reduced open probability compared with the WT. These observations are in agreement with the decrease in the strength of channel-PI(4,5)P2 interactions in these mutants, as compared with the WT Kir2.1 channel, that is expected to decrease the open probability of the channels. On the other hand, we have shown previously that although cholesterol significantly suppresses whole-cell currents of Kir2.1 it only has a very small effect on the open probability of the channel (∼5%) as detected by single channel analysis (8). We thus proposed that an increase in membrane cholesterol induces a conformational change of the channel protein that leads to a silent (nonactive) state of the channel that cannot be detected by single channel analysis. Thus, although in whole-cell configuration alterations in cholesterol sensitivity and in channel-PI(4,5)P2 interactions can cancel each other, in single channel recordings, only changes in channel-PI(4,5)P2 interactions are accounted for. As a result, the decrease in the strength of channel-PI(4,5)P2 interactions observed for the single mutants is manifested as a decrease in the open probability.
From a mechanistic point of view, molecular dynamics simulations indicated that the N251D mutation reverses the effect of the L222I mutation on the distance between the backbone central carbon of position 222 and those of a chain of residues in the cytosolic domain. This reversal residue chain seems to serve as the wiring between the two switches, connecting the proximal regions of positions 222 and 251 (Fig. 9B). This relationship between L222I and N251D provides two novel insights into the structure-function relationship of Kir channels. First, earlier structure-function studies have primarily explored the role of the regions of the cytosolic domain that are proximal to the transmembrane domain. Beyond these regions, however, the role of the complex design of the cytosolic domain in channel function remains mostly unexplored. This relationship demonstrates how the intricate arrangement of the β sheets connects distant regions of the channel in a manner that enables control of channel modulation. Second, these data indicate that there is a long distance functional connection between residues in the CD and EF loops that do not physically interact with each other. To our knowledge, this is the first time that a pair of non-interacting residues that are ∼24 Å away from each other and have the same effect on channel modulation when mutated separately cancels each other's effect when they are both mutated. Furthermore, identification of a reversal residue chain that connects Leu-222 and Asn-251 provides unique insight into the manner by which the three-dimensional structure of the cytosolic domain underlies a sophisticated molecular machine. These findings provide insights into the channel structure-function relationship that are independent of the specific effects of cholesterol and PI(4,5)P2.
Our results suggest that the continuation of the reversal residue chain from position 251 around the cytosolic pore of the channel from one subunit to its adjacent subunit wires the two switches to a critical hydrogen-bonding link between the βD and the βE strands (Fig. 9A). Specifically, our analysis suggests that the hydrogen bonding between the side chains of Lys-233 and Asp-246 that are located in these two strands is controlled by the combinations of the residues in positions 222 (Leu/Ile) and 251 (Asn/Asp) in correspondence with the effect of these combinations on the cholesterol sensitivity of the channel. Thus, in accord with our analysis, mutagenesis of Asp-246 to eliminate the hydrogen bonding between the side chains of these positions resulted in loss of cholesterol sensitivity and in reduction in the strength of interaction between the channel and PI(4,5)P2 but not in changes in single channel conductance. These observations suggest a critical role of this interstrand interaction in channel modulation.
In summary, our data suggest that mutations that are apart from one another in the three-dimensional structure (i.e. L222I and N251D) have similar effects on negating cholesterol and decreasing PI(4,5)P2 sensitivity. These residues are allosterically coupled as the double mutant (L222I/N251D) reverts the effects of each single mutant. Our simulations suggest that the two residues are connected via the βE strand, DE loop, and βD strand through a key salt bridge interaction between Lys-233 and Asp-246. The D246N mutation mimics each of the L222I and N251D mutations, supporting our modeling results that the two-way switch we have described proceeds through establishment of this critical salt bridge.
