Background: Regulation of integrin activation has important implications for tumor cell invasion and metastasis.
Results: EGF activates ERK/p90RSK and Rho/Rho kinase signaling in A431 and DiFi colon cancer cells, leading to phosphorylation of filamin A (FLNa) and inactivation of the α5β1 integrin receptor.
Conclusion: EGF promotes α5β1 inactivation through the p90RSK-dependent phosphorylation of FLNa.
Significance: We have identified a novel EGF-dependent mechanism controlling the α5β1 integrin activation state.
Keywords: Adhesion, Cancer Biology, Epidermal Growth Factor (EGF), Fibronectin, Filamin, Integrin
Abstract
Cell adhesion, motility, and invasion are regulated by the ligand-binding activity of integrin receptors, transmembrane proteins that bind to the extracellular matrix. Integrins whose conformation allows for ligand binding and appropriate functional activity are said to be in an active state. Integrin activation and subsequent ligand binding are dynamically regulated by the association of cytoplasmic proteins with integrin intracellular domains. In this study, we evaluated the role of EGF in the regulation of the activation state of the α5β1 integrin receptor for fibronectin. The addition of EGF to either A431 squamous carcinoma cells or DiFi colon cancer cells resulted in loss of α5β1-dependent adhesion to fibronectin but no loss of integrin from the cell surface. EGF activated the EGF receptor/ERK/p90RSK and Rho/Rho kinase signaling pathways. Blocking either pathway inhibited EGF-mediated loss of adhesion, suggesting that they work in parallel to regulate integrin function. EGF treatment also resulted in phosphorylation of filamin A (FLNa), which binds and inactivates β1 integrins. EGF-mediated FLNa phosphorylation was completely blocked by an inhibitor of p90RSK and partially attenuated by an inhibitor of Rho kinase, suggesting that both pathways converge on FLNa to regulate integrin function. A431 clonal cell lines expressing non-phosphorylated dominant-negative FLNa were resistant to the inhibitory effects of EGF on integrin function, whereas clonal cell lines overexpressing wild-type FLNa were more sensitive to the inhibitory effect of EGF. These data suggest that EGF-dependent inactivation of α5β1 integrin is regulated through FLNa phosphorylation and cellular contractility.
Introduction
The extracellular matrix regulates basic biological processes by providing the cell with chemical, structural, and mechanical information about the tissue microenvironment. Changes in matrix properties are perceived by the cell through complex structural linkages between transmembrane receptors and the cytoskeleton. These structural linkages are dynamic and regulated by a system of scaffolding proteins, adaptor molecules, enzymes, and lipids (1). The interaction of cells with the extracellular environment is dependent on integrins, a family of heterodimeric transmembrane proteins that represent the primary connection between the extracellular matrix and the intracellular cytoskeleton. The binding of integrins to the extracellular matrix regulates bidirectional signaling cascades controlling the basic cellular functions of survival, growth, and migration (2). The ability of integrins to bind extracellular matrix proteins is regulated intracellularly by proteins that bind to the integrin cytoplasmic domain, resulting in structural alterations in the ectodomain that regulate ligand accessibility (3). Integrins whose conformation promotes ligand binding and appropriate functional activity are said to be activated. Integrin activation is under the control of “inside-out signaling,” a process by which intracellular signaling networks regulate the association of integrin cytoplasmic tails with binding proteins that impact integrin conformation. In adherent cells, integrin activation states are dynamic and carefully regulated to coordinate the requisite binding and release of matrix proteins required for cell migration, cytokinesis, and tissue remodeling (4).
Cytoplasmic actin-binding proteins, such as talins and filamins, bind directly to integrin tails and positively or negatively regulate integrin function. Talin is a cytoskeletal adaptor protein that binds simultaneously to actin and integrin, thereby providing a structural continuum between the extracellular matrix and filamentous actin. The binding of talin to the integrin cytoplasmic domain causes changes in the integrin ectodomain that result in the integrin being in a high-affinity state and able to bind ligand (5). Filamin A (FLNa)2 is an actin-binding protein that cross-links actin into orthogonal networks. FLNa binds to the integrin cytoplasmic domain, but unlike talin, FLNa promotes an inactive non-ligand binding conformation (6). Several studies have provided evidence that FLNa is a negative regulator of integrin functions, such as cell adhesion and migration (7, 8). The FLNa monomer is a 240–280-kDa protein that consists of an N-terminal actin-binding domain and 24 Ig-like domains. FLNa and talin have overlapping binding sites on the β1 cytoplasmic tail, and FLNa can prevent the binding of talin to integrin, thereby keeping integrin in an inactive conformation (6). Integrin cytoplasmic domains bind directly to FLNa IgG domain 21, and this binding site is cryptic and appears to be under the regulation of cellular tension (9, 10).
Integrins can physically and functionally associate with growth factor receptor tyrosine kinases to promote reciprocal signaling mechanisms required for the regulation of proliferation, migration, and survival (11). Co-clustering of EGF receptor (EGFR) and integrins is seen during cell adhesion, where integrin ligation can initiate ligand-independent EGFR aggregation and signaling (12). Integrin-dependent adhesion also enhances growth factor-initiated signaling by increasing the strength and duration of the signal (13). However, the role that growth factor receptor tyrosine kinases, such as EGFR, play in the regulation of integrin activation through inside-out signaling is less well understood. Earlier studies have shown that EGF/EGFR can modulate cell adhesion in several cancer cell lines (14, 15); however, the mechanisms by which EGF regulates integrin function have been largely unexplored. In this study, we show that treatment of A431 squamous carcinoma cells or DiFi colon cancer cells with EGF prevents α5β1 integrin-dependent adhesion to fibronectin. The inhibitory effect of EGF on adhesion is due to the inactivation of the α5β1 integrin receptor. Integrin inactivation is dependent on the ERK/p90RSK and Rho/Rho kinase signaling pathways, which are activated in response to EGF and converge to regulate the phosphorylation of FLNa at Ser-2152. These data identify the p90RSK-dependent phosphorylation of FLNa as an essential step in the regulation of α5β1 integrin function by EGF.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Unless indicated otherwise, reagents were obtained from Sigma-Aldrich. Non-tissue culture-treated bacterial plates were from Greiner Bio-one North America (Monroe, NC). EGF was purchased from R&D Systems (Minneapolis, MN). The anti-α5 integrin (clone P1D6) and anti-β1 integrin (clone P5D2) blocking antibodies were from EMD Millipore Corp. (Billerica, MA), and control mouse IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA). Mouse monoclonal antibody to phospho-ERK; rabbit antibodies to phospho-Thr-18/Ser-19 myosin light chain 2 (MLC2), phospho-Ser-2152 FLNa, phospho-Ser-380 ribosomal S6 kinase (RSK), and total RSK; and the MEK inhibitor U0126 were purchased from Cell Signaling Technology (Danvers, MA). The MEK inhibitor PD184352 (also called CI-1040) and rabbit anti-ERK2 and anti-focal adhesion kinase (FAK) antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Anti-FLNa monoclonal antibody was from AbD Serotec (Raleigh, NC). The EGFR inhibitor AG1478 and the p90RSK inhibitor BI-D1870 were from Enzo Life Sciences (Plymouth Meeting, PA). The vectors expressing wild-type or mutant FLNa were obtained from Addgene (Cambridge, MA) and were initially deposited by John Blenis (16). The cell-permeable form of C3 transferase was obtained from Cytoskeleton (Denver, CO). Human plasma fibronectin was purified to homogeneity from a fibronectin- and fibrinogen-rich byproduct of Factor VIII production by ion exchange chromatography on DEAE-cellulose (Amersham Biosciences) as described previously (17).
