Background: Desensitization of FSH response by down-regulation of FSHR transcription is critical for FSH action.
Results: Chromatin modifier MTA2 participates in the down-regulation of FSHR transcription.
Conclusion: The FSH/Ar/MTA2 cascade may serve as an indispensable negative feedback mechanism to modulate FSH transduction events in Sertoli cells.
Significance: Our findings provide new insights into mechanisms by which FSH is deregulated in male infertile patients.
Keywords: Androgen Receptor, Endocrinology, Histone Deacetylase, Spermatogenesis, Testis, MTA2, Sertoli Cell (SC), Follicle-stimulating Hormone (FSH)
Abstract
The effect of follicle-stimulating hormone (FSH) on spermatogenesis is modulated at a fundamental level by controlling the number of competent receptors present at the surface of Sertoli cells (SCs). One underlying mechanism is the down-regulation of the expression levels of the FSH receptor (FSHR) gene after exposure to FSH. Here we report that metastasis-associated protein 2 (MTA2), a component of histone deacetylase and nucleosome-remodeling complexes, as a gene product induced directly by testosterone or indirectly by FSH, is exclusively expressed in SCs. Stimulation of SCs with FSH is accompanied by up-regulation of MTA2 expression and enhancement of deacetylase activity. This effect requires the integrity of functional androgen receptor. Furthermore, MTA2 is a potent corepressor of FSHR transcription, because it can recruit histone deacetylase-1 onto the FSHR promoter and participates in the down-regulation of FSHR expression upon FSH treatment. Abolishment of endogenous MTA2 by siRNA treatment disrupted the desensitization of the FSH response and thereafter impaired the FSH-dependent secretory function of SCs. From a clinical standpoint, deregulated expression of MTA2 in SCs of human pathological testes negatively correlates to the deregulated level of serum FSH. Overall, our present results provide the first evidence that the FSH/androgen receptor/MTA2 cascade may serve as an indispensable negative feedback mechanism to modulate the transduction events of SCs in response to FSH. These data also underscore an unexpected reproductive facet of MTA2, which may operate as a novel integrator linking synergistic actions of FSH and androgen signaling in SCs.
Introduction
Since the first description of the Sertoli cell (SC)4 in 1865, this cell has drawn much attention of researchers because it is the only somatic type possessing a close structural relationship with germ cells inside seminiferous tubules (1). The SC serves as a principal structural element to maintain the integrity of the impermeable and immunological blood-testis barrier, provide structural support, and secrete diverse functional glycoproteins and peptides in favor of normal germ cell development and maturation (2). SC function is tightly controlled by FSH and testosterone signaling. FSH helps to determine testicular size, germ cell numbers per testis, and spermatozoa output by regulating the proliferation and thereby the final number of SCs. In contrast to FSH, ablation of androgen signaling in total androgen receptor (Ar) knock-out or in SC-specific Ar knock-out mice results in complete sterility, suggesting that androgen is absolutely essential for the maintenance of spermatogenesis (3). Accumulated evidence supports an overlapping and synergistic action of these two pathways in SCs. For example, only SCs possess receptors for testosterone and FSH within the seminiferous tubules. FSH and testosterone induce phosphorylation of ERK kinase to similar levels, but FSH is about 2-fold more effective in phosphorylating CREB. Moreover, FSH and testosterone can both stimulate the level of Ca2+ (4). Nevertheless, the mechanisms whereby integration of these two pathways regulates reproductive function probably involve actions at different levels and remain to be fully established.
MTA2 (metastasis-associated protein 2), as well as the prototype family member MTA1, belongs to the nucleosome remodeling and histone deacetylation (NuRD) complex that is associated with ATP-dependent chromatin remodeling and histone deacetylase (HDAC) activity (5). MTA2 functions in conjunction with other components of NuRD to mediate transcriptional repression as it facilitates the association of repressor molecules with the chromatin (6). MTA2 is a repressor of ERα activity, and its overexpression leads to estrogen-independent growth of human breast cancer cells (7). Besides its malignancy-promoting effects, growing evidence strongly suggests that additional, as yet poorly characterized, peripheral actions of MTA2 are likely to take place. In this sense, novel involvement of MTA2 in proper imprinted expression of H19 and Peg3 during mouse preimplantation development has been reported very recently (8). Furthermore, in contrast to the restricted expression patterns of other MTA family members, MTA2 is ubiquitously expressed (9). However, the physiological relevance of MTA2 signaling in such peripheral systems remains to be fully delineated.
Testis is a complex endocrine organ where different cell types interplay to ensure male fertility, under the control of an array of extragonadal and intragonadal signaling. In recent years, it has become evident that different factors with key roles in the HDAC are potentially involved in the regulation of testicular function (10–12). The identification of MTA2 as an integral part of histone deacetylation complexes prompted us to evaluate whether this signal is expressed in testis. Moreover, hormonal regulation of testicular MTA2 expression, as well as the functional meaning of this regulation, was assessed using different experimental paradigms. Overall, the proposed analysis would pave the way for a better understanding of the potential role of this important chromatin modifier in testicular physiology.
EXPERIMENTAL PROCEDURES
Human Tissue Collection
All samples were obtained after patients gave written informed consent. Specifically, testicular biopsies from the following patients were analyzed: men with hypospermatogenesis (mean age 38.4 years; range 31–46 years, n = 11) and men with spermatogenic arrest at the level of round spermatids (mean age 34.2 years; range 28–42 years, n = 15) as well as Sertoli cell only syndrome (SCOS) (mean age 31.6 years; range 26–45 years, n = 9). We also obtained testicular tissues from normozoospermic patients who underwent testicular biopsy during genital surgery procedures for varicocele or epididymal cysts (mean age 33.1 years; range 28–46 years, n = 9). All participants underwent a complete physical examination and semen analysis (according to WHO 2001 criteria). Patients with abnormal karyotype, Y chromosome microdeletion, and chronic diseases, in addition to those undergoing hormonal treatments or who had been exposed to alcohol or drugs, were excluded from the study. Testicular tissues were fixed in Bouin's fixative for 8 h immediately after collection. The use of the human tissue in this study was approved by the Human Research Committee of the Fourth Military Medical University for Approval of Research Involving Human Subjects. The protocol employed strictly conformed to the standards set by the 2008 Revised Declaration of Helsinki.
Animals
Pregnant C57BL/6 mice at 14 days of gestation, male 10-week-old C57BL/6 mice, and adult Sprague-Dawley rats (4 months of age) were purchased from the Animal Research Center of our university. They were housed in plastic boxes individually and provided standard mouse food pellets and water ad libitum throughout the whole experimental period. The room temperature (20 °C) and light/dark cycles (light on from 08:00 a.m. to 08:00 pm.) were strictly controlled. At postnatal day (PD) 5, 14, 21, 28, 45, and 70, mice were sacrificed under diethyl ether anesthesia, followed by cervical dislocation. Adult male mice were hypophysectomized (Hypo) through a parapharyngeal approach under avertin (tribromoethyl alcohol) anesthesia (day of hypophysectomy = day 0). Animals were provided with water containing 5% sucrose and solid food pellets ad libitum after surgery. For hormone supplementary experiments, mice received an subcutaneous injection of either 0.2 unit of ovine FSH (oFSH) in 0.2 ml of normal saline or 2 mg of testosterone propionate in 0.2 ml of sesame oil (Sigma-Aldrich) or two hormones together on daily basis. The supplemental treatment lasted for 5 days. Flutamide (FLUT) (Sigma), dissolved in corn oil (Sigma), was administered subcutaneously to hypophysectomized mice at a dose of 0.012 μg/g body weight/day for 5 days. Animals receiving the vehicle (normal saline/sesame oil/corn oil) were served as control. Selective Leydig cell (LC) elimination was achieved by systemic administration of the cytotoxic drug ethylene dimethane sulfonate (EDS) (Sigma-Aldrich) (in a single dose of 75 mg/kg intraperitoneally) in rats as described previously (13). For histological studies, some testes were fixed in Bouin's solution for 24 h, embedded in paraffin, and processed into 5-μm-thick sections for hematoxylin-eosin (H&E) staining. The Ethics Committee for Animal Experiments of the Fourth Military Medical University approved all animal work, and the experimental protocols strictly complied with the institutional guidelines and the criteria outlined in the Guide for Care and Use of Laboratory Animals.
Hormone Assays
For human patients, serum FSH was measured by an immunoradiometric assay (Diagnostic Product Corp.). Serum testosterone was measured using a radioimmunoassay (Diagnostic Product Corp.) in blood samples obtained between 9 and 10 a.m. Rat blood was collected from the orbital sinus after animals had been anesthetized. Pooled serum samples from each group were measured for testosterone concentration by radioimmunoassay as described previously (14).
Cells and Treatment
TM3, TM4, GC-1 spg, and GC-2 spd(ts) cell lines were obtained from the American Type Culture Collection (Manassas, VA) and maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal calf serum (Invitrogen). SCs were isolated from 8-week-old mouse testes and cultured in enriched DMEM/F-12 supplemented with growth factors as described (15). After 48 h of incubation, SC cultures were hypotonically treated with 20 mm Tris (pH 7.4) for 2.5 min to lyse residual germ cells, followed by two successive washes with DMEM/F-12 to remove cell debris. The purity of these SC cultures was routinely analyzed by quantitative RT-PCR (QRT-PCR) (supplemental Table 1). SCs employed in the present study were all freshly isolated SCs unless otherwise indicated. oFSH was added to SC cultures at a concentration of 25 ng/ml in normal saline, and testosterone was added at 40 ng/ml in ethanol. This concentration of testosterone is thought to be equivalent to that available to the SC in vivo, whereas the FSH concentration used has been shown to stimulate SC function (16). In another experimental setting, SCs were incubated with 5 μg/ml actinomycin D and 25 ng/ml oFSH together. Some SCs were pretreated with FLUT (1 μm) for 4 h before stimulation with testosterone. Cultures of SCs as controls for FSH or testosterone treatment received an equivalent volume of saline or ethanol, respectively. In some cases, SCs were pretreated for 2 h with the signaling pathway inhibitor PP2 (10 μm; Calbiochem) or PD98059 (50 μm; Sigma). Cells treated with DMSO were served as controls. Cells were then harvested at different time points of post-treatment as indicated in single-strength PBS for further analysis. Mouse blastocysts were prepared as described elsewhere (8).
