Background: Thioredoxins (Trxs) play a crucial role in the oxidative stress response.
Results: A redox-sensing transcriptional regulator, RexT, controls expression of TrxA2, and TrxA2 regulates the DNA binding activity of RexT.
Conclusion: The RexT-TrxA2 regulatory system regulates gene expression in response to redox state.
Significance: This is the first report on a transcriptional regulator of the trx gene in cyanobacteria.
Keywords: Cyanobacteria, Oxidative Stress, Redox Regulation, Thioredoxin, Transcription Regulation
Abstract
Thioredoxins are ubiquitous proteins that catalyze thiol-disulfide redox reactions. They have a crucial role in the oxidative stress response as well as the redox regulation of metabolic enzymes. In cyanobacteria, little is known about the regulation of trx gene expression despite the importance of thioredoxins in cellular functions. In the present study, transcriptional regulation of the trx genes under oxidative stress conditions was investigated in the heterocystous cyanobacterium Anabaena sp. strain PCC 7120. When cells were exposed to H2O2, only the trxA2 gene (all1866) of seven trx genes was induced. Disruption of the rexT gene (alr1867), encoding a transcriptional regulator of the ArsR family, resulted in increased expression of trxA2. RexT bound to the region downstream of the transcription initiation site of trxA2. The DNA binding activity of RexT was impaired by H2O2 through the formation of an intramolecular disulfide bond, which induced expression of the trxA2 gene. The inactivated DNA binding activity of RexT was restored by reduced TrxA2. Hence, RexT is considered as a redox-sensing transcriptional repressor of trxA2. These results support the idea that the RexT-TrxA2 regulatory system is important for the oxidative stress response in this cyanobacterium.
Introduction
Cyanobacteria are a large group of eubacteria characterized by oxygen-evolving photosynthesis. Anabaena sp. strain PCC 7120 (hereafter Anabaena PCC 7120) is a filamentous cyanobacterium in which certain vegetative cells differentiate into heterocysts, which are specialized cells for nitrogen fixation (1, 2). Heterocysts provide the microoxic environment for the oxygen-labile nitrogenase complex, the enzyme responsible for nitrogen fixation (3). Heterocysts are unable to carry out photosynthesis and depend on vegetative cells for carbohydrate to generate reductant required for nitrogen fixation. The oxidative pentose phosphate pathway is assumed to be the major route for sugar catabolism and production of reductant in heterocysts. Glucose-6-phosphate dehydrogenase, which controls the entry of carbon into the oxidative pentose phosphate pathway, is essential for nitrogen fixation (4, 5). Glucose-6-phosphate dehydrogenase is reductively inactivated and oxidatively reactivated by thioredoxins (Trxs)2 to prevent a futile cycling, which would occur if the Calvin and oxidative pentose phosphate pathways operated simultaneously (6, 7). Although Trxs have been shown to be present in heterocysts, whether they operate in heterocysts is unclear (8).
Trxs are small disulfide-containing redox proteins that reduce disulfide bonds in other proteins. They can act both as a modulator of enzyme activity by reducing regulatory disulfide bonds in a target protein and as a reducing agent. Trxs regulate a number of Calvin cycle enzymes such as fructose-1,6-bisphosphatase, glyceraldehyde-3-phosphate dehydrogenase, sedoheptulose-1,7-bisphosphatase, and phosphoribulokinase as well as glucose-6-phosphate dehydrogenase in chloroplast (9, 10). Moreover, more than 50 proteins involved in various physiological functions have been indicated to interact with Trxs in the cyanobacterium Synechocystis sp. PCC 6803, suggesting their regulatory roles in a wide variety of cellular processes (11–13).
Transcriptional regulation of trx genes has been well studied in bacteria. In Escherichia coli, the transcriptional regulator OxyR, which is activated by H2O2, regulates genes necessary for the defense against oxidative stress, including trxC (14). The redox-sensing transcriptional regulator Spx of Bacillus subtilis activates the transcription of trxA (15). In actinobacteria such as Streptomyces coelicolor and Corynebacterium glutamicum, the trx gene is under the control of the extracytoplasmic function σ factors SigR and SigH, respectively (16, 17). In Synechocystis sp. PCC 6803, expression of the trx genes is not induced by oxidative stress (18, 19), whereas transcript levels of the trxA and trxB genes are rapidly decreased by transitions from light to dark and restored to the original levels by reillumination (13). It has been suggested that the redox state of the photosynthetic electron transport chain regulates the expression of trxA and trxB, but their molecular mechanisms such as acting as sensor proteins and transcriptional regulators have not been revealed.
In the genome of Anabaena PCC 7120, there are seven trx genes (20). The trxA2 gene (all1866) has been shown to be up-regulated by nitrogen deprivation (21). In this study, we investigated transcriptional regulation of the trx genes by oxidative stress. It was found that the trxA2 gene was induced by H2O2, and a redox-sensing transcriptional regulator encoded by the rexT gene (alr1867) controlled the expression of trxA2. The DNA binding activity of RexT was regulated through the formation of an intramolecular disulfide bond, and TrxA2 was able to reduce the disulfide bond of RexT. These data suggest that the RexT-TrxA2 system is important for the oxidative stress response in Anabaena PCC 7120. To our knowledge, this is the first report on a transcriptional regulator of the trx gene in cyanobacteria.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Culture Conditions
Anabaena sp. strain PCC 7120 and a rexT disruptant (DR1867K) were grown in BG-11 medium as described previously (21). Neomycin was added to the medium at a final concentration of 30 μg/ml when required. Oxidative stress conditions were set up by addition of 3 mm H2O2 to cultures in the midlogarithmic phase (OD750 of 0.4–0.5).
