Background: AKAP-Lbc is a scaffold protein that coordinates cardiac hypertrophic signaling.
Results: AKAP-Lbc interacts with Shp2, facilitating its regulation by PKA.
Conclusion: AKAP-Lbc integrates PKA and Shp2 signaling in the heart. Under pathological hypertrophic conditions Shp2 is phosphorylated by PKA, and phosphatase activity is inhibited.
Significance: Inhibition of Shp2 activity through AKAP-Lbc-anchored PKA is a previously unrecognized mechanism that may promote pathological cardiac hypertrophy.
Keywords: AKAP, Cardiac Hypertrophy, Protein Kinase A (PKA), Protein Phosphatase, Protein Phosphorylation, Scaffold Proteins, Signal Transduction
Abstract
Pathological cardiac hypertrophy (an increase in cardiac mass resulting from stress-induced cardiac myocyte growth) is a major factor underlying heart failure. Our results identify a novel mechanism of Shp2 inhibition that may promote cardiac hypertrophy. We demonstrate that the tyrosine phosphatase, Shp2, is a component of the A-kinase-anchoring protein (AKAP)-Lbc complex. AKAP-Lbc facilitates PKA phosphorylation of Shp2, which inhibits its protein-tyrosine phosphatase activity. Given the important cardiac roles of both AKAP-Lbc and Shp2, we investigated the AKAP-Lbc-Shp2 interaction in the heart. AKAP-Lbc-tethered PKA is implicated in cardiac hypertrophic signaling; however, mechanism of PKA action is unknown. Mutations resulting in loss of Shp2 catalytic activity are also associated with cardiac hypertrophy and congenital heart defects. Our data indicate that AKAP-Lbc integrates PKA and Shp2 signaling in the heart and that AKAP-Lbc-associated Shp2 activity is reduced in hypertrophic hearts in response to chronic β-adrenergic stimulation and PKA activation. Thus, while induction of cardiac hypertrophy is a multifaceted process, inhibition of Shp2 activity through AKAP-Lbc-anchored PKA is a previously unrecognized mechanism that may promote compensatory cardiac hypertrophy.
Introduction
Organization of the bewildering array of cell signaling proteins into coherent networks is facilitated by scaffold proteins (1). A-kinase-anchoring proteins (AKAPs)3 are a diverse family of scaffold proteins that form multiprotein complexes functioning to integrate cAMP signaling with other pathways (2–4). All members of the AKAP family possess a conserved PKA-anchoring domain as well as binding sites for other signaling components (5, 6). For example, to ensure that signaling is tightly regulated, AKAPs coordinate both signal activators (e.g. protein kinases) and signal terminators (e.g. protein phosphatases) (7–9). Here, we report an interaction of the protein-tyrosine phosphatase, Shp2 (PTPN11) with AKAP-Lbc (also termed AKAP13) and demonstrate that AKAP-Lbc integrates PKA and Shp2 signaling in the heart.
Localized regulation and integration of signal transduction are important for proper cardiac function, and perturbation of this leads to heart failure. We are now just beginning to understand the spatiotemporal aspects of AKAP-mediated signaling in the heart. Multiple cardiac AKAPs have been characterized and shown to perform a critical role in mediating the effects of neurohumoral stimulation on the heart by integrating PKA activity with additional enzymes (10–12).
A recent proteomic study suggests that differential expression of AKAPs coupled with alterations in the AKAP “interactome” may be critical factors in heart failure (13). Indeed, we have demonstrated previously that AKAP-Lbc expression is up-regulated under hypertrophic conditions in rats as well as in human heart failure samples, promoting cardiac hypertrophy through a protein kinase D1 (PKD1)-mediated mechanism (14).
Cardiac hypertrophy is initially a beneficial, compensatory process, decreasing wall stress and increasing cardiac output and stroke volume. However, prolonged hypertrophy is maladaptive, transitioning to decompensation and cardiac failure. Multiple pathological hypertrophic pathways converge on a set of transcriptional regulators, promoting initiation of a developmental gene-reprogramming paradigm (often termed the fetal gene response). These “fetal” cardiac genes encode proteins involved in contraction, calcium handling, and metabolism, and their activation accompanies cardiac hypertrophy (15, 16).
In addition to binding PKD1, AKAP-Lbc acts as Rho-guanine nucleotide exchange factor, implicated in cardiomyocyte hypertrophy (17), possibly through a p38α mitogen-activated protein kinase (MAPK) pathway (18). AKAP-Lbc also anchors protein kinase A (PKA) and protein kinase C (PKCα and PKCη isoforms) (19), and our previous data suggest a role for PKA in hypertrophic signaling (14), but downstream pathways are not clear.
Interestingly, Shp2 is also implicated in the modulation of myocyte size, cardiomyopathy, and heart failure (20–22). LEOPARD syndrome patients most commonly manifest hypertrophic cardiomyopathy due to mutations in the PTPN11 gene encoding Shp2 that generally results in impaired Shp2 catalytic activity (23, 24). Here, we observe similar results, showing diminished Shp2 activity associated with AKAP-Lbc in hypertrophic heart samples induced by chronic isoproterenol treatment to activate PKA. Mechanistically, our data suggest that AKAP-Lbc facilitates the phosphorylation of Shp2 by PKA, acting to inhibit Shp2 PTP activity, which may in turn promote cardiac hypertrophy.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Anti-V5-agarose, anti-FLAG-agarose and anti-FLAG antibody were from Sigma. Anti-V5 antibody (mouse, 1:5000) and purified recombinant PKD1 were from Invitrogen. Anti-Shp2 antibody (rabbit, 1:1000) was from Santa Cruz and was also kindly provided by Geng Sheng Feng (University of California San Diego) (rabbit, 1:20,000). Anti-phospho-PKA substrate (RRXS*/T*) antibody (1:1000) was from Cell Signaling Technology. Purified recombinant PKA was from Promega.
Bacterial and Mammalian Expression Constructs
cDNA for Shp2 expression was kindly provided by Geng-Sheng Feng. Bimolecular fluorescence complementation (BiFC) plasmids were originally from Chang-Deng Hu (Purdue University). Full-length AKAP-Lbc was expressed as a RFP and V5-tagged protein in pcDNA3.1, or as FLAG-AKAP-Lbc in pEGFP-N1. pEGFP-N1-AKAP-Lbc-ΔPKA expresses a mutant version of AKAP-Lbc that cannot bind the PKA-RII regulatory subunit (25).
Bacterial Expression
GST-AKAP-Lbc fusion proteins were produced in BL21(DE3)pLys as described previously (19).
Transfections, Co-immunoprecipitations, and Pulldowns
HEK293 cells were transfected and lysed as described previously (19). For phosphorylation experiments, the phosphatase inhibitor microcystin-LR was included (100 nm). Lysates were incubated on ice for 10 min and centrifuged at 20,000 × g for 15 min at 4 °C. Cleared lysates were incubated with antibodies for 1 h at 4 °C with rocking, followed by precipitation of antibody-antigen complexes with protein A/G-agarose. Immunoprecipitates were washed 5 × 1 ml in lysis buffer, eluted in SDS-PAGE sample buffer, and separated by SDS-PAGE. GST pulldowns were performed similarly, except that protein complexes were isolated by incubation with glutathione-Sepharose for 1 h at 4 °C. For endogenous protein co-immunoprecipitations (co-IPs) from heart, frozen mouse hearts were homogenized by Polytron in 20 mm HEPES, 150 mm NaCl, 5 mm EDTA, 1% Triton X-100, 0.5%, and protease inhibitors. The heart extract was clarified by centrifugation at 20,000 × g for 20 min and then used for IP.
Mass Spectrometry
Proteomics and informatics services were provided by the CBC-UIC Research Resources Center Mass spectrometry, Metabolomics and Proteomics Facility, established in part by a grant from The Searle Funds at the Chicago Community Trust to the Chicago Biomedical Consortium.
BiFC
BiFC expression constructs consisting of the N terminus or C terminus of Venus (VN and VC, respectively) were originally provided by Chang-Deng Hu. AKAP-Lbc was cloned into the VN vector with the Venus fragment fused to the N terminus. Shp2 was cloned into the VC vector with the Venus fragment at the C terminus. Cells were transiently transfected and then imaged ∼20 h after transfection so that the proteins were not highly overexpressed. CFP was expressed along with the BiFC constructs, as a marker for transfected cells.