In Vitro siRNA Treatment
In vitro siRNA treatment was carried out according to a previous report (17). In brief, TM4 cells or SCs were plated in 6-well culture plates in DMEM without antibiotics. The cells were allowed to grow to 50–60% confluence before being transfected with siRNA against Ar (sc-29203, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), siRNA against MTA1 (sc-35982, Santa Cruz Biotechnology, Inc.), siRNA against MTA2 (sc-35984, Santa Cruz Biotechnology, Inc.), or a control siRNA (sc-37007, Santa Cruz Biotechnology, Inc.). Subsequent incubation of cells in transfection medium along with the transfection reagent strictly followed the Santa Cruz Biotechnology protocol. The cells were incubated with the siRNA mixture for a period of 48 h before being subjected to other assays.
Plasmid Constructs, Transient Transfections, and Luciferase Assays
His-tagged full-length and truncated Ar mutant constructs depleted of ligand binding domain (LBD) (His-Ar and His-ArΔLBD) were amplified from a mouse testis cDNA library using PCR and subcloned into pcDNA3.1-His vector. FSHR(−100/+123) was generated by PCR amplification using rat genomic clone 54.111 as template according to a previous study (18). The amplified fragment was digested with SstI and XbaI and subcloned into the SstI/NheI sites of pGL3-Basic. Full-length MTA2 cDNA was isolated from a mouse testis cDNA library. MTA2 cDNA containing the entire reading frame of MTA2 was subcloned into pcDNA3.1-His vector using restriction sites EcoRI and ApaI to generate His-tagged MTA2. SCs were transfected with 0.5 μg of reporter plasmid in the presence or absence of 0.5 μg of empty expression vector or vectors expressing His-MTA2 using FUGENE reagent (Roche Applied Science) according to the manufacturer's instructions. Two days after transfection, the cells were stimulated with vehicle or oFSH (25 ng/ml) for 6 h, after which the cells were collected, and total cellular proteins were extracted using reporter lysis buffer (Promega, Madison, WI). Luciferase assays were performed using a Victor2 luminometer (PerkinElmer Life Sciences). Luciferase activities were normalized for total protein as determined by a Bradford assay.
Glutathione S-Transferase (GST) Fusion Protein Production
Different fusion proteins were constructed for MTA2 and mouse Ar, using the bacterial expression vector pGEX4T-1 (GE Healthcare). Amino acids 1–668, 1–419, and 1–278 for MTA2 and 1–899 and 1–650 for Ar were used as fusion parts. Proteins were expressed in Escherichia coli BL21DE3 cells and purified using glutathione-Sepharose 4B (Amersham Biosciences) as instructed by the manufacturer.
Pull-down Assay
The GST fusion full-length and truncated Ar proteins and His-tagged MTA2 protein were incubated for 1 h at room temperature by gentle shaking in a binding buffer containing 50 mm HEPES, pH 7.4, 150 mm NaCl, 10% glycerol, 1% Triton X-100, 1 mm EDTA, 10 mm NaF, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin in the absence or presence of testosterone. Beads were washed three times in the same buffer. Proteins were eluted from the beads with Laemmli sample buffer, separated by SDS-PAGE, and then subjected to immunoblotting analysis.
RT-PCR and QRT-PCR
Total RNA was extracted using an RNeasy minikit (Qiagen Inc., Valencia, CA) according to the manufacturer's instructions. Routine DNase (Applied Biosystems/Ambion, Austin, TX) treatment (1 unit of DNase I/μg of total RNA) was performed before reverse transcription. First-strand cDNA was synthesized using 1 μg of RNA with Superscript III (RNase H-reverse transcriptase, Invitrogen), according to the manufacturer's instructions, and PCR was set up according to Promega's reverse transcription system protocol. Amplification of 18S served as an internal control. Primer sequences used were as follows: MTA2, 5′-TGG TTA GAC GGA TTG AGG AG-3′ and 5′-TCA AAC TCC CGA GCA TTA CT-3′ (gene accession number NM_011842.3); rat MTA2, 5′-GGGTAGGAGATTATGTCTAT-3′ and 5′-ATTGGTTTAAGATATCCGTC-3′ (gene accession number NM_001100740.1); MTA1, 5′-CAG TGT CGC CTC TGC GCA TC-3′ and 5′-TCC ACT GCT CCG AGC TGG AA-3′ (17); 18S, 5′-CTC GCC GCG CTC TAC CTA CCTA-3′ and 5′-ATG AGC CAT TCG CAG TTT CAC TGTA-3′(17); FSHR, 5′- GGG ATC TGG ATG TCA TCA CT-3′ and 5′-GGA GAA CAC ATC TGC CTC TA-3′ (gene accession number BC137991.1); Ar, 5′-TAT GTG CCA GCA GAA ACG ATT GTA-3′ and 5′-CGG TAC TCA TTG AAA ACC AAG TCA-3′ (19); ABP, 5′-AAC CAT TAA CCA CCA AAT TA-3′ and 5′-ATC CAG TTT AAA CAT ATC CG-3′ (gene accession number GU269237.1). PCR products were then quantified by SYBR Green intercalation using the MiniOpticonTM system (Bio-Rad). The relative abundance of each target transcript was quantified using the comparative ΔΔCt method, with 18S as an internal control.
Western Blotting
Protein samples were prepared in ice-cold radioimmune precipitation assay buffer (50 mm Tris-HCl, 150 mm NaCl, 1% (v/v) Triton X-100, 1% (w/v) sodium deoxycholate, and 0.1% (w/v) SDS, pH 7.5) supplemented with Complete protease inhibitor mixture tablets (Roche Applied Science). Protein was separated on SDS-PAGE and transferred to nitrocellulose membrane (Millipore, Bedford, MA). Membranes were then incubated with primary antibodies, including anti-MTA1 (dilution 1:1000; Santa Cruz Biotechnology, Inc.), anti-β-actin (dilution 1:2000; Santa Cruz Biotechnology, Inc.), anti-MTA2 (dilution 1:1000; Santa Cruz Biotechnology, Inc.), anti-Ar (dilution 1:1000; Santa Cruz Biotechnology, Inc.), anti-His (Invitrogen), anti-GST (Abcam, New Territories, Hong Kong), anti-pSrc, anti-Src, anti-pERK42/44, and anti-ERK42/44 (Cell Signaling Technology) in blocking solution overnight at 4 °C. Positive signals were finally detected by using an ECL kit (Amersham Biosciences).
Immunohistochemistry
A Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) was used for immunohistochemical staining according to the protocol recommended by the manufacturer. The primary anti-MTA2 antibody was used at a dilution of 1:150. Control slides were incubated with a preabsorbed serum instead of primary antibody. Immunostaining was evaluated manually and graded by two pathologists as described previously (20).
Co-Immunoprecipitation (Co-IP)
Co-IP analysis was performed as reported before (17). Briefly, protein lysates were obtained using radioimmune precipitation assay buffer containing Complete protease inhibitor mixture tablets (Roche Applied Science) and centrifuged at 5000 × g at 4 °C for 10 min. The lysates were incubated with rabbit anti-Ar, goat anti-MTA2 antibodies, or control IgG antibodies at 4 °C overnight. On the following day, protein A-Sepharose (Pierce) was added into lysates, and the compound was incubated at 4 °C for another 2 h. Immunocomplexes were finally eluted from the Sepharose beads by boiling in Laemmli sample buffer and subjected to SDS-PAGE and immunoblotting analysis with goat-anti MTA2 or rabbit anti-HDAC1 antibody (Abcam, Shatin, Hong Kong, China).
Double ChIP
Double ChIP analysis was performed as described previously (21). Briefly, first round or control IgG antibodies were added to chromatin extracts and incubated overnight at 4 °C, followed by the addition of 60 ml of salmon sperm/protein A-agarose (Upstate Biotechnology) to recover immunocomplexes. The bound protein complexes were eluted by 10 mm dithiothreitol (DTT) at room temperature for 30 min, and the elution was then diluted 10 times with reChIP buffer (1% Triton X-100, 2 mm EDTA, 150 mm NaCl, 20 mm Tris (pH 8.1)) and subsequently reimmunoprecipitated by the addition of the second round antibody overnight at 4 °C. Recovery and preparation of DNA was performed and followed by PCR using primers for the FSHR promoter, 5′-CTT GAA GGA TAA GAC AGG TGC-3′ and 5′-CTG CTT TCT GCC TGC TCC-3′ (22). Single step ChIP assays for MTA2 and HDAC1 were carried out as controls.
Data Presentation and Statistical Analysis
Experiments were repeated at least three times, and one representative result from at least three similar results is presented. Correlation of relative MTA2 immunoreactive content in human testis to serum FSH level was determined based on Pearson's correlation coefficient with the aid of SPSS 15.0 software. Quantitative data are presented as mean ± S.D. Results were analyzed for statistically significant differences using analysis of variance, followed by Tukey's test. p < 0.05 was considered significant.