Cell Culture
Human epidermoid A431 carcinoma cells were grown in DMEM (Invitrogen) containing antibiotics (penicillin/streptomycin) and 10% FBS (HyClone Laboratories, Logan, UT). The DiFi colorectal carcinoma cells have been described previously (18) and were kindly provided by Dr. Zhen Fan (The University of Texas MD Anderson Cancer Center). DiFi cells were cultured in DMEM/medium F-12 containing 10% FBS plus antibiotics.
Isolation of Wild-type and Mutant FLNa-overexpressing A431 Cell Lines
Three μg of the expression vector pcDNA3 (control) or the vector expressing wild-type (pcDNA3-WT-FLNa) or mutant (pcDNA3-FLNa(S2152A)) FLNa were transfected into A431 cells (2 × 105 cells cultured overnight) using Lipofectamine 2000 (Invitrogen). Two days later, cells were trypsinized and placed in selection medium (500 μg/ml G418). Pools of G418-resistant A431 cells were obtained, and G418-resistant clones were isolated by limiting dilution. Two clones, each expressing wild-type FLNa, mutant FLNa(S2152A), or vector alone, were selected for expansion and used for experiments.
Cell Adhesion Assay
Polystyrene non-tissue culture-treated 48-well plates (Lab Planet) were coated with fibronectin (10 μg/ml, unless stated otherwise) in PBS overnight at 4 °C. Wells were then blocked at room temperature in PBS plus 3% (w/v) BSA for 2 h. For adhesion assays, A431 cells in DMEM containing 0.1% BSA and 15 mm Hepes were seeded onto fibronectin-coated wells. Control experiments showed that none of the cell lines used in the studies were adherent in the absence of fibronectin. Pharmacological inhibitors were added to cells prior to plating. EGF was added at the time of seeding. Specific treatment times and EGF doses are provided in the figure legends. Adherent cells were washed once in PBS, fixed in PBS containing 3% formaldehyde for 40 min, and stained with 0.05% toluidine blue for 1 h. Wells were then washed three times in water. Toluidine blue was extracted with 10% acetic acid, and its absorbance was measured at 650 nm. Measurements were corrected for light scattering by subtracting the absorbance obtained at 405 nm.
Immunoblotting
Signaling intermediates activated in response to EGF were identified by immunoblotting. For these experiments, cells in DMEM containing 0.1% BSA and 15 mm Hepes were seeded onto non-tissue culture-treated plates (12-well), which were coated overnight at 4 °C with poly-l-lysine (100 μg/ml). After 40 min at 37 °C, cells were stimulated with EGF (3 nm) for 20 min. When pharmacological inhibitors were used, cells were pretreated with inhibitors for 1 h prior to EGF treatment. After EGF treatment, cells were placed immediately on ice, washed once in cold PBS, and lysed with 2× hot sample buffer (0.12 m Tris (pH 6.8), 4% SDS, 20% glycerol, and 5% β-mercaptoethanol). In some experiments, nuclear free lysates were prepared. Cells on ice were lysed with cold lysis buffer (10 mm Tris-Cl (pH 7.4), 1% Triton X-100, 0.5% Nonidet P-40, 140 mm NaCl, 10 mm NaF, 1 mm Na3O4V, and Complete mini protease inhibitors (Roche Applied Science)). Cell lysates were cleared by centrifugation (14,000 rpm for 15 min at 4 °C), and protein concentrations were determined with a bicinchoninic acid protein assay reagent kit (Thermo Scientific, Rockford, IL) using BSA as a standard.
Samples were separated on SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (Schleicher & Schüll). Membranes were blocked in Tris-HCl (pH 7.4), 150 mm NaCl, and 0.1% Tween 20 containing 5% (w/v) BSA and incubated overnight with primary antibodies at 4 °C. Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies, and proteins were detected using an enhanced chemiluminescence reagent (GE Healthcare). For reprobing, membranes were incubated in 62.5 mm Tris-Cl (pH 6.8), 2% SDS, and 1% β-mercaptoethanol for 30 min at 60 °C to remove previously bound antibodies. ImageJ software was used to measure the intensity of Western blot bands.
RESULTS
EGF Inhibits Cell Adhesion
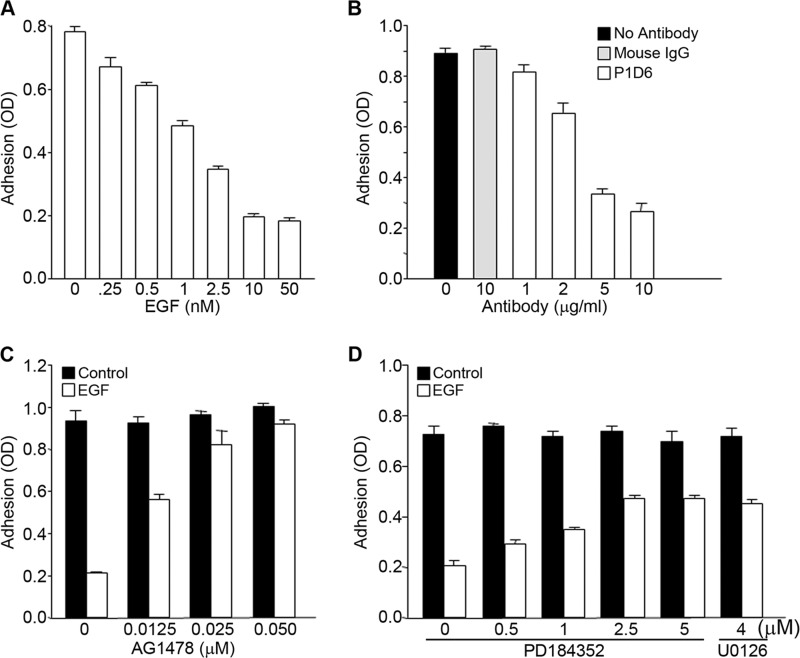
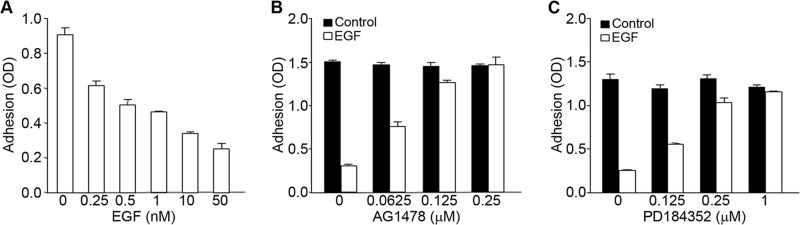
Earlier studies have shown that EGF can modulate adhesion in several cancer cell lines, suggesting that EGF signaling regulates integrin function (15). A431 is a metastatic squamous carcinoma cell line that expresses high levels of EGFR, the tyrosine kinase receptor for EGF (19). Experiments were performed to determine the role of EGFR in the regulation of the function of α5β1 integrin. Incubation of A431 cells with EGF inhibited the binding of cells to fibronectin. The effect of EGF on A431 cell adhesion was dose-dependent, as increasing amounts of EGF resulted in a progressive loss of cell adhesion to fibronectin (Fig. 1A). There was no adhesion of cells to control wells, which were not coated with fibronectin (data not shown). A431 cell adhesion to fibronectin was inhibited by a blocking antibody (clone P1D6) to the α5β1 integrin receptor (Fig. 1B). These data are consistent with EGF inhibiting the adhesive function of α5β1 integrin. The inhibitory