Mutant Construction
All primers used in this study (supplemental Table S1) were designed based on genome data from CyanoBase (22). The rexT gene was inactivated as follows. DNA fragments upstream and downstream of the rexT gene were amplified by PCR using the primer pairs 1867-5F and 1867-5R and 1867-3F and 1867-3R, respectively (supplemental Table S1). The upstream fragment was cloned between the SacI and BamHI sites of pBluescript II KS+ (Agilent Technologies), and then the downstream fragment was cloned between the BamHI and XhoI sites. A neomycin resistance cassette excised by digestion with BamHI from the plasmid pRL161 (23) was inserted into the BamHI site between the upstream and downstream fragments. The SacI-XhoI fragment was excised from the resultant plasmid and cloned between the SacI and XhoI sites of pRL271 (24) to construct pR1867K. pR1867K was transferred by conjugation into Anabaena PCC 7120 according to Elhai and Wolk (25) to construct the rexT mutant DR1867K.
Quantitative Reverse Transcription-PCR
Total RNA was extracted from whole filaments according to Pinto et al. (26) and treated with DNase I (Takara Bio, Shiga, Japan). cDNAs were synthesized with specific primers using the PrimeScript first strand cDNA synthesis kit (Takara Bio), and quantitative RT-PCR was performed as described previously (27). Relative transcript levels were normalized with the value for 16 S rRNA and are represented as means of triplicate experiments.
Mapping of Transcription Initiation Sites by Rapid Amplification of cDNA Ends-PCR
The transcription initiation site of the trxA2 gene was determined using the SMARTer RACE cDNA Amplification kit (Takara Bio) as described previously (27). The resulting PCR product was cloned into the pGEM-T Easy vector (Promega), and seven clones were sequenced.
Expression and Purification of His-RexT and His-TrxA2
For construction of an expression plasmid for the histidine-tagged RexT protein, a DNA fragment containing the alr1867 coding region was amplified by PCR using the primer pair 1867-F and 1867-R. The amplified DNA fragment was cloned between the NdeI and EcoRI sites of the pCold II expression vector (Takara Bio) to construct pC1867. E. coli BL21(DE3) harboring pC1867 was grown at 37 °C in 200 ml of Luria-Bertani medium supplemented with carbenicillin (50 μg/ml). The recombinant gene was expressed in exponentially growing cells (A600 of 0.4) by rapidly chilling the culture medium to 15 °C and adding 0.1 mm isopropyl β-d-thiogalactopyranoside. After 24 h of incubation at 15 °C, the cells were harvested by centrifugation. His-RexT was purified with the Ni-NTA Fast Start kit (Qiagen). The elution fraction containing the purified protein was loaded onto a PD MidiTrap G-25 column (GE Healthcare) equilibrated with 20 mm Tris-HCl (pH 8.0), 0.1 m NaCl, 10% glycerol, and the protein was eluted with the same buffer. Cysteine residues at position 40, 41, and 105 of RexT were replaced with serine by site-directed mutagenesis using the PrimeSTAR mutagenesis basal kit (Takara Bio). Serine-substituted RexT proteins were expressed and purified as described for His-RexT.
His-TrxA2 was expressed from expression plasmid pCTrxA2 and purified as described for His-RexT. pCTrxA2 was constructed as follows. A DNA fragment amplified by PCR using the primer pair TrxA2-F and TrxA2-R was cloned between the NdeI and SalI sites of the pCold II vector. A PD MidiTrap G-25 column equilibrated with 25 mm Tris-HCl (pH 7.9), 150 mm NaCl, 10% glycerol was used for buffer exchange of purified His-TrxA2.
Gel Mobility Shift Assay
Gel mobility shift assays were carried out using His-RexT and 0.2 pmol of a DNA probe including the intergenic region between trxA2 and rexT in 20 μl of binding buffer (20 mm Tris-HCl (pH 8.0), 100 mm NaCl, 10 mm MgCl2, 1 mm dithiothreitol (DTT), 10% glycerol). The mixtures were incubated for 30 min at room temperature and then subjected to electrophoresis on a native 5% polyacrylamide gel. DNA probes were visualized by staining with ethidium bromide.
Thiol Redox State Analyses
His-RexT and its derivatives (10 μg) were reduced by incubation with 1 mm DTT and then oxidized with 3 mm H2O2. Proteins were precipitated by addition of TCA at a final concentration of 10% (w/v) and dissolved in 200 mm Tris-HCl (pH 8.0) containing 1% SDS and 15 mm 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS) (Invitrogen). After incubation for 2 h in the dark, 1-μg aliquots of proteins were separated by non-reducing SDS-PAGE.
