Background: AMPK activation promotes glucose and lipid metabolism.
Results: Hepatic AMPK activities were decreased in fatty liver from lipodystrophic mice, and leptin activated the hepatic AMPK via the α-adrenergic effect.
Conclusion: Leptin improved the fatty liver possibly by activating hepatic AMPK through the central and sympathetic nervous systems.
Significance: Hepatic AMPK plays significant roles in the pathophysiology of lipodystrophy and metabolic action of leptin.
Keywords: Adrenergic Receptor, AMP Kinase, AMP-activated Kinase (AMPK), Leptin, Lipodystrophy, Liver Metabolism, Fatty Liver, Sympathetic Nervous System
Abstract
Leptin is an adipocyte-derived hormone that regulates energy homeostasis. Leptin treatment strikingly ameliorates metabolic disorders of lipodystrophy, which exhibits ectopic fat accumulation and severe insulin-resistant diabetes due to a paucity of adipose tissue. Although leptin is shown to activate 5′-AMP-activated protein kinase (AMPK) in the skeletal muscle, the effect of leptin in the liver is still unclear. We investigated the effect of leptin on hepatic AMPK and its pathophysiological relevance in A-ZIP/F-1 mice, a model of generalized lipodystrophy. Here, we demonstrated that leptin activates hepatic AMPK through the central nervous system and α-adrenergic sympathetic nerves. AMPK activities were decreased in the fatty liver of A-ZIP/F-1 mice, and leptin administration increased AMPK activities in the liver as well as in skeletal muscle with significant reduction in triglyceride content. Activation of hepatic AMPK with A769662 also led to a decrease in hepatic triglyceride content and blood glucose levels in A-ZIP/F-1 mice. These results indicate that the down-regulation of hepatic AMPK activities plays a pathophysiological role in the metabolic disturbances of lipodystrophy, and the hepatic AMPK activation is involved in the therapeutic effects of leptin.
Introduction
Leptin is an adipocyte-derived hormone that regulates energy homeostasis mainly through the hypothalamus (1, 2). In addition to food intake and energy expenditure, leptin regulates glucose and lipid metabolism. Indeed, the usefulness of leptin treatment in various types of diabetes, including type 1, type 2, and lipoatrophic diabetes, has been demonstrated in rodent models (3–8). The clinical application of leptin treatment has already begun (9–12), especially in lipoatrophic diabetes that develops with lipodystrophy.
Lipodystrophy is a disease characterized by a paucity of adipose tissue that leads to leptin deficiency. Patients with lipodystrophy generally suffer severe insulin-resistant diabetes. Although the molecular mechanism by which insulin resistance develops in lipodystrophy is not fully understood, ectopic fat accumulation in insulin target tissues such as skeletal muscle and liver is thought to be one of the major causes for insulin resistance. The pathological condition in which ectopically accumulated fat exerts adverse effects against the cellular function is referred to as “lipotoxicity” (13). The amount of fat accumulated in tissues is known to correlate with the severity of insulin resistance (14). Lipoatrophic patients frequently develop severe fatty liver and excess fat accumulation in the skeletal muscle (15).
We and others have demonstrated that leptin effectively improves insulin sensitivity accompanied by dramatic reduction of fat content in the liver and skeletal muscle in patients with lipodystrophy (3, 9–12). Using rodent models, it was demonstrated that leptin activates AMPK3 in the skeletal muscle through both central and direct pathways (16). AMPK is a heterotrimeric enzyme that is conserved from yeast to humans and functions as a “fuel gauge” to monitor the status of cellular energy. AMPK potently stimulates fatty acid oxidation by inhibiting the activity of acetyl-CoA carboxylase (17). Thus, AMPK activation by leptin is a plausible mechanism by which leptin reduces ectopic fat in the skeletal muscle.
In addition to the skeletal muscle, recent studies have shown the physiological significance of AMPK in the liver (18–20). However, the effect of leptin on hepatic AMPK activity remains to be determined. The role of AMPK in the pathogenesis of metabolic abnormalities in lipodystrophy also remains unclear. In this study, we investigated the effect of leptin on hepatic AMPK activities and the pathophysiological role of AMPK in A-ZIP/F-1 mice, a well established mouse model of generalized lipodystrophy (21).
EXPERIMENTAL PROCEDURES
Materials and Animals
All reagents were analytic grade and obtained from Sigma unless otherwise stated. C57BL/6J mice and Wistar rats were purchased from Japan SLC, Inc. The F1 mice analyzed in Fig. 4 were obtained by crossing male A-ZIP/F-1 mice on the FVB/N background with female leptin transgenic mice on the C57BL/6J background (3, 22). A-ZIP/F-1 and the F1 mice were studied with appropriate littermate controls. Mice and rats were housed in an animal facility maintained at 20 °C with a 12:12-h light/dark cycle, allowed free access to water and standard rodent chow, and were randomly assigned to experimental groups. The mice were analyzed at the age of 9–10 weeks (C57BL/6J) or 15 weeks (A-ZIP/F-1, F1). Kyoto University Graduate School of Medicine Committee on Animal Research approved all experimental procedures.