effects of EGF on cell adhesion were prevented when cells were pretreated with inhibitors of EGFR kinase or ERK activation (Fig. 1, C and D), indicating a role for EGFR signaling in mediating the inhibitory effects of EGF on cell adhesion. Although the addition of the EGFR kinase inhibitor AG1478 nearly completely reversed the inhibitory effect of EGF on adhesion, inhibition of MEK using PD184352 significantly attenuated but did not completely inhibit the effects of EGF on cell adhesion. This partial reversal was seen with another inhibitor of MEK activity, U0126 (Fig. 1D), suggesting that EGFR may also activate ERK-independent pathways that contribute to the inhibitory effects of EGF on cell adhesion. Similar results were seen using the colon carcinoma cell line DiFi (18). These cells also express high levels of EGFR and were inhibited from binding to fibronectin in the presence of EGF (Fig. 2A). The inhibitory effects of EGF were dose-dependent, and the effective range of inhibition was similar to that seen in A431 cells (compare Figs. 1A and 2A). EGF-dependent inhibition of cell adhesion in DiFi cells was reversed when cells were pretreated with AG1478, an inhibitor of EGFR kinase (Fig. 2B), or with PD184352, an inhibitor of MEK (Fig. 2C).
FIGURE 1.
EGF signaling inhibits α5β1 integrin-dependent A431 cell adhesion to fibronectin. A, A431 cells were allowed to adhere to fibronectin (10 μg/ml)-coated wells in the presence of the indicated concentrations of EGF. B, cell suspensions were pretreated for 1 h with either control mouse IgG or anti-α5 integrin blocking antibody P1D6 at the indicated concentrations prior to addition to fibronectin-coated wells for 1 h. C and D, cells were pretreated for 1 h with the indicated amounts of either the EGFR kinase inhibitor AG1478 (C) or the MEK inhibitors PD184352 and U0126 (D) prior to the addition of 3 nm EGF and allowed to adhere to fibronectin-coated wells for 1 h. Cells were then fixed in formaldehyde, and cell adhesion was assessed by staining with toluidine blue. The dye was extracted with 10% acetic acid, and its optical density was measured at 650 nm. Error bars indicate S.D. of triplicate samples for one of three representative experiments.
FIGURE 2.
EGF signaling inhibits α5β1 integrin-dependent adhesion of DiFi colon cancer cells to fibronectin. A, DiFi cells were seeded onto fibronectin(10 μg/ml)-coated wells for 75 min in the presence of the indicated concentrations of EGF. Cell adhesion was assayed as described in the legend to Fig. 1. B and C, DiFi cells were pretreated for 1 h with the EGFR kinase inhibitor AG1478 (B) or the MEK inhibitor PD184352 (C) at the indicated concentrations prior to assay with or without EGF (10 nm). Error bars indicate S.D. of triplicate samples for one of three representative experiments.
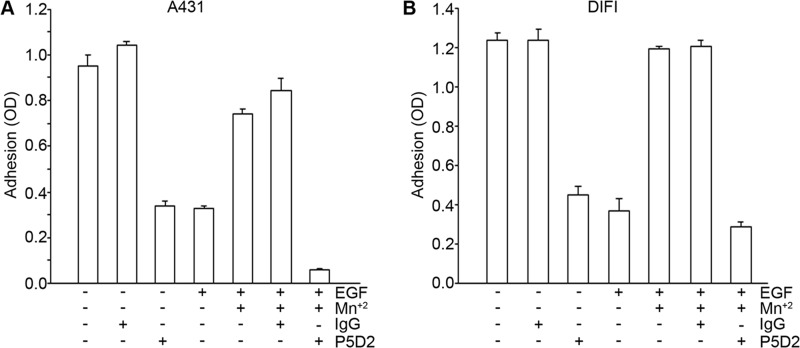
To determine the mechanism by which EGF inhibits cell adhesion to fibronectin, we considered whether EGF causes a loss of integrin from the cell surface or whether EGF induces a change in the activation state of the integrin. Flow cytometry studies showed that there was no loss of β1 integrin from the cell surface in either A431 or DiFi cells following the addition of EGF (data not shown). To determine whether EGF causes a decrease in the activation state of the integrin, cells were pretreated with Mn2+, which stabilizes integrins in an active conformation. As shown in Fig. 3, pretreatment of cells with Mn2+ prevented the inhibitory effect of EGF on cell adhesion in both A431 and DiFi cells. The Mn2+-dependent adhesion of both cell types was completely inhibited by a blocking antibody to β1 integrin (clone P5D2), indicating that the effects of Mn2+ on adhesion are dependent on β1 integrin. The addition of a control IgG had no effect on Mn2+-induced adhesion. These data suggest that EGF inhibits cell adhesion through changes in integrin activation state and that stabilization of the high-affinity, ligand binding conformation of the integrin with Mn2+ prevents EGF-dependent inactivation. These data are consistent with a mechanism in which EGF inhibits cell adhesion by causing a decrease in the affinity of α5β1 integrin for fibronectin. Taken together, these data suggest that EGF regulates the interaction of cells with fibronectin by regulating intracellular signaling pathways that impact on the activation state of α5β1 integrin.
FIGURE 3.
Mn2+ prevents EGF inhibition of α5β1 integrin function in A431 (A) and DiFi (B) cells. Cells were pretreated for 1 h at room temperature with solvent control, 0.5 mm MnCl2, control mouse IgG (20 μg/ml), or anti-β1 integrin blocking antibody P5D2 (20 μg/ml). Cells were then seeded onto fibronectin-coated wells in the presence of EGF (10 nm) for 60 min (A431 cells) or 75 min (DiFi cells) at 37 °C. Cell adhesion was assessed as described in the legend to Fig. 1. Additions to the medium were as indicated. Error bars indicate S.D. of triplicate samples for one of three representative experiments.