RESULTS
Distinctive Expression of MTA2 in SCs
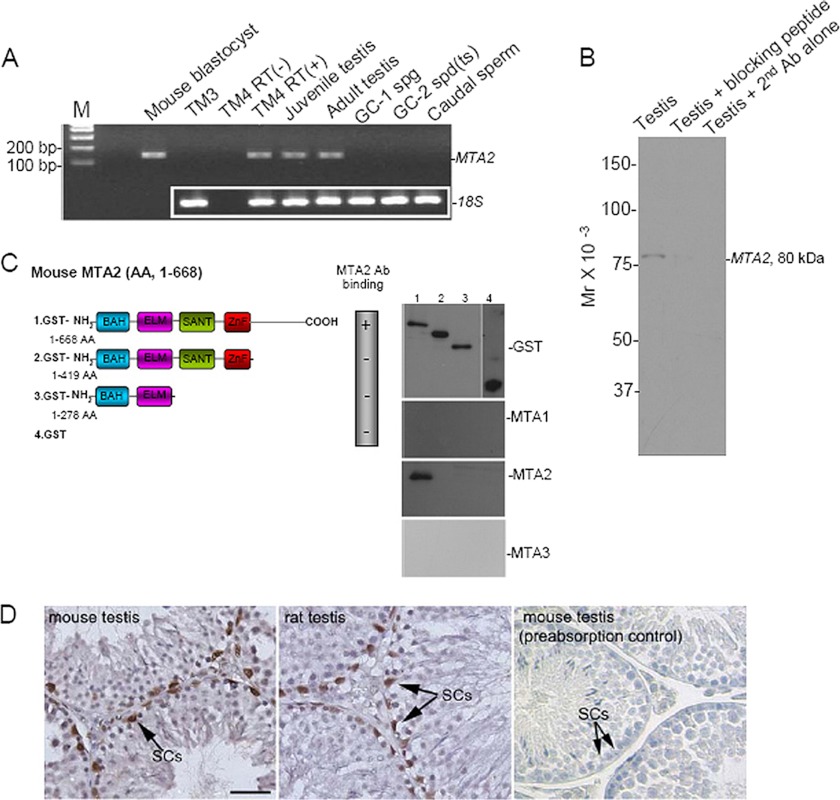
As a first step to understand the physiological role(s) of MTA2 in the testis, we sought to establish the cellular distribution of the target protein within mouse testis. Expression of the gene encoding MTA2 was evaluated in different spermatogenic cell lines and in the mouse testis at different stages of postnatal development. Our RT-PCR assay demonstrated an exclusive expression of MTA2 mRNA in the TM4 SC line and in the testis. In contrast, no amplification of the target gene was found in the TM3 LC line, the GC-1 spg cell line (corresponding to a stage between the type B spermatogonia and the primary spermatocytes), the spermatocyte-derived GC-2 spd(ts) cell line, and caudal sperms. Meanwhile, using similar RT-PCR conditions, MTA2 mRNA was detected in mouse blastocyst samples, used as the positive control (8). As expected, omitting the RT reaction resulted in no bands after amplification in TM4 cells, confirming the specificity of the assay (Fig. 1A). In another experimental setting, immunoblotting analysis demonstrated a single band of the target protein in the whole blot, and negative controls consisting of incubating samples with the preabsorbed primary antibody or omitting the primary antibody demonstrated an abolished expression of testicular MTA2, suggesting that the commercial antibody employed in the present study did not cross-react with other proteins (Fig. 1B). We also examined the binding abilities of MTA antibodies to different regions of the mouse MTA2 protein. It turned out that only MTA2 antibody could detect the carboxyl-terminal portion of the target protein, further confirming the specificity of the antibody (Fig. 1C). Immunolocalization of MTA2 protein was then carried out in adult rodent testicular sections by means of immunohistochemistry. The result evidenced the predominant presence of specific MTA2 immunostaining in SCs inside the seminiferous tubules of the rodent testis. Of note, in all positive cells, MTA2 immunoreactivity showed specific nuclear location. Other testicular cell types, such as germ cells and LC, were all negative for MTA2 at all stages studied (Fig. 1D).
FIGURE 1.
MTA2 is exclusively expressed in SCs. A, expression profile of MTA2 was evaluated in different spermatogenic cell lines and in mouse testis at different stages of postnatal development using RT-PCR. Amplification of MTA2 mRNA in mouse blastocyst samples served as a positive control. 18S was used as a loading control. B, immunoblotting analysis demonstrated a single band of MTA2 protein in the testicular lysates, which was absent when samples were incubated with preabsorbed primary antibody or without primary antibody. C, different fusion proteins were constructed for MTA2 as depicted in the left panel and were then subjected to immunoblotting analysis using antibodies against MTA1, MTA2, and MTA3 (right panel). AA, amino acids. D, immunohistochemical analysis in rodent testes revealed a distinct nuclear localization of MTA2 in SCs (arrows). Replacement of the primary antibody with preabsorbed primary antibody abolished the immunostaining, confirming the specificity of the assay. Bar, 25 μm.
Pattern of Cellular Expression of MTA2 in Mouse Testis during Postnatal Development
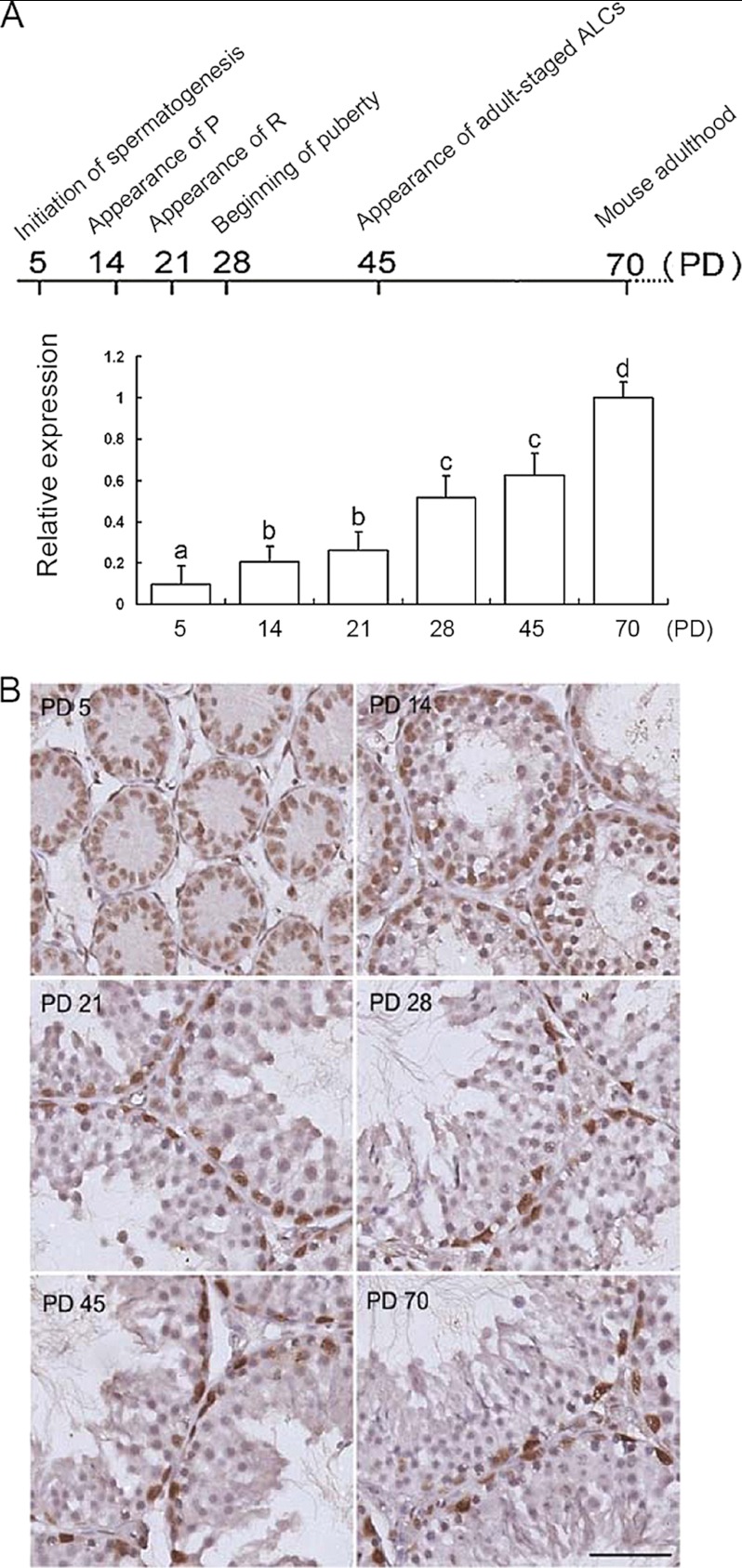
Assessment of MTA2 mRNA expression by QRT-PCR analysis demonstrated persistent expression of the gene in mouse testis throughout postnatal development. In detail, six representative stages of development were explored: initiation of spermatogenesis (PD 5), appearance of pachytene spermatocytes (PD 14), appearance of round spermatids (PD 21), beginning of puberty (PD 28), appearance of adult-staged Leydig cells (PD 45), and adulthood (PD 70). Among them, PD 5 and PD 14 correspond to infancy, PD 21 corresponds to prepuberty, PD 28 and PD 45 correspond to puberty, and PD 70 corresponds to adulthood, respectively (23). The available data revealed that the expression levels of MTA2 in mouse testis changed along the study period, with the highest values being detected during the adult period (Fig. 2A). In addition, clear-cut MTA2 immunostaining was observed in SCs inside the seminiferous tubules using a goat anti-MTA2 polyclonal antibody. In contrast, negligible staining in other cell types was impossible to differentiate from background and was considered negative (Fig. 2B).
FIGURE 2.
Developmental profile of MTA2 expression in murine testis throughout postnatal maturation. A, QRT-PCR analysis of MTA2 transcripts in mouse developing postnatal testis. Top, diagram of the stages of mouse spermatogenesis; P, pachytene spermatocytes; R, round spermatids; ALCs, adult Leydig cells. Bottom, QRT-PCR analysis of MTA2 mRNA level. a, b, c, and d denote groups that are statistically different (p < 0.05; analysis of variance followed by Tukey's test). B, immunohistochemical detection of MTA2 expression in various developmental stages of mouse testes. Bar, 25 μm. Error bars, S.D.