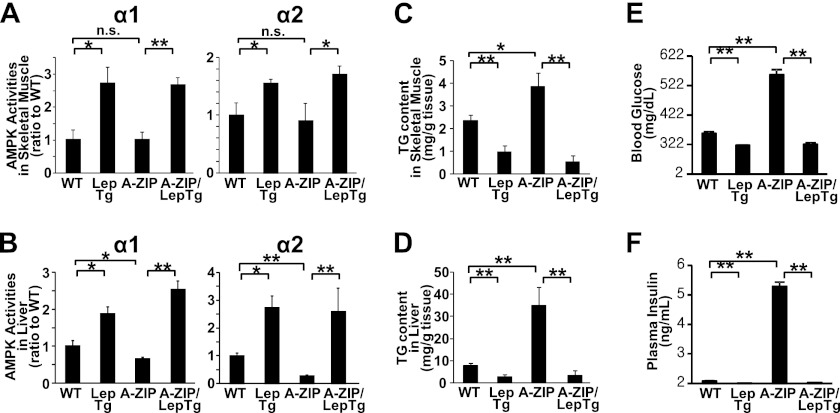
FIGURE 4.
AMPK activation and reduction in ectopic triglyceride accumulation in skeletal muscle and liver from leptin transgenic mice and double transgenic A-ZIP/LepTg mice. AMPK activities in gastrocnemius muscle (A) and liver (B), triglyceride content in gastrocnemius muscle (C) and liver (D), and blood glucose levels (E) and plasma insulin levels (F) from F1 mice obtained by crossing A-ZIP/F-1 mice and leptin transgenic mice are shown. Data are shown as ratios to +/+ (mean ± S.E.). n = 4–7. *, p < 0.05; **, p < 0.01; n.s., not significant.
Drug Administration
For continuous treatment, leptin was administered for 6 days using a subcutaneously implanted osmotic pump (Durect) at the dose of 0.65 mg/kg/day. For single intraperitoneal and intracerebroventricular (i.c.v.) injection, the dose of leptin was 1 mg/kg and 1 μg/mouse, respectively. Prazosin (2.5 mg/kg/day) or propranolol (1 mg/kg/day) was continuously co-administered with leptin for 6 days using an independently implanted osmotic pump. A769662 was administered once daily by intraperitoneal injection at the dose of 30 mg/kg/day for 4 days.
Primary Hepatocyte
Hepatocytes were isolated from male Wistar rats (100–150 g) by a two-step collagenase perfusion. The portal vein was cannulated under chloral hydrate anesthesia, and the liver was perfused with hepatocyte liver perfusion medium and digest medium (Invitrogen). After perfusion, hepatocytes were purified by filtration (100-μm mesh) and centrifuged (100 × g, 1 min, four times) and seeded onto 6-well culture plates coated with type I collagen (Iwaki) (1 × 106 cells/well). Cells were cultured in DMEM containing 10% FBS, 100 nm insulin, 100 nm dexamethasone, 30 mg/liter kanamycin, and 5 units/ml aprotinin for 12 h, and the medium were replaced with DMEM containing 10% FBS, 1 nm insulin, 1 nm dexamethasone, 30 mg/liter kanamycin, and 5 units/ml aprotinin for 6 h prior to stimulation. The cells were stimulated by 100 ng/ml leptin, 1 mm 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside or 0.5 mm 2,4-dinitrophenol for the indicated times.
Hepatic Vagotomy
Hepatic vagotomy was performed as described previously (23, 24) with modifications. Briefly, a hepatic branch of the ventral subdiaphragmatic vagal trunk was cleft using micro scissors under ether anesthesia, and the abdominal muscle wall and skin incision was closed with silk sutures. Drugs were introduced 1 week after the surgery. Accomplishment of the amputation was visually confirmed when sampling.
Chemical Sympathectomy
C57BL/6J mice were chemically sympathectomized by continuous infusion of guanethidine (30 mg/kg/day) for 6 days as described above.
Tissue Sampling and Biochemical Analysis
Tissues were rapidly isolated and frozen in liquid nitrogen by freeze-clamping (25) under chloral hydrate anesthesia after starvation for 6 h. Mice had been starved for 4 h previous to and during the study in a single administration study. Blood glucose was determined by reflectance glucometer under ad libitum feeding conditions. Plasma leptin was measured by RIA (Linco). Plasma insulin (Morinaga), adiponectin (Linco), and interleukin-6 (R&D Systems) were measured by ELISA. Triglyceride content was determined by E-test kit (Wako) in 2-propanol/heptane extract of the tissues. Homeostasis model assessment insulin resistance (HOMA-IR) was calculated on the assumption that the titer of murine insulin is as much as that of human insulin.
Isoform-specific AMPK Activity
AMPK activities were determined as described previously (26). Briefly, frozen tissues were homogenized in Hepes/Triton-based lysis buffer and then centrifuged. The supernatants were immunoprecipitated with protein A-Sepharose beads and isoform-specific antibodies against AMPK α1 or α2 (Millipore). Kinase activities in the immune complex were determined by the phosphorylation of the SAMS peptide using [γ-32P]ATP.