Effects of EGF on α5β1 Integrin Function Are Mediated through p90RSK and Rho Signaling
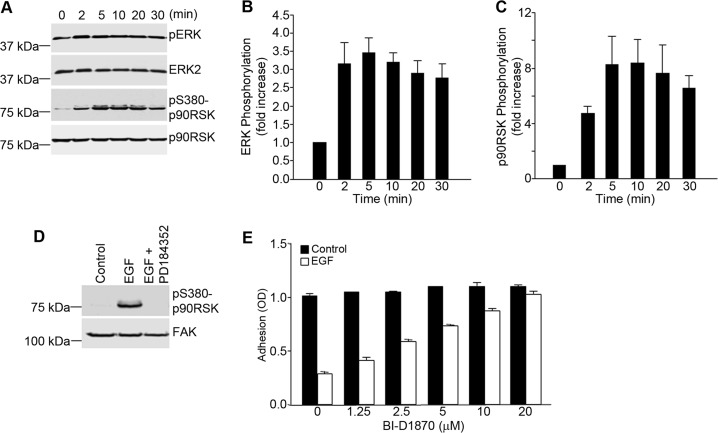
To understand how EGF might regulate the activation state of α5β1 integrin, a detailed analysis of the molecular events regulating integrin adhesive function was carried out in A431 cells. As ERK signaling was shown to modulate integrin function by EGF (Fig. 1), experiments were done to address whether p90RSK, a major target of ERK in the cytoplasm, is involved in the regulation of integrin function by EGF. As shown in Fig. 4A, phosphorylation of both ERK and p90RSK could be seen within 2–5 min after the addition of EGF to A431 cells. Quantitation of Western blots of EGF-treated A431 cell lysates showed a rapid parallel increase in the phosphorylation of both ERK and p90RSK within 5 min (Fig. 4, B and C). ERK activity was required for p90RSK activation, as inhibition of ERK activity with PD184352 completely blocked the phosphorylation of p90RSK in response to EGF (Fig. 4D). To determine whether p90RSK is required for the inhibitory effects of EGF on cell adhesion, A431 cells were preincubated with the p90RSK inhibitor BI-D1870 prior to incubation with EGF. As shown in Fig. 4E, BI-D1870 prevented the inhibitory effect of EGF on cell adhesion, thus confirming that p90RSK activation is required for the inhibitory effect of EGF on α5β1 integrin.
FIGURE 4.
EGF-dependent inhibition of α5β1 integrin function in A431 cells requires the MEK/ERK/p90RSK pathway. A, A431 cells were seeded onto poly-l-lysine-coated wells and stimulated with EGF (3 nm) for the indicated times. Nuclear free cell lysates were prepared and analyzed by Western blotting for the phosphorylation of ERK and p90RSK. Staining for total ERK and total p90RSK served as loading controls. pS380-p90RSK, phospho-Ser-380 p90RSK. B and C, the bands shown in A were quantified and normalized to ERK2 (B) and p90RSK (C), respectively. Densitometry measurements were performed using ImageJ software. Values are means ± S.D. of three separate experiments. D, A431 cells were seeded onto poly-l-lysine-coated wells, pretreated with PD184352 (2.5 μm), and incubated with EGF (3 nm) for 20 min. Cell lysates were prepared and analyzed by Western blotting using antibodies to phosphorylated p90RSK. Staining for total FAK served as a loading control. E, A431 cell suspensions were pretreated for 1 h with an inhibitor of p90RSK (BI-D1870) at the indicated concentrations prior to cell adhesion to fibronectin-coated wells in the absence or presence of EGF (3 nm). Cell adhesion was assessed as described in the legend to Fig. 1. Error bars indicate S.D. of triplicate samples for one of three representative experiments.
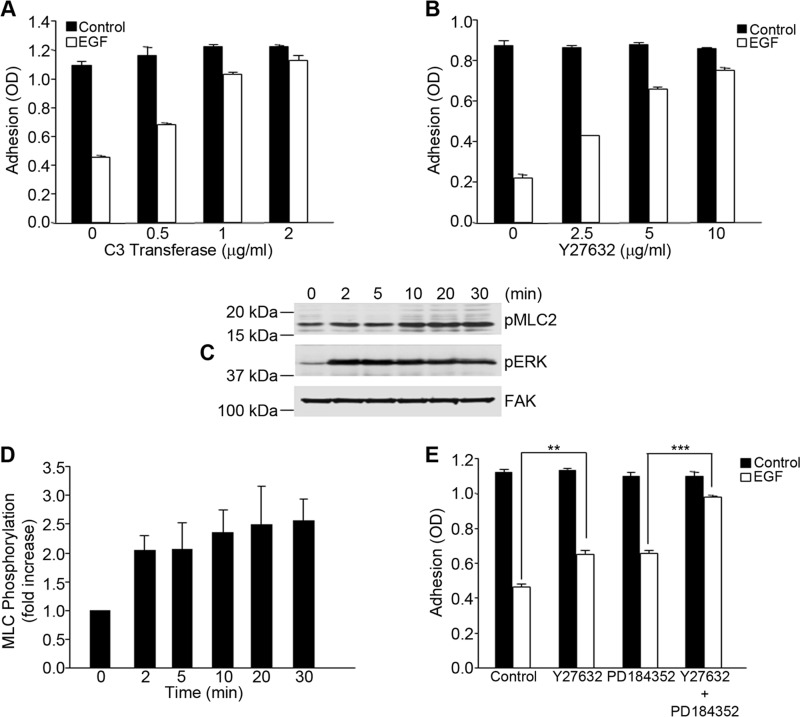
EGF is a known regulator of cytoskeletal dynamics that can also impact on integrin function. Therefore, experiments were done to determine whether Rho signaling might be involved in the regulation of integrin function by EGF. To evaluate this possibility, A431 cells were preincubated with inhibitors of the Rho signaling pathway prior to treatment with EGF. The addition of increasing amounts of the Rho inhibitor C3 transferase nearly completely prevented the loss of cell adhesion in response to EGF (Fig. 5A). Similar results were obtained when cells were pretreated with Y27632, an inhibitor of the Rho effector Rho kinase, which also attenuated the inhibitory effect of EGF on cell adhesion (Fig. 5B). Western blot analysis showed that EGF stimulated the phosphorylation of MLC (Fig. 5C), which could be completely inhibited by pretreatment of cells with Y27632 (data not shown). Quantitation of the Western blot data showed a 2.5-fold increase in MLC phosphorylation within 10 min of treatment with EGF (Fig. 5D). These data suggest that cellular contractility pathways contribute to the inactivation of integrin function by EGF. Interestingly, the effects of the Rho kinase inhibitor Y27632 and the MEK inhibitor PD184352 were additive when combined at suboptimal amounts (Fig. 5E), suggesting that contractility and EGFR signaling pathways work in parallel to regulate integrin function.
FIGURE 5.
Inhibition of α5β1 integrin function by EGF is dependent on Rho/Rho kinase. A and B, A431 cells were pretreated for 6 h with the indicated amounts of cell-permeable C3 transferase (A) or the Rho kinase inhibitor Y27632 for 1 h (B) prior to adhesion to fibronectin with or without EGF (3 nm). Error bars indicate S.D. of triplicate samples for one of three representative experiments. C, A431 cells were stimulated with EGF (3 nm) for the designated times, and protein phosphorylation was assessed by Western blotting using specific antibodies to phospho-MCL2 (pMLC2) and phospho-ERK (pERK). Staining for total FAK served as a loading control. D, bands for phospho-MLC2 were quantified and normalized to FAK. Values are means ± S.D. of three separate experiments. E, A431 cells were pretreated for 1 h with a suboptimal concentration of Y27632 (2.5 μm) or PD184352 (0.5 μm). Cell adhesion to fibronectin was assayed as described in the legend to Fig. 1. Statistical analysis to determine significance was performed using Student's t test. **, p < 0.01; ***, p < 0.001. Error bars indicate S.D. of triplicate samples for one of three representative experiments.