Confocal Microscopy
Confocal images were acquired using a Carl Zeiss LSM 510 mounted on an Axiovert 100 M microscope. Images were obtained using a 514-nm argon laser for YFP and 458 nm for CFP with a Plan-Apochromat 63×/1.4 oil immersion objective lens. A 531–595-nm wavelength bandpass filter was used for YFP emission, and a 470–500-nm wavelength bandpass filter was used for CFP, with a pinhole of 0.7 Airy units, which provides a z resolution of ∼0.6 μm.
In Vitro PTP Activity Assay
Following immunoprecipitation of either AKAP-Lbc or Shp2, immune complexes were washed five times with IP buffer (10 mm sodium phosphate buffer, pH 6.95, 150 mm NaCl, 5 mm EDTA, 5 mm EGTA, 1% Triton X-100) before being resuspended in phosphatase assay buffer (50 mm HEPES, 100 mm NaCl, 5 mm DTT, 2 mm Na2EDTA, 0.01% Brij-35, pH 7.5). The phosphatase assay was carried out in a total reaction volume of 50 μl using 30 μm fluorescein diphosphate as substrate. After a 20-min incubation at 30 °C, supernatant was transferred to a 96-well plate, and phosphatase activity was measured using a PHERAstar FS microplate reader, with excitation at 485 nm and emission at 520 nm.
For calibration of PTP activity using this assay, T cell PTP (New England Biolabs) was serially diluted and used for assay as described above. Fluorescence intensity was measured for known amounts of enzyme ranging from 0 to 500 milliunits of specific activity. One unit is defined as the amount of enzyme that hydrolyzes 1 nmol of p-nitrophenyl phosphate (50 mm) in 1 min at 30 °C in a total reaction volume of 50 μl.
In Vitro Shp2 Phosphorylation
Immunoprecipitated Shp2 was phosphorylated in vitro in kinase assay buffer (25 mm Tris, pH 7.5, 0.1 mm EGTA, 0.1 mm Na3VO4, 0.03% Brij-35, 10 mm MgAc2, 100 mm ATP, 1 mCi of [γ-32P]ATP) supplemented with bacterially purified recombinant PKA C-subunit (0.2 mg), or recombinant PKD1 (0.2 mg), for 20 min at 30 °C. Reactions were terminated by washing twice with fresh kinase buffer prior to resuspension in Laemmli sample buffer or PTP activity assay.
Chronic Infusion of Isoproterenol and Measurement of Cardiac Hypertrophy in Mice
16-week-old, male FVB mice received continuous subcutaneous administration of isoproterenol (25 μg/g per day for 30 days) or saline control via minipump (Alzet) implantation. Hearts were assessed before and after treatment by M-mode echocardiography using a VisualSonics Vevo 770 ultrasound instrument.
Histology
Hearts were removed from mice, and a small section of the left ventricle was fixed in paraformaldehyde and embedded in paraffin. Paraffin-embedded sections were stained with hematoxylin and eosin.
Statistical Analysis of Data
All data are expressed as means ± S.E. Differences in quantitative variables were examined by one-way analysis of variance (ANOVA) or an unpaired two-tailed t test. A p value < 0.05 was considered significant (*), a p value < 0.01 was considered very significant (**), and a p value < 0.001 was considered extremely significant (***). All analyses were performed using InStat.
RESULTS
Shp2 Is a Novel Component of the AKAP-Lbc Complex
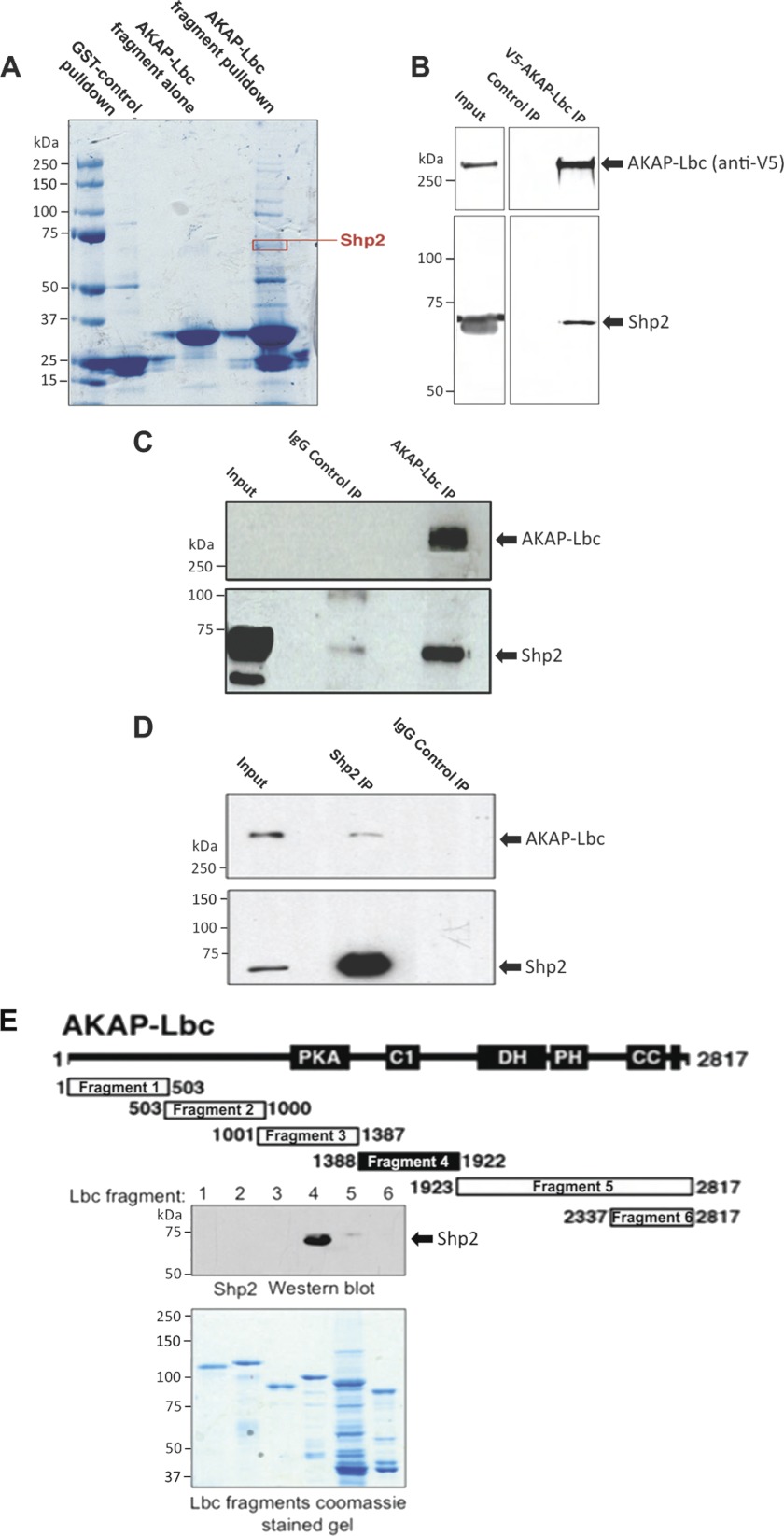
The AKAP-Lbc signaling complex is composed of multiple protein kinases (18, 26); therefore, we wondered whether AKAP-Lbc may also bind protein phosphatases. To identify novel binding partners, we performed multiple proteomic screens using GST-tagged AKAP-Lbc fragments to purify associated proteins, which were identified by tandem MS. This approach identified the tyrosine phosphatase Shp2 as an AKAP-Lbc associated protein (Fig. 1A). The AKAP-Lbc-Shp2 interaction was validated in HEK293 cells by co-precipitation of endogenous Shp2 with AKAP-Lbc (Fig. 1B). In addition, endogenous Shp2 was co-purified with endogenous AKAP-Lbc from heart extract and vice versa (Fig. 1, C and D). Next, we performed mapping studies to define the Shp2 site of interaction with AKAP-Lbc. Binding of Shp2 to a family of immobilized GST-AKAP-Lbc fragments detected an interaction with a central portion of AKAP-Lbc (residues 1388–1922; Fig. 1E).
FIGURE 1.