Western Blotting Analysis
40 μg of protein per each sample was subjected to SDS-PAGE using 4–12% BisTris gel (Bio-Rad). Antibodies were from Cell Signaling Technology or Merck. ECL Plus (GE Healthcare) and LAS-1000 image analyzer (Fuji film) were used for detection and quantification.
Quantitative Analysis of Gene Expressions
Total RNA was prepared using Isogen (Molecular Research Center). mRNA levels were quantified by real time PCR with the Taqman method (ABI Prism 7300). Primer sets and probes were as follows: 18 S, CGCGCAAATTACCCACTCCCGA, CGGCTACCACATCCAAGGA, and CCAATTACAGGGCCTCGAAA; AMPKα1, TGCAAAGATAGCCGACTTTGGTCTTTCA, GAACGTCCTGCTTGATGCACACAT, and TGGGTGAGCCACAGCTTGTTCTTA; and AMPKα2, TGATTCCAGCACAGCTGAGAACCACT, AAGCATCGATGATGAGGTGGTGGA, and ACAAAGTGCTGCCAGTCAAAGAGC (probe, forward, and reverse, respectively) Relative amounts of mRNAs were normalized with the ribosomal 18 S RNA.
Statistical Analyses
Two groups were compared by Student's t test. Comparisons between multiple groups were evaluated by analysis of variance. p < 0.05 was considered statistically significant.
RESULTS
Effect of Leptin Treatment on AMPK α1 and α2 Activities in Skeletal Muscle and Liver
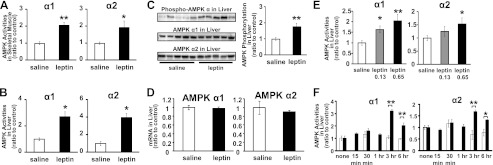
The isoform-specific AMPK activities in skeletal muscles and liver were determined in leptin- and saline-treated mice. Both AMPK α1 and α2 activities in skeletal muscle were increased 2-fold in leptin-treated mice compared with saline-treated mice (Fig. 1A). AMPK α1 and α2 activities in the liver were also increased 2.5- and 3.5-fold, respectively (Fig. 1B). The phosphorylation of AMPK α was also increased in leptin-treated mice compared with saline-treated mice (Fig. 1C). Meanwhile, protein or mRNA expressions of AMPK α1 and α2 in the liver were not significantly different between leptin- and saline-treated mice (Fig. 1, C and D). Therefore, the increase of AMPK activities in the liver from leptin-treated mice was not due to the increase in their expression levels.
FIGURE 1.
AMPK activation in skeletal muscle and liver by leptin administration. Isoform-specific AMPK activities in gastrocnemius muscle (A) and liver (B) from C57BL/6J mice after continuous saline or leptin administration are shown. Western blot analyses for phospho-AMPKα, AMPK α1 and α2 (C), and AMPK α1 and α2 mRNA levels normalized to 18 S ribosomal RNA (D) in liver are shown. AMPK activities in liver after 0.13 and 0.65 mg/kg/day continuous leptin infusion (E) are shown. AMPK activities in liver 15 min to 6 h after single intraperitoneal injection of saline or leptin (F) are shown. Data are shown as ratios to saline or quiescent control (mean ± S.E.). □, saline; ■, leptin. n = 4–6. *, p < 0.05; **, p < 0.01 versus saline.
When leptin was administered continuously, the activation of AMPK in the liver was dose-dependent (Fig. 1E). After intraperitoneal single leptin injection, the activation of both AMPK α1 and α2 was detected from 3 h while no activation was observed within 1 h (Fig. 1F).
Mechanism of Hepatic AMPK Activation by Leptin
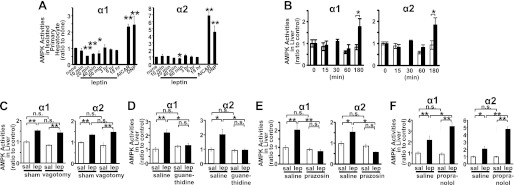
To clarify whether leptin acts directly on hepatocytes, we examined AMPK activities in isolated primary rat hepatocytes with or without leptin (Fig. 2A). The addition of leptin into the culture medium increased neither AMPK α1 nor α2 activities. Next, we examined the effect of leptin i.c.v. injection on AMPK activities in the liver (Fig. 2B). The activation of both AMPK α1 and α2 was detected 3 h after leptin injection at the dose that did not cause any effect when administered peripherally. These results indicate that leptin activates AMPK in the liver mainly through the CNS.
FIGURE 2.
Mechanism of hepatic AMPK activation by leptin administration. AMPK activities in isolated rat primary hepatocytes 10 min to 18 h after stimulation by leptin, 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) (40 min), or 2,4-dinitrophenol (DNP) (15 min) (A) are shown. AMPK activities in liver 15 min to 3 h after i.c.v. administration of saline (sal) or leptin (lep) (B). The effects of hepatic vagotomy (C), chemical sympathectomy (D), co-administration of antagonists against α1-adrenoreceptors (E) or β-adrenoreceptors (F) on hepatic AMPK activation by leptin are shown. Data are shown as ratios to saline or quiescent control (mean ± S.E.). n = 4–6 (A, and C–E); n = 3–6 (B). *, p < 0.05; **, p < 0.01 versus control. n.s., not significant.