Androgen-regulated Expression of MTA2 in SCs
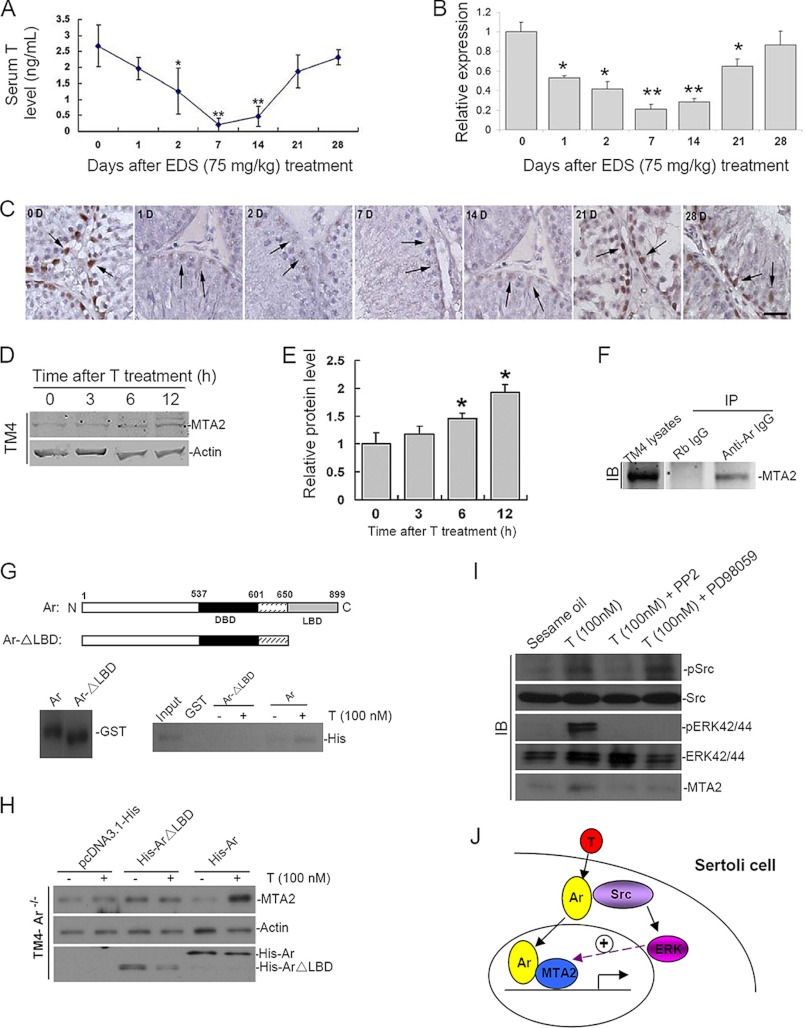
Given that the expression pattern of MTA2 in rodent testis is conservative, we thereafter employed a well established androgen manipulation model, namely EDS-treated rat testis, to further explore the possible upstream modulation of MTA2. Adult male rats received a single EDS injection (75 mg of EDS in DMSO/kg of body), and LC elimination was confirmed by measurement of circulated testosterone concentrations in EDS-treated groups, which dropped to nearly undetectable values at days 7 and 14 and began to restore at day 21 after EDS injection (Fig. 3A). In line with the hormonal change, the relative expression of rat MTA2 mRNA began to decrease 1 day after EDS treatment, with the minimal level observed at days 7 and 14 post-EDS, as revealed by QRT-PCR analysis (Fig. 3B). Parallel immunohistochemical staining, conducted at different time points after selective LC elimination, revealed a persistent expression of MTA2 protein in SCs throughout a 28-day period after EDS administration, with a significant decrease in MTA2 expression observed at days 7 and 14 post-EDS, which was followed by a gradual reappearance of MTA2-positive staining from post-EDS day 21 onward (Fig. 3C). Thus, the expression profile of MTA2 in EDS-treated rat testes coincided well with the process of LC repopulation. The androgen-regulated expression of MTA2 was also confirmed in the cultured TM4 cell line (Fig. 3, D and E). Moreover, the co-IP assay demonstrated a direct interaction between endogenous MTA2 and Ar in TM4 cells upon testosterone stimulation (Fig. 3F). Because the LBD of Ar is responsible for binding to some co-factors during transcription regulation (4), we were curious whether the LBD serves as the binding region for MTA2. An in vitro GST pull-down assay performed using GST fusion full-length and truncated Ar proteins and His-tagged MTA2 protein clearly showed that the interaction between Ar and MTA2 upon testosterone treatment was enhanced only in the presence of the LBD (Fig. 3G). We next tried to confirm the importance of the LBD for the interaction between Ar and MTA2 from a reverse angle. Because TM4 cells are Ar-positive, we knocked down Ar by siRNA treatment (supplemental Fig. 1) and then transfected cells with His-tagged full-length and truncated Ar mutant constructs. Interestingly, up-regulation of MTA2 expression in response to androgen stimulation could only be achieved when cells were rescued with full-length Ar; thus, the LBD is definitely required for the interaction between Ar and MTA2 (Fig. 3H). In another experiment, we tested whether the stimulatory effect of testosterone on MTA2 expression was mediated through a non-classical androgen action. Treatment of SCs with PP2, a broad-spectrum inhibitor of Src, or PD98059, a well characterized inhibitor of MAPK activity, could both abolish the elevated expression of MTA2 induced by testosterone treatment, suggesting that T-dependent activation of MTA2 requires activated Src and MAPKs (Fig. 3I). Taken together, the available data indicated that the androgen regulation of MTA2 expression may be achieved though the non-classical testosterone actions. Upon testosterone stimulation, Ar translocates from cytoplasm to nucleus and interacts with MTA2. This intimate association may help to sequester MTA2 in situ and thereafter enhance its up-regulation by the non-classical androgen signaling (Fig. 3J).
FIGURE 3.
Specific expression of MTA2 in SCs is regulated by androgen signaling. A, serum testosterone (T) level (ng/ml) in rats during androgen manipulation. B, MTA2 mRNA level in rat testis at different time points after administration of the cytotoxic drug EDS was evaluated by QRT-PCR. Data were presented as the mean ± S.D. of at least three determinations (*, p < 0.05; **, p < 0.01, when compared with control). C, SC expression of MTA2 protein (arrows) in rat testis at different time points after administration of the cytotoxic drug EDS was illustrated using immunohistochemical staining. Bar, 25 μm. D, time-dependent up-regulation of MTA2 expression in TM4 cells by testosterone (40 ng/ml) treatment was assessed by Western blotting. E, immunoblot data were densitometrically scanned and compared. Bars, mean ± S.D. (error bars) of n = 3, normalized against actin, wherein the control was arbitrarily set at 1, against which one-way analysis of variance was performed (*, p < 0.05). F, after treatment with testosterone (40 ng/ml) for 6 h, TM4 cells were harvested, and lysates were subjected to a co-IP assay followed by immunoblot analysis to demonstrated the association of endogenous AR with MTA2. G, different GST fusion proteins were constructed for Ar as depicted in the top panel and were then subjected to in vitro pull-down assay along with His-tagged MTA2 protein in the absence or presence of testosterone supplement. H, after Ars were knocked down using siRNA, TM4 cells were transfected with different Ar plasmids and were then incubated with testosterone (100 nm) for 1 h. Immunoblotting analysis were employed to assess the expression level of MTA2. Actin served as loading control. I, primary SCs were pretreated for 2 h with DMSO, PP2 (10 μm), or PD98059 (50 μm) before stimulation for 1 h with EtOH (Vehicle Ctrl) or 100 nm T. Immunoblot analysis of whole-cell extracts was first performed using the antisera against MTA2, pSrc, or pERK followed by reprobing the blots with antisera against total Src or ERK. The figure shown is representative of three experiments. J, up-regulation of MTA2 by the non-classical testosterone signaling pathway in Sertoli cells and the potential enhancement of the interaction between MTA2 and Ar by this process.
FSH Stimulation of MTA2 Expression in SCs
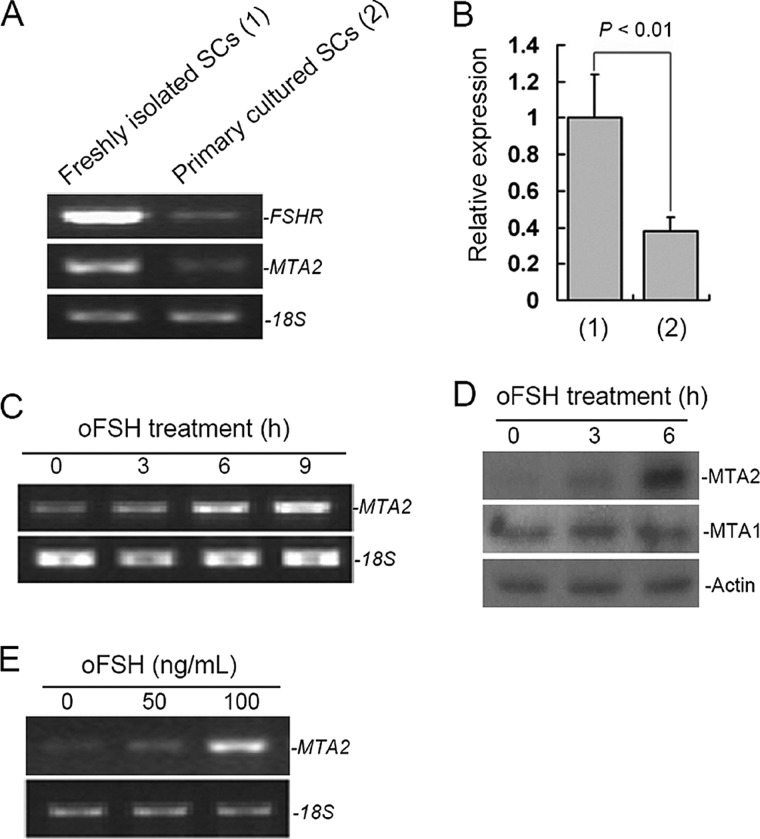
Because the occurrence of normal spermatogenesis requires the proper integration of FSH and testosterone signals, we then investigated whether FSH could regulate the expression of MTA2. Unlike freshly prepared SCs, which expressed abundant FSH receptors, primary cultured Sertoli cell lines express a very low level of FSHR (24). In line with this, the expression level of MTA2 in primary cultured SCs was relatively lower than that in freshly prepared SCs, as shown by an RT-PCR assay (Fig. 4A). Normalized expression level of MTA2 using QRT-PCR revealed a 62.03% reduction in the primary cultured SCs (p < 0.01, n = 3) (Fig. 4B). Furthermore, analysis of RNAs from freshly prepared SCs by RT-PCR demonstrated a time-dependent stimulation of MTA2 messenger RNA by FSH (Fig. 4C). Western blotting with an anti-MTA2 antibody also showed that the level of MTA2 of relative molecular mass 80,000 (Mr 80,000) was significantly increased in FSH-treated SCs. In contrast, FSH had no effect on expression of MTA1 protein (Fig. 4D). Because mouse SCs cease to proliferate around postnatal day 17 (25), the observed effect of FSH on MTA2 expression may not be reflective of the proliferative status of the cell. In addition, induction of MTA2 expression by FSH was also dose-dependent (Fig. 4E).