Shp2 interacts with AKAP-Lbc. A, identification of Shp2 co-purifying with AKAP-Lbc by mass spectrometry. Heart extract was used for GST-AKAP-Lbc fragment (amino acid residues 1756–1801) pulldowns (2 mg of heart extract per pulldown). The resulting material was eluted from the GST-agarose and concentrated. Proteins were resolved by SDS-PAGE and Coomassie staining and identified by tandem MS. B, co-IP of endogenous Shp2 with V5-AKAP-Lbc. HEK293 cells were transfected for expression of V5-tagged AKAP-Lbc. AKAP-Lbc was immunoprecipitated with anti-V5-agarose from cell lysates. Parallel control IPs were performed using an equal amount of lysate where V5-AKAP-Lbc was not expressed (Control IP). IPs were washed, and the bound proteins were separated by SDS-PAGE and transferred to nitrocellulose. Detection of Shp2 and AKAP-Lbc was carried out by immunoblotting. C, co-IP of endogenous Shp2 with endogenous AKAP-Lbc in heart. AKAP-Lbc was immunoprecipitated from mouse heart extract (5 mg of total protein). Parallel control IgG IPs were carried out using an equal amount of heart extract. IPs were washed, and the bound proteins were separated by SDS-PAGE and transferred to nitrocellulose. Detection of AKAP-Lbc and co-purifying Shp2 were carried out by immunoblotting. D, co-IP of endogenous AKAP-Lbc with endogenous Shp2 in heart. Shp2 was immunoprecipitated from mouse heart extract, and detection of AKAP-Lbc and co-purifying Shp2 were carried out by immunoblotting (as described for C). E, mapping of Shp2 binding to AKAP-Lbc indicating that Shp2 binds to amino acid residues 1388–1922 of AKAP-Lbc. Diagram shows GST-AKAP-Lbc fragments used for pulldown experiments. Shaded area indicates Shp2 binding fragment as determined by immunoblot detection of Shp2 co-purifying with AKAP-Lbc fragments by GST pulldown from heart extract. Coomassie-stained gel indicates equal expression of the AKAP-Lbc fragments that were used for GST pulldown.
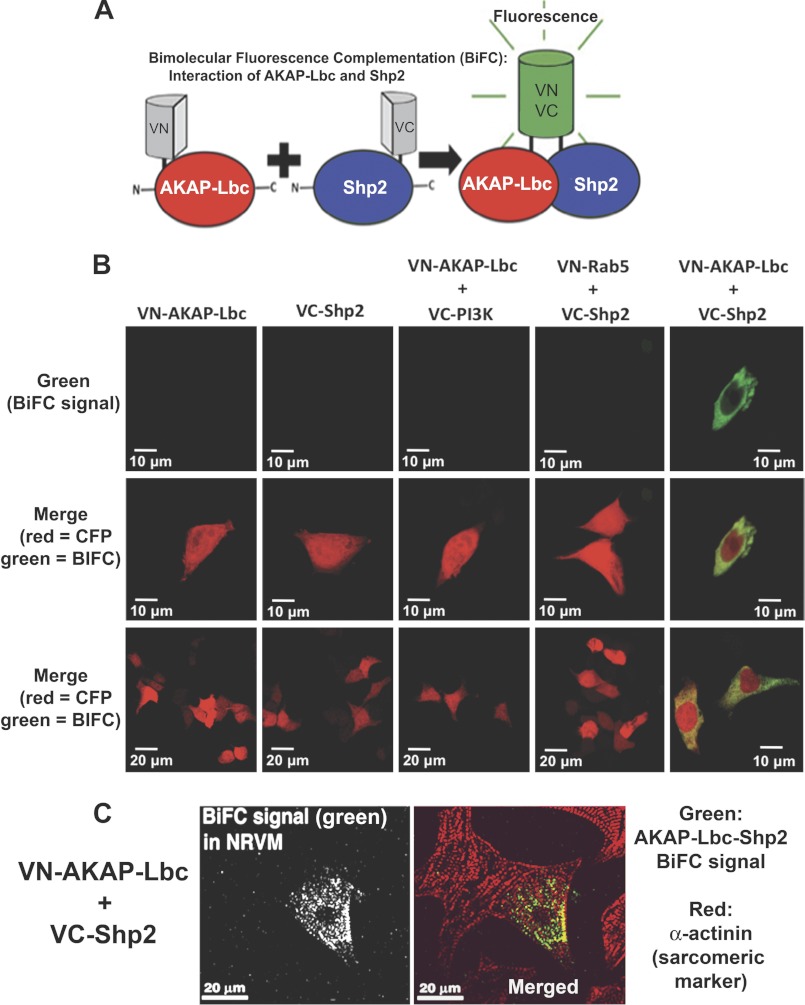
To visualize the interaction of AKAP-Lbc and Shp2 inside cells, we used BiFC. This technique has several advantages over more traditional immunostaining or fluorescent protein localization. In particular, the BiFC method enables us to specifically visualize the Shp2-AKAP-Lbc interaction at potentially low levels of protein expression, without background (i.e. fluorescence is observed only for Shp2-AKAP-Lbc complex formation) (27, 28). A nonfluorescent fragment (VN) from a split Venus fluorescent protein was fused to AKAP-Lbc (VN-AKAP-Lbc), and a nonfluorescent VC fragment was fused to Shp2 fragment (VC-Shp2). Interaction of AKAP-Lbc and Shp2 brings the two nonfluorescent fragments into close proximity, thereby reconstituting a functional fluorescent protein, resulting in fluorescence. Results in Fig. 2 show that Shp2 specifically interacts with AKAP-Lbc in the cytoplasm of HEK293 cells (Fig. 2B). In Fig. 2C (merged image), yellow indicates overlap of AKAP-Lbc-Shp2 complex BiFC signal (green) with α-actinin (red), suggesting that a portion of the AKAP-Lbc-Shp2 complex has a sarcomeric and sarcolemmal localization in cardiac myocytes. We did not observe significant differences in AKAP-Lbc-Shp2 complex formation and localization by BiFC in myocytes under basal and agonist treatment. No fluorescence was observed when VN-AKAP-Lbc was expressed alone or with a noninteracting control VC-protein (VC-PI3K). Similarly, no fluorescence was observed with expression of VC-Shp2 alone or with a control VN-protein (VN-Rab5). Western blot analysis of protein expression confirmed that all proteins were similarly expressed and were not degraded. Collectively, our biochemical studies and imaging data demonstrate that Shp2 is a component of the AKAP-Lbc signaling complex.
FIGURE 2.
Visualization of AKAP-Lbc-Shp2 interaction inside cells by BiFC. A, schematic diagram illustrates the BiFC assay. The Venus (green) fluorescent protein is split into two nonfluorescent halves which are fused to AKAP-Lbc and Shp2. Specific protein-protein interaction results in a functional Venus (green) fluorescent protein. B, HEK293 cells were co-transfected with VN-AKAP-Lbc and VC-Shp2 and CFP (pseudo-colored red), as a marker for transfected cells. Cells were fixed 20 h after transfection and imaged. No BiFC (green) fluorescence is observed in cells expressing either VN-AKAP-Lbc or VC-Shp2 alone or when VN-AKAP-Lbc is co-expressed with a noninteracting control VC-protein (VC-PI3K). Similarly, no BiFC (green) fluorescence is observed when VC-Shp2 is co-expressed with a control VN-protein (VN-Rab5), whereas specific interaction of VC-Shp2 with VN-AKAP-Lbc results in fluorescence. Equal protein expression was determined by Western blotting (data not shown). C, neonatal rat ventricular cardiac myocytes were electroporated for expression of VN-AKAP-Lbc and VC-Shp2. After 48-h treatment with phenylephrine (10 μm), cells were fixed, permeabilized, and immunostained for the sarcomeric marker protein α-actinin (red in merged image). BiFC signal is shown in green in the merged image.