Therefore, we examined the involvement of autonomic nerves in the effect of leptin on AMPK activation in the liver. Hepatic vagotomy did not show any effect on AMPK activation by leptin in the liver (Fig. 2C). In contrast, chemical sympathectomy by guanethidine treatment completely inhibited the activation of both AMPK α1 and α2 by leptin in the liver (Fig. 2D). We further investigated the involvement of the subtype-specific sympathetic nervous system. Administration of propranolol, a β-antagonist, did not suppress AMPK activation in the liver, whereas prazosin, an α1-antagonist, completely inhibited the activation of both AMPK α1 and α2 by leptin in the liver (Fig. 2, E and F).
AMPK α1 and α2 Activities in Skeletal Muscle and Liver from A-ZIP/F-1 Mice
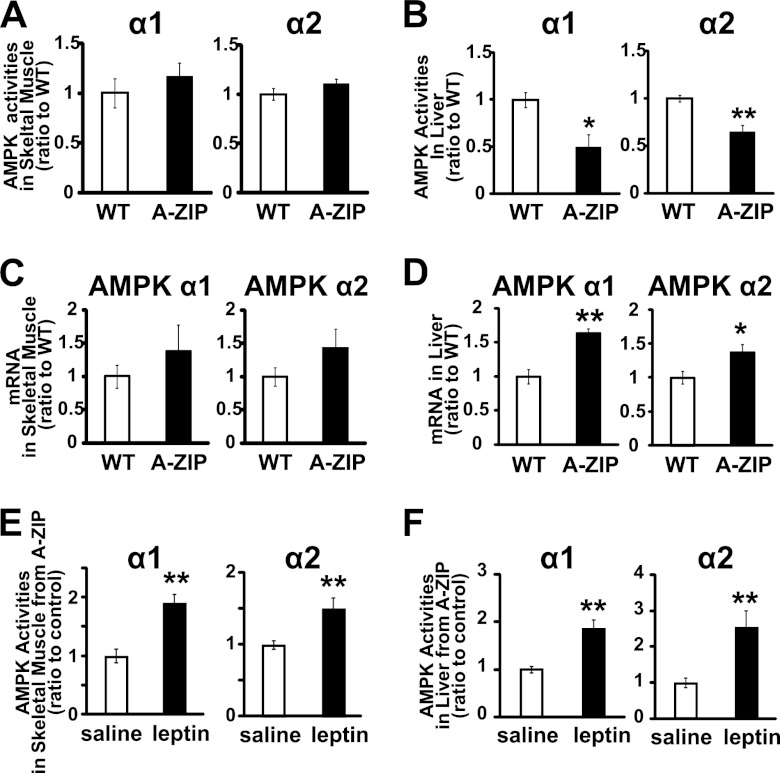
To explore the pathophysiological role of AMPK in lipodystrophy, we examined AMPK α1 and α2 activities in skeletal muscle and liver from A-ZIP/F-1 (A-ZIP) mice. Characteristics of A-ZIP mice used in this study are shown in Table 1. Consistent with previous studies (21), A-ZIP mice showed hyperglycemia and hyperinsulinemia, suggesting insulin resistance, and also showed increased liver weight, suggesting fatty liver. Plasma leptin and adiponectin levels were markedly decreased, but the plasma interleukin-6 level was not significantly different from that in WT mice. In these A-ZIP mice, both AMPK α1 and α2 activities in the liver were apparently decreased compared with WT mice, although those in the skeletal muscle were not significantly different from WT mice (Fig. 3, A and B). AMPK α1 and α2 mRNA expressions in the skeletal muscle were not significantly different in A-ZIP mice, and those in the liver were rather increased compared with WT mice (Fig. 3, C and D). Therefore, the decrease in AMPK activities in the liver from A-ZIP mice was not due to the change in their mRNA expressions. However, leptin treatment effectively increased AMPK α1 and α2 activities in the liver as well as in the skeletal muscle from A-ZIP mice (Fig. 3, E and F).
TABLE 1.
Characteristics of A-ZIP/F-1 mice used in this study
Values are as follows: n = 8–11 (glucose); n = 3–5 (leptin); n = 5–7 (other adipocytokines).
| WT | A-ZIP/F-1 mice | |
|---|---|---|
| Body weight | 30.7 ± 0.8 g | 32.5 ± 0.4 g |
| Glucose | 167 ± 5.8 mg/dl | 331 ± 39 mg/dla |
| Insulin | 0.24 ± 0.0 ng/ml | 0.46 ± 0.1 ng/mlb |
| Leptin | 6.15 ± 1.3 ng/ml | 1.25 ± 0.5 ng/mlb |
| Adiponectin | 6.27 ± 0.4 μg/ml | 1.46 ± 0.5 μg/mla |
| Interleukin-6 | 5.3 ± 0.8 pg/ml | 6.5 ± 1.0 pg/ml |
| Liver weight | 1.19 ± 0.0 g | 1.98 ± 0.2 ga |
a p < 0.01 versus WT. Data are expressed as means ±S.E.
b p < 0.05 versus WT. Data are expressed as means ±S.E.
FIGURE 3.