Mass Spectrometry (MS) Analysis
H2O2-oxidized His-RexT was incubated with 10 mm N-ethylmaleimide (NEM) for 15 min at room temperature. The alkylated His-RexT was subjected to non-reducing SDS-PAGE, and Coomassie Brilliant Blue-stained bands were excised. The protein was in-gel digested with trypsin, and analyzed by MALDI-TOF MS (Voyager-DE STR, Applied Biosystems).
RESULTS
Expression of trxA2 Is Induced by Hydrogen Peroxide and Negatively Regulated by RexT
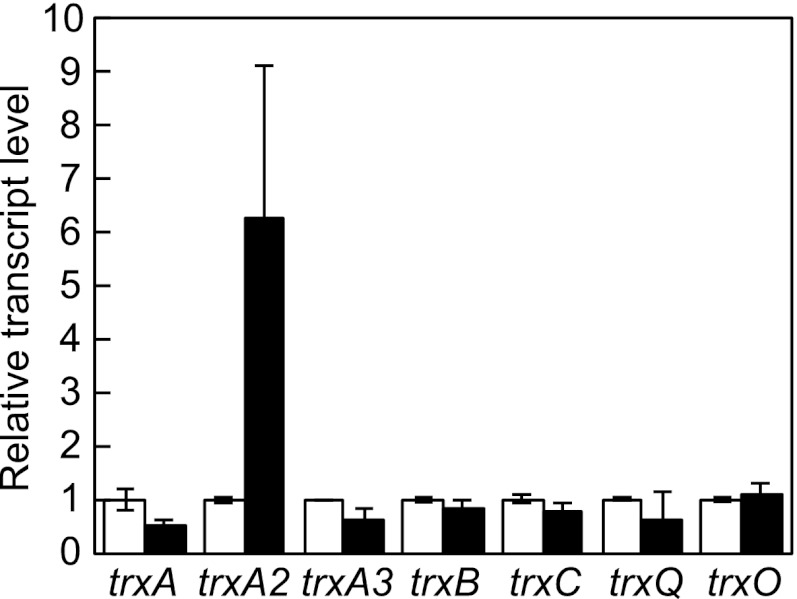
The complete sequence of the Anabaena PCC 7120 genome revealed seven trx genes (20, 28). According to the phylogenetic analyses of trx genes (28, 29) and the nomenclature of trx genes of Synechocystis PCC 6803 (13), we designated trx genes of Anabaena PCC 7120 as follows: alr0052 (trxA), all1866 (trxA2), all2367 (trxA3), all2341 (trxB), alr3955 (trxC), all1893 (trxQ), and alr2205 (trxO). The products of trxA, trxA2, and trxA3 are related to the plant m-type Trx, and trxB and trxQ encode an x- and y-type Trx, respectively (28, 29). The transcript levels of trx genes are known to be up-regulated by oxidative stress in various bacteria such as E. coli, B. subtilis, and S. coelicolor (14, 16, 30). Changes in expression of the trx genes in response to oxidative stress were investigated in Anabaena PCC 7120. Filaments in the midlogarithmic phase were subjected to 3 mm H2O2 for 10 min, and the transcript levels of the trx genes were determined by quantitative RT-PCR (Fig. 1). Only expression of trxA2 was induced by H2O2. The transcript level of trxA2 was increased about 6-fold after H2O2 treatment. A lower concentration of H2O2 (1 mm) did not induce trxA2 expression (data not shown).
FIGURE 1.
Changes in the transcript levels of the trx genes in response to H2O2. The relative transcript levels of the trx genes before (white bars) and 10 min after addition of 3 mm H2O2 (black bars) were determined by quantitative RT-PCR. The transcript level before the H2O2 addition was taken as 1 for each gene. Means ± S.D. (error bars) of three independent experiments are shown.
The alr1867 gene, encoding a transcriptional regulator of the ArsR family, is located upstream of trxA2 in the opposite direction (Fig. 2). This genetic organization is also conserved among other cyanobacteria such as Anabaena variabilis ATCC 29413, Nostoc punctiforme PCC 73102, Cyanothece sp. PCC 7425, and Acaryochloris marina MBIC11017. To ascertain the physiological role of Alr1867 protein as a transcriptional regulator of trxA2, the alr1867 gene was deleted from the genome of Anabaena PCC 7120 to construct an alr1867 disruptant, DR1867K. Expression of trxA2 was drastically increased in DR1867K. The transcript level of trxA2 in DR1867K was more than 100-fold higher (122.1 ± 10.9) than the wild-type level under normal growth conditions, whereas expression of the other trx genes was not affected by disruption of alr1867 (data not shown). These results indicate that Alr1867 protein is a negative regulator of trxA2. We designated the alr1867 gene as rexT (redox-sensing transcriptional regulator of thioredoxin A2).
FIGURE 2.
The nucleotide sequence of the intergenic region between trxA2 and rexT. The coding regions of trxA2 and rexT are shaded in gray. A bent arrow and boldface letters indicate the identified transcription initiation site of trxA2 and the −10 and −35 promoter regions, respectively. The entire sequence corresponds to DNA probe F1 used for gel mobility shift assays in Fig. 3. DNA probes F2 and F3 are shown with dotted lines. Boxes D1–D4 are regions deleted from probe F1 in probes D1 to D4, respectively. The inverted repeat sequence found within the RexT-binding site is indicated with arrows.