EGF Regulates α5β1 Integrin Activation State through Phosphorylation of FLNa
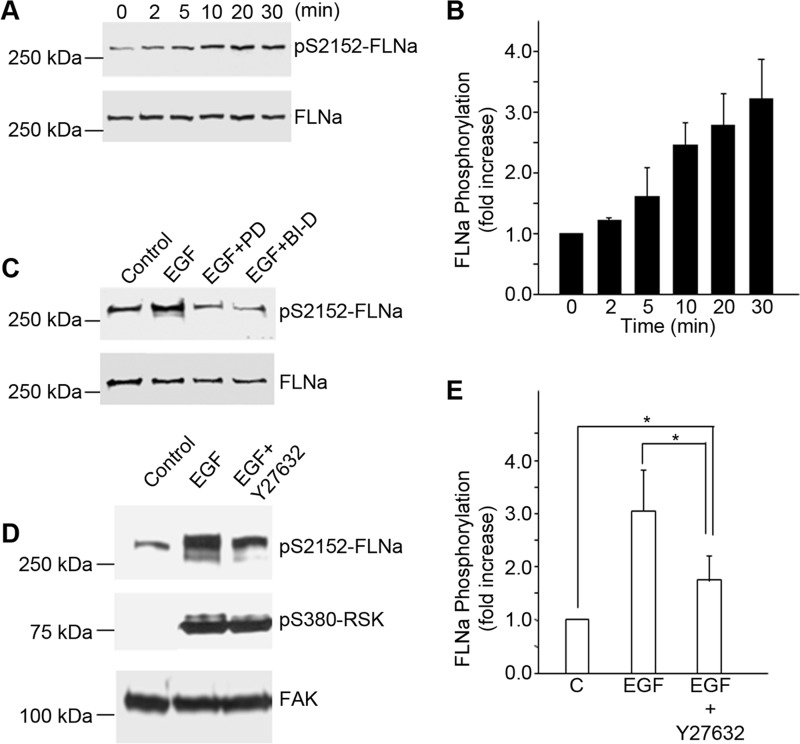
FLNa is a cytoskeleton-associated protein that binds integrin cytoplasmic domains and is known to regulate integrin activation states. FLNa is a direct target of p90RSK that phosphorylates FLNa at Ser-2152 (16). Western blot analysis of EGF-treated A431 cell lysates showed that phosphorylation of FLNa at Ser-2152 began 5–10 min following treatment of cells with EGF (Fig. 6A) and was increased by 3-fold within 30 min (Fig. 6B). FLNa phosphorylation in response to EGF was completely blocked by specific inhibitors of MEK (PD184352) and RSK (BI-D1870) (Fig. 6C), confirming that FLNa is a downstream target of an ERK/p90RSK signaling pathway in these cells. FLNa phosphorylation was also partially inhibited by the Rho kinase inhibitor Y27632 (Fig. 6D). Quantitation of the data from four separate experiments showed that inhibition of Rho kinase reduced the phosphorylation of FLNa by about half after EGF treatment (Fig. 6E). These data indicate that although the FLNa phosphorylation in response to EGF was dependent on p90RSK, the extent of phosphorylation could be modulated by Rho kinase. These findings suggest that the ERK/RSK and Rho/Rho kinase pathways converge on FLNa to effect changes in α5β1 function. Parallel experiments done on DiFi colon cancer cells showed that EGF also activated the same pathways, leading to the phosphorylation of FLNa and the inhibition of α5β1 function (data not shown).
FIGURE 6.
EGF-mediated phosphorylation of FLNa is dependent on MEK/ERK/p90RSK and Rho/Rho kinase pathways. A, A431 cells were stimulated with EGF (3 nm) at the indicated times. Nuclear free cell lysates were immunoblotted using antibodies to phosphorylated FLNa. Staining for total FLNa served as a loading control. pS2152-FLNa, phospho-Ser-2152 FLNa. B, bands for phospho-FLNa were quantified and normalized to total FLNa. Values are means ± S.D. of three separate experiments. C, A431 cells were pretreated for 1 h with an inhibitor of MEK, PD184352 (PD; 2.5 μm), or with an inhibitor of p90RSK, BI-D1870 (BI-D; 10 μm), before stimulation with EGF (3 nm) for 20 min. Lysates were prepared and analyzed by Western blotting using antibodies to phosphorylated FLNa. Staining with antibodies to FLNa served as a loading control. This experiment is representative of three separate experiments. D and E, A431 cells were pretreated for 1 h with an inhibitor of Rho kinase, Y27632 (10 μm), before stimulation with EGF (3 nm) for 20 min. Lysates were prepared and analyzed by Western blotting (D) using antibodies to phosphorylated FLNa or phosphorylated p90RSK. pS380-p90RSK, phospho-Ser-380 p90RSK. Staining with antibodies to FAK served as a loading control (C). Bands for phosphorylated FLNA were quantified (E) and normalized to FAK. Values are means ± S.D. of four separate experiments. Statistical analysis to determine significance was performed using Student's t test. *, p < 0.05.