AMPK activities and mRNA expressions in a mouse model of lipoatrophic diabetes. α1- and α2-isoform-specific AMPK activities in the soleus muscle (A) and liver (B) are shown. AMPK α1 and α2 mRNA levels in the soleus muscle (C) and liver (D) normalized to 18 S ribosomal RNA are shown. AMPK activities in gastrocnemius muscle (E) and liver (F) from A-ZIP/F-1 mice after continuous leptin administration are shown. Data are shown as ratios to WT or saline control (mean ± S.E.). □, WT; ■, A-ZIP/F-1. n = 4–5. (A–D). □, saline; ■, leptin. n = 9–10 (E and F). *, p < 0.05; **, p < 0.01 versus control.
Effect of Transgenic Overexpression of Leptin on AMPK α1 and α2 Activities in Skeletal Muscle and Liver from A-ZIP/F-1 Mice
To explore the chronic effect of leptin, we crossed transgenic mice overexpressing leptin (LepTg) and A-ZIP mice, producing mice of four genotypes as follows: WT, LepTg, A-ZIP, and A-ZIP/LepTg. AMPK α1 and α2 activities in both the skeletal muscle and liver were markedly increased in LepTg mice (Fig. 4, A and B). At this time, triglyceride contents in skeletal muscle and liver in LepTg mice were reduced to more than half of those in WT mice (Fig. 4, C and D). AMPK activities were unchanged in the skeletal muscle but were apparently decreased in the liver from A-ZIP mice when compared with WT mice (Fig. 4, A and B). As to triglyceride contents, the apparent increment was observed in both the skeletal muscle and liver in A-ZIP mice (Fig. 4, C and D). However, AMPK activities were increased, and triglyceride content was decreased in A-ZIP/LepTg mice as well as in LepTg mice in both the skeletal muscle and liver (Fig. 4, A–D). In accordance with our previous report, blood glucose and plasma insulin levels were lower in LepTg mice than in WT mice, and severe hyperglycemia and hyperinsulinemia in A-ZIP mice were strikingly ameliorated by transgenic overexpression of leptin (Fig. 4, E and F) (3).
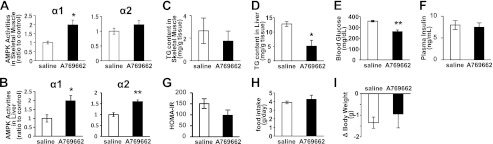
Effect of AMPK Activator, A769662, on AMPK Activities and Triglyceride Content in Skeletal Muscle and Liver from A-ZIP/F-1 Mice
After intraperitoneal single injection of A769662, an AMPK-specific activator, the activity of AMPK α1 was increased but that of α2 was not significantly increased in the skeletal muscle (Fig. 5A). The activity of both AMPK α1 and α2 was clearly increased in the liver (Fig. 5B). Although repetitive injection of A769662 for 4 days did not significantly reduce triglyceride content in the skeletal muscle, it effectively reduced triglyceride content to one-third of that in saline-treated mice in the liver (Fig. 5, C and D). At this time, the blood glucose level was significantly decreased, and HOMA-IR, an index of insulin resistance, tended to be decreased although plasma insulin level was not significantly decreased (Fig. 5, E–G). Food intake and body weight were not affected by A769662 (Fig. 5, H and I).
FIGURE 5.
Resolution of fatty liver in A-ZIP/F-1 mice by AMPK activation. AMPK activities in gastrocnemius muscle (A) and liver (B) 30 min after a single intraperitoneal injection of AMPK activator, A769662 (30 mg/kg) are shown. Triglyceride contents in gastrocnemius muscle (C) and liver (D), blood glucose (E), plasma insulin (F), and calculated HOMA-IR (G) after the repetitive A769662 administration (30 mg/kg/day for 4 days) are shown. Food intake (H) and weight change (I) during the study period are shown.
DISCUSSION
This is the first report clearly demonstrating that leptin activates hepatic AMPK through the central nervous system and an α-adrenergic effect in vivo. It had long been unclear whether leptin activates hepatic AMPK in vivo. It was reported that adenovirus-induced leptin overexpression failed to increase hepatic AMPK activities (27). However, leptin-induced suppression of gluconeogenesis was abolished in liver-specific AMPK α2 knock-out mice, suggesting that leptin suppresses gluconeogenesis through hepatic AMPK activation (18). Furthermore, a slight increase in hepatic AMPK activity 45 min after leptin administration was reported in mice, although it has been deemed as an artificial effect (28, 29). In this study, we demonstrated that hepatic AMPK activation by leptin is dose-dependent (Fig. 1E), and hepatic AMPK activity clearly increases from 3 h after leptin administration in mice (Fig. 1F).
In the skeletal muscle, AMPK is reported to be activated by leptin both directly on skeletal muscles and indirectly through the hypothalamic relay (16). In the liver, this study demonstrated that leptin activates AMPK mainly through the CNS and that leptin has no direct AMPK-activating effect on hepatocytes (Fig. 2, A and B). There is a report showing the increase in AMPK phosphorylation by leptin using Huh7 human hepatoma cells overexpressing leptin receptors; however, it was observed only in the receptor-overexpressing cells (30). AMPK activation in the skeletal muscle by leptin is biphasic, and the former phase, which occurred in 15 min, is caused by direct muscle stimulation (16). Meanwhile, hepatic AMPK activation was detected only from 3 h after leptin administration, supporting the notion that leptin activates hepatic AMPK mainly through the CNS.