PTP Activity Is Associated with AKAP-Lbc
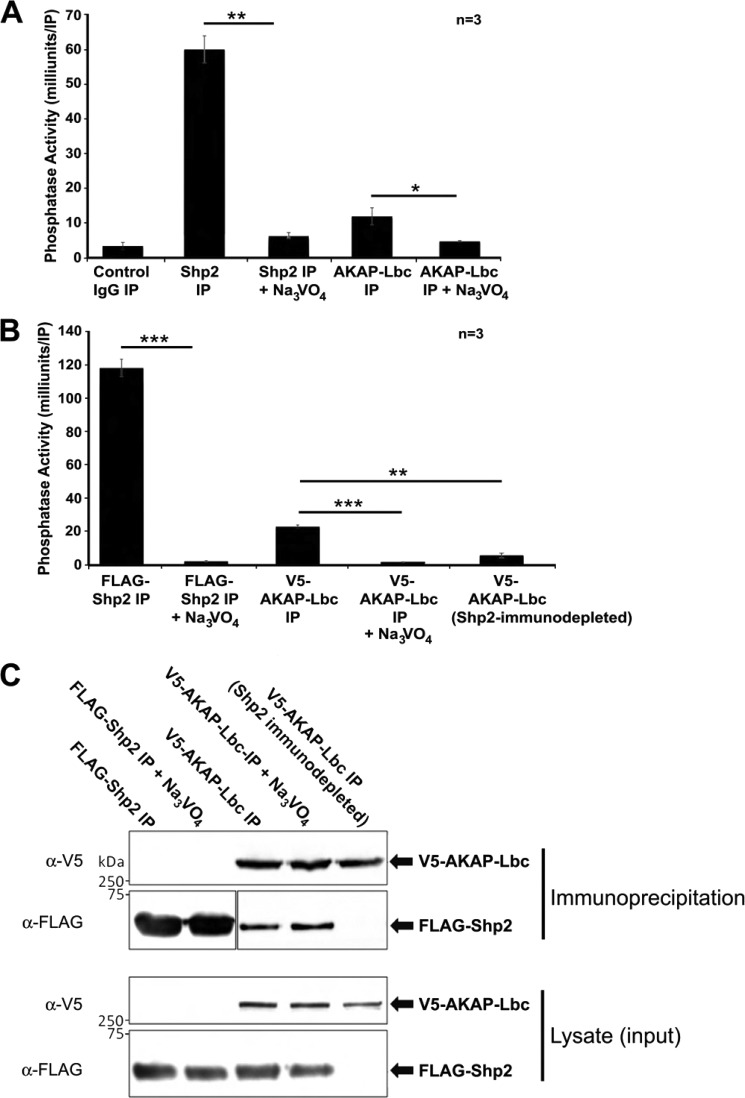
Immunoprecipitation of endogenous AKAP-Lbc from mouse heart extract followed by in vitro PTP activity assay demonstrates that tyrosine phosphatase activity co-purifies with AKAP-Lbc (Fig. 3A). Shp2 IPs were used as a positive control in these experiments, and sodium orthovanadate was used to inhibit tyrosine phosphatase activity, demonstrating that we are specifically measuring PTP activity. Additionally, our results show that PTP activity is associated with V5-tagged AKAP-Lbc, expressed in HEK293 cells (Fig. 3B). Using two different antibodies in these experiments (anti-AKAP-Lbc and anti-V5) gives us confidence that the phosphatase activity measured is specifically associated with AKAP-Lbc. Importantly, we also carried out measurements of AKAP-Lbc-associated PTP activity from samples where Shp2 had been previously immunodepleted. Results presented in Fig. 3B show a dramatic reduction of PTP activity associated with AKAP-Lbc, suggesting that we are measuring Shp2 activity in the AKAP-Lbc complex. Western blots indicating corresponding levels of AKAP-Lbc and Shp2 from samples used in these assays are shown in Fig. 3C.
FIGURE 3.
Measurement of PTP activity co-purifying with AKAP-Lbc. A, measurement of PTP activity co-purifying with AKAP-Lbc from mouse heart. AKAP-Lbc complexes were isolated from mouse heart extract using anti-AKAP-Lbc antibody. Immunoprecipitates were washed, and PTP activity was measured by a fluorometric in vitro assay using fluorescein diphosphate as substrate. Sodium orthovanadate (100 μm working concentration) was added to inhibit PTP activity, confirming that released phosphate was due to phosphatase activity and not proteolytic activity. Parallel negative control IgG IP assays and positive control Shp2 IP assays were also were carried out. Results indicate mean PTP activity per IP ± S.E. (error bars). All assays were performed in triplicate for three independent experiments. B, measurement of PTP activity co-purifying with AKAP-Lbc expressed in HEK293 cells. V5-AKAP-Lbc complexes were isolated from HEK293 cells using anti-V5-agarose. Immunoprecipitates from cell lysates (2 mg of total protein) were washed, and PTP activity was measured as described above. As a control, PTP activity co-purifying with AKAP-Lbc expressed in HEK293 cells previously immunodepleted for Shp2 was measured. Results presented show mean PTP activity per IP ± S.E. after control IgG IP background activity has been subtracted. All assays were performed in triplicate for three independent experiments. Differences in quantitative variables were examined by ANOVA. p = 0.05 is considered significant (*), p = 0.01 is considered very significant (**), and p = 0.001 is considered extremely significant (***). C, Western blots showing corresponding levels of AKAP-Lbc and Shp2 in samples used for PTP activity measurement in Fig. B.
Shp2 Is a PKA Substrate
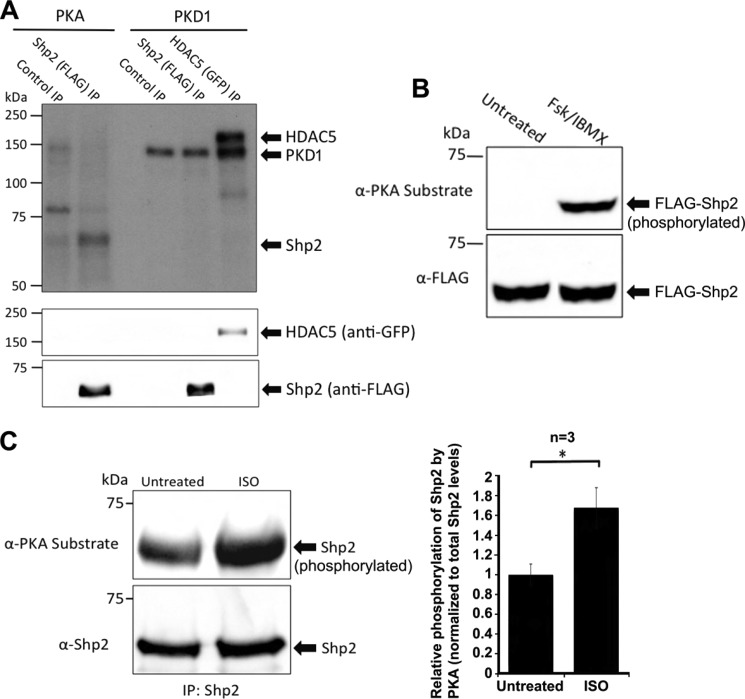
Our results demonstrate that Shp2 is a component of the AKAP-Lbc complex; however, the function and regulation of Shp2 in the AKAP-Lbc complex are unclear. To determine whether Shp2 is a substrate for either PKA and/or PKD1, we performed in vitro phosphorylation assays using [γ-32P]MgATP with immunopurified Shp2 and purified recombinant PKA and PKD1 (Fig. 4A). Following SDS-PAGE and transfer to nitrocellulose, autoradiography results demonstrate that Shp2 is phosphorylated by PKA but not by PKD1. PKD1 autophosphorylation as well as a positive control using HDAC5 (a well characterized PKD1 substrate) indicates that the PKD1 was active in this assay (Fig. 4A, top panel). Western blotting for Shp2 confirmed that equivalent levels of Shp2 were present in both the PKA and PKD1 reaction (Fig. 4A, bottom panel).
FIGURE 4.
Shp2 is a PKA, but not a PKD1 substrate. A, PKA, but not PKD1, phosphorylates Shp2 in vitro. Immunoprecipitated FLAG-tagged Shp2 from HEK293 cells was phosphorylated in vitro in kinase assay buffer supplemented with [γ-32P]ATP and bacterially purified recombinant PKA catalytic subunit (0.2 mg), or recombinant PKD1 (0.2 mg). Reactions were for 20 min at 30 °C and were terminated by washing twice with fresh kinase buffer prior to resuspension in Laemmli sample buffer. Incorporation of phosphate was determined by autoradiography following SDS-PAGE and transfer to nitrocellulose. GFP-HDAC5 (a well characterized PKD1 substrate) was used as a positive control in this experiment. Additionally, autophosphorylation of PKD1 is observed, indicating that PKD1 was active in this experiment. Immunoblotting confirmed similar levels of Shp2 in both the PKA and PKD1 phosphorylation reactions. A control FLAG-IP was also carried out using cell lysate where FLAG-Shp2 was not expressed. B, activation of PKA promotes Shp2 phosphorylation in HEK293 cells. Cells were treated with either DMSO (untreated) or forskolin (20 μm) and IBMX (75 μm) for 20 min to activate PKA. Western blotting using an anti-PKA-phosphosubstrate antibody was carried out following immunoprecipitation of FLAG-Shp2, SDS-PAGE, and transfer to nitrocellulose. The immunoblot was stripped and then probed for total levels of Shp2 with anti-FLAG antibody. C, activation of PKA promotes Shp2 phosphorylation in cardiac myocytes. Neonatal rat ventricular myocytes were treated with either DMSO (untreated) or isoproterenol (10 μm isoproterenol for 20 min) to activate PKA. Western blotting using an anti-PKA-phosphosubstrate antibody was carried out following immunoprecipitation of endogenous Shp2, SDS-PAGE, and transfer to nitrocellulose. The immunoblot was stripped and then probed for total levels of Shp2 with anti-Shp2 antibody. The relative increase in Shp2 phosphorylation by PKA in response to isoproterenol was quantified over three independent experiments. Error bars, indicate S.E. A p value of 0.05 is considered significant (*).