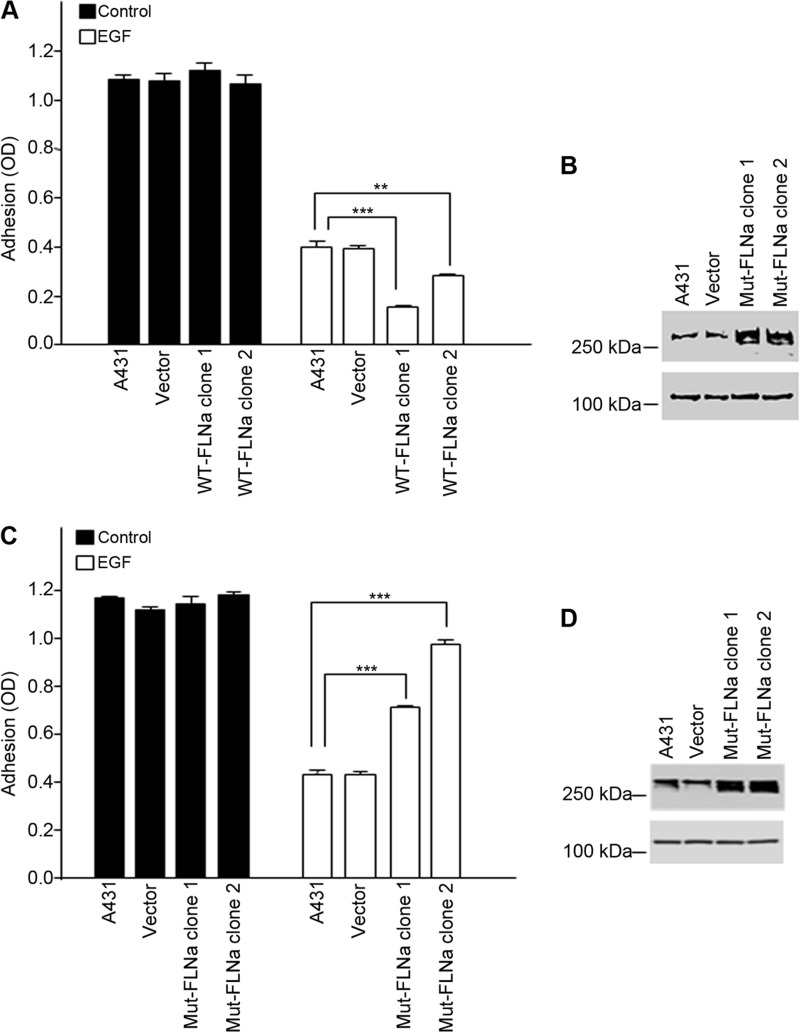
To address the role of FLNa phosphorylation in the regulation of integrin activity, stable A431 cell lines that expressed either Myc-tagged WT-FLNa or the non-phosphorylatable mutant S2152A (Mut-FLNa) were constructed. Two clonal cell lines expressing either wild-type FLNa (WT-FLNa clones 1 and 2) or mutant FLNa (Mut-FLNa clones 1 and 2) were selected for further analysis. In the absence of EGF, all cell lines attached to fibronectin (Fig. 7, A and C, black bars). Following the addition of EGF, cells expressing WT-FLNa were significantly less adherent to fibronectin than controls (Fig. 7A, white bars), whereas cells expressing FLNa(S2152A) were more adherent than control cells (Fig. 7C, white bars). As shown in Fig. 7B, cells transfected with WT-FLNa expressed 2–3-fold more FLNa than control cells, suggesting that the increased amount of FLNa had sensitized the cells to the inhibitory effects of EGF. Fig. 7D shows that cells expressed mutant FLNa in amounts equal to or slightly greater than endogenous levels, suggesting that the FLNa mutant functions as a dominant-negative to prevent EGF-dependent effects on integrin function. These findings suggest that in response to EGF, A431 cells regulate the functional activity of α5β1 integrin through the phosphorylation of FLNa.
FIGURE 7.
EGF inhibition of α5β1 function is mediated by phosphorylation of FLNa. A and C, clonal cell lines expressing either WT-FLNa (A) or the mutant S2152A (Mut-FLNa) (C) were compared in fibronectin-based adhesion assays in the absence (black bars) or presence (white bars) of EGF (3 nm) as described in the legend to Fig. 1. Parental cells (A431) or clones (vector) expressing only pcDNA3 served as controls. Statistical analysis to determine significance was performed using Student's t test, **, p < 0.01; ***, p < 0.001. Error bars indicate S.D. of triplicate samples for one of three representative experiments. B and D, lysates from parental cells (A431), clonal cells expressing only pcDNA3 (vector), or clonal cell lines expressing either WT-FLNa (B) or the S2152A mutant (Mut-FLNa) (D) were immunoblotted for FLNa and FAK. FAK staining served as a loading control. Western blots are representative of three separate experiments.
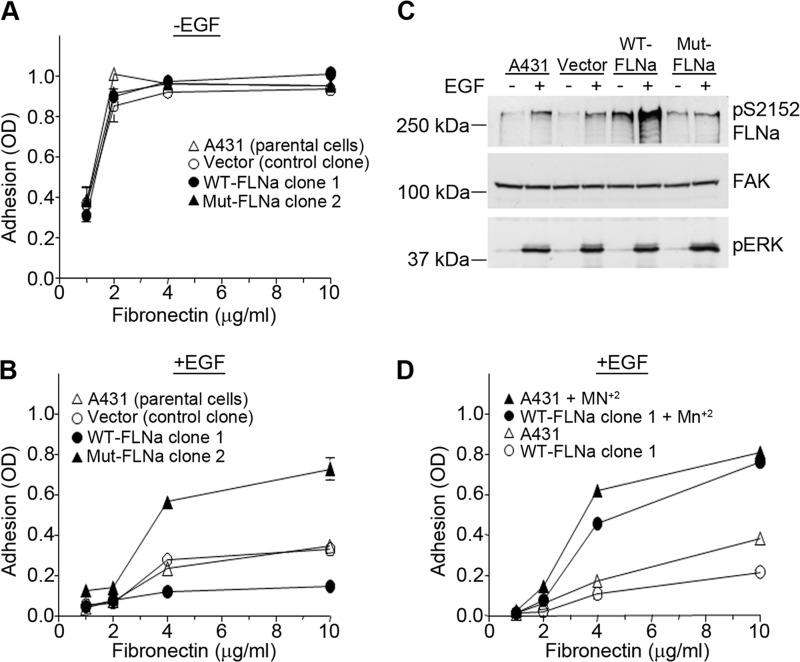
To characterize the effect of FLNa phosphorylation on integrin affinity, cell lines expressing wild-type or mutant FLNa were compared for their ability to adhere to substrates coated with increasing amounts of fibronectin. As shown in Fig. 8A, all cell lines adhered equally to all doses of fibronectin, indicating that ectopic expression of wild-type or mutant FLNa does not affect the base-line affinity of α5β1 integrin for fibronectin. In the presence of EGF, however, the cell lines demonstrated differential adhesion to fibronectin (Fig. 8B). The cell line expressing WT-FLNa was significantly less adherent than either the A431 parental or vector control cells. In contrast, the cell line expressing the non-phosphorylatable S2152A mutant exhibited significantly greater adhesion to fibronectin than control cells. These data indicate that in the presence of EGF, FLNa modulates the efficiency of cell adhesion to fibronectin, consistent with a role for FLNa in regulating the affinity of α5β1 integrin for fibronectin. To evaluate the amount of phosphorylated FLNa in each cell line, cell extracts were analyzed by Western blotting. As shown in Fig. 8C, EGF treatment resulted in an increase in phosphorylation of FLNa in control cells (A431 and vector) and in cells expressing WT-FLNa. However, EGF treatment did not result in an increase in phosphorylation in cells expressing mutant FLNa, suggesting that the non-phosphorylatable FLNa mutant was acting as a dominant-negative to block the phosphorylation of endogenous FLNa, thereby preventing the EGF-dependent inactivation of α5β1 integrin. The addition of 0.5 mm Mn2+ to cells expressing WT-FLNa rescued adhesion of cells to fibronectin (Fig. 8D), consistent with a role for FLNa in regulating integrin function by changing its activation state. Taken together, our data are consistent with a model in which EGF signaling activates the Rho/Rho kinase and ERK/p90RSK signaling pathways, which converge on FLNa to promote FLNa binding to and inactivation of α5β1 integrin.
FIGURE 8.