The parasympathetic and sympathetic nervous systems between the hypothalamus and liver play an important role in regulating metabolism (31). In this study, chemical sympathectomy completely inhibited hepatic AMPK activation by leptin although hepatic vagotomy did not, indicating that leptin activates hepatic AMPK mainly through the sympathetic nervous system (Fig. 2, C and D). Moreover, we demonstrated that hepatic AMPK activation by leptin was mainly dependent on the α-adrenergic effect but not on the β-adrenergic effect (Fig. 2, E and F). Not the β- but the α1-adrenoreceptor stimulation was shown to activate AMPK in isolated skeletal muscle, L6 myotubes, H9C2 cardiomyocyte, and rat heart (16, 32, 33), although not the α1- but the β-adrenoreceptors mediate AMPK activation in brown and white adipocytes (32, 34). The physiological significance of this adrenoreceptor tissue specificity will be an issue in the future.
Recent reports have revealed some adipocytokines harbor the potential to activate AMPK in the liver or skeletal muscle. AMPK potently stimulates fatty acid oxidation by inhibiting the activity of acetyl-CoA carboxylase (17). Thus, we hypothesized that AMPK might play a pathophysiological role in ectopic fat accumulation and marked insulin resistance developed in lipodystrophy. Indeed, analysis of A-ZIP mice revealed the decrease in AMPK activities in the liver, suggesting the pathophysiological significance of hepatic AMPK in the development of fatty liver in lipodystrophy (Fig. 3B).
It is interesting that AMPK activities in the skeletal muscle were not decreased in A-ZIP mice when compared with WT mice. Basal AMPK activities in the skeletal muscle in fa/fa rats were also not different from those from control rats (35, 36), although AMPK activities in the liver in fa/fa rats and ob/ob mice were decreased (37). Although it is unknown what determines the difference between the skeletal muscle and the liver, some factors may counteract the decrease of AMPK activities brought by leptin deficiency in the skeletal muscle.
We previously showed that transgenic overexpression of leptin strikingly improves metabolic abnormalities in A-ZIP mice (3). Insulin-stimulated PI3K activity in the skeletal muscle and liver were amplified in LepTg mice (22). However, the underlying molecular mechanisms of metabolic action of leptin have not been fully clarified. Although leptin was reported to activate AMPK in the skeletal muscle (16), the effect of leptin on hepatic AMPK activity had been unclear. We found that leptin activates both isoforms of AMPK not only in the skeletal muscle but also in the liver in association with the reduction of tissue triglyceride content in A-ZIP mice (Fig. 4). These results indicated the therapeutic role of AMPK in the metabolic improvement by leptin in lipodystrophy.
To confirm the therapeutic role of hepatic AMPK in the improvement of fatty liver by leptin in lipodystrophy, we investigated the effect of A769662, an AMPK-specific activator on liver triglyceride content in A-ZIP mice. A769662 was shown to activate AMPK and decrease acetyl-CoA carboxylase activity and triglyceride in the liver of ob/ob mice (38). It was also reported that A769662 preferably works on the liver in vivo due to the preference of tissue distribution of A769662 after injection (38). Indeed, although A769662 significantly activated only AMPK α1 and did not significantly decrease triglyceride content in the skeletal muscle, it effectively activated both AMPK α1 and α2 and reduced triglyceride content to one-third in the liver from A-ZIP mice (Fig. 5, A–D). These results indicated that hepatic AMPK activation is involved in the improvement of fatty liver by leptin in lipodystrophy. We could not find obvious decrease in the insulin levels. but the HOMA-IR tended to decrease, suggesting hepatic AMPK activation leads to improvement of insulin resistance in lipodystrophy (Fig. 5, F and G).
In conclusion, this study demonstrates that leptin activates AMPK not only in the skeletal muscle but also in the liver in mice. Leptin activates hepatic AMPK mainly through the CNS and α-adrenergic effects of sympathetic nerves. This study also indicates that hepatic AMPK is involved in the development of metabolic disorders and their improvement by leptin in A-ZIP mice. This study provides the useful notion to understand the molecular mechanism by which leptin regulates energy metabolism and will guide the development of novel metabolic pharmaceuticals.
Acknowledgments
We thank Yoko Koyama and Mayumi Nagamoto for secretarial and technical assistance and Drs. Shuichi Koda, Hideki Matsumoto, and Fumihiko Yokoya for helpful technical advice.
This work was supported in part by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Ministry of Health, Labor and Welfare of Japan, The Takeda Medical Research Foundation, The Japan Foundation of Applied Enzymology, Eli Lilly and Co., and The Nakatomi Foundation.
- AMPK
- 5′-AMP-activated protein kinase
- A-ZIP
- A-ZIP/F-1 mice
- LepTg
- transgenic mice overexpressing leptin
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- HOMA-IR
- homeostasis model assessment insulin resistance
- i.c.v.