FIGURE 4.
FSH regulation of MTA2 expression in SCs. A, RT-PCR analysis of MTA2 expression in freshly isolated and primary cultured SCs. 18S was used as a loading control. B, PCR products from A were then quantified by SYBR Green intercalation as described under “Experimental Procedures.” Quantitative data in terms of MTA2 expression levels were normalized to those of the internal control 18S. Values are the mean ± S.D. (error bars) of at least three determinations. C, RT-PCR analysis of MTA2 expression in SCs was carried out at different time points of oFSH treatment. D, expression of MTA1 and MTA2 in SCs at different time points of oFSH treatment was evaluated at the translational level by Western blotting. E, dose-dependent up-regulation of MTA2 mRNA in SCs upon oFSH treatment. Parallel amplification of 18S mRNA served as internal control.
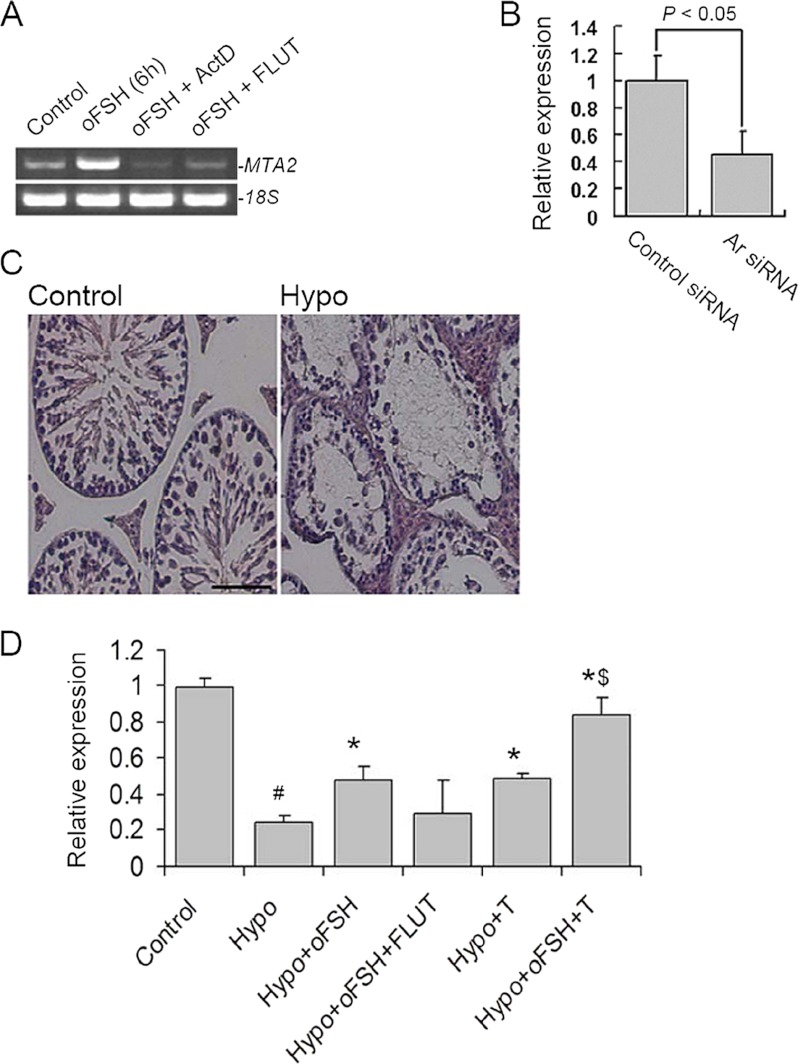
Up-regulation of MTA2 Expression by FSH in SCs Is Mediated via Ar Signaling
Within seminiferous tubules, SCs are the only cell types that possess receptors for testosterone and FSH, and thus these cells are the major targets of both hormonal signals that regulate spermatogenesis (4). To this end, we were interested to know whether MTA2 functions in the cross-talk between these two pathways. Treatment of SCs with actinomycin D, an inhibitor of transcription, completely inhibited FSH-mediated induction of MTA2 mRNA. The same effect was also observed in the SCs treated with FLUT, a specific anti-androgenic toxicant (Fig. 5A). To further illustrate the indispensable involvement of Ar signaling in the FSH-induced increase of MTA2 expression, we checked the expression level of MTA2 in TM4 cells depleted of endogenous Ar. Quantitative RT-PCR analyses revealed a 54.82% reduction of FSH-induced up-regulation of MTA2 in TM4 after Ar ablation (Fig. 5B). To evaluate FSH modulation of MTA2 via the Ar pathway in vivo, we employed the Hypo mouse as the experimental model. Long term (4-week) hypophysectomy resulted in a clear cut decrease of the atrophy of all testicular compartments, with atrophic LC in the interstitial space and regressing seminiferous epithelium with apparent arrest of spermatogenesis within the tubules (Fig. 5C). Quantitative RT-PCR analyses demonstrated that 4-week hypophysectomy induced a dramatic decrease in MTA2 mRNA levels (0.2436 ± 0.0373, p < 0.01 versus control), a response that was partially prevented by replacement with exogenous oFSH (0.4754 ± 0.078, p < 0.01 versus Hypo + oFSH). However, similar responses to hypophysectomy and gonadotropin replacement were undetectable for testicular MTA2 mRNA levels when treatment with oFSH was applied simultaneously with FLUT (0.2946 ± 0.1862, p > 0.05 versus Hypo). As expected, treatment with oFSH together with testosterone resulted in a more dramatic up-regulation of the MTA2 expression (0.8359 ± 0.1041, p < 0.05 versus Hypo, versus Hypo + oFSH, or versus Hypo + testosterone, respectively) (Fig. 5D). Taken together, these findings indicate the possible existence of MTA2 in the cross-talk between FSH and Ar signaling.
FIGURE 5.
Up-regulation of MTA2 expression by FSH in SCs is mediated via AR signaling. A, regulation of MTA2 mRNA by FSH in SCs was inhibited by blockage in AR signaling. ActD, actinomycin D. B, after being treated with Ar siRNA or control siRNA for 48 h, TM4 cells were subjected to oFSH treatment for 6 h, followed by QRT-PCR analysis of MTA2 expression levels. Quantitative data in terms of MTA2 expression levels were normalized to those of the internal control 18S. Values are the mean ± S.D. (error bars) of at least three determinations. C, effect of hypophysectomy on mouse testicular morphology was evaluated in H&E-stained transverse testis sections. Bar, 25 μm. D, hypophysectomized mice were treated with oFSH with or without FLUT, oFSH, and testosterone (T), as described under “Experimental Procedures,” and the expression level of MTA2 mRNA was then determined by QRT-PCR. Values are the mean ± S.D. of at least three determinations. #, p < 0.05 when compared with control group; *, p < 0.05 when compared with Hypo group; $, p < 0.05 when compared with the Hypo + oFSH or Hypo + testosterone group.