These observations clearly have implications beyond the scope of the modulation of Kir2 channels by cholesterol. Lys-233 and Asp-246 are located at the crossroad that connects three loops, which are critical for channel gating: the CD loop, the DE loop, and the EF loop. The CD loop that includes Leu-222 in Kir2.1 affects the cytosolic gate of the channel (29, 57), the G loop, and plays a critical role in the modulation of Kir channel function by cholesterol (23, 24, 29), PI(4,5)P2 (39), and sodium (38, 54). The DE loop has been shown to be involved in the binding of a modulator that enhances the interaction of Kir3 channels with PI(4,5)P2, namely the βγ subunits of the G-protein (40), indicating that the conformation of this loop plays a critical role in gating. Finally, the EF loop includes Asn-251 in Kir2.1 and as we have shown in this study plays a key role in both cholesterol and PI(4,5)P2 modulation of channel function. Our data suggest that the interaction between Lys-233 and Asp-246 couples these regions together and that removal of the hydrogen bonding uncouples them. Accordingly, it is likely that the interaction between Lys-233 and Asp-246 acts as the relay that communicates changes in the loops to affect channel gating.
Supplementary Material
Acknowledgments
We thank Heikki Vaananen and Sophia Gruszecki (Virginia Commonwealth University) for oocyte preparation.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-073965, HL-083298 (to I. L.), and HL-059949 (to D. E. L.). This work was also supported by American Heart Association Scientist Development Grant 11SDG5190025 (to A. R.-D.).

This article contains supplemental Figs. 1–4 and Table 1.
P. W. Fowler, A. Ivetac, S. Khalid, Y. Mokrab, P. J. Stansfeld, K. Tai, and M. S. P. Sansom, a database of potassium ion channel homology models and molecular dynamics simulations.
- Kir
- inwardly rectifying potassium
- MβCD
- methyl-β-cyclodextrin
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- diC8-PI(4,5)P2
- dioctanoyl phosphatidylinositol (4,5) bisphosphate
- DPBS
- Dulbecco's phosphate-buffered saline containing calcium and magnesium.
REFERENCES
- 1. Levitan I., Fang Y., Rosenhouse-Dantsker A., Romanenko V. (2010) Cholesterol and ion channels. Subcell. Biochem. 51, 509–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenhouse-Dantsker A., Mehta D., Levitan I. (2012) Regulation of ion channels by membrane lipids. Compr. Physiol. 2, 31–68 [DOI] [PubMed] [Google Scholar]
- 3. Levitan I. (2009) Cholesterol and Kir channels. IUBMB Life 61, 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolotina V., Omelyanenko V., Heyes B., Ryan U., Bregestovski P. (1989) Variations of membrane cholesterol alter the kinetics of Ca2+-dependent K+ channels and membrane fluidity in vascular smooth muscle cells. Pflugers Arch. 415, 262–268 [DOI] [PubMed] [Google Scholar]
- 5. Heaps C. L., Tharp D. L., Bowles D. K. (2005) Hypercholesterolemia abolishes voltage-dependent K+ channel contribution to adenosine-mediated relaxation in porcine coronary arterioles. Am. J. Physiol. Heart Circ. Physiol. 288, H568–H576 [DOI] [PubMed] [Google Scholar]
- 6. Martens J. R., Navarro-Polanco R., Coppock E. A., Nishiyama A., Parshley L., Grobaski T. D., Tamkun M. M. (2000) Differential targeting of Shaker-like potassium channels to lipid rafts. J. Biol. Chem. 275, 7443–7446 [DOI] [PubMed] [Google Scholar]
- 7. Martens J. R., Sakamoto N., Sullivan S. A., Grobaski T. D., Tamkun M. M. (2001) Targeting of Kv1.5 to caveolae. J. Biol. Chem. 276, 8409–8414 [DOI] [PubMed] [Google Scholar]