RexT Protein Binds to the Promoter Region of trxA2
Gel mobility shift assays were carried out with purified His-RexT protein and DNA probe F1 including the intergenic region between trxA2 and rexT (Figs. 2 and Fig. 3A). His-RexT reduced the electrophoretic mobility of probe F1, and the amount of the RexT-F1 complex increased in proportion to the concentration of His-RexT (Fig. 3A). No interaction between His-RexT and DNA probe F2 or F3, which is an internal fragment of the coding region of trxA2 or rexT, respectively (Fig. 2), was observed (Fig. 3A). These observations led to the conclusion that RexT binds to the intergenic region between trxA2 and rexT in a sequence-specific manner.
FIGURE 3.
Gel mobility shift assays with His-RexT and the promoter region of the trxA2 gene. A, probe F1 (10 nm) was mixed with His-RexT in the amounts indicated above each lane, and then the mixtures were subjected to electrophoresis. Probe F2 (lanes 1–4) or probe F3 (lanes 5–8) was added in a final concentration of 30 nm as a competitor. B, the binding of His-RexT to DNA probes D1, D2, D3, and D4 was determined. The sequences of DNA probes are shown in Fig. 2. White, gray, and black arrowheads indicate free probes, competitor DNA, and protein-DNA complexes, respectively.
The transcription initiation site of trxA2 was determined by rapid amplification of cDNA ends-PCR experiments. The sequence of cloned PCR products was determined by the dye terminator method. The 5′-end of five clones of seven clones sequenced was situated at position −40 with respect to the translation start site of the trxA2 gene (Fig. 2). Putative −10 and −35 promoter regions were found upstream of the transcription initiation site (Fig. 2). To define the promoter region interacting with RexT, four DNA probes, D1 to D4, in which a 20-bp portion of the intergenic region was deleted were prepared (Fig. 2). Although RexT was able to bind to probes D1, D3, and D4, affinity of RexT to probe D2 was markedly reduced (Fig. 3B), suggesting that the region from −18 to −37 with respect to the translation start site is important for the binding of RexT to the promoter region of trxA2.
DNA Binding Activity of RexT Is Regulated through the Formation of an Intramolecular Disulfide Bond
Expression of the trxA2 was induced by H2O2 (Fig. 1). The effects of H2O2 on the DNA binding activity of RexT were determined by gel mobility shift assays (Fig. 4). Binding of RexT to probe F1 was prevented by addition of H2O2 (Fig. 4A). Addition of an excess of the reducing agent DTT restored the DNA binding activity of RexT that was inactivated by H2O2 (Fig. 4B), indicating that the effects of oxidation and reduction on the DNA binding activity of RexT are reversible.
FIGURE 4.
Redox-sensitive control of the DNA binding activity of RexT. His-RexT (1 μm) and probe F1 were incubated in the presence of 1 mm DTT (A) or 1 mm H2O2 (B) for 15 min, and then H2O2 (A) or DTT (B) was added in the amounts indicated above each lane. After 15 min, the mixtures were subjected to electrophoresis. Lane 1, His-RexT was not added.
The RexT protein contains 3 Cys residues, Cys-40, Cys-41, and Cys-105. These Cys residues are likely to be involved in the redox-sensitive control of the DNA binding activity. When the His-RexT protein oxidized with H2O2 was subjected to non-reducing SDS-PAGE, only one band of a molecular mass of ∼14 kDa, which corresponded to the monomeric form of His-RexT (14.1 kDa), was observed (Fig. 5A), indicating that oxidized His-RexT does not undergo dimerization. We next investigated whether the thiol groups of Cys residues of His-RexT are modified by treatment with H2O2 using AMS. AMS covalently modifies free thiol groups, retarding electrophoretic mobility in proportion to the number of free thiol groups in proteins. The electrophoretic mobility of His-RexT treated with DTT was retarded by AMS modification (Fig. 5B, lanes 1 and 2). His-RexT treated with H2O2 was also modified by AMS, although it migrated faster than reduced His-RexT (Fig. 5B, lanes 2–4). The molecular masses of His-RexT modified with one, two, or three AMS molecules are 14.6, 15.2, and 15.7 kDa, respectively. Compared with molecular mass markers and RexT derivatives that contain 1 or 2 Cys residues (Fig. 5B), it was indicated that 1 Cys residue of RexT was free when it was oxidized. These results support the conclusion that RexT is inactivated by oxidation through the formation of an intramolecular disulfide bond.
FIGURE 5.
Oxidation of Cys residues of RexT. A, purified His-RexT proteins (1 μg) incubated with 1 mm DTT (lane 1) or 1 mm H2O2 (lane 2) were separated by non-reducing SDS-PAGE. Lane M, molecular mass markers. B, oxidation of Cys residues of RexT was determined as follows. Proteins (1 μg) treated with 1 mm DTT were further incubated with (+) or without (−) 3 mm H2O2, and then free thiol groups were modified with AMS. The modified samples were separated by non-reducing SDS-PAGE. Open arrowheads indicate the shifted band of proteins modified with one, two, or three AMS molecules. Red, reduction; Ox, oxidation.