To determine whether PKA phosphorylates Shp2 in vivo, we expressed FLAG-Shp2 in HEK293 cells and then treated these cells with forskolin and IBMX to activate PKA, prior to lysis. Following immunoprecipitation of Shp2, SDS-PAGE and transfer to nitrocellulose, we performed Western blotting using an anti-PKA-phosphosubstrate antibody. This antibody recognizes PKA substrate phosphorylation with the consensus sequence R-R-X-pS/pT. Results in Fig. 4B show that Shp2 phosphorylation is barely detected under basal conditions, whereas Shp2 is phosphorylated in response to PKA activation. Similarly, Shp2 is phosphorylated by PKA in cardiac myocytes in response to isoproterenol stimulation (Fig. 4C). Overall, these results indicate that Shp2 is a PKA substrate.
PKA Phosphorylation of Shp2 Inhibits Its PTP Activity in the AKAP-Lbc Complex
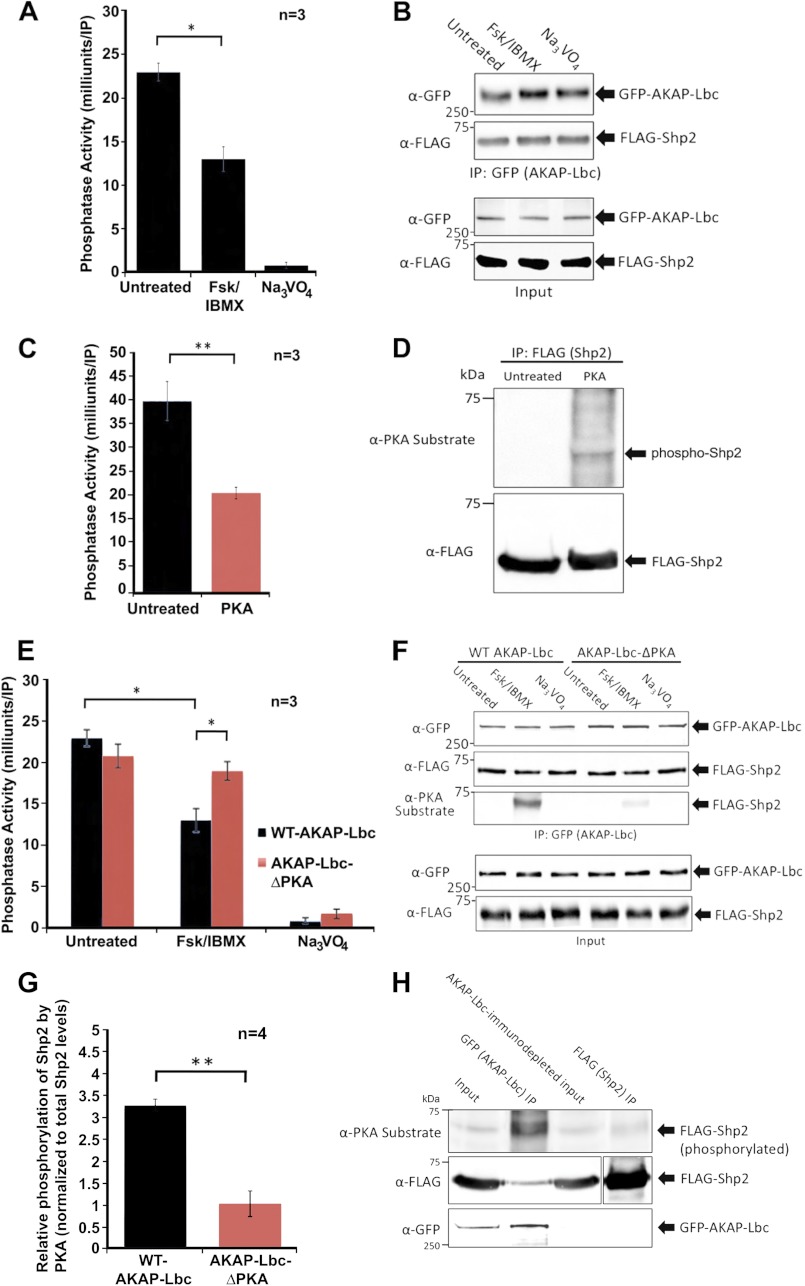
Next, we determined whether PKA activation and subsequent Shp2 phosphorylation modulate Shp2 activity. HEK293 cells were untreated or treated with forskolin/IBMX for 20 min prior to lysis, AKAP-Lbc immunoprecipitation, and PTP assay. Activation of PKA reduces AKAP-Lbc-associated PTP activity, suggesting that PKA inhibits Shp2 activity in the AKAP-Lbc complex (Fig. 5A). The levels of AKAP-Lbc and associated Shp2 in these assays were determined by Western blotting, indicating similar levels of AKAP-Lbc and co-immunoprecipitating Shp2 in all conditions (Fig. 5B). Together, these results indicate that PKA does not affect the association of Shp2 with AKAP-Lbc, but acts to inhibit Shp2 catalytic activity in the AKAP-Lbc complex.
FIGURE 5.
Shp2 activity in the AKAP-Lbc complex is inhibited by PKA. A, PKA activation promotes a decrease in Shp2 activity in the AKAP-Lbc complex. HEK293 cells transfected for expression of AKAP-Lbc and FLAG-Shp2 were treated for 20 min with either DMSO (untreated), or with forskolin (20 μm) and IBMX (75 μm) to activate PKA. Subsequent AKAP-Lbc immunoprecipitations were used for in vitro measurement of PTP activity. All assays were performed in triplicate for three independent experiments. Sodium orthovanadate was used in all assays to determine that PTP activity was being measured. B, Western blot loading controls demonstrate even expression of AKAP-Lbc and Shp2. To confirm even levels of AKAP-Lbc and co-immunoprecipitating Shp2 under all conditions, each IP was equally divided into four tubes; three tubes were used for PTP assay, and the fourth was used for SDS-PAGE and Western blotting, probed with anti-GFP antibodies for AKAP-Lbc detection and anti-FLAG antibodies for Shp2 detection. C, in vitro phosphorylation of Shp2 by PKA inhibits Shp2 activity. Immunoprecipitated Shp2 was phosphorylated in vitro in kinase assay buffer supplemented with bacterially purified recombinant PKA C-subunit (0.2 mg) for 20 min at 30 °C. Parallel control reactions were performed where no PKA was added. Reactions were terminated by washing twice with fresh kinase buffer prior to PTP activity assay. All assays were performed in triplicate for three independent experiments. D, Western blot loading controls confirm phosphorylation of Shp2 by PKA and that equal amounts of Shp2 were present in all assay conditions. E, AKAP-Lbc-anchored PKA promotes a decrease in Shp2 activity. HEK293 cells were transfected for the expression of wild-type (WT)-AKAP-Lbc and FLAG-Shp2 or AKAP-Lbc-ΔPKA (a mutant that cannot bind PKA) and FLAG-Shp2. Prior to lysis, cells were treated for 20 min with either DMSO (untreated), or with forskolin/IBMX to activate PKA. AKAP-Lbc was immunoprecipitated, and immune complexes were used for in vitro measurement of PTP activity. All assays were performed in triplicate for three independent experiments. Sodium orthovanadate was used in all assays to confirm that PTP activity was being measured. F, Western blot loading controls demonstrate comparable expression of AKAP-Lbc-WT, AKAP-Lbc-ΔPKA, and associated Shp2 for all assays; however, PKA phosphorylation of Shp2 (assessed using the anti-PKA-phosphosubstrate antibody) is reduced in cells expressing AKAP-Lbc-ΔPKA compared with AKAP-Lbc-WT. G, Shp2 phosphorylation by AKAP-Lbc-anchored PKA is quantified using ImageJ software. H, Shp2 not associated with AKAP-Lbc is minimally phosphorylated by PKA, in contrast to Shp2 in the AKAP-Lbc complex. Prior to lysis, HEK293 cells expressing GFP-AKAP-Lbc and FLAG-Shp2 were treated for 20 min with forskolin/IBMX to activate PKA. AKAP-Lbc was immunoprecipitated, and subsequent AKAP-Lbc-immunodepleted lysate was used for immunoprecipitation of Shp2. Immune complexes were subjected to SDS-PAGE and transfer to nitrocellulose. Immunoblotting was performed with anti-PKA-phosphosubstrate antibody to determine the extent of Shp2 phosphorylation. Membrane was stripped and re-probed with anti-FLAG and anti-GFP antibodies to confirm the levels of Shp2 and AKAP-Lbc in each experimental condition. A shorter exposure is shown for total FLAG-Shp2 in the FLAG (Shp2) IP, compared with the other lanes (indicated by separation of the two exposures), due to relative high levels of total FLAG-Shp2 immunoprecipitated. Error bars, indicate S.E. p = 0.05 is considered significant (*), and p = 0.01 is considered very significant (**).