EGF modulates a5β1 integrin affinity through phosphorylation of FLNa. A and B, A431 parental cells, a vector control clone, WT-FLNa clone 1, and Mut-FLNa clone 2 were allowed to adhere to wells coated with increasing concentrations of fibronectin in the absence (A) or presence (B) of 3 nm EGF. Cell adhesion assays were performed as described in the legend to Fig. 1. Error bars indicate S.D. of triplicate samples for one of three representative experiments. C, A431 parental cells, a clone containing only the pcDNA3 vector, WT-FLNa clone 1, and Mut-FLNa clone 2 were treated with EGF (3 nm) for 20 min. Cell lysates were prepared, and phosphorylated FLNa was visualized by Western blotting using an antibody to phosphorylated FLNa. FAK was used as a loading control, and phospho-ERK (pERK) served as a positive control for EGF treatment. Western blots are representative of three separate experiments. pS2152 FLNa, phospho-Ser-2152 FLNa. D, A431 parental cells and WT-FLNa clone 1 were pretreated for 1 h at room temperature with or without 0.5 mm MnCl2. Cells were then seeded onto wells coated with increasing concentrations of fibronectin in the presence of 3 nm EGF. Cell adhesion was assessed as described in the legend to Fig. 1. Error bars indicate S.D. of triplicate samples for one of three representative experiments.
DISCUSSION
In this study, we defined a molecular pathway by which EGF controls the activation state of integrin receptors. Our data show that in colon and squamous cancer cells, EGF activates EGFR signaling, which leads to loss of α5β1 integrin function. Further investigation of the molecular mechanisms underlying this phenomenon indicated that in A431 as well as DiFi cells, EGF activates both the ERK/p90RSK and Rho/Rho kinase signaling pathways, which converge on FLNa to regulate inactivation of α5β1 integrin. These findings suggest that the partnering of FLNa with the β1 integrin cytoplasmic domain is regulated by both contractile forces and phosphorylation. An earlier analysis of the crystal structure of a three-domain FLNa fragment containing the primary integrin-binding site (IgFLNa19–21) showed that the binding of integrin cytoplasmic domains to FLNa is regulated by an autoinhibition mechanism whereby the primary integrin-binding site on IgFLNa21 is sterically blocked by the partially unfolded IgFLNa20 domain (9). Molecular dynamics simulations based on this structure have predicted that mechanical forces can unfold FLNa to expose the integrin-binding site and that phosphorylation of Ser-2152 within IgFLNa20 will facilitate this unfolding by lowering the force threshold required (10, 20). By preventing the association of activating molecules, such as talin, with the integrin cytoplasmic tail, FLNa helps to maintain the integrin in an inactive state (6). Our data showing that inactivation of α5β1 integrin in response to EGF is dependent on both FLNa phosphorylation and cellular contractility provide the first in vivo evidence in support of this model.
Dysregulation of signaling through EGFR and other ErbB family members is seen in many cancers. The molecular basis for this dysregulation varies considerably among different tumor types, and the impact of these altered signaling pathways on tumor progression, treatment, and subsequent relapse is not well understood but remains a highly important question in the design of EGFR-based treatment protocols (21, 22). Earlier studies have shown that adhesion of various tumor cell lines to the extracellular matrix can be modified either positively or negatively by EGF (14, 15). These studies provided suggestive evidence that in some tumors, EGFR signaling could regulate the activation state of the integrin. More recent studies have now demonstrated that EGF signaling can either positively or negatively control the activation state and function of several integrin receptors. Studies indicate that EGF can regulate integrin function by several mechanisms, including changing the amount of integrins on the cell surface, activating inside-out signaling pathways to affect ligand binding, and regulating outside-in signaling to selectively control integrin functions. In keratinocytes and squamous carcinoma, EGF signaling promotes α6β4 integrin inactivation and hemidesmosome disassembly (23, 24). In contrast, EGF positively regulates α5β1 and αvβ5 integrin activation in ovarian and pancreatic cancer cells to promote invasion and metastasis (25, 26). These data suggest that EGF regulation of integrin function is likely to be both integrin- and cell type-specific.
EGF-mediated inactivation of α5β1 in A431 cells is dependent on phosphorylation of FLNa by p90RSK. p90RSK is a member of the RSK family of Ser/Thr kinases and is a substrate for ERK1/2. In response to several different stimuli, including EGF, p90RSK is activated at the plasma membrane and controls a number of cellular processes, including cell growth, motility, and survival. These same cellular processes are also regulated by integrins, suggesting cross-talk between RSK signaling and integrin function. However, data that specifically address the details of this cross-talk are lacking. In response to mitogen stimulation, the canonical pathway to p90RSK activation proceeds through sequential ERK-dependent and PDK1-dependent phosphorylation events that are required for complete activation (27). Our data indicate that inhibition of p90RSK catalytic activity completely restores integrin function in EGF-treated cells. However, inhibition of ERK signaling only partially restores integrin activity in A431 cells. The inability of ERK inhibitors to completely restore integrin function suggests that ERK1/2-independent pathways to p90RSK activation may function in these cells. Such alternative mechanisms to p90RSK activation include membrane translocation and phosphorylation by p38 or ERK5 (28–30). Our findings indicate that FLNa is phosphorylated by p90RSK in A431 and DiFi cells and that, in these cancer cell lines, p90RSK-dependent phosphorylation of FLNa at Ser-2152 promotes the inactivation of α5β1 integrin in response to EGF.
The tumor microenvironment, particularly contact with the extracellular matrix, is now recognized as an essential regulator of tumor dormancy, a state of prolonged latency in which disseminated tumor cells remain in secondary sites, such as bone marrow and lymph nodes, for years with no clinical manifestation of malignancy (reviewed in Ref. 31). Loss of cell adhesion is thought to induce a state of dormancy in which the tumor cell is non-proliferative but capable of activating survival pathways, allowing it to avoid apoptosis and remain dormant. Recent studies have provided evidence that activation of β1 integrin plays a critical role in the release of metastatic tumor cells from dormancy. Three-dimensional culture systems used to model conditions of tumor dormancy have shown that ligation of β1 integrins and subsequent organization of the actin cytoskeleton are required for the conversion of quiescent tumor cells into proliferating cells (32–34). Identifying the mechanisms by which the integrin activation state regulates metastatic cell growth represents an important avenue of investigation. Understanding the complex interactions between the microenvironment and the tumor cell, particularly those that regulate the switch between proliferation and dormancy, may provide important clues toward developing strategies designed to control metastatic disease by maintaining the dormant state. Our studies define a molecular pathway by which metastatic cells may remain dormant under conditions in which prevailing levels of EGF are sufficient to activate EGFR kinase.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 CA58626.
- FLNa
- filamin A
- EGFR
- EGF receptor
- MLC2
- myosin light chain 2
- RSK
- ribosomal S6 kinase
- FAK
- focal adhesion kinase.