To determine which Cys residues of RexT are involved in the formation of a disulfide bond, each Cys residue was replaced with a Ser residue to generate C40S, C41S, and C105S proteins, which were designated as 2-Cys mutants. 2-Cys mutant proteins decreased the electrophoretic mobility of probe F1 (Fig. 6A). Binding of 2-Cys mutant proteins to probe F1 was also prevented by addition of H2O2, although concentrations required for inactivation were higher than that of the wild-type protein (Figs. 4A and 6A). Addition of DTT restored the DNA binding activity of 2-Cys mutant proteins that were inactivated by 3 mm H2O2, indicating that Cys residues were not overoxidized to sulfinic or sulfonic acids (Fig. 6B). The oxidized C40S and C105S proteins were not modified with AMS, but C41S proteins were not fully oxidized by 3 mm H2O2 (Fig. 6C), consistent with the partial inactivation of C41S by 3 mm H2O2 (Fig. 6A). These results indicate that 3 Cys residues of RexT are capable of forming a disulfide bond between any two of them.
FIGURE 6.
Redox response of 2-Cys mutant proteins of RexT. The effect of H2O2 (A) and DTT (B) on the DNA binding activities of 2-Cys mutant proteins was determined by gel mobility shift assays as described in Fig. 4. C, oxidation of cysteine residues of RexT and 2-Cys mutant proteins was determined as described in Fig. 5B.
To further investigate the correlation between the inactivation of RexT and the formation of a disulfide bond, we prepared 1-Cys mutant proteins of RexT, C41S,C105S, C40S,C105S, and C40S,C41S, which contain only 1 Cys residue. 1-Cys mutant proteins bound to probe F1 in a sequence-specific manner (Fig. 7A). However, the DNA binding activities of 1-Cys mutant proteins were not impaired by H2O2 (Fig. 7B). A thiol group of C40S,C105S and C40S,C41S proteins was free after treatment with H2O2, and half of C41S,C105S proteins were oxidized by H2O2 (Fig. 7C). These results confirm that RexT is inactivated through the formation of an intramolecular disulfide bond.
FIGURE 7.
Redox response of 1-Cys mutant proteins of RexT. A, gel mobility shift assays with 1-Cys mutant proteins were carried out as described in Fig. 3A. B and C, the effect of H2O2 on the DNA binding activities and oxidation of cysteine residues of 1-Cys mutant proteins were determined as described in Fig. 6, A and C.
Mass Spectrometric Analysis of Oxidized RexT
To ascertain whether all 3 Cys residues are indeed involved in the disulfide bond formation, oxidized RexT was analyzed by MS. The oxidized RexT was alkylated by NEM before trypsin digestion and MALDI-TOF MS analysis. Cys residues that are present in a disulfide bond are not modified with NEM. The results of MS analysis are summarized in Table 1 and Fig. 8. Peptide 1 containing Cys-40 and Cys-41 was identified; its mass was lower by 2.03 Da than the predicted mass. The identification of peptide 3 containing Cys-105 modified with NEM suggested that some oxidized RexT proteins would contain a disulfide bond between Cys-40 and Cys-41 and free Cys-105. Moreover, peptide 5 with a mass corresponding to the sum of two tryptic peptides, 37–50 and 98–112, and one NEM modification was identified. The observed mass was 2.01 Da lower than that predicted for the sum of the individual peptides, indicating that the 37–50 and 98–112 peptides were cross-linked by a disulfide bond. Although it was not possible to distinguish whether a disulfide bond was formed between Cys-105 and Cys-40 or Cys-41 from this analysis, it was indicated that Cys-105 was also involved in the disulfide bond formation. Peptides 2 and 4 contained a free Cys residue, which could result from reduction of a disulfide bond during MS analysis or might be protected from NEM modification.
TABLE 1.
Mass spectrometry results of oxidized RexT
| Peptide | Sequence | NEMa |
Mr |
Intensity | |
|---|---|---|---|---|---|
| Calb | Expc | ||||
| 1 (37–50) | GEQCCAEFDFAIAK | 0 | 1,531.66 | 1,529.63 | 143,313 |
| 2 (98–112) | SAQPLLTCQQSAIVK | 0 | 1,586.86 | 1,586.85 | 20,828 |
| 3 (98–112) | SAQPLLTCQQSAIVK | 1 | 1,711.90 | 1,711.89 | 18,998 |
| 4 (37–50 + 98–112) | GEQCCAEFDFAIAK + SAQPLLTCQQSAIVK | 0 | 3,117.50 | 3,115.48 | 7,974 |
| 5 (37–50 + 98–112) | GEQCCAEFDFAIAK + SAQPLLTCQQSAIVK | 1 | 3,242.55 | 3,240.54 | 68,154 |
a Number of cysteine residues modified with NEM.
b Relative molecular mass based on the matched peptide sequence.
c Experimental m/z.
FIGURE 8.
Mass spectrometry analysis of oxidized RexT. Oxidized RexT was analyzed by MALDI-TOF MS after tryptic digestion. Cys residues forming a disulfide bond and modified with NEM are indicated with an asterisk and +, respectively. Because it was not possible to distinguish whether a disulfide bond was formed between Cys-105 and Cys-40 or Cys-41 from this analysis, both residues are indicated with an asterisk in parentheses.