For direct evidence that PKA phosphorylation of Shp2 inhibits Shp2 catalytic activity we phosphorylated Shp2 in vitro with purified PKA and then performed a PTP activity assay. Results indicate that Shp2 is phosphorylated (Fig. 5D) and displays reduced PTP activity compared with untreated Shp2 (Fig. 5C).
Next, we tested whether AKAP-Lbc facilitates PKA phosphorylation of Shp2 by anchoring PKA. We used a mutant form of AKAP-Lbc that cannot bind PKA, termed AKAP-Lbc-ΔPKA. The AKAP-Lbc-ΔPKA mutant has two amino acid substitutions (A1251P/I1260P) in the PKA-RII binding region that disrupt the amphipathic helix structure required for hydrophobic binding to the regulatory subunit of PKA, thereby disrupting PKA association (29). HEK293 cells expressing wild-type AKAP-Lbc or the AKAP-Lbc-ΔPKA mutant were untreated or treated with forskolin/IBMX for 20 min prior to lysis, AKAP-Lbc immunoprecipitation, and PTP assay. Activation of PKA significantly reduces wild-type AKAP-Lbc (WT-AKAP-Lbc)-associated PTP activity, but not PTP activity associated with the AKAP-Lbc-ΔPKA mutant (Fig. 5E). Western blot analysis demonstrates equal expression of both WT-AKAP-Lbc and AKAP-Lbc-ΔPKA and equal co-immunoprecipitation of Shp2 (Fig. 5F). Using the PKA-phosphosubstrate antibody we examined the extent of Shp2 phosphorylation in Shp2 associated with either WT-AKAP-Lbc or the AKAP-Lbc-ΔPKA mutant. Results show that PKA phosphorylation of Shp2 associated with AKAP-Lbc is barely detectable under basal conditions. In response to forskolin/IBMX treatment, Shp2 phosphorylation is significantly diminished when Shp2 is co-expressed with the AKAP-Lbc-ΔPKA mutant compared with the WT-AKAP-Lbc complex (Fig. 5, F and G). By carrying out an additional experiment, where we precleared AKAP-Lbc (and associated Shp2) from cell lysate prior to Shp2 immunoprecipitation, our results demonstrate that the Shp2 not associated with AKAP-Lbc is minimally phosphorylated by PKA, in contrast to the Shp2 that is associated with AKAP-Lbc (Fig. 5H). Collectively, these data suggest that AKAP-Lbc plays an important role in the regulation of Shp2 activity by facilitating phosphorylation of Shp2 by PKA. In summary, phosphorylation of Shp2 by AKAP-Lbc-anchored PKA inhibits Shp2 activity and does not affect the association of Shp2 with AKAP-Lbc.
Shp2 PTP Activity Is Depressed in Cardiac Hypertrophy Induced by Isoproterenol
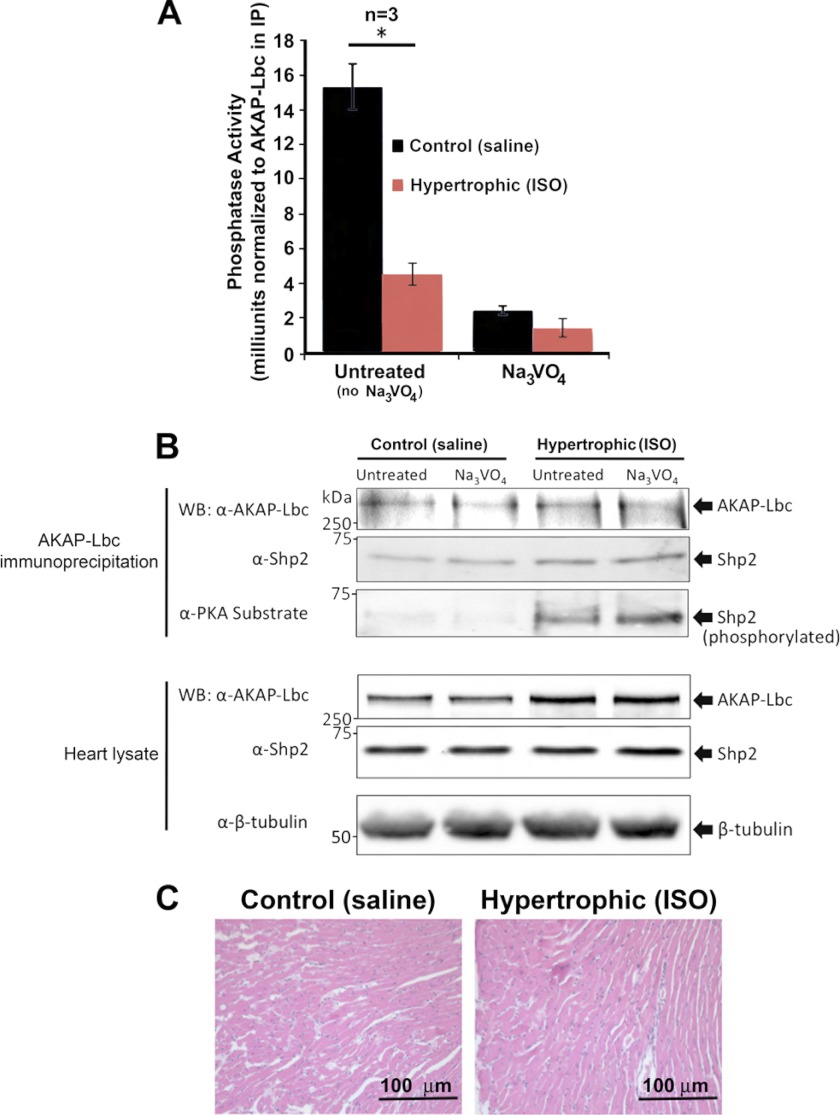
Both AKAP-Lbc and Shp2 are implicated in pathological cardiac hypertrophy and heart failure. We have previously demonstrated that AKAP-Lbc-anchored PKA plays a role in the induction of hypertrophy (14), and recent publications demonstrate that Shp2 loss of function or knock-out in mice results in hypertrophic cardiomyopathy (30–32). Therefore, we hypothesized that inhibition of Shp2 activity in the AKAP-Lbc complex by PKA may be a previously unrecognized factor in the induction of cardiac hypertrophy. To test this hypothesis, we induced cardiac hypertrophy in mice by chronic infusion of isoproterenol (25 μg/g per day for 30 days) using a minipump (33). Heart morphology and function were assessed immediately by echocardiography prior to minipump implantation and after 30 days. Additionally, a small section of the left ventricle was sectioned and stained with hematoxylin. Results indicate that isoproterenol treatment significantly induced cardiac hypertrophy compared with control animals (infused with saline). Using these samples we observed that PTP activity associated with AKAP-Lbc is significantly decreased in hypertrophic heart compared with control heart (Fig. 6A and Table 1). Consistent with previous reports (14, 17), we observed a 1.4 ± 0.1-fold increase in AKAP-Lbc expression in hypertrophic hearts; therefore, PTP activity was normalized to AKAP-Lbc expression for all IP assays. No change in Shp2 expression levels under hypertrophic conditions was observed. As in previous experiments, the levels of Shp2 co-immunoprecipitating with AKAP-Lbc were consistent in hypertrophic and control conditions, and importantly, Shp2 is phosphorylated by PKA under isoproterenol-induced hypertrophic conditions (Fig. 6B). Overall, these results suggest that chronic activation of PKA in the heart promotes the inhibition of Shp2 activity associated with AKAP-Lbc. This is a previously unrecognized mechanism that may contribute to the induction of cardiac hypertrophy (depicted in Fig. 7).
FIGURE 6.