- 8. Romanenko V. G., Fang Y., Byfield F., Travis A. J., Vandenberg C. A., Rothblat G. H., Levitan I. (2004) Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys. J. 87, 3850–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ambudkar I. S. (2004) Cellular domains that contribute to Ca2+ entry events. Sci. STKE 2004, pe32. [DOI] [PubMed] [Google Scholar]
- 10. Bowles D. K., Heaps C. L., Turk J. R., Maddali K. K., Price E. M. (2004) Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J. Appl. Physiol. 96, 2240–2248 [DOI] [PubMed] [Google Scholar]
- 11. Lockwich T. P., Liu X., Singh B. B., Jadlowiec J., Weiland S., Ambudkar I. S. (2000) Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 275, 11934–11942 [DOI] [PubMed] [Google Scholar]
- 12. Lundbaek J. A., Birn P., Girshman J., Hansen A. J., Andersen O. S. (1996) Membrane stiffness and channel function. Biochemistry 35, 3825–3830 [DOI] [PubMed] [Google Scholar]
- 13. Toselli M., Biella G., Taglietti V., Cazzaniga E., Parenti M. (2005) Caveolin-1 expression and membrane cholesterol content modulate N-type calcium channel activity in NG108–15 cells. Biophys. J. 89, 2443–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lundbaek J. A., Birn P., Hansen A. J., Søgaard R., Nielsen C., Girshman J., Bruno M. J., Tape S. E., Egebjerg J., Greathouse D. V., Mattice G. L., Koeppe R. E., 2nd, Andersen O. S. (2004) Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of micelle-forming amphiphiles and cholesterol. J. Gen. Physiol. 123, 599–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu C. C., Su M. J., Chi J. F., Chen W. J., Hsu H. C., Lee Y. T. (1995) The effect of hypercholesterolemia on the sodium inward currents in cardiac myocyte. J. Mol. Cell. Cardiol. 27, 1263–1269 [DOI] [PubMed] [Google Scholar]
- 16. Levitan I., Christian A. E., Tulenko T. N., Rothblat G. H. (2000) Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J. Gen. Physiol. 115, 405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Romanenko V. G., Rothblat G. H., Levitan I. (2004) Sensitivity of volume-regulated anion current to cholesterol structural analogues. J. Gen. Physiol. 123, 77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hutter O. F., Noble D. (1960) Rectifying properties of heart muscle. Nature 188, 495. [DOI] [PubMed] [Google Scholar]
- 19. Sanguinetti M. C., Tristani-Firouzi M. (2010) in Cardiac Electrophysiology from Cell to Bedside (Zipes D. P., Jalife J., eds) 5th Ed., pp. 105–114, Elsevier/W. B. Saunders Co., Philadelphia [Google Scholar]
- 20. Zaritsky J. J., Eckman D. M., Wellman G. C., Nelson M. T., Schwarz T. L. (2000) Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ. Res. 87, 160–166 [DOI] [PubMed] [Google Scholar]
- 21. Olesen S. P., Clapham D. E., Davies P. F. (1988) Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature 331, 168–170 [DOI] [PubMed] [Google Scholar]
- 22. Fang Y., Schram G., Romanenko V. G., Shi C., Conti L., Vandenberg C. A., Davies P. F., Nattel S., Levitan I. (2005) Functional expression of Kir2.x in human aortic endothelial cells: the dominant role of Kir2.2. Am. J. Physiol. Cell Physiol. 289, C1134–C1144 [DOI] [PubMed] [Google Scholar]
- 23. Epshtein Y., Chopra A. P., Rosenhouse-Dantsker A., Kowalsky G. B., Logothetis D. E., Levitan I. (2009) Identification of a C-terminus domain critical for the sensitivity of Kir2.1 to cholesterol. Proc. Natl. Acad. Sci. U.S.A. 106, 8055–8060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenhouse-Dantsker A., Leal-Pinto E., Logothetis D. E., Levitan I. (2010) Comparative analysis of cholesterol sensitivity of Kir channels: role of the CD loop. Channels 4, 63–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romanenko V. G., Rothblat G. H., Levitan I. (2002) Modulation of endothelial