REFERENCES
- 1. Geiger B., Yamada K. M. (2011) Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 3, a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 3. Anthis N. J., Campbell I. D. (2011) The tail of integrin activation. Trends Biochem. Sci. 36, 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shattil S. J., Kim C., Ginsberg M. H. (2010) The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 11, 288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H., Calderwood D. A. (2003) Talin binding to integrin β tails: a final common step in integrin activation. Science 302, 103–106 [DOI] [PubMed] [Google Scholar]
- 6. Kiema T., Lad Y., Jiang P., Oxley C. L., Baldassarre M., Wegener K. L., Campbell I. D., Ylänne J., Calderwood D. A. (2006) The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell 21, 337–347 [DOI] [PubMed] [Google Scholar]
- 7. Calderwood D. A., Huttenlocher A., Kiosses W. B., Rose D. M., Woodside D. G., Schwartz M. A., Ginsberg M. H. (2001) Increased filamin binding to β-integrin cytoplasmic domains inhibits cell migration. Nat. Cell Biol. 3, 1060–1068 [DOI] [PubMed] [Google Scholar]
- 8. Takala H., Nurminen E., Nurmi S. M., Aatonen M., Strandin T., Takatalo M., Kiema T., Gahmberg C. G., Ylänne J., Fagerholm S. C. (2008) β2 integrin phosphorylation on Thr-758 acts as a molecular switch to regulate 14–3-3 and filamin binding. Blood 112, 1853–1862 [DOI] [PubMed] [Google Scholar]
- 9. Lad Y., Kiema T., Jiang P., Pentikäinen O. T., Coles C. H., Campbell I. D., Calderwood D. A., Ylänne J. (2007) Structure of three tandem filamin domains reveals autoinhibition of ligand binding. EMBO J. 26, 3993–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pentikäinen U., Ylänne J. (2009) The regulation mechanism for the autoinhibition of binding of human filamin A to integrin. J. Mol. Biol. 393, 644–657 [DOI] [PubMed] [Google Scholar]
- 11. Cabodi S., Di Stefano P., Leal Mdel P., Tinnirello A., Bisaro B., Morello V., Damiano L., Aramu S., Repetto D., Tornillo G., Defilippi P. (2010) Integrins and signal transduction. Adv. Exp. Med. Biol. 674, 43–54 [DOI] [PubMed] [Google Scholar]
- 12. Moro L., Venturino M., Bozzo C., Silengo L., Altruda F., Beguinot L., Tarone G., Defilippi P. (1998) Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 17, 6622–6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyamoto S., Teramoto H., Gutkind J. S., Yamada K. M. (1996) Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135, 1633–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Genersch E., Schuppan D., Lichtner R. B. (1996) Signaling by epidermal growth factor differentially affects integrin-mediated adhesion of tumor cells to extracellular matrix proteins. J. Mol. Med. 74, 609–616 [DOI] [PubMed] [Google Scholar]
- 15. Genersch E., Schneider D. W., Sauer G., Khazaie K., Schuppan D., Lichtner R. B. (1998) Prevention of EGF-modulated adhesion of tumor cells to matrix proteins by specific EGF receptor inhibition. Int. J. Cancer 75, 205–209 [DOI] [PubMed] [Google Scholar]
- 16. Woo M. S., Ohta Y., Rabinovitz I., Stossel T. P., Blenis J. (2004) Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol. Cell. Biol. 24, 3025–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKeown-Longo P. J., Mosher D. F. (1985) Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J. Cell Biol. 100, 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gross M. E., Zorbas M. A., Danels Y. J., Garcia R., Gallick G. E., Olive M., Brattain M. G., Boman B. M., Yeoman L. C. (1991) Cellular growth response to epidermal growth factor in colon carcinoma cells with an amplified epidermal growth factor receptor derived from a familial adenomatous polyposis patient. Cancer Res. 51, 1452–1459 [PubMed] [Google Scholar]
- 19. Masui H., Castro L., Mendelsohn J. (1993) Consumption of EGF by A431 cells: evidence for receptor recycling. J. Cell Biol. 120, 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen H. S., Kolahi K. S., Mofrad M. R. (2009) Phosphorylation facilitates the integrin binding of filamin under force. Biophys. J. 97, 3095–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carter C. A., Giaccone G. (2012) Treatment of nonsmall cell lung cancer: overcoming the resistance to epidermal growth factor receptor inhibitors. Curr. Opin. Oncol. 24, 123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehra R., Serebriiskii I. G., Dunbrack R. L., Jr., Robinson M. K., Burtness B., Golemis E. A. (2011) Protein-intrinsic and signaling network-based sources of resistance to EGFR- and ErbB family-targeted therapies in head and neck cancer. Drug Resist. Updat. 14, 260–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frijns E., Kuikman I., Litjens S., Raspe M., Jalink K., Ports M., Wilhelmsen K., Sonnenberg A. (2012) Phosphorylation of threonine 1736 in the C-terminal tail of integrin β4 contributes to hemidesmosome disassembly. Mol. Biol. Cell 23, 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kashyap T., Germain E., Roche M., Lyle S., Rabinovitz I. (2011) Role of β4 integrin phosphorylation in human invasive squamous cell carcinoma: regulation of hemidesmosome stability modulates cell migration. Lab. Invest. 91, 1414–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lau M. T., So W. K., Leung P. C. (2012) Integrin β1 mediates epithelial growth factor-induced invasion in human ovarian cancer cells. Cancer Lett. 320, 198–204 [DOI] [PubMed] [Google Scholar]
- 26. Ricono J. M., Huang M., Barnes L. A., Lau S. K., Weis S. M., Schlaepfer D. D., Hanks S. K., Cheresh D. A. (2009) Specific cross-talk between epidermal growth factor receptor and integrin αvβ5 promotes carcinoma cell invasion and metastasis. Cancer Res. 69, 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Callaghan D. S., O'Donnell D., O'Connell F., O'Byrne K. J. (2010) The role of inflammation in the pathogenesis of non-small cell lung cancer. J. Thorac. Oncol. 5, 2024–2036 [DOI] [PubMed] [Google Scholar]
- 28. Richards S. A., Dreisbach V. C., Murphy L. O., Blenis J. (2001) Characterization of regulatory events associated with membrane targeting of p90 ribosomal S6 kinase 1. Mol. Cell. Biol. 21, 7470–7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zaru R., Ronkina N., Gaestel M., Arthur J. S., Watts C. (2007) The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat. Immunol. 8, 1227–1235 [DOI] [PubMed] [Google Scholar]
- 30. Ranganathan A., Pearson G. W., Chrestensen C. A., Sturgill T. W., Cobb M. H. (2006) The MAP kinase ERK5 binds to and phosphorylates p90RSK. Arch. Biochem. Biophys. 449, 8–16 [DOI] [PubMed] [Google Scholar]
- 31. Klein C. A. (2011) Framework models of tumor dormancy from patient-derived observations. Curr. Opin. Genet. Dev. 21, 42–49 [DOI] [PubMed] [Google Scholar]
- 32. Shibue T., Weinberg R. A. (2009) Integrin β1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. 106, 10290–10295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barkan D., Kleinman H., Simmons J. L., Asmussen H., Kamaraju A. K., Hoenorhoff M. J., Liu Z. Y., Costes S. V., Cho E. H., Lockett S., Khanna C., Chambers A. F., Green J. E. (2008) Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barkan D., Chambers A. F. (2011) β1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clin. Cancer Res. 17, 7219–7223 [DOI] [PubMed] [Google Scholar]