- intracerebroventricular.
REFERENCES
- 1. Halaas J. L., Boozer C., Blair-West J., Fidahusein N., Denton D. A., Friedman J. M. (1997) Physiological response to long term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. U.S.A. 94, 8878–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campfield L. A., Smith F. J., Guisez Y., Devos R., Burn P. (1995) Recombinant mouse OB protein. Evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549 [DOI] [PubMed] [Google Scholar]
- 3. Ebihara K., Ogawa Y., Masuzaki H., Shintani M., Miyanaga F., Aizawa-Abe M., Hayashi T., Hosoda K., Inoue G., Yoshimasa Y., Gavrilova O., Reitman M. L., Nakao K. (2001) Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes 50, 1440–1448 [DOI] [PubMed] [Google Scholar]
- 4. Miyanaga F., Ogawa Y., Ebihara K., Hidaka S., Tanaka T., Hayashi S., Masuzaki H., Nakao K. (2003) Leptin as an adjunct of insulin therapy in insulin-deficient diabetes. Diabetologia 46, 1329–1337 [DOI] [PubMed] [Google Scholar]
- 5. Naito M., Fujikura J., Ebihara K., Miyanaga F., Yokoi H., Kusakabe T., Yamamoto Y., Son C., Mukoyama M., Hosoda K., Nakao K. (2011) Therapeutic impact of leptin on diabetes, diabetic complications, and longevity in insulin-deficient diabetic mice. Diabetes 60, 2265–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang M. Y., Chen L., Clark G. O., Lee Y., Stevens R. D., Ilkayeva O. R., Wenner B. R., Bain J. R., Charron M. J., Newgard C. B., Unger R. H. (2010) Leptin therapy in insulin-deficient type I diabetes. Proc. Natl. Acad. Sci. U.S.A. 107, 4813–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimomura I., Hammer R. E., Ikemoto S., Brown M. S., Goldstein J. L. (1999) Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401, 73–76 [DOI] [PubMed] [Google Scholar]
- 8. Kusakabe T., Tanioka H., Ebihara K., Hirata M., Miyamoto L., Miyanaga F., Hige H., Aotani D., Fujisawa T., Masuzaki H., Hosoda K., Nakao K. (2009) Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high fat diet. Diabetologia 52, 675–683 [DOI] [PubMed] [Google Scholar]
- 9. Ebihara K., Kusakabe T., Hirata M., Masuzaki H., Miyanaga F., Kobayashi N., Tanaka T., Chusho H., Miyazawa T., Hayashi T., Hosoda K., Ogawa Y., DePaoli A. M., Fukushima M., Nakao K. (2007) Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J. Clin. Endocrinol. Metab. 92, 532–541 [DOI] [PubMed] [Google Scholar]
- 10. Farooqi I. S., Jebb S. A., Langmack G., Lawrence E., Cheetham C. H., Prentice A. M., Hughes I. A., McCamish M. A., O'Rahilly S. (1999) Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 [DOI] [PubMed] [Google Scholar]
- 11. Oral E. A., Simha V., Ruiz E., Andewelt A., Premkumar A., Snell P., Wagner A. J., DePaoli A. M., Reitman M. L., Taylor S. I., Gorden P., Garg A. (2002) Leptin replacement therapy for lipodystrophy. N. Engl. J. Med. 346, 570–578 [DOI] [PubMed] [Google Scholar]
- 12. Petersen K. F., Oral E. A., Dufour S., Befroy D., Ariyan C., Yu C., Cline G. W., DePaoli A. M., Taylor S. I., Gorden P., Shulman G. I. (2002) Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 109, 1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee Y., Hirose H., Ohneda M., Johnson J. H., McGarry J. D., Unger R. H. (1994) Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc. Natl. Acad. Sci. U.S.A. 91, 10878–10882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perseghin G., Scifo P., De Cobelli F., Pagliato E., Battezzati A., Arcelloni C., Vanzulli A., Testolin G., Pozza G., Del Maschio A., Luzi L. (1999) Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans. A 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48, 1600–1606 [DOI] [PubMed] [Google Scholar]
- 15. Kim J. K., Gavrilova O., Chen Y., Reitman M. L., Shulman G. I. (2000) Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J. Biol. Chem. 275, 8456–8460 [DOI] [PubMed] [Google Scholar]
- 16. Minokoshi Y., Kim Y. B., Peroni O. D., Fryer L. G., Müller C., Carling D., Kahn B. B. (2002) Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415, 339–343 [DOI] [PubMed] [Google Scholar]
- 17. Winder W. W., Wilson H. A., Hardie D. G., Rasmussen B. B., Hutber C. A., Call G. B., Clayton R. D., Conley L. M., Yoon S., Zhou B. (1997) Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J. Appl. Physiol. 82, 219–225 [DOI] [PubMed] [Google Scholar]
- 18. Andreelli F., Foretz M., Knauf C., Cani P. D., Perrin C., Iglesias M. A., Pillot B., Bado A., Tronche F., Mithieux G., Vaulont S., Burcelin R., Viollet B. (2006) Liver adenosine monophosphate-activated kinase-α2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology 147, 2432–2441 [DOI] [PubMed] [Google Scholar]