TrxA2 Regulates the DNA Binding Activity of RexT
Because Trx catalyzes disulfide-dithiol exchange reactions, we determined whether TrxA2 was able to reduce the disulfide bond in the oxidized form of RexT. The DNA binding activity of RexT that was inactivated by 1 mm H2O2 was not restored by addition of 0.5 mm DTT (Fig. 9). However, incubation with various concentrations of TrxA2 revealed that as the concentration of TrxA2 was increased the DNA binding activity of RexT was gradually restored (Fig. 9). As shown above, RexT is inactivated through the formation of an intramolecular disulfide bond. Thus, TrxA2 is able to reduce the intramolecular disulfide bond of RexT.
FIGURE 9.
Effects of TrxA2 on the DNA binding activity of RexT. His-RexT (1 μm) and probe F1 were incubated in the presence of 1 mm H2O2 for 15 min, and then DTT and TrxA2 were added in the amounts indicated above each lane. After 15 min, the mixtures were subjected to electrophoresis. Lane 1, His-RexT was not added.
DISCUSSION
In the present study, transcriptional regulation of the trx genes under oxidative stress conditions was investigated in Anabaena PCC 7120. We demonstrated that only the trxA2 gene was induced by H2O2 (Fig. 1), and RexT was a redox-sensing transcriptional regulator of the trxA2 gene. Disruption of the rexT gene resulted in increased expression of trxA2, and the DNA binding activity of RexT was inhibited by H2O2 through the formation of an intramolecular disulfide bond (Figs. 4 and 8), suggesting that expression of trxA2 was derepressed by inactivation of the transcriptional repressor RexT. Moreover, the DNA binding activity of oxidized RexT was restored by incubation with reduced TrxA2 (Fig. 9). Thus, TrxA2 controls expression of its structural gene by regulating the DNA binding activity of RexT depending on redox state.
The RexT protein contains 3 Cys residues. An intramolecular disulfide bond was formed between any 2 of 3 Cys residues, and every disulfide bond inactivated the DNA binding activity of RexT (Figs. 6 and 8). Moreover, the mutant proteins that contained only 1 Cys residue were not sensitive to H2O2, indicating that the formation of a disulfide bond was essential for the inactivation of RexT protein (Fig. 7). It is worth noting that C41S mutant protein was not fully oxidized and inactivated with 3 mm H2O2, suggesting that Cys-41 is highly susceptible to oxidation (Fig. 6). Cys-41 is conserved among RexT orthologues of cyanobacteria and is predicted to be positioned in the helix-turn-helix domain (supplemental Fig. S1). Orthologous proteins of RexT in A. variabilis and N. punctiforme also contain Cys-40, and Cys-105 is characteristic of Anabaena spp. Orthologous proteins in Cyanothece PCC 7425 and A. marina have another Cys residue at position 68 and 46, respectively. Thus, the formation of a disulfide bond between Cys-41 and another Cys residue is likely to be the fundamental mechanism inactivating RexT by oxidation. It has been shown that formation of an intramolecular disulfide bond induces conformational changes that remarkably alter the topology of the DNA-binding interface in an ArsR family transcriptional regulator (31).
The 20-bp region from −18 to −37 with respect to the translation start site was shown to be important for the binding of RexT to the promoter region of trxA2 (Fig. 3B). Because the transcription initiation site of trxA2 was located at position −40 (Fig. 2), the binding of RexT could hinder elongation of the trxA2 transcript, which is coincident with the function of RexT as a transcriptional repressor. Transcriptional regulators of the ArsR family are known to bind DNA as a dimer, and dimerized DNA-binding proteins often bind to inverted repeat sequences (32, 33). The inverted repeat sequence 5′-ATTCGN15CGAAT-3′ was found within the RexT-binding region (Fig. 2). This sequence is a candidate for the RexT recognition sequence.
Transcriptome analyses of Synechocystis PCC 6803 show that the trx genes are not induced under oxidative stress conditions in contrast to other bacteria such as E. coli and B. subtilis (18, 19). Moreover, cyanobacteria do not have homologues of the redox-sensing transcriptional regulators OxyR and Spx (34). However, we found that the trxA2 gene was induced by H2O2 in Anabaena PCC 7120 (Fig. 1). The antioxidant system of Synechocystis PCC 6803 relies mainly on a high catalase activity (35, 36). In contrast, in Anabaena PCC 7120, the antioxidant mechanism does not rely on catalase, but the 2-Cys peroxiredoxin plays a more prominent role, which is remarkably similar to the chloroplast system (35). Peroxiredoxins are thiol-based peroxidases that catalyze the reduction of H2O2 by the concerted action of 2 Cys residues, which form the intermolecular disulfide bond during catalysis (37). Recycling of peroxiredoxin is achieved by reduced Trx. Thus, increased expression of TrxA2 after treatment with H2O2 could contribute to scavenging H2O2, although it remains to be shown whether the level of TrxA2 protein is increased concomitantly with up-regulation of the trxA2 transcription.
Despite the microoxic environment in heterocysts, H2O2 is actively generated in heterocysts through the reactions catalyzed by superoxide dismutase and nitrogenase reductase and inactivates nitrogenase (38, 39). It has been demonstrated that rubrerythrin, which is preferentially expressed in heterocysts, decomposes H2O2 generated within heterocysts (39). The transcript level of the rbrA gene, encoding rubrerythrin, was not affected by the rexT disruption (data not shown). In addition, DNA microarray analyses showed that no trx genes were up-regulated in heterocysts.3 These observations suggest that the RexT-TrxA2 system might not be active in heterocysts under normal growth conditions, but under oxidative stress conditions such as high light or low temperature, this system could regulate expression of redox-responsive genes not only in vegetative cells but also in heterocysts.