inward-rectifier K+ current by optical isomers of cholesterol. Biophys. J. 83, 3211–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh D. K., Rosenhouse-Dantsker A., Nichols C. G., Enkvetchakul D., Levitan I. (2009) Direct regulation of prokaryotic Kir channel by cholesterol. J. Biol. Chem. 284, 30727–30736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh D. K., Shentu T. P., Enkvetchakul D., Levitan I. (2011) Cholesterol regulates prokaryotic Kir channel by direct binding to channel protein. Biochim. Biophys. Acta 1808, 2527–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D'Avanzo N., Hyrc K., Enkvetchakul D., Covey D. F., Nichols C. G. (2011) Enantioselective protein-sterol interactions mediate regulation of both prokaryotic and eukaryotic inward rectifier K+ channels by cholesterol. PLoS One 6, e19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenhouse-Dantsker A., Logothetis D. E., Levitan I. (2011) Cholesterol sensitivity of KIR2.1 is controlled by a belt of residues around the cytosolic pore. Biophys. J. 100, 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pegan S., Arrabit C., Zhou W., Kwiatkowski W., Collins A., Slesinger P. A., Choe S. (2005) Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat. Neurosci. 8, 279–287 [DOI] [PubMed] [Google Scholar]
- 31. Hilgemann D. W., Feng S., Nasuhoglu C. (2001) The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE 2001, re19. [DOI] [PubMed] [Google Scholar]
- 32. Lopes C. M., Zhang H., Rohacs T., Jin T., Yang J., Logothetis D. E. (2002) Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron 34, 933–944 [DOI] [PubMed] [Google Scholar]
- 33. Logothetis D. E., Jin T., Lupyan D., Rosenhouse-Dantsker A. (2007) Phosphoinositide-mediated gating of inwardly rectifying K+ channels. Pflugers Arch. 455, 83–95 [DOI] [PubMed] [Google Scholar]
- 34. Rosenhouse-Dantsker A., Logothetis D. E. (2007) Molecular characteristics of phosphoinositide binding. Pflugers Arch. 455, 45–53 [DOI] [PubMed] [Google Scholar]
- 35. He C., Yan X., Zhang H., Mirshahi T., Jin T., Huang A., Logothetis D. E. (2002) Identification of critical residues controlling G protein-gated inwardly rectifying K+ channel activity through interactions with the βγ subunits of G proteins. J. Biol. Chem. 277, 6088–6096 [DOI] [PubMed] [Google Scholar]
- 36. Santiago J., Guzmàn G. R., Rojas L. V., Marti R., Asmar-Rovira G. A., Santana L. F., McNamee M., Lasalde-Dominicci J. A. (2001) Probing the effects of membrane cholesterol in the Torpedo californica acetylcholine receptor and the novel lipid-exposed mutation alpha C418W in Xenopus oocytes. J. Biol. Chem. 276, 46523–46532 [DOI] [PubMed] [Google Scholar]
- 37. Zidovetzki R., Levitan I. (2007) Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta 1768, 1311–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hilgemann D. W. (1995) in Single-Channel Recording (Sakmann B., Neher E., eds) pp. 307–327, Plenum, New York [Google Scholar]
- 39. Zhang H., He C., Yan X., Mirshahi T., Logothetis D. E. (1999) Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat. Cell Biol. 1, 183–188 [DOI] [PubMed] [Google Scholar]
- 40. Yokogawa M., Osawa M., Takeuchi K., Mase Y., Shimada I. (2011) NMR analyses of the Gβγ binding and conformational rearrangements of the cytoplasmic pore of G protein-activated inwardly rectifying potassium channel 1 (GIRK1). J. Biol. Chem. 286, 2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tai K., Stansfeld P. J., Sansom M. S. (2009) Ion-blocking sites of the Kir2.1 channel revealed by multiscale modeling. Biochemistry 48, 8758–8763 [DOI] [PubMed] [Google Scholar]