Shp2 activity is decreased in isoproterenol-induced hypertrophic hearts. A, AKAP-Lbc complexes were isolated from healthy (Control; saline-treated) and hypertrophic (ISO-treated) mouse heart extract by immunoprecipitation using anti-AKAP-Lbc antibody. Immunoprecipitates were washed, and PTP activity was measured as described previously. Results presented show PTP activity ± S.E. measured in triplicate from three hypertrophic hearts and three age-matched control healthy hearts. Control IgG IP background activity has been subtracted, and the PTP activity was normalized to AKAP-Lbc expression in the immunoprecipitation. Untreated refers to no Na3VO4. B, Western blotting of AKAP-Lbc and associated Shp2 levels in the IPs used for PTP assay. Bottom panels show levels of AKAP-Lbc and Shp2 in heart lysate used for IP. C, histological analysis of mouse hearts corresponding to samples used for PTP assay. Hematoxylin/Eosin staining of left ventricle sections indicates isoproterenol-induced hypertrophic myocytes (Table 1). Echocardiography characteristics indicate ventricular dilation and hypertrophy.
TABLE 1.
Echocardiographic data of mice receiving saline solution or isoproterenol (ISO) for 30 days
HR, heart rate; LV FS, left ventricular fractional shortening; EF, ejection fraction; LV ESV, left ventricular end-systolic volume; LV EDV, left ventricular end-diastolic volume; LV mass, left ventricular mass; LVAWd, left ventricular anterior wall thickness during diastole; LVPWd, left ventricular posterior wall thickness during diastole.
| Echocardiographic parameters | Before saline (n = 3) | Control (saline) (n = 3) | Before ISO (n = 3) | Hypertrophic (ISO) (n = 3) |
|---|---|---|---|---|
| HR, bpm | 525 ± 12 | 512 ± 15 | 530 ± 10 | 595 ± 22a |
| LV FS, % | 31.71 ± 2.87 | 38.24 ± 4.77 | 35.46 ± 4.39 | 57.42 ± 3.38a |
| EF, % | 59.76 ± 4.02 | 67.82 ± 5.93 | 63.93 ± 5.56 | 87.56 ± 2.33a |
| LV ESV, μl | 61.81 ± 3.67 | 77.86 ± 5.08 | 55.19 ± 5.46 | 7.25 ± 1.48a |
| LV EDV, μl | 24.79 ± 2.87 | 25.10 ± 2.92 | 24.73 ± 3.31 | 57.45 ± 5.2a |
| LV mass, mg | 117.50 ± 11.95 | 125.26 ± 4.41 | 117.50 ± 11.95 | 177.30 ± 8.52a |
| LVAWd, mm | 0.87 ± 0.09 | 0.87 ± 0.06 | 0.97 ± 0.04 | 1.24 ± 0.08a |
| LVPWd, mm | 0.82 ± 0.05 | 0.80 ± 40.05 | 0.77 ± 0.04 | 1.11 ± 0.12a |
a p < 0.05 vs. all other groups.
FIGURE 7.
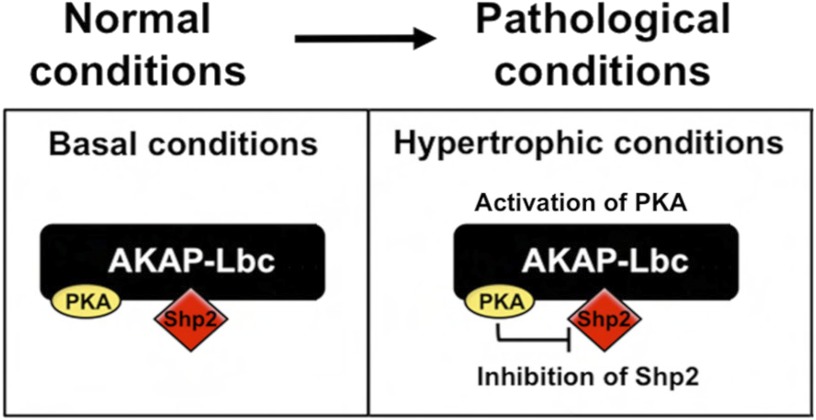
Model showing the role of AKAP-Lbc in the regulation of Shp2 activity by PKA. AKAP-Lbc assembles a signaling complex composed of PKA and Shp2 in cardiac myocytes. Stress conditions (e.g. chronic activation of the β-adrenergic receptor) lead to PKA activation, thereby promoting inhibition of Shp2 activity, which may contribute to the induction of cardiac hypertrophy.
DISCUSSION
In this report we identify the tyrosine phosphatase Shp2 as a previously unappreciated component of the AKAP-Lbc signaling complex. Our mapping experiments show that Shp2 binds predominantly to a central region of AKAP-Lbc encompassing amino acid residues 1388–1923. We show that Shp2 is a PKA substrate and that PKA phosphorylation of Shp2 inhibits its PTP activity. Furthermore, by using a mutant form of AKAP-Lbc that is unable to bind PKA, we demonstrate that AKAP-Lbc-anchored PKA modulates Shp2 phosphorylation and activity in the AKAP-Lbc complex.
Because AKAP-Lbc also scaffolds and promotes the activation of PKD1, we examined whether PKD1 could phosphorylate Shp2. Our results demonstrate that although PKA and PKD1 are basophilic protein kinases with similar substrate specificity that can phosphorylate shared cardiac substrates, PKD1 does not phosphorylate Shp2. These results indicate that inhibition of Shp2 is specific to PKA signaling and does not occur downstream of PKD. We are currently mapping the site(s) of PKA phosphorylation in Shp2. By performing in vitro PKA phosphorylation reactions using fragments of Shp2, our current data indicate multiple sites of phosphorylation. Using point mutations we are investigating effects on phosphatase activity to pinpoint critical amino acid residues.
Given the important cardiac roles of both AKAP-Lbc and Shp2, we performed experiments to investigate the AKAP-Lbc-Shp2 interaction in the heart. Several lines of evidence suggest that AKAP-Lbc interacts with Shp2 in the heart, which may be important for regulation of cardiac function. First, we demonstrate the co-IP of endogenous Shp2 with endogenous AKAP-Lbc from heart extract. Second, we are able to visualize this interaction using a BiFC approach, indicating a cytoplasmic Shp2-AKAP-Lbc interaction, with possible localization at the plasma membrane and association with the cytoskeleton. Third, our in vitro PTP activity assay data indicate that Shp2-PTP activity is associated with endogenous AKAP-Lbc in the heart.
Mutations resulting in loss of Shp2 catalytic activity are associated with congenital heart defects and cardiac hypertrophy (23, 24). AKAP-Lbc is also implicated in cardiac hypertrophic signaling, and through knockdown/rescue experiments we previously showed that AKAP-Lbc-tethered PKA is important for the induction of cardiac myocyte hypertrophy (14); however, the mechanism of PKA action is unknown. To induce PKA activation and cardiac hypertrophy in vivo, mice were subjected to chronic β-adrenergic stimulation through isoproterenol infusion. Under these hypertrophic conditions, our results indicate that AKAP-Lbc-associated Shp2 activity is reduced. Thus, while induction of hypertrophy is a multifaceted process, inhibition of Shp2 activity through enhanced PKA signaling in response to chronic β-adrenergic stimulation may be a previously unrecognized mechanism promoting compensatory cardiac hypertrophy. Importantly, our Shp2 activity data using a form of AKAP-Lbc that is unable to bind PKA (AKAP-Lbc-ΔPKA) supports a model where AKAP-Lbc facilitates the phosphorylation and inhibition of Shp2 through PKA (Fig. 5). Interestingly, we and others have previously observed up-regulation of AKAP-Lbc expression under hypertrophic conditions (14, 17). We speculate that this may facilitate the integration of PKA and Shp2 signaling in the heart. Striated muscle- and cardiac-specific Shp2-null mice show cardiac defects due to decreased ERK1/2 activation and increased Rho, Akt, and mTOR activation (30, 34, 35); therefore, we plan to examine the effects of Shp2 inhibition by PKA on these downstream pathways.
Experiments presented here were performed using a model of compensated hypertrophy, and from our echocardiography data it is clear that the hearts are not in the decompensatory phase leading to heart failure. Therefore it will be of interest to determine Shp2 activity at different stages progressing to heart failure.
This work was supported by American Heart Association Grant 11SDG5230003 (to G.K.C.) and National Center for Advancing Translational Science-University of Illinois at Chicago Center for Clinical and Translational Sciences Grant UL1TR000050.
- AKAP
- A-kinase-anchoring protein
- BiFC
- bimolecular fluorescence complementation
- CFP
- cyan fluorescent protein
- DMSO
- dimethyl sulfoxide
- IBMX
- isobutylmethylxanthine
- IP
- immunoprecipitation
- PTP
- protein-tyrosine phosphatase
- Shp2
- Src homology domain-containing phosphatase 2
- VC
- C terminus of Venus
- VN
- N terminus of Venus.