Supplementary Material
This work was supported by PRESTO of the Japan Science and Technology Agency (to S. E.) and Grant-in-aid for Scientific Research (C) 23603005 from the Japan Society for the Promotion of Science (to S. E.).

This article contains supplemental Fig. S1 and Table S1.
S. Ehira and M. Ohmori, unpublished data.
- Trx
- thioredoxin
- AMS
- 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid
- NEM
- N-ethylmaleimide
- RexT
- redox-sensing transcriptional regulator of thioredoxin A2.
REFERENCES
- 1. Flores E., Herrero A. (2010) Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 8, 39–50 [DOI] [PubMed] [Google Scholar]
- 2. Kumar K., Mella-Herrera R. A., Golden J. W. (2010) Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2, a000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolk C. P., Ernst A., Elhai J. (1994) in The Molecular Biology of Cyanobacteria (Bryant D. A., ed) pp. 769–823, Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 4. Summers M. L., Wallis J. G., Campbell E. L., Meeks J. C. (1995) Genetic evidence of a major role for glucose-6-phosphate dehydrogenase in nitrogen fixation and dark growth of the cyanobacterium Nostoc sp. strain ATCC 29133. J. Bacteriol. 177, 6184–6194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lechno-Yossef S., Fan Q., Wojciuch E., Wolk C. P. (2011) Identification of ten Anabaena sp. genes that under aerobic conditions are required for growth on dinitrogen but not for growth on fixed nitrogen. J. Bacteriol. 193, 3482–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cossar J. D., Rowell P., Stewart W. D. (1984) Thioredoxin as a modulator of glucose-6-phosphate dehydrogenase in a N2-fixing cyanobacterium. J. Gen. Microbiol. 130, 991–998 [Google Scholar]
- 7. Udvardy J., Borbely G., Juhåsz A., Farkas G. L. (1984) Thioredoxins and the redox modulation of glucose-6-phosphate dehydrogenase in Anabaena sp. strain PCC 7120 vegetative cells and heterocysts. J. Bacteriol. 157, 681–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heping D., Kentemich T., Schmitz K., Müller B., Bothe H. (1992) Distribution of thioredoxins in heterocysts and vegetative cells of cyanobacteria. J. Photochem. Photobiol. B Biol. 16, 285–295 [Google Scholar]
- 9. Montrichard F., Alkhalfioui F., Yano H., Vensel W. H., Hurkman W. J., Buchanan B. B. (2009) Thioredoxin targets in plants: the first 30 years. J. Proteomics 72, 452–474 [DOI] [PubMed] [Google Scholar]
- 10. Buchanan B. B., Holmgren A., Jacquot J. P., Scheibe R. (2012) Fifty years in the thioredoxin field and a bountiful harvest. Biochim. Biophys. Acta 1820, 1822–1829 [DOI] [PubMed] [Google Scholar]
- 11. Lindahl M., Florencio F. J. (2003) Thioredoxin-linked processes in cyanobacteria are as numerous as in chloroplasts, but targets are different. Proc. Natl. Acad. Sci. U.S.A. 100, 16107–16112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perez-Perez M. E., Florencio F. J., Lindahl M. (2006) Selecting thioredoxins for disulphide proteomics: target proteomes of three thioredoxins from the cyanobacterium Synechocystis sp. PCC 6803. Proteomics 6, Suppl. 1, S186–S195 [DOI] [PubMed] [Google Scholar]
- 13. Pérez-Pérez M. E., Martín-Figueroa E., Florencio F. J. (2009) Photosynthetic regulation of the cyanobacterium Synechocystis sp. PCC 6803 thioredoxin system and functional analysis of TrxB (Trx x) and TrxQ (Trx y) thioredoxins. Mol. Plant 2, 270–283 [DOI] [PubMed] [Google Scholar]
- 14. Ritz D., Patel H., Doan B., Zheng M., Aslund F., Storz G., Beckwith J. (2000) Thioredoxin 2 is involved in the oxidative stress response in Escherichia coli. J. Biol. Chem. 275, 2505–2512 [DOI] [PubMed] [Google Scholar]
- 15. Nakano S., Erwin K. N., Ralle M., Zuber P. (2005) Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol. Microbiol. 55, 498–510 [DOI] [PubMed] [Google Scholar]
- 16. Paget M. S., Kang J. G., Roe J. H., Buttner M. J. (1998) σR, an RNA polymerase σ factor that modulates expression of the thioredoxin system in response to oxidative stress in Streptomyces coelicolor A3(2). EMBO J. 17, 5776–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ehira S., Teramoto H., Inui M., Yukawa H. (2009) Regulation of Corynebacterium glutamicum heat shock response by the extracytoplasmic-function σ factor SigH and transcriptional regulators HspR and HrcA. J. Bacteriol. 191, 2964–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi M., Ishizuka T., Katayama M., Kanehisa M., Bhattacharyya-Pakrasi M., Pakrasi H. B., Ikeuchi M. (2004) Response to oxidative stress involves a novel peroxiredoxin gene in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 45, 290–299 [DOI] [PubMed] [Google Scholar]