Association of MTA2 with HDAC and FSHR
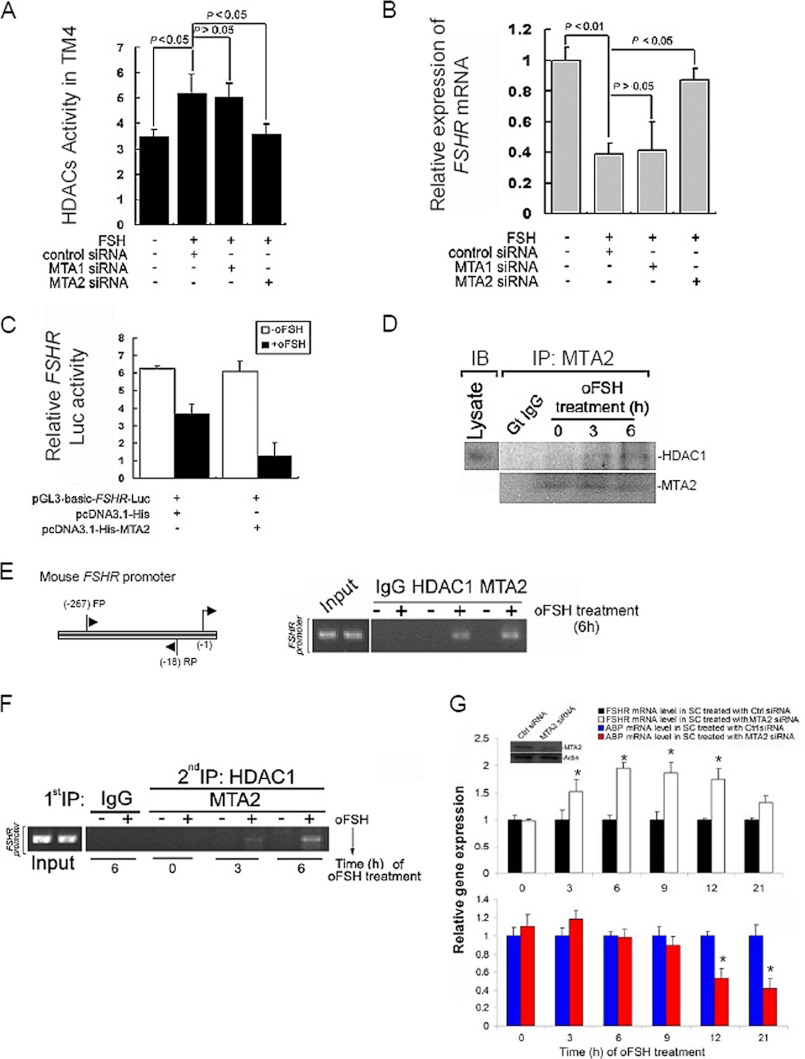
Because MTA2, a component of the NuRD complex, was induced by FSH, we next examined the influence of FSH on the status of HDAC activity. We treated TM4 cells for 6 h with or without oFSH after we had knocked down the endogenous expression of MTA1 or MTA2 and then evaluated the status of HDAC activity using a colorimetric assay. The addition of oFSH to cells was accompanied by an expected increase in the HDAC activity (5.178 ± 0.743 versus 3.478 ± 0.267, p < 0.05 when compared with control group), which was compromised in the cells treated with MTA2 siRNA (3.578 ± 0.404 versus 5.178 ± 0.743, p < 0.05 when compared with control siRNA). Ablation of MTA1 had no effect on the increase of HDAC activity induced by FSH (Fig. 6A). The action of FSH during spermatogenesis is regulated at a fundamental level by controlling the number of competent receptors present at the surface of SCs. One mechanism of control is the down-regulation of the steady state levels of the FSHR gene after exposure to FSH by recruitment of HDAC (26). We observed that ablation of MTA2 expression significantly reversed the down-regulation of FSHR mRNA level by FSH treatment. This was a specific effect of MTA2 siRNA, because inhibition of MTA1 produced no such reversal effect (Fig. 6B). Because MTA2 has been shown to have HDAC activity, and because FSH induces MTA2 expression as well as histone deacetylation, we hypothesized that MTA2, in conjunction with HDAC complex, may repress FSHR transcription and may thus provide a molecular explanation for the reported suppression of the FSHR mRNA by FSH. As shown in Fig. 6C, co-transfection of FSHR-luciferase with either FSH or overexpressing His-MTA2, but not with control vector, was accompanied by significant suppression of FSHR transcription, lending strong support to our hypothesis. To determine whether the observed repression of FSHR transcription by MTA2 was associated with recruitment of HDAC complexes in vivo, we next examined the association between endogenous MTA2 and the components of HDAC by co-immunoprecipitation and Western blotting. It was found that MTA2 structurally interacted with HDAC1 in SCs, and this interaction was gradually enhanced with the incubation of exogenous FSH (Fig. 6D). To confirm that the FSHR gene chromatin is a direct target of MTA2, we performed ChIP assays, using primers encompassing 249 bp of the FSHR promoter region. MTA2 and HDAC1 were both recruited to FSHR promoter in the presence of FSH stimulation (Fig. 6E). To further demonstrate the physical interaction of the MTA2-histone deacetylase complex with the FSHR chromatin, we performed a double ChIP in SCs in the presence of oFSH. Initial ChIP was done with anti-MTA2 antibody to immunoprecipitate the MTA2-bound DNA sequences, and the second ChIP was done with the anti-HDAC1 antibody. We observed simultaneous co-association of MTA2 and HDAC1 with the FSHR chromatin, and its recruitment was gradually augmented when FSH treatment progressed (Fig. 6F). These results collectively suggested that MTA2 represses FSHR expression in an HDAC-dependent manner in SCs, providing an explanation for the repression of FSHR transcription at the molecular level. The available data led us to speculate on the implications of the interaction between MTA2 and FSHR for SC biology. Production of testicular androgen-binding protein (ABP) is significantly up-regulated by FSH stimulation. Therefore, ABP response to FSH is believed to be a useful parameter of FSH-dependent secretory function in SCs (4). We knocked down the MTA2 in primary cultured SCs and incubated the cells with oFSH for different durations. As expected, FSHR expression was elevated in MTA2 knockdown SCs upon FSH stimulation and persisted along the study period. Interestingly, ABP expression was significantly decreased during the late phase of FSH treatment (Fig. 6G). Thus, the homologous down-regulation of FSHR expression by MTA2 modifier may be required for the persistence of FSH response in SCs.
FIGURE 6.
MTA2 participates in the down-regulation of FSHR expression upon FSH treatment by directly recruiting HDAC1 into FSHR promoter. A, effects of MTA1 siRNA or MTA2 siRNA treatment on HDAC activity in TM4 cells in response to oFSH stimulation. B, expression level of FSHR mRNA in MTA1 siRNA- or MTA2 siRNA-treated TM4 cells upon oFSH stimulation was determined using QRT-PCR. Values are the mean ± S.D. (error bars) of at least three determinations. C, SCs were transfected with FSHR(−100/+123) Luc and expression vectors for His-MTA2 (0.5 μg) and empty vector as indicated. Two days after transfection, the cells were stimulated with vehicle or oFSH (25 ng/ml) for 6 h, after which the cells were harvested, and luciferase activities were normalized for total protein as determined by a Bradford assay. Results represent the mean ± S.D. luciferase activity for four independent experiments using two replicates. D, direct association of MTA2 and HDAC1 in SCs was illustrated by a co-IP assay followed by Western blotting analysis (IB) at different time points after oFSH treatment. E, ChIP analysis showing recruitment of MTA1 and HDAC1 onto the mouse FSHR promoter in SCs upon FSH stimulation. F, double ChIP analysis of MTA2-HDAC1 complex onto the FSHR promoter in SCs at different time points after oFSH treatment. G, after SCs had been deprived of endogenous MTA2 by siRNA treatment, cells were incubated with oFSH for different durations. QRT-PCR analysis was then used to evaluate the MTA2 and ABP mRNA levels at different time points after oFSH treatment. Values are the mean ± S.D. of at least three determinations. *, p < 0.05 when compared with control group. The efficiency of MTA2 deletion was also monitored by immunoblotting analysis (inset).
Disrupted Expression of MTA2 in Human Pathological Testes Correlates to the Deregulated Level of Serum FSH
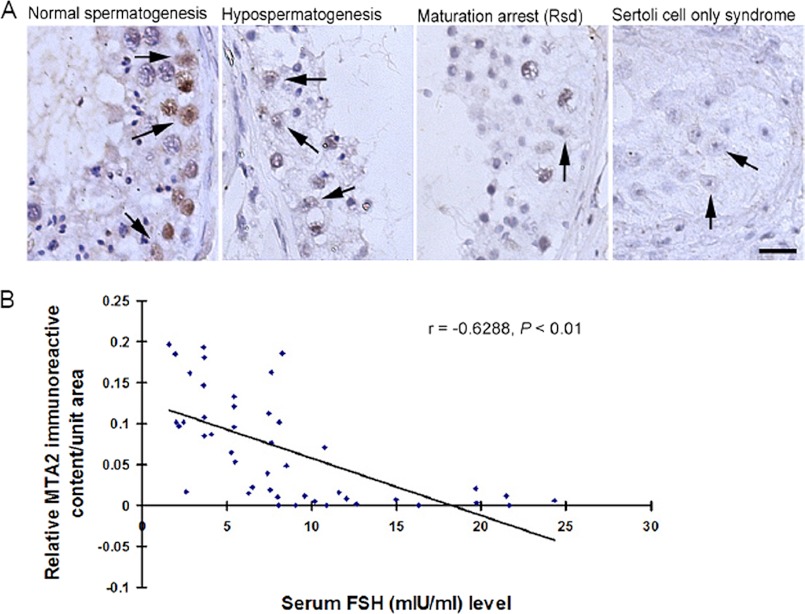
Deregulated expression of HDAC components has been frequently reported in male infertile patients (27). We therefore investigated the localization of MTA2 immunoexpression in testicular tissues of patients with impairment of spermatogenesis and in controls. A notable immunoreaction was detected in the nuclei of SCs during normal spermatogenesis. In contrast, a relatively weaker staining was found in the SCs of hypospermatogenesis. SCs in the seminiferous tubules from spermatogenic arrest at the round spermatid level and from SCOS possessed negligible stainings, respectively (Fig. 7A and supplemental Table 3). In addition, we observed that the ratio of MTA2-immunostained SCs in all groups correlated negatively with the concentration of serum FSH (r = −0.6288; p < 0.01; Fig. 7B and supplemental Table 2).
FIGURE 7.
Deregulated testicular expression of MTA2 negatively correlates with serum FSH level in human infertile patients. A, immunohistochemical analysis of MTA2 expression in human pathological testes. The arrows denote the positive staining of MTA2 in SCs. Bar, 15 μm. B, correlation of relative MTA2 immunoreactive content in human testis with serum FSH level was determined based on Pearson's correlation coefficient with the aid of SPSS 15.0 software.
DISCUSSION
Among the many chromatin modifiers identified, the NuRD complex is unique because it possesses both nucleosome remodeling and HDAC activities (28). HDACs are known to play an important role for the regulation of gene expression in SCs during spermatogenesis (29). For example, in vivo application of the HDAC inhibitor trichostatin A results in murine male infertility due to an impairment of meiosis. Gene expression analysis suggests an indirect mechanism involving SC deregulation as a probable etiology (29). Among various members of the HDAC family, HDAC1 is known to represent the predominant HDACs within the testis (30). HDAC1 is localized in the nuclei of spermatogonia and SCs. Interestingly, compelling evidence has established a close link between HDAC1 and MTA2 (31). In this context, the present study demonstrated an exclusive and development-regulated expression of MTA2 in SCs, strongly indicating that the existence of MTA2 in SCs may be functionally associated with HDAC1, which might play a unique role in this somatic cell.