- 19. Assifi M. M., Suchankova G., Constant S., Prentki M., Saha A. K., Ruderman N. B. (2005) AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am. J. Physiol. Endocrinol. Metab. 289, E794–E800 [DOI] [PubMed] [Google Scholar]
- 20. Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moitra J., Mason M. M., Olive M., Krylov D., Gavrilova O., Marcus-Samuels B., Feigenbaum L., Lee E., Aoyama T., Eckhaus M., Reitman M. L., Vinson C. (1998) Life without white fat. A transgenic mouse. Genes Dev. 12, 3168–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ogawa Y., Masuzaki H., Hosoda K., Aizawa-Abe M., Suga J., Suda M., Ebihara K., Iwai H., Matsuoka N., Satoh N., Odaka H., Kasuga H., Fujisawa Y., Inoue G., Nishimura H., Yoshimasa Y., Nakao K. (1999) Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes 48, 1822–1829 [DOI] [PubMed] [Google Scholar]
- 23. German J., Kim F., Schwartz G. J., Havel P. J., Rhodes C. J., Schwartz M. W., Morton G. J. (2009) Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 150, 4502–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pocai A., Obici S., Schwartz G. J., Rossetti L. (2005) A brain-liver circuit regulates glucose homeostasis. Cell Metab. 1, 53–61 [DOI] [PubMed] [Google Scholar]
- 25. Davies S. P., Carling D., Munday M. R., Hardie D. G. (1992) Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur. J. Biochem. 203, 615–623 [DOI] [PubMed] [Google Scholar]
- 26. Miyamoto L., Toyoda T., Hayashi T., Yonemitsu S., Nakano M., Tanaka S., Ebihara K., Masuzaki H., Hosoda K., Ogawa Y., Inoue G., Fushiki T., Nakao K. (2007) Effect of acute activation of 5′-AMP-activated protein kinase on glycogen regulation in isolated rat skeletal muscle. J. Appl. Physiol. 102, 1007–1013 [DOI] [PubMed] [Google Scholar]
- 27. Lee Y., Yu X., Gonzales F., Mangelsdorf D. J., Wang M. Y., Richardson C., Witters L. A., Unger R. H. (2002) PPARα is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proc. Natl. Acad. Sci. U.S.A. 99, 11848–11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brabant G., Müller G., Horn R., Anderwald C., Roden M., Nave H. (2005) Hepatic leptin signaling in obesity. FASEB J. 19, 1048–1050 [DOI] [PubMed] [Google Scholar]
- 29. Dzamko N. L., Steinberg G. R. (2009) AMPK-dependent hormonal regulation of whole-body energy metabolism. Acta Physiol. 196, 115–127 [DOI] [PubMed] [Google Scholar]
- 30. Uotani S., Abe T., Yamaguchi Y. (2006) Leptin activates AMP-activated protein kinase in hepatic cells via a JAK2-dependent pathway. Biochem. Biophys. Res. Commun. 351, 171–175 [DOI] [PubMed] [Google Scholar]
- 31. Uyama N., Geerts A., Reynaert H. (2004) Neural connections between the hypothalamus and the liver. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 280, 808–820 [DOI] [PubMed] [Google Scholar]
- 32. Hutchinson D. S., Bengtsson T. (2006) AMP-activated protein kinase activation by adrenoceptors in L6 skeletal muscle cells. Mediation by α1-adrenoceptors causing glucose uptake. Diabetes 55, 682–690 [DOI] [PubMed] [Google Scholar]
- 33. Xu M., Zhao Y. T., Song Y., Hao T. P., Lu Z. Z., Han Q. D., Wang S. Q., Zhang Y. Y. (2007) α1-adrenergic receptors activate AMP-activated protein kinase in rat hearts. Sheng Li Xue Bao 59, 175–182 [PubMed] [Google Scholar]
- 34. Koh H. J., Hirshman M. F., He H., Li Y., Manabe Y., Balschi J. A., Goodyear L. J. (2007) Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem. J. 403, 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnes B. R., Ryder J. W., Steiler T. L., Fryer L. G., Carling D., Zierath J. R. (2002) Isoform-specific regulation of 5′-AMP-activated protein kinase in skeletal muscle from obese Zucker (fa/fa) rats in response to contraction. Diabetes 51, 2703–2708 [DOI] [PubMed] [Google Scholar]
- 36. Bergeron R., Previs S. F., Cline G. W., Perret P., Russell R. R., 3rd, Young L. H., Shulman G. I. (2001) Effect of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes 50, 1076–1082 [DOI] [PubMed] [Google Scholar]
- 37. Yu X., McCorkle S., Wang M., Lee Y., Li J., Saha A. K., Unger R. H., Ruderman N. B. (2004) Leptinomimetic effects of the AMP kinase activator 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia 47, 2012–2021 [DOI] [PubMed] [Google Scholar]
- 38. Cool B., Zinker B., Chiou W., Kifle L., Cao N., Perham M., Dickinson R., Adler A., Gagne G., Iyengar R., Zhao G., Marsh K., Kym P., Jung P., Camp H. S., Frevert E. (2006) Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3, 403–416 [DOI] [PubMed] [Google Scholar]