- 19. Li H., Singh A. K., McIntyre L. M., Sherman L. A. (2004) Differential gene expression in response to hydrogen peroxide and the putative PerR regulon of Synechocystis sp. strain PCC 6803. J. Bacteriol. 186, 3331–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaneko T., Nakamura Y., Wolk C. P., Kuritz T., Sasamoto S., Watanabe A., Iriguchi M., Ishikawa A., Kawashima K., Kimura T., Kishida Y., Kohara M., Matsumoto M., Matsuno A., Muraki A., Nakazaki N., Shimpo S., Sugimoto M., Takazawa M., Yamada M., Yasuda M., Tabata S. (2001) Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8, 205–213; 227–253 [DOI] [PubMed] [Google Scholar]
- 21. Ehira S., Ohmori M. (2006) NrrA, a nitrogen-responsive response regulator facilitates heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 59, 1692–1703 [DOI] [PubMed] [Google Scholar]
- 22. Nakao M., Okamoto S., Kohara M., Fujishiro T., Fujisawa T., Sato S., Tabata S., Kaneko T., Nakamura Y. (2010) CyanoBase: the cyanobacteria genome database update 2010. Nucleic Acids Res. 38, D379–D381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elhai J., Wolk C. P. (1988) A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene 68, 119–138 [DOI] [PubMed] [Google Scholar]
- 24. Black T. A., Cai Y., Wolk C. P. (1993) Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 9, 77–84 [DOI] [PubMed] [Google Scholar]
- 25. Elhai J., Wolk C. P. (1988) Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 167, 747–754 [DOI] [PubMed] [Google Scholar]
- 26. Pinto F. L., Thapper A., Sontheim W., Lindblad P. (2009) Analysis of current and alternative phenol based RNA extraction methodologies for cyanobacteria. BMC Mol. Biol. 10, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ehira S., Ohmori M. (2011) NrrA, a nitrogen-regulated response regulator protein, controls glycogen catabolism in the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. J. Biol. Chem. 286, 38109–38114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hisabori T., Motohashi K., Hosoya-Matsuda N., Ueoka-Nakanishi H., Romano P. G. (2007) Towards a functional dissection of thioredoxin networks in plant cells. Photochem. Photobiol. 83, 145–151 [DOI] [PubMed] [Google Scholar]
- 29. Florencio F. J., Pérez-Pérez M. E., López-Maury L., Mata-Cabana A., Lindahl M. (2006) The diversity and complexity of the cyanobacterial thioredoxin systems. Photosynth. Res. 89, 157–171 [DOI] [PubMed] [Google Scholar]
- 30. Nakano S., Küster-Schöck E., Grossman A. D., Zuber P. (2003) Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A. 100, 13603–13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guimarães B. G., Barbosa R. L., Soprano A. S., Campos B. M., de Souza T. A., Tonoli C. C., Leme A. F., Murakami M. T., Benedetti C. E. (2011) Plant pathogenic bacteria utilize biofilm growth-associated repressor (BigR), a novel winged-helix redox switch, to control hydrogen sulfide detoxification under hypoxia. J. Biol. Chem. 286, 26148–26157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ye J., Kandegedara A., Martin P., Rosen B. P. (2005) Crystal structure of the Staphylococcus aureus pI258 CadC Cd(II)/Pb(II)/Zn(II)-responsive repressor. J. Bacteriol. 187, 4214–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao H., Volkov A., Veldore V. H., Hoch J. A., Varughese K. I. (2010) Crystal structure of the transcriptional repressor PagR of Bacillus anthracis. Microbiology 156, 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Latifi A., Ruiz M., Zhang C. C. (2009) Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 33, 258–278 [DOI] [PubMed] [Google Scholar]
- 35. Pascual M. B., Mata-Cabana A., Florencio F. J., Lindahl M., Cejudo F. J. (2010) Overoxidation of 2-Cys peroxiredoxin in prokaryotes: cyanobacterial 2-Cys peroxiredoxins sensitive to oxidative stress. J. Biol. Chem. 285, 34485–34492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tichy M., Vermaas W. (1999) In vivo role of catalase-peroxidase in Synechocystis sp. strain PCC 6803. J. Bacteriol. 181, 1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chae H. Z., Uhm T. B., Rhee S. G. (1994) Dimerization of thiol-specific antioxidant and the essential role of cysteine 47. Proc. Natl. Acad. Sci. U.S.A. 91, 7022–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao W., Guo Q., Zhao J. (2007) A membrane-associated Mn-superoxide dismutase protects the photosynthetic apparatus and nitrogenase from oxidative damage in the cyanobacterium Anabaena sp. PCC 7120. Plant Cell Physiol. 48, 563–572 [DOI] [PubMed] [Google Scholar]
- 39. Zhao W., Ye Z., Zhao J. (2007) RbrA, a cyanobacterial rubrerythrin, functions as a FNR-dependent peroxidase in heterocysts in protection of nitrogenase from damage by hydrogen peroxide in Anabaena sp. PCC 7120. Mol. Microbiol. 66, 1219–1230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.