In a previous study, we have demonstrated that the expression of MTA1, the founding member of the MTA family, is partially modulated by androgen signaling in murine epididymis (32). Similarly, MTA2 expression became undetectable in rat testis after selective withdrawal of adult-type LC by administration of the cytotoxic compound EDS. Conversely, repopulation of this cell type was associated with recovery of testicular MTA2 signal. Overall, our present results are strongly indicative of an androgen regulation of testicular MTA2 expression. Mechanistically, the ligand-bound AR, once in the nucleus, binds chromatin and recruits coactivators and chromatin remodeling complexes, resulting in the recruitment of RNA polymerase II for initiation of transcription. Many of the AR coactivators either themselves possess histone acetyltransferase activity or recruit proteins with histone acetyltransferase activity to the AR-regulated promoters, as is the case for many other nuclear receptors (33). Together with our findings that Ar interacted with MTA2 via its LBD in response to androgen stimulation, it is possible that as an important HDAC recruiter and a transcriptional cofactor, MTA2 may directly participate in the regulation of Ar activity because gene expression is usually tightly controlled by the balance between acetylation and deacetylation (34). Testosterone actions present an interesting paradox in that numerous genes and proteins are up-regulated in response to stimulation, but few genes have been characterized that are known to be induced with this steroid through the classical mechanism of Ar binding to specific promoter elements (4). Similarly, we did not detect any androgen response elements in the MTA2 promoter region (data not shown). Therefore, along with our in vitro results (Fig. 3I), the regulation of MTA2 expression is probably mediated through the non-classical testosterone actions requiring both MAPK and Src kinase activity (35).
The regulation of testicular MTA2 expression by FSH was assessed, given its major role in the control of SC development and function. Our results showed that FSH participates in the tuning of MTA2 expression in mouse testis, but this modulation appears to be mediated via androgen signaling. Actually, it has been reported that FSH induces the expression of AR and regulates the androgen responsiveness of SCs, although there are differences in the actions of FSH and testosterone. FSH alone can support SC proliferation, determine the final SC number during the testicular development, and thereby affect the spermatogenic potential. In contrast, testosterone but not FSH is essential for the maintenance of normal adult spermatogenesis (4). On this basis, it is tempting to consider that the regulation of androgen signaling may serve as an indispensable complementary pathway for FSH action because testosterone is apparently more effective for the stimulation of spermatogenic differentiation, and SC function is critically influenced by the local testosterone level (3). Of note, MTA2 might be the key point to better understand the complicated cross-talk between these two pathways. This leads to the discussion of the following question. What is the function of the up-regulation of MTA2 in response to FSH stimulation?
The direct action of FSH on SCs is transient. Therefore, continuous stimulation of SCs with FSH leads to a desensitization of the cells to FSH. Desensitization of the FSH response in SCs involves multiple steps in signal transduction, among which the down-regulation of the transcription of the FSHR gene is of great importance (36). Previous study has shown that suppression of HADC activity by trichostatin A treatment completely prevents the homologous down-regulation of the transcription of the FSH receptor gene (26). Consistently, we have demonstrated that MTA2 represses FSH-mediated FSHR transcription by recruiting HDAC1. Our observation that the sensitivity of SCs in response to FSH stimulation (indicated by the secretory marker ABP expression level in the current study) was substantially compromised in the absence of endogenous MTA2 strengthened the notion that down-regulation of FSHR by deacetylation operates as an indispensable self-regulating mechanism for the continuity of FSH response in SCs. Mechanistically, recruitment of HDAC to promoters has emerged as a general mechanism of transcription repression of target genes. For example, recruitment of the HDAC complex to target genes of the retinoic acid receptor represses transcription and prevents differentiation, and treatment with retinoic acid induces differentiation by displacing the HDAC complex from PML-RAR-α. Similarly, transcription repressor complexes containing HDAC have been demonstrated in the HRG/ER-α (37) and Six3/rhodopsin pathways (37). Our observation that MTA2 can directly recruit HDAC1 into the promoter region of FSHR in response to FSH stimulation is important because it reveals that the reported association between MTA2 and HDACs in the NuRD complex may reflect direct MTA2 interaction. Furthermore, it supports the idea that the FSHR gene could utilize a mode of regulation that links the control of gene expression by signal transduction and chromatin structure.
Mammalian seminiferous epithelium consists of SCs and germ cells. Its renewal and functioning, which underlie spermatogenesis, require the expression and precise coordination of a multitude of genes in both SCs and germ cells. Dysfunction of such genetic factors is often associated with disturbed spermatogenesis and is suspected to be a frequent cause of male infertility (38). In our study, assessment of SC-related MTA2 expression in human pathological testis by immunohistochemistry demonstrated a clear cut attenuated level of this histone modifier in the SCs of patients with impaired or arrested spermatogenesis, indicating that MTA2 expression in SCs may be associated with human spermatogenic failure. The complete abolishment of MTA2 immunoreactivity in the SCs of SCOS tubules is in good agreement with the previous report that in spermatogenesis impairment where spermatocytes were absent, the nuclei of all or most SCs showed an intense acetylation of histone H4, regardless of the origin of the pathology (39). In addition, an abnormally high level of serum FSH has been frequently reported in male infertile patients (40). Given that the concentration of FSH in the serum of non-seasonal breeding males is relatively constant, the regulation of the number of FSH receptors and their competency to bind FSH and transduce signal may be an important level of control of the action of FSH in males. As described above, once the homologous down-regulation of the FSHR gene was disrupted, persistent exposure of FSHR to FSH stimulation may easily lead to abnormal desensitization of SCs, and thereafter an irresponsive reaction of the cells to FSH may possibly occur. This may explain the observation that MTA2 expression correlated negatively with concentration of serum FSH level because HDAC plays an important role in the FSH-induced repression of the FSHR gene in SCs. Our study also points to the diagnostic potential of MTA2 for male infertility.
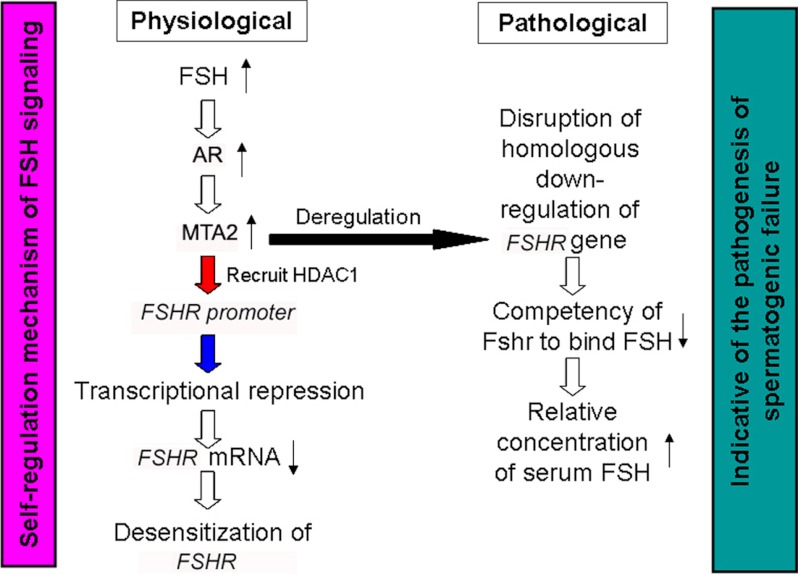
In summary, we propose a novel role of SCs expressing MTA2. GnRH signal-induced FSH binds to FSHR and subsequently activates a series of downstream signals to facilitate normal spermatogenic differentiation. Simultaneously, FSH induces Ar activation, followed by an up-regulation of MTA2 expression via the non-classical androgen signaling. HDAC complexes associated with the MTA2 corepressor bind to the FSHR gene and mediate its transcriptional repression in response to FSH. Conversely, deregulated MTA2 expression may result in the disruption of the homologous down-regulation of the FSHR gene and the inevitable reduction of competency of FSHR to bind FSH, followed by an unusual elevation of the serum FSH level (Fig. 8). Overall, the FSH/Ar/MTA2 cascade may serve as an important negative feedback mechanism to modulate the timing and magnitude of subsequent signal transduction of SCs in response to FSH.
FIGURE 8.
Summary diagram of the possible mechanisms related to endogenous MTA2 function contributing to the down-regulation of FSHR expression upon FSH treatment in SCs.
Supplementary Material
Acknowledgment
We thank Hui Wang for careful assistance during preparation of the manuscript.
This work was supported by National Natural Science Foundation of China Grants 31271248, 30800395, and 31171154.

This article contains supplemental Tables 1–3 and Fig. 1.
- SC
- Sertoli cell
- Ar
- androgen receptor
- HDAC
- histone deacetylase
- SCOS
- Sertoli cell only syndrome
- PD
- postnatal day
- Hypo
- hypophysectomized
- oFSH
- ovine FSH
- FLUT
- flutamide
- LC
- Leydig cell
- EDS
- ethylene dimethane sulfonate
- QRT-PCR
- quantitative RT-PCR
- LBD
- ligand binding domain
- IP
- immunoprecipitation
- ABP
- androgen-binding protein
- NuRD
- nucleosome remodeling and histone deacetylation.