REFERENCES
- 1. Shi F., Lemmon M. A. (2011) Biochemistry: KSR plays CRAF-ty. Science 332, 1043–1044 [DOI] [PubMed] [Google Scholar]
- 2. Carnegie G. K., Means C. K., Scott J. D. (2009) A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life 61, 394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong W., Scott J. D. (2004) AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 5, 959–970 [DOI] [PubMed] [Google Scholar]
- 4. Beene D. L., Scott J. D. (2007) A-kinase anchoring proteins take shape. Curr. Opin. Cell Biol. 19, 192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newlon M. G., Roy M., Morikis D., Carr D. W., Westphal R., Scott J. D., Jennings P. A. (2001) A novel mechanism of PKA anchoring revealed by solution structures of anchoring complexes. EMBO J. 20, 1651–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pidoux G., Taskén K. (2010) Specificity and spatial dynamics of protein kinase A signaling organized by A-kinase-anchoring proteins. J. Mol. Endocrinol. 44, 271–284 [DOI] [PubMed] [Google Scholar]
- 7. Schillace R. V., Scott J. D. (1999) Association of the type 1 protein phosphatase PP1 with the A-kinase anchoring protein AKAP220. Curr. Biol. 9, 321–324 [DOI] [PubMed] [Google Scholar]
- 8. Cardone L., Carlucci A., Affaitati A., Livigni A., DeCristofaro T., Garbi C., Varrone S., Ullrich A., Gottesman M. E., Avvedimento E. V., Feliciello A. (2004) Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of Src signaling. Mol. Cell. Biol. 24, 4613–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alto N., Carlisle Michel J. J., Dodge K. L., Langeberg L. K., Scott J. D. (2002) Intracellular targeting of protein kinases and phosphatases. Diabetes 51, S385–388 [DOI] [PubMed] [Google Scholar]
- 10. Mauban J. R., O'Donnell M., Warrier S., Manni S., Bond M. (2009) AKAP-scaffolding proteins and regulation of cardiac physiology. Physiology 24, 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diviani D., Dodge-Kafka K. L., Li J., Kapiloff M. S. (2011) A-kinase anchoring proteins: scaffolding proteins in the heart. Am. J. Physiol. Heart Circ. Physiol. 301, H1742–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carnegie G. K., Burmeister B. T. (2011) A-kinase anchoring proteins that regulate cardiac remodeling. J. Cardiovasc. Pharmacol. 58, 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aye T. T., Soni S., van Veen T. A., van der Heyden M. A., Cappadona S., Varro A., de Weger R. A., de Jonge N., Vos M. A., Heck A. J., Scholten A. (2012) Reorganized PKA-AKAP associations in the failing human heart. J. Mol. Cell. Cardiol. 52, 511–518 [DOI] [PubMed] [Google Scholar]
- 14. Carnegie G. K., Soughayer J., Smith F. D., Pedroja B. S., Zhang F., Diviani D., Bristow M. R., Kunkel M. T., Newton A. C., Langeberg L. K., Scott J. D. (2008) AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol. Cell 32, 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frey N., Olson E. N. (2003) Cardiac hypertrophy: the good, the bad, and the ugly. Annu. Rev. Physiol. 65, 45–79 [DOI] [PubMed] [Google Scholar]
- 16. Heineke J., Molkentin J. D. (2006) Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 7, 589–600 [DOI] [PubMed] [Google Scholar]
- 17. Appert-Collin A., Cotecchia S., Nenniger-Tosato M., Pedrazzini T., Diviani D. (2007) The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates α1-adrenergic receptor-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 104, 10140–10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cariolato L., Cavin S., Diviani D. (2011) A-kinase anchoring protein (AKAP)-Lbc anchors a PKN-based signaling complex involved in α1-adrenergic receptor-induced p38 activation. J. Biol. Chem. 286, 7925–7937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carnegie G. K., Smith F. D., McConnachie G., Langeberg L. K., Scott J. D. (2004) AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol. Cell 15, 889–899 [DOI] [PubMed] [Google Scholar]
- 20. Nakamura T., Colbert M., Krenz M., Molkentin J. D., Hahn H. S., Dorn G. W., 2nd, Robbins J. (2007) Mediating ERK1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. J. Clin. Invest. 117, 2123–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krenz M., Gulick J., Osinska H. E., Colbert M. C., Molkentin J. D., Robbins J. (2008) Role of ERK1/2 signaling in congenital valve malformations in Noonan syndrome. Proc. Natl. Acad. Sci. U.S.A. 105, 18930–18935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakamura T., Gulick J., Colbert M. C., Robbins J. (2009) Protein-tyrosine phosphatase activity in the neural crest is essential for normal heart and skull development. Proc. Natl. Acad. Sci. U.S.A. 106, 11270–11275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kontaridis M. I., Swanson K. D., David F. S., Barford D., Neel B. G. (2006) PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J. Biol. Chem. 281, 6785–6792 [DOI] [PubMed] [Google Scholar]
- 24. Stewart R. A., Sanda T., Widlund H. R., Zhu S., Swanson K. D., Hurley A. D., Bentires-Alj M., Fisher D. E., Kontaridis M. I., Look A. T., Neel B. G. (2010) Phosphatase-dependent and -independent functions of Shp2 in neural crest cells underlie LEOPARD syndrome pathogenesis. Dev. Cell 18, 750–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diviani D., Soderling J., Scott J. D. (2001) AKAP-Lbc anchors protein kinase A and nucleates Gα12-selective Rho-mediated stress fiber formation. J. Biol. Chem. 276, 44247–44257 [DOI] [PubMed] [Google Scholar]
- 26. Smith F. D., Langeberg L. K., Cellurale C., Pawson T., Morrison D. K., Davis R. J., Scott J. D. (2010) AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade. Nat. Cell Biol. 12, 1242–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerppola T. K. (2006) Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat. Protoc. 1, 1278–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shyu Y. J., Suarez C. D., Hu C. D. (2008) Visualization of ternary complexes in living cells by using a BiFC-based FRET assay. Nat. Protoc. 3, 1693–1702 [DOI] [PubMed] [Google Scholar]
- 29. Diviani D., Scott J. D. (2001) AKAP signaling complexes at the cytoskeleton. J. Cell Sci. 114, 1431–1437 [DOI] [PubMed] [Google Scholar]
- 30. Kontaridis M. I., Yang W., Bence K. K., Cullen D., Wang B., Bodyak N., Ke Q., Hinek A., Kang P. M., Liao R., Neel B. G. (2008) Deletion of Ptpn11 (Shp2) in cardiomyocytes causes dilated cardiomyopathy via effects on the extracellular signal-regulated kinase/mitogen-activated protein kinase and RhoA signaling pathways. Circulation 117, 1423–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ishida H., Kogaki S., Narita J., Ichimori H., Nawa N., Okada Y., Takahashi K., Ozono K. (2011) LEOPARD-type SHP2 mutant Gln510Glu attenuates cardiomyocyte differentiation and promotes cardiac hypertrophy via dysregulation of Akt/GSK-3β/β-catenin signaling. Am. J. Physiol. Heart Circ. Physiol. 301, H1531–1539 [DOI] [PubMed] [Google Scholar]
- 32. Marin T. M., Clemente C. F., Santos A. M., Picardi P. K., Pascoal V. D., Lopes-Cendes I., Saad M. J., Franchini K. G. (2008) Shp2 negatively regulates growth in cardiomyocytes by controlling focal adhesion kinase/Src and mTOR pathways. Circ. Res. 103, 813–824 [DOI] [PubMed] [Google Scholar]
- 33. Taglieri D. M., Monasky M. M., Knezevic I., Sheehan K. A., Lei M., Wang X., Chernoff J., Wolska B. M., Ke Y., Solaro R. J. (2011) Ablation of p21-activated kinase-1 in mice promotes isoproterenol-induced cardiac hypertrophy in association with activation of ERK1/2 and inhibition of protein phosphatase 2A. J. Mol. Cell. Cardiol. 51, 988–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Princen F., Bard E., Sheikh F., Zhang S. S., Wang J., Zago W. M., Wu D., Trelles R. D., Bailly-Maitre B., Kahn C. R., Chen Y., Reed J. C., Tong G. G., Mercola M., Chen J., Feng G. S. (2009) Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death. Mol. Cell. Biol. 29, 378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marin T. M., Keith K., Davies B., Conner D. A., Guha P., Kalaitzidis D., Wu X., Lauriol J., Wang B., Bauer M., Bronson R., Franchini K. G., Neel B. G., Kontaridis M. I. (2011) Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J. Clin. Invest. 121, 1026–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]