- 42. Nishida M., Cadene M., Chait B. T., MacKinnon R. (2007) Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 26, 4005–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jo S., Lim J. B., Klauda J. B., Im W. (2009) CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J. 97, 50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., Klein M. L. (1983) Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 [Google Scholar]
- 45. Noskov S. Y., Bernèche S., Roux B. (2004) Control of ion selectivity in potassium channels by electrostatic and dynamic properties of carbonyl ligands. Nature 431, 830–834 [DOI] [PubMed] [Google Scholar]
- 46. Phillips J. C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R. D., Kalé L., Schulten K. (2005) Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brooks B. R., Brooks C. L., 3rd, Mackerell A. D., Jr., Nilsson L., Petrella R. J., Roux B., Won Y., Archontis G., Bartels C., Boresch S., Caflisch A., Caves L., Cui Q., Dinner A. R., Feig M., Fischer S., Gao J., Hodoscek M., Im W., Kuczera K., Lazaridis T., Ma J., Ovchinnikov V., Paci E., Pastor R. W., Post C. B., Pu J. Z., Schaefer M., Tidor B., Venable R. M., Woodcock H. L., Wu X., Yang W., York D. M., Karplus M. (2009) CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Humphrey W., Dalke A., Schulten K. (1996) VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- 49. McDonald I. K., Thornton J. M. (1994) Satisfying hydrogen bonding potential in proteins. J. Mol. Biol. 238, 777–793 [DOI] [PubMed] [Google Scholar]
- 50. Mirshahi T., Logothetis D. E., Rosenhouse-Dantsker A. (2006) Hydrogen-bonding dynamics between adjacent blades in G-protein β-subunit regulates GIRK channel activation. Biophys. J. 90, 2776–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zerangue N., Schwappach B., Jan Y. N., Jan L. Y. (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22, 537–548 [DOI] [PubMed] [Google Scholar]
- 52. Yin H. L., Janmey P. A. (2003) Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 65, 761–789 [DOI] [PubMed] [Google Scholar]
- 53. Rosenhouse-Dantsker A., Logothetis D. E. (2006) New roles for a key glycine and its neighboring residue in potassium channel gating. Biophys. J. 91, 2860–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rosenhouse-Dantsker A., Sui J. L., Zhao Q., Rusinova R., Rodríguez-Menchaca A. A., Zhang Z., Logothetis D. E. (2008) A sodium-mediated structural switch that controls the sensitivity of Kir channels to PtdIns(4,5)P2. Nat. Chem. Biol. 4, 624–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tikku S., Epshtein Y., Collins H., Travis A. J., Rothblat G. H., Levitan I. (2007) Relationship between Kir2.1/Kir2.3 activity and their distributions between cholesterol-rich and cholesterol-poor membrane domains. Am. J. Physiol. Cell Physiol. 293, C440–C550 [DOI] [PubMed] [Google Scholar]
- 56. Du X., Zhang H., Lopes C., Mirshahi T., Rohacs T., Logothetis D. E. (2004) Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J. Biol. Chem. 279, 37271–37281 [DOI] [PubMed] [Google Scholar]
- 57. Hansen S. B., Tao X., MacKinnon R. (2011) Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tao X., Avalos J. L., Chen J., MacKinnon R. (2009) Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 Å resolution. Science 326, 1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang C. L., Feng S., Hilgemann D. W. (1998) Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature 391, 803–806 [DOI] [PubMed] [Google Scholar]
- 60. Soom M., Schönherr R., Kubo Y., Kirsch C., Klinger R., Heinemann S. H. (2001) Multiple PIP2 binding sites in Kir2.1 inwardly rectifying potassium channels. FEBS Lett. 490, 49–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.