REFERENCES
- 1. Grinspon R. P., Rey R. A. (2010) Anti-Müllerian hormone and Sertoli cell function in paediatric male hypogonadism. Horm. Res. Paediatr. 73, 81–92 [DOI] [PubMed] [Google Scholar]
- 2. Petersen C., Soder O. (2006) The sertoli cell. A hormonal target and “super” nurse for germ cells that determines testicular size. Horm. Res. 66, 153–161 [DOI] [PubMed] [Google Scholar]
- 3. Loss E. S., Jacobus A. P., Wassermann G. F. (2007) Diverse FSH and testosterone signaling pathways in the Sertoli cell. Horm. Metab. Res. 39, 806–812 [DOI] [PubMed] [Google Scholar]
- 4. Walker W. H., Cheng J. (2005) FSH and testosterone signaling in Sertoli cells. Reproduction 130, 15–28 [DOI] [PubMed] [Google Scholar]
- 5. Toh Y., Nicolson G. L. (2009) The role of the MTA family and their encoded proteins in human cancers. Molecular functions and clinical implications. Clin. Exp. Metastasis 26, 215–227 [DOI] [PubMed] [Google Scholar]
- 6. Lu X., Kovalev G. I., Chang H., Kallin E., Knudsen G., Xia L., Mishra N., Ruiz P., Li E., Su L., Zhang Y. (2008) Inactivation of NuRD component Mta2 causes abnormal T cell activation and lupus-like autoimmune disease in mice. J. Biol. Chem. 283, 13825–13833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui Y., Niu A., Pestell R., Kumar R., Curran E. M., Liu Y., Fuqua S. A. (2006) Metastasis-associated protein 2 is a repressor of estrogen receptor α whose overexpression leads to estrogen-independent growth of human breast cancer cells. Mol. Endocrinol. 20, 2020–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma P., Lin S., Bartolomei M. S., Schultz R. M. (2010) Metastasis tumor antigen 2 (MTA2) is involved in proper imprinted expression of H19 and Peg3 during mouse preimplantation development. Biol. Reprod. 83, 1027–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manavathi B., Singh K., Kumar R. (2007) MTA family of coregulators in nuclear receptor biology and pathology. Nucl. Recept. Signal. 5, e010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou W., Dong Y., Zhang J., Yin Z., Wen H., Xiong L., Li W. (2012) Hypoxia-induced deacetylation is required for tetraploid differentiation in response to testicular ischemia-reperfusion (IR) injury. J. Androl., in press [DOI] [PubMed] [Google Scholar]
- 11. Fenic I., Hossain H. M., Sonnack V., Tchatalbachev S., Thierer F., Trapp J., Failing K., Edler K. S., Bergmann M., Jung M., Chakraborty T., Steger K. (2008) In vivo application of histone deacetylase inhibitor trichostatin-a impairs murine male meiosis. J. Androl. 29, 172–185 [DOI] [PubMed] [Google Scholar]
- 12. Kim D. H., Shim J. S., Kwon H. J. (2005) Coordinated transcriptional regulation of calmegin, a testis-specific molecular chaperon, by histone deacetylase and CpG methyltransferase. Exp. Mol. Med. 37, 492–496 [DOI] [PubMed] [Google Scholar]
- 13. O'Shaughnessy P. J., Morris I. D., Baker P. J. (2008) Leydig cell regeneration and expression of cell signaling molecules in the germ cell-free testis. Reproduction 135, 851–858 [DOI] [PubMed] [Google Scholar]
- 14. Chamkhia N., Sakly M., Rhouma K. B. (2006) Male reproductive impacts of styrene in rat. Toxicol. Ind. Health 22, 349–355 [DOI] [PubMed] [Google Scholar]
- 15. Chang Y. F., Lee-Chang J. S., Panneerdoss S., MacLean J. A., 2nd, Rao M. K. (2011) Isolation of Sertoli, Leydig, and spermatogenic cells from the mouse testis. BioTechniques 51, 341–342, 344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maguire S. M., Tribley W. A., Griswold M. D. (1997) Follicle-stimulating hormone (FSH) regulates the expression of FSH receptor messenger ribonucleic acid in cultured Sertoli cells and in hypophysectomized rat testis. Biol. Reprod. 56, 1106–1111 [DOI] [PubMed] [Google Scholar]
- 17. Li W., Wu Z. Q., Zhao J., Guo S. J., Li Z., Feng X., Ma L., Zhang J. S., Liu X. P., Zhang Y. Q. (2011) Transient protection from heat-stress induced apoptotic stimulation by metastasis-associated protein 1 in pachytene spermatocytes. PLoS One 6, e26013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heckert L. L., Daggett M. A., Chen J. (1998) Multiple promoter elements contribute to activity of the follicle-stimulating hormone receptor (FSHR) gene in testicular Sertoli cells. Mol. Endocrinol. 12, 1499–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foxley G. J., Dong Q., Handelsman D. J. (2001) Quantitative reverse transcriptase polymerase chain reaction assay for mouse androgen receptor mRNA. Endocrine 15, 193–198 [DOI] [PubMed] [Google Scholar]
- 20. Huang W., Sun L., Lü B., Wang W., Pu R., Chen L., Xia Y. (2004) Localization and in situ quantification of 5-hydroxytryptamine and its receptor in rat submaxillary gland. J. Mol. Histol. 35, 47–53 [DOI] [PubMed] [Google Scholar]
- 21. Manavathi B., Peng S., Rayala S. K., Talukder A. H., Wang M. H., Wang R. A., Balasenthil S., Agarwal N., Frishman L. J., Kumar R. (2007) Repression of Six3 by a corepressor regulates rhodopsin expression. Proc. Natl. Acad. Sci. U.S.A. 104, 13128–13133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hermann B. P., Hornbaker K., Rice D. A., Sawadogo M., Heckert L. L. (2008) In vivo regulation of follicle-stimulating hormone receptor by the transcription factors upstream stimulatory factor 1 and upstream stimulatory factor 2 is cell specific. Endocrinology 149, 5297–5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu X., Arumugam R., Zhang N., Lee M. M. (2010) Androgen profiles during pubertal Leydig cell development in mice. Reproduction 140, 113–121 [DOI] [PubMed] [Google Scholar]
- 24. Chuang C. K., Lee K. H., Fan C. T., Su Y. S. (2007) FSH-sensitive murine Sertoli cell lines immortalized by human telomerase gene hTERT express the androgen receptor in response to TNF-α stimulation. Biosci. Rep. 27, 403–411 [DOI] [PubMed] [Google Scholar]
- 25. Mendis S. H., Meachem S. J., Sarraj M. A., Loveland K. L. (2011) Activin A balances Sertoli and germ cell proliferation in the fetal mouse testis. Biol. Reprod. 84, 379–391 [DOI] [PubMed] [Google Scholar]
- 26. Griswold M. D., Kim J. S., Tribley W. A. (2001) Mechanisms involved in the homologous down-regulation of transcription of the follicle-stimulating hormone receptor gene in Sertoli cells. Mol. Cell. Endocrinol. 173, 95–107 [DOI] [PubMed] [Google Scholar]
- 27. Saito M., Kumamoto K., Robles A. I., Horikawa I., Furusato B., Okamura S., Goto A., Yamashita T., Nagashima M., Lee T. L., Baxendale V. J., Rennert O. M., Takenoshita S., Yokota J., Sesterhenn I. A., Trivers G. E., Hussain S. P., Harris C. C. (2010) Targeted disruption of Ing2 results in defective spermatogenesis and development of soft-tissue sarcomas. PLoS One 5, e15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lai A. Y., Wade P. A. (2011) Cancer biology and NuRD. A multifaceted chromatin remodelling complex. Nat. Rev. Cancer 11, 588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fenic I., Sonnack V., Failing K., Bergmann M., Steger K. (2004) In vivo effects of histone-deacetylase inhibitor trichostatin-A on murine spermatogenesis. J. Androl. 25, 811–818 [DOI] [PubMed] [Google Scholar]
- 30. Hazzouri M., Pivot-Pajot C., Faure A. K., Usson Y., Pelletier R., Sèle B., Khochbin S., Rousseaux S. (2000) Regulated hyperacetylation of core histones during mouse spermatogenesis. Involvement of histone deacetylases. Eur. J. Cell Biol. 79, 950–960 [DOI] [PubMed] [Google Scholar]
- 31. Yao Y. L., Yang W. M. (2003) The metastasis-associated proteins 1 and 2 form distinct protein complexes with histone deacetylase activity. J. Biol. Chem. 278, 42560–42568 [DOI] [PubMed] [Google Scholar]
- 32. Ma L., Li W., Zhu H. P., Li Z., Sun Z. J., Liu X. P., Zhao J., Zhang J. S., Zhang Y. Q. (2010) Localization and androgen regulation of metastasis-associated protein 1 in mouse epididymis. PLoS One 5, e15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yong E. L., Loy C. J., Sim K. S. (2003) Androgen receptor gene and male infertility. Hum. Reprod. Update 9, 1–7 [DOI] [PubMed] [Google Scholar]
- 34. Popov V. M., Wang C., Shirley L. A., Rosenberg A., Li S., Nevalainen M., Fu M., Pestell R. G. (2007) The functional significance of nuclear receptor acetylation. Steroids 72, 221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng J., Watkins S. C., Walker W. H. (2007) Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in Sertoli cells. Endocrinology 148, 2066–2074 [DOI] [PubMed] [Google Scholar]
- 36. George J. W., Dille E. A., Heckert L. L. (2011) Current concepts of follicle-stimulating hormone receptor gene regulation. Biol. Reprod. 84, 7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mazumdar A., Wang R. A., Mishra S. K., Adam L., Bagheri-Yarmand R., Mandal M., Vadlamudi R. K., Kumar R. (2001) Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat. Cell Biol. 3, 30–37 [DOI] [PubMed] [Google Scholar]
- 38. Devi Y. S., Sarda K., Stephen B., Nagarajan P., Majumdar S. S. (2006) Follicle-stimulating hormone-independent functions of primate Sertoli cells. Potential implications in the diagnosis and management of male infertility. J. Clin. Endocrinol. Metab. 91, 1062–1068 [DOI] [PubMed] [Google Scholar]
- 39. Faure A. K., Pivot-Pajot C., Kerjean A., Hazzouri M., Pelletier R., Péoc'h M., Sèle B., Khochbin S., Rousseaux S. (2003) Misregulation of histone acetylation in Sertoli cell-only syndrome and testicular cancer. Mol. Hum. Reprod. 9, 757–763 [DOI] [PubMed] [Google Scholar]
- 40. Lardone M. C., Parada-Bustamante A., Ebensperger M., Valdevenito R., Kakarieka E., Martínez D., Pommer R., Piottante A., Castro A. (2011) DAX-1 and DAX-1A expression in human testicular tissues with primary spermatogenic failure. Mol. Hum. Reprod. 17, 739–746 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.